Abstract
While rare globally, blooms of the toxic cyanobacteria Nodularia spumigena are a recurring problem in a few estuaries, such as the Baltic Sea and several southern Australian estuaries. Here, we document recurring Nodularia spumigena Mertens blooms in the Gippsland Lakes, S.E. Australia; a temperate lagoon system with episodic, winter-spring dominated catchment inflows. Physico-chemical conditions exerted a strong influence over bloom development, with blooms consistently occurring at surface water salinities between 9 and 20 (average = 15), inorganic nitrogen concentrations <0.4 μM, and inorganic nitrogen to reactive phosphorus ratios <5. There was a positive correlation between average annual chlorophyll a and total phosphorus (TP) load in years when there was no Nodularia bloom, but this relationship broke down in Nodularia bloom years, even though there was a strong correlation between in-lake TP and chlorophyll a during these years; this highlights the importance of internal sources of phosphorus to bloom development. Large catchment derived nitrate and nitrite (NOx) inputs following wildfires and floods in 2007, led to high concentrations of NOx within the surface waters of the Gippsland Lakes through the second half of 2007 and the start of 2008. We hypothesise that these high NOx concentrations were a key factor leading to an unprecedented Synechococcus sp. bloom that developed in the austral summer of 2007–2008, despite conditions that would otherwise favour a Nodularia bloom.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication of estuaries is an increasing problem globally; one which is largely caused by increased nutrient loads derived from urban and agricultural sources. A consequence of eutrophication is an increase in the occurrence of harmful algal blooms (Cloern 2001). Of particular concern are blooms of cyanobacteria which are often toxic. While rare globally, blooms of the cyanobacteria Nodularia sp. are well documented (Francis 1878; Sellner 1997) and have been a recurring problem in estuaries such as the Baltic Sea and the Peel-Harvey Estuary in Western Australia (Sellner 1997). Factors thought to favour Nodularia blooms in estuaries include low salinity (<15), strong stratification, warm temperatures (>17°C), low inorganic nitrogen and high phosphorus in the surface waters (Sellner 1997). Turbulence including small scale shear and vertical mixing are known to inhibit the growth of heterocystous cyanobacteria including Nodularia (Moisander et al. 2002; Moisander and Paerl 2000). Nodularia may, however, be absent from estuaries which have seemingly favourable physico-chemical conditions, leading to speculation that other factors—such as the absence of trace elements, or geographic isolation—may play a role in its occurrence (Moisander and Paerl 2000).
Within the Baltic Sea, Nodularia blooms typically occur when P rich hypolimnetic water mixes into the warm, calm euphotic zone by currents, eddies and upwelling (Sellner 1997). The Peel-Harvey Estuary has high rates of P loading, which are derived from intensive fertiliser application and low P retention within the predominantly sandy soils of the catchment (McComb and Humphries 1992). These inputs are utilised by a spring diatom bloom which breaks down in the benthos, leading to a release of P during late spring that triggers the Nodularia blooms (Hearn and Robson 2000).
The Gippsland Lakes, a large series of interconnected coastal lagoons in S.E. Australia are another estuarine system afflicted by Nodularia spumigena Mertens blooms. The first record of a Nodularia bloom in the Gippsland Lakes was in 1965 (Solly 1966). The most severe bloom on record occurred in the Austral summer of 1987–1988 and was reported throughout most of the lake system, and resulted in a severe loss of revenue from tourism which is one of the major industries in the region. Sporadic Nodularia blooms have since occurred, with the most recent being in 2002. A recent study of in-lake biogeochemical dynamics following a flood event suggests that these blooms are predominantly driven by phosphorus released from the sediment rather than catchment derived loads (Cook et al. 2010). In the Austral summer of 2007–2008 a severe Synechococcus bloom occurred which was the first recorded instance of this species in the Gippsland Lakes.
This study was undertaken to review and synthesise the physico-chemical factors that give rise to cyanobacterial blooms within the Gippsland Lakes. Based on previous studies in the literature and a detailed study of one Nodularia bloom in the Gippsland Lakes (Cook et al. 2010), we expected that:
-
1.
The onset of Nodularia blooms would be strongly favoured by a low availability of inorganic nitrogen relative to phosphorus, low salinity and associated strong stratification.
-
2.
Phytoplankton biomass would be decoupled from catchment nutrient loads in years of Nodularia blooms as a consequence of high internal phosphorus loading and the ability of these organisms to derive their nitrogen requirements from atmospheric N2 fixation.
