Abstract
Observed increases in the mineralization rate of labile organic carbon (LOC) in the presence of black carbon (BC) have led to speculation that corresponding decreases in non-pyrogenic (i.e. non-BC) soil organic carbon (npSOC) could significantly reduce or negate the C sequestration benefit of BC in soils. Here we show that the potential effect of an increased LOC decomposition rate on long-term npSOC stocks is negligible, even when using assumptions that would favour large losses, potentially causing no more than 3–4 % loss of npSOC over 100 years if 50 % of above-ground crop residues were converted to BC annually. Conversely, if the BC-stimulated enhanced stabilization of npSOC that has been observed in laboratory trials is extrapolated to the long-term, it would greatly increase soil carbon stocks by 30–60 %. Annual additions of BC derived from crop residues would increase total SOC (including both BC and npSOC) by an amount five times greater than the potential increase from enhanced stabilization and an order of magnitude greater than losses of npSOC caused by annual removals of biomass to provide BC feedstock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Black carbon (BC) is an important component of the global carbon cycle, its recalcitrance and ubiquity in soils making it a significant contributor to slow-cycling terrestrial carbon pools (Masiello 2004; Preston and Schmidt 2006), while lowering the susceptibility of soil organic carbon (SOC) to climate-change induced loss (Lehmann et al. 2008). However, observations of enhanced loss rates of boreal Pinus sylvestris forest litter in the presence of BC have given rise to speculation that the C sequestration potential of BC might be substantially reduced, or even negated, by induced losses of non-pyrogenic SOC (npSOC) (Wardle et al. 2008a, b; Lehmann and Sohi 2008).
Alteration of the turnover rates of npSOC pools by addition of a new substrate is referred to as ‘priming’, with increased or decreased turnover rates being defined as positive or negative priming, respectively. This terminology dates back at least to Bingeman et al. (1953) who investigated the effect of fresh plant matter additions on the decomposition of an organic soil. Since then, use of the term priming has most often been associated with the effect of more-labile substrate additions on turnover of less-labile or recalcitrant soil C and N pools, for example, the impact of mineral-N fertilizer on organic-N turnover in soils (Jenkinson et al. 1985), or the effect of highly bioavailable organic C in the form of glucose on more-stable SOC fractions (Dalenberg and Jager 1981). Decomposition of BC, which is generally recognized as being a highly recalcitrant fraction of SOC (Kuhlbusch and Crutzen 1995; Schmidt and Noack 2000), can also be primed by the addition of labile substrates (Hamer et al. 2004; Kuzyakov et al. 2009). In this paper, however, we are concerned instead with the reverse interaction, whereby BC may alter the turnover rates of less-recalcitrant SOC fractions. Interactions of this type have also been widely described as ‘priming’ in the recent literature (see, for example, Jones et al. 2011; Zimmerman et al. 2011, and also many of the references in the next paragraph), a terminology that we adopt here, defining priming in its broadest sense as any change (positive or negative, persistent or ephemeral) in the turnover rate of soil organic matter caused by the addition of a new substrate (whether it be labile or recalcitrant).
Observations of both positive and negative priming of npSOC by BC (Kuzyakov et al. 2009; Spokas and Reicosky 2009; Liang et al. 2010; Jones et al. 2011; Keith et al. 2011; Zimmerman et al. 2011) indicate that at least two distinct mechanisms occur in soils. Positive priming of npSOC can be caused by enhanced microbial respiration of labile organic carbon (LOC) in the presence of BC (Kuzyakov et al. 2009). Previous studies do not, however, show any evidence of increased respiration of stable npSOC in the presence of BC (Cross and Sohi 2011; Jones et al. 2011). On the contrary, the respiration rate of stable soil carbon can be suppressed (Jones et al. 2011), and the fraction of added LOC that is stabilized by complexation with soil minerals (Liang et al. 2010) and/or BC (Zimmerman et al. 2011) can be increased, giving rise to negative priming. The different time scales at which the processes of LOC decomposition and building of stable C stocks operate may contribute to observed positive priming during early stages of incubation studies, followed by reduced or negative priming later (Keith et al. 2011; Zimmerman et al. 2011).
Despite this growing interest in BC-induced priming, no-one has yet attempted to quantify the cumulative impact of priming on total carbon stocks in the long-term. The central aim of this study was to estimate, by modeling, the long-term (centennial) implications of the recent and rapidly expanding body of evidence regarding interactions between BC and non-BC organic carbon in mineral soils.
The largest accumulation of BC in soil, and hence the greatest impact on npSOC stocks due to BC, would occur if large concentrations of BC were intentionally added to soils (for example, for the purposes of C sequestration or soil improvement, in which case BC is typically referred to as ‘biochar’) (Lehmann 2007; Woolf et al. 2010). It is important for risk analysis to estimate the maximum induced loss of npSOC that might be incurred in such a system. Therefore, to ensure that our study investigated the upper range of the potential impact on SOC stocks by deliberate or natural BC additions to soil, we modeled these impacts in the context of a biochar system designed to ensure rapid accumulation of BC stocks through the utilization of residues from a high net-primary-production (NPP) crop (Zea mays L.) and the thermochemical conversion of this biomass to BC under process conditions engineered to ensure high yields of high-stability BC (see “BC production” section in “Methods” section).
In addition to priming interactions, the production of BC together with its addition to soils can influence npSOC stocks in three other ways: (1) by diverting plant-C, which would otherwise have been directly added to soil, into consumption by fire or biochar feedstock provision; (2) by altering (typically increasing) crop NPP in BC-amended soils; and (3) by organic products of BC decomposition entering npSOC pools. These feedbacks were predicted by coupling the SOC model to a crop-production model and to a BC-production and -decomposition model (see “Methods” section). Although considerable uncertainty remains in the magnitudes of priming, altered NPP, and BC-decomposition parameters, and in their variation with soil type, crop and climate, nonetheless sufficient data exist to estimate the ranges of their plausible values. The results reported here, therefore, investigate the long-term impact of BC on npSOC stocks under a range of assumptions representing the uncertainty in these parameters.
Methods
Overview
The effect of BC production and addition to soils on npSOC was calculated by a model comprised of three coupled sub-models:
-
(1)
SOC turnover
-
(2)
Phytomass production
-
(3)
BC production and decomposition.
The model was calculated at monthly iterations for 100 years. Although many input parameters to the model (such as temperature, rainfall, crop-type, planting and harvesting schedule, and harvest index [HI] etc.) could change over the course of a century in response to climatic/social/technological change, allowing all of these parameters to vary interannually during the simulation would obscure the temporal effect of BC alone on npSOC. Therefore, to elucidate those feedbacks directly attributable to BC, the simulation was run using the same monthly-climate and cropping-regime data for each year of the simulation. Only those parameters that varied in response to BC accumulation in soils (such as crop NPP and priming of npSOC turnover) were allowed to change over the course of the simulation.
In order to ensure that the range of our results included the high end of potential long-term effects of BC on npSOC (because we wish to know how large these effects might potentially be), we used a set of assumptions designed to bracket this maximum plausible impact on npSOC. This was achieved by testing the implications of (1) priming effects up to and including the largest that have been measured in any published short-term study; (2) assuming that priming effects persist long-term; and (3) assuming that BC stocks accumulate in soil at a high rate (because they are produced from the abundant residues from a high-NPP crop; the BC is produced in an engineered pyrolysis system that gives high yields of BC per unit biomass feedstock; and the BC is produced under controlled conditions which ensure that it decomposes only slowly).
SOC turnover model
The soil carbon turnover model was a version of the RothC version 26.3 soil C model (Coleman and Jenkinson 2008) modified such that the turnover rates of soil LOC and the fraction of LOC that is stabilized were altered by priming factors proportional to the concentration of BC in soil (measured in kg C m−2; note that wherever we discuss quantities of BC we always refer to the C content alone, whereas biochar refers to the complete substance that contains ash, H, O and other elements in addition to C). RothC defines four active soil carbon pools. Inputs of raw phytomass are partitioned between two relatively-labile C pools, “decomposable plant material” (DPM) and “resistant plant material” (RPM), with DPM decomposing more rapidly than RPM. Although no strict definition exists of how rapidly organic matter must decay before it is termed labile, in the context of the centennial time scale considered here, both DPM and RPM, being mineralized within a few years or less, can be considered as labile. Together DPM and RPM carbon pools are referred to here as labile added organic carbon (LAOC). Decomposing LAOC is partitioned between a biological soil C pool (BIO), a relatively-stable soil C pool (HUM) and mineralized CO2. BIO and HUM soil C pools also decompose in turn, with their decomposed C being partitioned between CO2, BIO and HUM. Decomposition rates and partition factors in RothC are calculated monthly, based on mean air temperature, precipitation, open-pan evaporation, soil texture (clay content) and soil cover (bare fallow or with plant cover). A detailed account of how the rates and partition factors are calculated in RothC is given in Coleman and Jenkinson (2008), with the description here limited to how these rates were modified to account for priming by BC.
Soil carbon in agricultural soils is typically not in a state of equilibrium, but rather may still be responding to historical changes in land-cover, land-management and climate (Bellamy et al. 2005; Smith et al. 2007). If the initial state of the modelled system were taken at a non-equilibrium point, then it would be impossible to discriminate between changes in soil C caused by ongoing response to historical factors and those caused by the perturbations being studied here. For this reason we adopted the standard practice of using a pseudo-equilibrium (calculated by running the RothC model with constant input parameters for a period of 1,000 years) as the initial state. The changes in npSOC (ΔnpSOC) and in total SOC (ΔtSOC) resulting from BC production and its addition to soil over 100 years were then calculated relative to this initial equilibrium value.
Few soil carbon models have, to-date, explicitly incorporated priming effects (Kuzyakov 2010), and to our knowledge only two such models have been experimentally verified (Blagodatsky et al. 2010; Neill 2011). A simple approach to modeling of priming that can readily be incorporated into existing first-order models (such as RothC) is to introduce a new rate-modifying factor (i.e. a coefficient by which decay rate constants are multiplied) that depends upon abundance of the substrate causing the priming (Kuzyakov 2010; Neill 2011). Accordingly, this is the approach that has been taken here. Positive priming of LOC decomposition by BC was modeled by increasing the RPM and DPM decomposition rate coefficients predicted by RothC (k r and k d , respectively; see Coleman and Jenkinson 2008 for a detailed account of how these rates are calculated in the unmodified RothC) by an amount proportional to the concentration of BC in the soil, according to
where P l is the the priming coefficient for LOC decomposition priming, and [BC] is the concentration of BC in the soil, measured in kg C m−2.