Methods
Site description
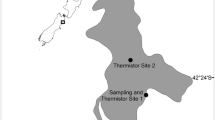
The Gippsland Lakes, S.E. Australia, is Australia’s largest estuarine system, with its three main lakes occupying 354 km2 (Fig. 1). Lake Wellington in the west is shallow (2.6 m deep on average) and well mixed; the central Lake Victoria and Lake King to the east are deeper (on average 4.8 and 5.4 m respectively) and are usually salinity stratified. In their natural state, the Lakes had an ephemeral sea inlet, but in 1889 a permanent, artificial sea-entrance was constructed, and this has led to increased salinity throughout the lakes, with resulting ecological change including changes in sea grass species and the loss of fringing vegetation such as the reed Phragmites australis (Harris et al. 1998).
Top panel shows the Gippsland lakes catchment and its location in S.E. Australia, with forested areas light grey, and the area burned in the 2006–2007 wildfires in dark grey (adapted from a map produced by the Victorian Department of Sustainability and Environment), and the gauging stations on the Latrobe (LR), Thomson (TH), Avon (AV), Mitchell (MI), Nicholson (NI) and Tambo (TA) Rivers. Bottom panel shows the sample sites used in the Gippsland Lakes. White circles indicate long-term EPA monitoring sites and black circles are recently installed EPA continuous monitoring stations (Note: some locations are combinations of the above site types)
The Gippsland Lakes has a catchment of approximately 20,500 km2, and consists of seven major rivers, which combine into two super-catchments; Lake Wellington receives waters from the Latrobe (4,900 km2), Thomson/Macalister (3,660 km2) and Avon (2,000 km2) Rivers, while Lake King North receives the Mitchell (5,500 km2), Nicholson (600 km2) and Tambo (3,700 km2) Rivers (Fig. 1). Other inputs into the lakes include several small creeks and the Wellington Main Drain, but these are minor in comparison to the rivers mentioned above.
Gippsland is a temperate region, which often experiences extreme weather events, such as floods, droughts and wild-fires. Winter and spring (June–December) typically receive the highest rainfall, and on average 75% of inflows occur during this period. The lakes have a high residence time, with the average flushing time estimated to be 206 days between 1975 and 1999 (Webster et al. 2001). Tidal influences are low, and seawater intrusion is primarily through seasonal changes in the sea level (typically ~30 cm).
Recently, the lakes have suffered from a series of extreme events: generally dry conditions between 1999 and 2007, with particularly low flows into the lakes in 1999–2000, 2002–2003 and 2006–2007; large-scale wildfires through much of the catchment in the summer of 2006–2007, which burned the catchments of the Latrobe (2%), Thomson/Macalister (57%), Mitchell (41%) and the Tambo/Nicholson (21%) rivers, equating to a total of 32% of the catchment of the Gippsland Lakes; and finally, major flooding in all of the major source-rivers (apart from the Latrobe River) in June 2007.
Data sources
The majority of the data came from the Victorian Water Resources Data Warehouse (www.vicwaterdata.net). Other data has been provided by the Victorian Environment Protection Authority (EPA) and Fisheries Victoria, Fisheries Research Branch (FRB). The data sets used here were derived from three monitoring programs. Long term river monitoring (1986–2008) commissioned by the Victorian Department of Sustainability and Environment, long term in-lake monitoring undertaken by the Victorian EPA (1986–2008), and short term intense in-lake monitoring undertaken by FRB (1997–2000). A summary of water quality parameters and their analytical methods is as follows. Total Kjeldahl nitrogen (TKN) and total phosphorus (TP) samples were either stored on ice or frozen in the field, while inorganic nitrogen (IN, NOx + NH4 +) and reactive phosphorus (RP) samples were frozen on-site, with the river samples filtered through 0.45 μm filters and the lake samples remaining unfiltered. Nutrients were analysed in the laboratory using standard colorimetric methods (APHA 1992; Grasshoff 1983). We note that river samples were not generally analysed for NH4 +. Ammonia samples were collected from selected rivers for a period from late 2002 to mid-2003, and during this time ammonia made up on average only 21% of total inorganic nitrogen. While not ideal, we believe, therefore, that nitrate and nitrite measurements capture the majority of inorganic nitrogen entering the lakes. Water samples were filtered onto glass fibre filters (Whatman GF/C), which were frozen in the field and later analysed for chlorophyll a spectrophotometrically, using the method of Strickland and Parsons (1972), and the equations of Jeffrey and Humphrey (1975). We note that during the Synechococcus bloom, the concentrations of chlorophyll a may have been underestimated because the cell size of this organism is smaller than the nominal pore size of these filters. We also note that surface samples were taken from the top 0.5 m of the water column and as a consequence the density of buoyant phytoplankton such as Nodularia may not be representative of the entire mixed layer. The dominant phytoplankton taxa were identified microscopically by the EPA or the Regional Water Quality Officer from the Victorian Department of Natural Resources and Environment using standard National Association of Testing Authorities (NATA) accredited methods. Our definition of a reported cyanobacterial bloom is when there are >2,000 cells/ml, which is the threshold at which the Victorian Department of Sustainability and Environment is notified of a bloom. Salinity was measured using a calibrated water quality probe in situ and results are reported on the practical salinity scale. River discharge was calculated from stage height which was measured daily using automated gauges.
There are several periods of high resolution in-lake monitoring, specifically 1997–2000 and 2004–2008, while there are also some notable gaps in the data, especially during the 1980s. In cases where a particular site had missing data and data for this missing period was available from another, nearby or related site, this alternate data was inserted into the data set, with adjustments made for catchment size if necessary. Likewise, if two or more agencies used sites that were close together, the data was pooled into one site. Annual data are reported as June 1–31 May. The reason for this is that the system is very event driven, and careful inspection of the flow data indicates that major flow events often occurred in June or July (but never before this), and these flow events strongly influence phytoplankton dynamics in the lakes up until the following June, when any blooms have usually subsided, and the cycle begins again.
Load estimates
Loads into the Gippsland Lakes were calculated from flows and nutrient concentrations from the gauged catchments of the Tambo, Nicholson, Mitchell, Avon, Thomson and Latrobe Rivers as well as the Wellington Main Drain. Loads were calculated using the Kendall Ratio method (Kendall et al. 1983) stratified for flow using a spreadsheet routine (Tan et al. 2005), which also calculated the standard deviation of the loads (Cooper and Watts 2002). Loads for a given year were only calculated if there was a minimum of three concentration measurements, but in general >10 measurements were used. Flow was stratified for each catchment based on visual inspection of the data, which divided flows into base flow, high flow and flood events. To ensure the accuracy of our estimates, a number of cross checks were made with load estimates previously made by the EPA; on average, these were found to be within a factor of 1.06 of our calculations. We report loads as loading rates per m2 of lake as is the convention throughout the literature.
Simultaneous load estimates for all catchments are only available for 3 years, 2005–2007, except for the Wellington Main Drain for which we only have 2 years of data. The Wellington Main drain was found to contribute <2% of TN and TP gauged loads respectively and so we made no further effort to estimate loads from this source. The combined loads of the Mitchell and Latrobe rivers accounted for 48–88% (mean 67%) of the gauged TN load and 45–87% (mean 64%) of the gauged TP load. There was an almost continuous load data set available for these two rivers, so the total loads into the Gippsland Lakes were calculated as the measured loads from the Mitchell and Latrobe rivers plus the loads measured in the other catchments, where available. When no load data was available from the other catchments, they were estimated based on annual log flow log load regressions from years where loads had been calculated (r2 = 0.60–0.84, mean r2 = 0.75, n > 6). We made no attempt to estimate inputs downstream of gauged areas or in ungauged catchments or atmospheric deposition. It has been estimated that between 1975 and 1999 ~81% of TN and TP entering the Gippsland Lakes was derived from the gauged catchments (Grayson et al. 2001), and so our load estimates are likely to be slightly below the true loads. Atmospheric deposition was ignored in the load estimates as no data are available for the Gippsland Lakes. Previous measurements of wet atmospheric deposition rates within the Latrobe Valley gave deposition rates of 1.4 to 3 and 0.3 kg ha−1 year−1 for inorganic nitrogen and reactive phosphorus respectively (Ayers et al. 1995; Pooley 1978). In general, these rates are <10% of the river derived TN and TP loads except in low flow years when these rates of deposition would be equivalent to ~30% of the river derived loads of TN and TP.
Annual lake averages
Continuous—although sometimes sparse—data from in-lake monitoring is available from 1986 to 2008 for surface waters, and from 1989 to 2008 for bottom waters. Average concentrations of key nutrients were calculated as an average of the four long-term monitoring sites in Lakes Victoria and King, i.e. Lake Victoria Central (LVC), Lake King South (LKS), Lake King North (LKN) and Lake King East (LKE, Fig. 1). Lake Wellington data were excluded from these averages because it has different limnological characteristics, including low salinity and little or no stratification, and because nuisance cyanobacterial blooms virtually always have very different dynamics to those observed in Lakes King and Victoria. Data were often sporadic, and so in order to ensure that high frequency data collection did not bias the data towards a particular time of year, each value was weighted based on the time between the measurements taken before and after it.