That is, it was assumed that, within the range of [BC] being considered, the priming effect was proportional to [BC], with the reaction rates defaulting to the unmodified k r and k d when [BC] = 0.
The range of P l was estimated from published measurements of LOC-mineralization priming by BC. Positive LOC-mineralization priming has been reported by Abiven and Andreoli (2010), Cross and Sohi (2011), Hamer et al. (2004), Jones et al. (2011), Keith et al. (2011), Liang et al. (2010), Luo et al. (2011), Novak et al. (2010), Spokas and Reicosky (2009), and Zimmerman et al. (2011). The greatest positive specific priming effect (i.e. priming per unit concentration of BC) on LOC decomposition that has been reported was a 135 % increase in LOC-mineralization rate at a BC concentration of 50 g kg−1 (Luo et al. 2011). This mass-fraction of BC in soil was converted to an application rate in kg m−2 by assuming a soil density of 1.3 Mg m−3 (the global mean topsoil bulk density; Batjes 1996), and BC addition to the top 0.15 m of the soil profile, yielding a value of P l = 13.8 % (kg BC m−2)−1. At the low end of the range of reported decomposition priming, in some cases no priming effect of BC on npSOC was found (Abiven and Andreoli 2010; Cross and Sohi 2011). Accordingly, results for LOC-mineralization priming are given for P l ranging from zero (no priming of LOC decomposition) to a maximum of 13.8 % (kg m−2)−1, i.e. P 1 = 6.9 ± 6.9 % (kg m−2)−1.
Negative priming of LOC-mineralization has been reported by Keith et al. (2011), Kuzyakov et al. (2009), Liang et al. (2010), Spokas and Reicosky (2009), and Zimmerman et al. (2011). Negative priming has been found to correspond with an increase in the fraction of LOC transferred to the stable organo-mineral-soil fraction (Liang et al. 2010; Cross and Sohi 2011). Therefore, negative priming of LAOC mineralization was modeled as an increase in the fraction of LAOC that is transferred to HUM rather than mineralized to CO2. We use the term “stabilization priming” to refer to negative priming resulting from this mechanism whereby the fraction of LAOC that becomes stabilized is increased. Following 1.5 years incubation, Liang et al. (2010) found a surplus of 3–8 % of added organic matter C in the organo-mineral fraction of BC-rich Anthrosols compared to adjacent lower-BC soils. Expressed relative to the excess BC in the Anthrosols versus the adjacent soils, this represented an increase of 3.6 ± 2.5 % (kg BC m−2)−1 in the fraction of LAOC transferred to HUM rather than mineralized to CO2.
In addition to the uncertainties in the priming parameters expressed above, the RothC model itself has an implicit uncertainty in its prediction of SOC values. The uncertainty in SOC stocks predicted by RothC has been estimated to be ±6.8–8.5 % when site-specific data on climate, soil, and NPP are available (Falloon and Smith 2003). The high end of this range (8.5 %) was assumed as the uncertainty in SOC change due to biomass residue additions or removals.
Phytomass production
The calculations presented here were based continuous maize (Zea mays L.) production. NPP was partitioned between above- and below-ground production by the root/shoot ratio. Above-ground production was partitioned between grain and residue by the HI, defined as dry mass of grain divided by total above-ground dry mass. Maize grain, stover and root biomass were all assumed to be 42 % C on a dry mass basis (ECN 2012).
The initial equilibrium npSOC pools were calculated by assuming that 100 % of non-grain crop residues were returned to the soil annually at harvest. It has been widely shown that sustainably maintaining soil structure and function, and preventing soil erosion requires that a substantial fraction of crop residues are returned to the soil annually (Lal 2005; Andrews 2006; Blanco-Canqui and Lal 2007, 2009; Wall 2008; Lafond et al. 2009). Therefore, the scenarios in which crop residues were used for production of BC or bioenergy assumed that 50 % of residues were removed annually, and 50 % of residues were returned to the soil immediately after harvest. It was assumed that all below-ground NPP and none of the grain were returned to the soil.
Biochar has been shown to have the potential to increase crop productivity, particularly in less fertile soils (Lehmann et al. 2003; Oguntunde and Ajayi 2004; Yamato et al. 2006; Rondon et al. 2007; Steiner et al. 2007; Kimetu et al. 2008; Major et al. 2010; Van Zwieten et al. 2010; Noguera et al. 2010; Vaccari et al. 2011). The effect of BC on NPP was modeled according to the method of Woolf et al. (2010), in which NPP response to BC was greatest on the least fertile soils and was zero on the most fertile soils, with a maximum response being reached at a BC application rate of 5 kg m−2. Soil fertility was graded according to the IIASA and FAO classification scheme (2000). The estimated uncertainty in the NPP response to BC additions was assumed to be ±50 % (Woolf et al. 2010).
The coupling between soil BC and NPP in the model creates a positive-feedback loop in which increasing soil-BC stocks give rise to increasing NPP, which in turn leads to increased crop-residue production and thus increased rates of BC production.