Results
Catchment nutrient loads
River flow exerted an overwhelming control over nutrient loads over the 22 year data set, with the highest loading rates generally occurring in the wetter period from 1986 to 1995, followed by generally lower loads in the drier period from 1999 to 2008. For total nitrogen (TN), total phosphorus (TP) and NOx, the highest loading rates (1.2, 0.08 and 0.4 mmol m−2 year−1 respectively) were observed in the flood year of 2007–2008 (Fig. 2) following one of the largest wildfires on record, which burned the upper catchments of the Avon, Thomson, Mitchell, Nicholson and Tambo Rivers. High TP loading rates were also observed in 1995–1996 (0.05 mmol m−2), and were predominantly derived from the Latrobe River (data not shown), which flooded in that year. The average molar TN:TP ratio of the loads was 22. The lowest TN:TP ratios occurred in the 1995–1996 Latrobe River flood year. The NOx:reactive P (RP) load ratios were generally in excess of 40 and were on average 50 (NB: NH4 + data are not available for the catchments, and if included, the IN:RP ratios would be even higher). Interestingly, the year with the second highest NOx:RP loading ratio was 2007–2008, which was also one of the years with the lowest TN:TP ratios.
Total annual inflow, and loads of total phosphorus (TP), total nitrogen (TN), filterable reactive phosphorus (RP) and NOx, as well as the molar TN:TP and NOx:RP ratios, entering the Gippsland Lakes from 1985 to 2008; there is no RP data available before 1990. Loads are normalised to the surface area of the lakes. Error bars represent the standard deviation of the loads. Shading indicates whether a summer bloom occurred
Historical flows and phytoplankton blooms
Flow into the lakes follows a seasonal pattern, with high winter-spring flows, and low summer-autumn flows (Fig. 3). For simplicity we have only presented in-lake time series data from the study site ‘Lake King South’ which we judge to be broadly representative of both Lake King and Victoria. In support of this judgement, virtually all the blooms reported from Lakes King and Victoria (the vertical lines in Fig. 3) coincided with a spike in chlorophyll a at this site. We note, however, that records of algal blooms in the Gippsland Lakes are incomplete, and detailed information about location, size and persistence are generally lacking. Given the major socio-economic impact of Nodularia blooms, however, we are confident that these particular blooms have all been documented. Cyanobacterial blooms always occurred in the summer and only two of the 10 cyanobacterial blooms recorded were not Nodularia; a Microcystis sp. bloom in January 1993 and a Synechococcus sp. bloom from December 2007 to July 2008. The temperature data indicate that cyanobacterial blooms only occur when the air temperature exceeds 20°C. It appears that average summer temperatures are sufficient for a cyanobacterial bloom to occur; above average temperatures are not required.
Long-term monitoring data for Lake King South (site LKS). Total flow is the summed flow recorded at all of the major catchment rivers, and maximum air temperature is from Bairnsdale Airport. The vertical lines represent the start of reported blooms (labelled N for Nodularia, M for Microcystis and S for Synechococcus). The horizontal grey line shows our estimated thresholds for salinity (20) and IN (0.4 μM), below which a Nodularia bloom is likely to occur
Water quality time series and bloom development
A time-series of salinity, inorganic nitrogen (IN) and RP data from site LKS for the period 1986–2008 reveals information about the onset of cyanobacterial blooms (Fig. 3). Nodularia blooms only occurred when the surface salinity was in the range of 9–20 (average = 15). The Synechococcus bloom, likewise, occurred when the salinity was ~17, whereas the Microcystis bloom occurred at a much lower salinity, of ~5. Stratification (as seen in the salinity difference between surface and bottom waters) was generally quite high when cyanobacteria blooms occurred, although short-term mixing events are likely to have been missed, given the one to two-month sampling regime that usually occurred. IN was usually low (<0.4 μM) in the period preceding Nodularia blooms, and in several cases, IN then increased dramatically following the onset of a bloom (e.g. 1988, 1990, 1997 and 1999); this was likely a result of leakage or recycling of N fixed by the Nodularia. Surface water RP concentrations were in the range 0.04–0.5 μM prior to Nodularia blooms. Bottom water RP concentrations were generally elevated (>1 μM) around the times of Noduluaria blooms.