BC production and decomposition
Raw biomass is converted to BC by thermal decomposition in an oxygen-limited environment (pyrolysis), a process that can occur in wildfires when there is insufficient airflow for complete combustion, or in engineered pyrolysers where biomass is heated in a vessel that excludes air. Engineered pyrolysis processes are often classified into two major types, fast and slow, according to the rate at which the biomass is heated. Fast pyrolysis, with biomass residence times of up to a few seconds, generates more volatile products and less BC than slow-pyrolysis for which biomass residence times can range from tens of minutes to days. In order to investigate systems in which large stocks of BC are produced (for the reasons given in the overview above), slow-pyrolysis with its higher biochar yield was assumed as the conversion process here.
In addition to heating rate and residence time, the other most important process parameter influencing both yield and chemical composition of biochar is the maximum temperature during pyrolysis (Neves et al. 2011). Increasing temperature leads to reduced yields of biochar. Increasing temperature also enriches biochar in C, giving lower O:C and H:C ratios (Antal and Grønli 2003; Neves et al. 2011). The O:C ratio is, in turn, an indicator of the recalcitrance of the biochar in soils (Spokas 2010). Thus, BC produced at low temperatures is relatively unstable with a shorter half-life, compared to more-stable BC produced at higher temperatures. These opposing effects of pyrolysis temperature on biochar yield and stability approximately counteract each other, with the yield of fixed-carbon (from so-called proximate analysis, often assumed to indicate a relatively-stable fraction of BC) being nearly constant over the temperature range 400–800 °C (Antal and Grønli 2003). Here, pyrolysis yield was based on an assumed 500 °C pyrolysis temperature, at which temperature 37 % of maize-stover organic carbon was assumed to remain in BC following pyrolysis (Demirbas 2001).
It is evident from incubation studies in which decay rates are initially rapid but slow down over time (Kuzyakov et al. 2009; Smith et al. 2010) and from the long mean residence times of BC in some soils (Lehmann et al. 2008) that biochar consists of a mixture of compounds with different decay kinetics in soil. For simplicity, biochar was accordingly modeled as a two-phase material with a labile and a recalcitrant component, each following an exponential decay curve as expressed by the equation
where M bc is the mass of BC at time t, M 0 is the initial mass of BC, labile fraction, R is the recalcitrant fraction of BC, L is the labile fraction of BC, k l is the labile decay rate-constant, and k r is the recalcitrant decay rate-constant.
Achieving reliable estimates of biochar half-life in soils is subject to considerable methodological difficulties. Estimates derived from studies of historic BC in soils are confounded by the difficulty of distinguishing between mineralization and other loss processes such as leaching, combustion and erosion (Bird et al. 1999; Czimczik et al. 2003; Ohlson et al. 2009) and by uncertainty in the quantities of BC that were historically added to the soil (Cheng et al. 2008). Incubation studies, on the other hand, are limited to short timescales, and are unreliable indicators of behavior in soils in situ (Hammes et al. 2008; Lehmann et al. 2009). Published estimates of the half-life of BC in soils are highly variable, with estimates including <140 years (Bird et al. 1999), 9,500 years (Preston and Schmidt 2006), 130–380 years (Hammes et al. 2008), 930 years (Cheng et al. 2008), 6 years (Nguyen et al. 2008), several centuries to several millennia (Liang et al. 2008), 900–1,800 years (Lehmann et al. 2008), 1,400 years (Kuzyakov et al. 2009), 930–13,000 years for biochar produced at 525 °C (Zimmerman 2010), and 40–110,000 years depending on O:C ratio (Spokas 2010). The wide variance in these estimates may reflect differences in the importance and rates of the various loss mechanisms (including erosion and leaching that do not constitute a net loss from bio-hydrosphere) in different environments (Cheng et al. 2008) and also variations in the chemical and physical properties of different biochars (Preston and Schmidt 2006; Spokas 2010). Importantly, this variation may reflect differences in the degree of carbonization of the initial BC stock as BC includes materials on a spectrum from slightly to highly charred with a corresponding variation in recalcitrance (Baldock and Smernik 2002; Masiello 2004; Spokas 2010). All the studies cited above which suggested BC half-lives of <400 years (Hammes et al. 2008; Bird et al. 1999; Nguyen et al. 2008) referred to BC formed under uncontrolled conditions during wildfires without the ability to separately quantify physical losses (erosion, leaching), which is therefore likely to have contained considerable quantities of lightly charred (and thus labile) material that may have been moved in the landscape. This is supported by the fact that Nguyen et al. (2008) observed that initial mass-loss over the first 30 years was rapid after which BC concentration stabilized, implying both physical loss or a high labile content of the biochar and also a long half-life of its recalcitrant fraction. Those studies that considered BC formed under controlled conditions at >500 °C, on the other hand, all suggested a half-life of >900 years. Given the large uncertainty in the half-life of BC in soil, we performed a sensitivity analysis of the results using half-lives of the recalcitrant fraction of biochar in the range 500–50,000 years with a half-life of 1,000 years assumed for the baseline results.
Notwithstanding its overall longevity, a fraction of BC is typically mineralized rapidly. This labile fraction of BC falls significantly with increasing pyrolysis temperature (Bruun et al. 2011). Measurements of the labile fraction are typically based on short-term mineralization during incubation. Published measurements of labile-BC mineralization losses (for BC produced at close to 500 °C) include 1–3 % mass-loss over 1 year for biochars produced at 400 °C and 0.5–1.2 % mass-loss for 650 °C biochars (Zimmerman 2010); 7.5 % mass-loss over 115 days for wheat-straw fast-pyrolysis biochar produced at 500 °C (Bruun et al. 2011); and (8 ± 1.6)%, mass-loss over 3 years during incubation of biochars produced at 450–550 °C (Whitman 2011). The calculations here were based on an assumed 7.5 % labile fraction with a sensitivity conducted for the range 5–10 %. The half-life of the labile-BC fraction was assumed to be 3 years, comparable to the half-life of RPM in the RothC model. The assumed yield and decomposition rates of biochar are summarized in Table 1.
During decomposition, C lost from biochar is not entirely mineralized to CO2, but is also transferred to stable (Brodowski et al. 2007; Knicker 2007; Kramer et al. 2004) and biological (Kuzyakov et al. 2009) SOC pools. It was assumed that decomposition products of BC were partitioned between stable (HUM), biological (BIO) and CO2 products in the same proportions as defined for humus decomposition by RothC (Coleman and Jenkinson 2008).
Study regions
The three study locations, selected to represent a diversity of agroecological zones in which maize is grown for grain, were (A) South Nandi in the Western Kenyan highlands, (B) Cedar Falls, Black Hawk County, Iowa, USA, and (C) CIAT-CORPOICA research station, Carimagua, Colombia. The locations were characterized by the data given in Table 2.
Results
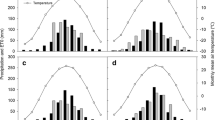
Figure 1 shows the predicted net change in npSOC and tSOC (which includes both npSOC and BC), for each of three study regions, over 100 years of removing 50 % of crop residues from the soil. In all three regions, removing crop residues without returning BC to the soil (as, for example, in bioenergy production) causes a gradual depletion of npSOC (Fig. 1a–c, orange-shaded confidence intervals), with npSOC being reduced by 21–28 % after 100 years. But if the removed plant residues were instead converted into biochar which was returned to the soil, the evolution of npSOC stocks would depend on the magnitude (and sign) of BC’s priming effects on npSOC and NPP. In the absence of any such interactions (i.e. if BC caused no priming of mineralization or stabilization and did not alter crop NPP) npSOC would decay in the same way as if residues were simply removed without BC being returned.
Predicted net change in npSOC and tSOC (including both BC and npSOC) over 100 years of annually removing 50 % of above-ground crop residues, with and without BC being returned to the soil. Results are shown for continuous maize production in each of three contrasting study regions (Colombian Savanna, West Kenyan highlands, and Black Hawk County, Iowa, USA). In each case, the mean change predicted by the model is shown together with both 68 and 95 % confidence intervals (CI) calculated by Monte Carlo analysis (n = 3,000) using the parameter ranges in Table 1. a–c Change in npSOC. Orange-shaded confidence intervals: no BC is produced and returned to the soil (or the equivalent case in which no interactions exist between BC and npSOC). Blue-shaded confidence intervals: change in npSOC when BC is added and causes priming effects on LOC decomposition and stabilization, and on plant NPP. d–f Change in tSOC including BC additions to the soil
However, when priming interactions between BC and npSOC, and effects of BC on NPP are considered, addition of biochar to the soil would reverse the initial decline of npSOC over time if accumulating stocks of BC in soil were to prime the stabilization of LOC, with npSOC showing a net increase over 100 years (Fig. 1a–c, blue-shaded confidence intervals). Thus, adding a fraction of plant-residue C to the soil as BC not only increases tSOC by 4–8 kg m−2 over 100 years (Fig. 1d–f), but may also increase npSOC.
It is evident from the analysis of contributions to the change in npSOC over 100 years that npSOC is highly insensitive to LOC-mineralization priming by BC (Fig. 2, orange bars), even in the worst-case scenario (when positive npSOC priming is as high as it plausibly might be), making this effect substantially irrelevant to the long-term stocks of npSOC. Organic products of BC decomposition also contribute <2 % to the change in npSOC (Fig. 2, purple bars). The largest predicted effect on npSOC was due to increased stabilization of added LOC (Fig. 2, blue bars), which increased npSOC by 35–60 %, more than enough to offset any losses due to residue extraction and positive priming. The contribution of BC-enhanced NPP (Fig. 2, green bars) was highly variable between study locations, with the greatest impact of BC on NPP being expected on the least fertile soil in Colombia (10–30 % increase in npSOC) and no improvement on yields being expected on the highly-fertile soil in Iowa.
Percentage change in npSOC after 100 years is broken down by contributions due to (a) annual removal of 50 % of above-ground crop residues to provide BC feedstock; (b) mineralization priming; (c) increased NPP due to improved fertility of BC-amended soils; (d) stabilization priming (stab. priming in the legend); and (e) organic products of BC decomposition. Error bars show the estimated uncertainty in these contributions
Discussion and conclusions
Given the paucity and variability of existing data on priming effects by BC, together with the challenges inherent in extrapolating from short-term laboratory incubations to long-term effects in a natural environment, some caution needs to be exercised in how these results should be interpreted. It is often the case with early conceptual-type modeling studies such as this that the most important conclusions to be drawn concern not the absolute values predicted by the model, which remain rather uncertain, but regard the sensitivities of the model to various parameters. Here, we have found long-term npSOC to be highly insensitive to positive priming of LOC-mineralization. More importantly, we have shown this to be the case in a system in which this effect is as large as it could reasonably be, because: (1) the assumed positive priming effect was as large as has been measured in any published short-term study, (2) the priming effect was assumed to persist long-term, and (3) BC stocks were assumed to accumulate in soil at an extremely high rate (because they were produced from the abundant residues from a high-NPP crop; the BC was produced in an engineered pyrolysis system that gives high yields of BC per unit biomass feedstock; and the BC was produced at a high enough temperature to ensure that it decomposed only slowly). We found that, even under these set of assumptions, LOC mineralisation priming had a negligible impact on long-term npSOC. If, in a scenario in which this effect is as large as it plausibly could be, the effect is still small, it is entirely reasonable to conclude that long-term npSOC stocks would be highly insensitive to BC-induced positive priming of LOC-mineralization as a general rule in mineral soils. Thus, the concern expressed by Wardle et al. (2008b) that positive priming of npSOC turnover may constitute a threat to the C sequestration potential of BC is unlikely to be realized in a mineral-soil environment. This somewhat counterintuitive result is readily explained by considering that, over the course of 100 years, the vast majority of LOC is either mineralized, or converted to a more-stable form by physical or chemical change, whether or not LOC-mineralization priming occurs.
Conversely, it is the fraction of LOC that is stabilized in organo-mineral assemblages which is of primary importance in determining long-term stocks of npSOC (as demonstrated by the high sensitivity of npSOC to negative priming by LOC stabilization shown in Fig. 2). The conceptual diagram shown in Fig. 3 is helpful to clarify how the interplay of increased LOC-mineralization rates (positive priming) and increased SOC stabilization (negative priming) could both coexist in the same soil-BC system and give rise to an overall effect in which early net positive priming gives way to net negative priming over time, in the manner observed by Zimmerman et al. (2011). Figure 3 also helps to clarify why it is the stabilization fraction rather than the LOC-mineralization rate that is most important in determining long-term npSOC stocks. The solid line (Fig. 3) shows cumulative mineralization as a fraction of the initial added LOC, in the absence of any priming effects. The dashed line shows cumulative mineralization of added plant-C with both positive LOC-priming and increased stabilization. In the early phase (shaded green), primed cumulative mineralization is higher than the baseline without priming, because the added LOC has been mineralized more rapidly. In the later phase (shaded blue), however, the amount of added plant-C that remains depends primarily on the fraction of added plant-C that has been stabilized. The available evidence suggests that, at least under some conditions, OC stabilization may be significantly increased by the incorporation of BC into mineral soil (Liang et al. 2010; Zimmerman et al. 2011), and that soils which have contained high concentrations of BC for long periods can show greatly increased levels of npSOC relative to adjacent soils without BC (Glaser et al. 2000). However, the underlying physical, chemical and biological processes by which C is stabilized in mineral soils and by which stable C is mineralized are still not well understood (Schmidt et al. 2011). It is clear from this modeling study that an improved understanding of the mechanisms underlying SOC stabilization should be a research priority in determining how incorporation of BC into soil would impact long-term npSOC levels.
Conceptual plot showing cumulative mineralization of initial added organic matter over time for a soil system in which positive priming of LOC-mineralization and an increase in the fraction of LOC that becomes stabilized both occur (dashed line), relative to a baseline system with no priming effects (solid line). This plot is purely conceptual and does not use real data, therefore no units are given on the time axis
References
Abiven S, Andreoli R (2010) Charcoal does not change the decomposition rate of mixed litters in a mineral cambisol: a controlled conditions study. Biol Fertil Soils. doi:10.1007/s00374-010-0489-1
Anderson EL (1988) Tillage and N fertilization effects on maize root growth and root:shoot ratio. Plant Soil 108:245–251. doi:10.1007/BF02375655
Andrews S (2006) Crop residue removal for biomass energy production: effects on soils and recommendations. US Department of Agriculture, Natural Resource Conservation Service
Antal M, Grønli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42:1619–1640. doi:10.1021/ie0207919
Baldock JA, Smernik RJ (2002) Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org Geochem 33:1093–1109. doi:10.1016/S0146-6380(02)00062-1
Basamba TA, Barrios E, Amézquita E et al (2006) Tillage effects on maize yield in a Colombian Savanna oxisol: soil organic matter and P fractions. Soil Tillage Res 91:131–142. doi:10.1016/j.still.2005.11.010
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163. doi:10.1111/j.1365-2389.1996.tb01386.x
Bellamy PH, Loveland PJ, Bradley RI et al (2005) Carbon losses from all soils across England and Wales 1978–2003. Nature 437:245–248. doi:10.1038/nature04038
Bingeman CW, Varner JE, Martin WP (1953) The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci Soc Am J 17:34–38
Bird MI, Moyo C, Veenendaal EM et al (1999) Stability of elemental carbon in a Savanna soil. Glob Biogeochem Cycles 13:923–932
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. doi:10.1016/j.soilbio.2010.04.005
Blanco-Canqui H, Lal R (2007) Soil and crop response to harvesting corn residues for biofuel production. Geoderma 141:355–362. doi:10.1016/j.geoderma.2007.06.012
Blanco-Canqui H, Lal R (2009) Corn stover removal for expanded uses reduces soil fertility and structural stability. Soil Sci Soc Am J 73:418–426. doi:10.2136/sssaj2008.0141
Brodowski S, Amelung W, Haumaier L, Zech W (2007) Black carbon contribution to stable humus in German arable soils. Geoderma 139:220–228. doi:10.1016/j.geoderma.2007.02.004
Bruun EW, Hauggaard-Nielsen H, Ibrahim N et al (2011) Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 35:1182–1189. doi:10.1016/j.biombioe.2010.12.008
Cheng C-H, Lehmann J, Thies JE, Burton SD (2008) Stability of black carbon in soils across a climatic gradient. J Geophys Res 113:G02027. doi:10.1029/2007JG000642
Coleman K, Jenkinson D (2008) ROTHC-26.3. Rothamsted Research, Harpenden, Herts
Cross A, Sohi SP (2011) The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol Biochem 43:2127–2134. doi:10.1016/j.soilbio.2011.06.016
Czimczik C, Preston C, Schmidt M, Schulz E (2003) How surface fire in Siberian Scots pine forests affects soil organic carbon in the forest floor: stocks, molecular structure, and conversion to black carbon (charcoal). Glob Biogeochem Cycles. doi:10.1029/2002GB001956
Dalenberg JW, Jager G (1981) Priming effect of small glucose additions to 14C-labelled soil. Soil Biol Biochem 13:219–223
Demirbas A (2001) Carbonization ranking of selected biomass for charcoal, liquid and gaseous products. Energy Convers Manag 42:1229–1238
ECN (2012) Phyllis, database for biomass and waste. Energy Research Centre of the Netherlands. http://ecn.nl/phyllis. Accessed 7 Dec 2011
Falloon P, Smith P (2003) Accounting for changes in soil carbon under the Kyoto Protocol: need for improved long-term data sets to reduce uncertainty in model projections. Soil Use Manag 19:265–269. doi:10.1111/j.1475-2743.2003.tb00313.x
FAO (2005) New Locclim climate database and interpolation software. FAO, Rome
Fischer R, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50:S-85. doi:10.2135/cropsci2009.10.0564
Glaser B, Balashov E, Haumaier L et al (2000) Black carbon in density fractions of anthropogenic soils of the Brazilian Amazon region. Org Geochem 31:669–678. doi:10.1016/S0146-6380(00)00044-9
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralisation. Org Geochem 35:823–830. doi:10.1016/j.orggeochem.2004.03.003
Hammes K, Torn MS, Lapenas AG, Schmidt MWI (2008) Centennial black carbon turnover observed in a Russian steppe soil. Biogeosciences 5:1339–1350
IIASA and FAO (2000) GAEZ global agro-ecological zones. http://www.iiasa.ac.at/Research/LUC/GAEZ/index.htm. Accessed 15 Dec 2011
ISRIC (1995) Soil and terrain database for Kenya (KENSOTER). http://www.isric.org/projects/soter-kenya-kensoter. Accessed 23 Nov 2011
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen—the so-called “priming” effect. J Soil Sci 36:425–444
Jones DL, Murphy DV, Khalid M et al (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731. doi:10.1016/j.soilbio.2011.04.018
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45:9611–9618. doi:10.1021/es202186j
Kimetu J, Lehmann J, Ngoze S et al (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726–739. doi:10.1007/s10021-008-9154-z
Knicker H (2007) How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 85:91–118. doi:10.1007/s10533-007-9104-4
Kramer RW, Kujawinski EB, Hatcher PG (2004) Identification of black carbon derived structures in a volcanic ash soil humic acid by Fourier transform ion cyclotron resonance mass spectrometry. Environ Sci Technol 38:3387–3395. doi:10.1021/es030124m
Kuhlbusch TAJ, Crutzen PJ (1995) Toward a global estimate of black carbon in residues of vegetation fires representing a sink of atmospheric CO2 and a source of O2. Glob Biogeochem Cycles 9:491–501. doi:10.1029/95GB02742
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. doi:10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Subbotina I, Chen H et al (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219. doi:10.1016/j.soilbio.2008.10.016
Lafond GP, Stumborg M, Lemke R et al (2009) Quantifying straw removal through baling and measuring the long-term impact on soil quality and wheat production. Agron J 101:529–537. doi:10.2134/agronj2008.0118x
Lal R (2005) World crop residues production and implications of its use as a biofuel. Environ Int 31:575–584. doi:10.1016/j.envint.2004.09.005
Lehmann J (2007) A handful of carbon. Nature 447:143–144. doi:10.1038/447143a
Lehmann J, Sohi SP (2008) Comment on “fire-derived charcoal causes loss of forest humus”. Science 321:1295c. doi:10.1126/science.1160005
Lehmann J, Pereira da Silva J, Steiner C et al (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357. doi:10.1023/A:1022833116184
Lehmann J, Skjemstad J, Sohi SP et al (2008) Australian climate-carbon cycle feedback reduced by soil black carbon. Nat Geosci 1:832–835. doi:10.1038/ngeo358
Lehmann J, Czimczik C, Laird D, Sohi SP (2009) Chapter 11: stability of biochar in soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan Ltd., London
Liang B, Lehmann J, Solomon D et al (2008) Stability of biomass-derived black carbon in soils. Geochim Cosmochim Acta 72:6078–6096. doi:10.1016/j.gca.2008.09.028
Liang B, Lehmann J, Sohi SP et al (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213. doi:10.1016/j.orggeochem.2009.09.007
Luo Y, Durenkamp M, De Nobili M et al (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314. doi:10.1016/j.soilbio.2011.07.020
Major J, Rondon M, Molina D et al (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian Savanna oxisol. Plant Soil 333:117–128. doi:10.1007/s11104-010-0327-0
Masiello CA (2004) New directions in black carbon organic geochemistry. Mar Chem 92:201–213. doi:10.1016/j.marchem.2004.06.043
Miller A, Fenton T, Oneal B et al (2010) Iowa soil properties and interpretations database (ISPAID) ver. 7.3. Iowa State University. http://www.extension.iastate.edu/soils/ispaid. Accessed 29 Nov 2011
Neill C (2011) Impacts of crop residue management on soil organic matter stocks: a modelling study. Ecol Model 222:2751–2760. doi:10.1016/j.ecolmodel.2011.04.029
Neves D, Thunman H, Matos A et al (2011) Characterization and prediction of biomass pyrolysis products. Prog Energy Combust Sci 37:611–630. doi:10.1016/j.pecs.2011.01.001
Nguyen B, Lehmann J, Kinyangi J et al (2008) Long-term black carbon dynamics in cultivated soil. Biogeochemistry 89:295–308. doi:10.1007/s10533-008-9220-9
Noguera D, Rondón M, Laossi K-R et al (2010) Contrasted effect of biochar and earthworms on rice growth and resource allocation in different soils. Soil Biol Biochem 42:1017–1027. doi:10.1016/j.soilbio.2010.03.001
Novak JM, Busscher WJ, Watts DW et al (2010) Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 154:281–288. doi:10.1016/j.geoderma.2009.10.014
Oguntunde PG, Fosu M, Ajayi AE, Giesen N (2004) Effects of charcoal production on maize yield, chemical properties and texture of soil. Biol Fertil Soil 39:295–299. doi:10.1007/s00374-003-0707-1
Ohlson M, Dahlberg B, Økland T et al (2009) The charcoal carbon pool in boreal forest soils. Nat Geosci 2:692–695. doi:10.1038/ngeo617
Preston CM, Schmidt MWI (2006) Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 3:397–420
Prince SD, Haskett J, Steininger M et al (2001) Net primary production of U.S. Midwest croplands from agricultural harvest yield data. Ecol Appl 11:1194–1205. doi:10.1890/1051-0761(2001)011[1194:NPPOUS]2.0.CO;2
Rondon M, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soil 43:699–708. doi:10.1007/s00374-006-0152-z
Schmidt MWI, Noack AG (2000) Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Glob Biogeochem Cycles 14:777–793
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. doi:10.1038/nature10386
Smith P, Chapman SJ, Scott WA et al (2007) Climate change cannot be entirely responsible for soil carbon loss observed in England and Wales, 1978–2003. Glob Change Biol 13:2605–2609. doi:10.1111/j.1365-2486.2007.01458.x
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347. doi:10.1016/j.soilbio.2010.09.013
Spokas K (2010) Review of the stability of biochar in soils: predictability of O:C molar ratios. Carbon Manag 1:289–303. doi:10.1016/j.carbpol.2010.10.007
Spokas KA, Reicosky DC (2009) Impacts of sixteen different biochars on soil greenhouse gas production. Ann Environ Sci 3:179–193
Steiner C, Teixeira WG, Lehmann J et al (2007) Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291:275–290. doi:10.1007/s11104-007-9193-9
Stumpe JM, Vlek PLG (1991) Acidification induced by different nitrogen sources in columns of selected tropical soils. Soil Sci Soc Am J 55:145–151
Vaccari FP, Baronti S, Lugato E et al (2011) Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur J Agron 34:231–238. doi:10.1016/j.eja.2011.01.006
Van Zwieten L, Kimber S, Morris S et al (2010) Effects of biochar from slow pyrolysis of paper mill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. doi:10.1007/s11104-009-0050-x
Wall A (2008) Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. For Ecol Manag 256:1372–1383. doi:10.1016/j.foreco.2008.06.044
Wardle DA, Nilsson M-C, Zackrisson O (2008a) Fire-derived charcoal causes loss of forest humus. Science 320:629. doi:10.1126/science.1154960
Wardle DA, Nilsson M-C, Zackrisson O (2008b) Response to comment on “fire-derived charcoal causes loss of forest humus”. Science 321:1295d. doi:10.1126/science.1160750
Whitman T (2011) Biochar as a carbon sequestration mechanism: decomposition, modelling, and policy. Thesis, Cornell University
Whitman T, Nicholson CF, Torres D, Lehmann J (2011) Climate change impact of biochar cook stoves in Western Kenyan farm households: system dynamics model analysis. Environ Sci Technol 45:3687–3694. doi:10.1021/es103301k
Woolf D, Amonette JE, Street-Perrott FA et al (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:1–9. doi:10.1038/ncomms1053
Yamato M, Okimori Y, Wibowo IF et al (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495. doi:10.1111/j.1747-0765.2006.00065.x
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301. doi:10.1021/es903140c
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179. doi:10.1016/j.soilbio.2011.02.005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woolf, D., Lehmann, J. Modelling the long-term response to positive and negative priming of soil organic carbon by black carbon. Biogeochemistry 111, 83–95 (2012). https://doi.org/10.1007/s10533-012-9764-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9764-6