In-lake nutrients and chlorophyll a
Annual average chlorophyll a concentrations varied between 2.5 μg l−1 in 2006–2007 up to 25 μg l−1 in 2007–2008 and 28 μg l−1 in 1987–1988, coinciding with the Synechococcus bloom and a Nodularia bloom, respectively (Fig. 4). Other notably high chlorophyll a concentrations occurred in the years 1996–1997, 1998–1999 and 2001–2002, which were also years in which Nodularia blooms occurred. There was no clear trend in chlorophyll a concentration over time in the data set. Annual average TN concentrations within the Gippsland Lakes generally ranged between 25 and 45 μM (Fig. 4). The Nodularia bloom year of 1987–1988, and the post-fire flood year of 2007–2008 stood out, having much higher TN concentrations, >70 μM. Other years with notably high TN concentrations—particularly in the surface waters—were 1995–1996, 1996–1997, 1998–1999 and 2001–2002, all of which coincided with blooms of Nodularia. The concentrations of TN were generally similar within the surface and bottom waters. TP concentrations generally fell within the range of 1–2.5 μM and were almost always slightly higher in the bottom waters (Fig. 4). As for TN, 2007–2008 stood out as having extremely high TP concentrations, >4.1 μM in the surface waters. Surface IN concentrations were highly variable, ranging from <1 μM in dry years up to 9.9 μM in the surface waters in the post-fire flood year of 2007–2008. IN concentrations were always highest in the bottom waters, with the highest bottom water concentration being observed in the cyanobacterial bloom years 1989–1990, 1995–1996, 1998–1999 and 2007–2008, when the average IN concentration was between 10 and 13 μM (Fig. 4). RP concentrations ranged from <0.1 up to 1.7 μM and showed a very similar pattern to IN, with the highest concentrations being observed in the bottom waters (Fig. 4). Average IN:RP ratios were always 16 or below in the bottom waters and were generally <10 (Fig. 4). The average IN:RP ratio in the surface water was also generally less than 10, although it exceeded the Redfield ratio in the surface waters in 2007–2008.
Average annual concentrations of chlorophyll a, total phosphorus, (TP), total nitrogen (TN), filterable reactive phosphorus (RP), dissolved inorganic nitrogen (IN) as well as TN:TP and IN:RP ratios in the surface and bottom waters of the Gippsland Lakes in each hydraulic year (July–June) from 1985–2008. Error bars show the standard error about the mean. Nodularia (N), Microcystis (M) and Synechococcus (S) bloom years are indicated
There was a weak, but significant positive correlation between annual average chlorophyll a and total annual (1 June–31 May) inflows into the lakes, excluding years with Nodularia blooms (Fig. 5). Excluding Nodularia bloom years, annual loads of TN and TP had a strong relationship with chlorophyll a although this was clearly non-linear, with chlorophyll a concentrations tending to plateau at high nutrient loads. A logarithmic fit to the data showed that flow, TN and TP loads explained 35, 61 and 67% of the variance in chlorophyll a respectively. For years in which Nodularia blooms occurred, there was no relationship between chlorophyll a and flow, TN or TP loads, except that there were critical thresholds of ~1600 GL, 0.2 and 0.01 mol m−2 respectively, below which blooms did not occur. Between the years 1985 and 2008, Nodularia blooms were recorded in 8 out of the 14 highest TN and TP load years. In general, chlorophyll a concentrations were low relative to nitrogen loading rates when compared to other temperate lagoon systems (Boynton et al. 1996).
Average surface chlorophyll a concentrations within the Gippsland Lakes plotted against annual (June 1–May 31) a total river inflow, b total nitrogen and c total phosphorus loads entering the Gippsland Lakes between 1985 and 2008. Symbols differentiate years when Nodularia, Synechococcus and Microcystis blooms occurred. Error bars for chlorophyll a show the standard error about the mean, and for the loads show the standard deviation from the mean. The curved lines are logarithmic lines of best fit, excluding Nodularia bloom years. The straight line in (b) is the linear regression of chlorophyll a versus total nitrogen load in Maryland coastal bays from Boynton et al. (1996). Note: The chlorophyll concentrations in the Synechococcus bloom year are a minimum estimate as a consequence of them potentially not being fully retained on the GF/C filters used in this study
Discussion
Physico chemical conditions conducive to Nodularia blooms
Nodularia spumigena blooms occurred in the Gippsland Lakes under conditions broadly similar to those previously identified in the literature as being favourable to this species, but with some site-specific differences. We identify salinity and N-limitation as being the critical factors that trigger the development of Nodularia blooms with the Gippsland Lakes. Warm temperatures (>17°C) and stratified conditions are also required for bloom initiation (Sellner 1997), however, this water temperature was exceeded every summer and the water column was continuously salinity stratified (Fig. 3) in the Gippsland Lakes and we therefore ignore these as a critical factors controlling the development of summer Nodularia blooms here.
Salinity is known to exert a strong influence on the development of Nodularia blooms and the onset of blooms was observed at salinities in the range 9–20 (Fig. 3). This is slightly higher than the salinity range of 5–15 reported for the optimum growth of Nodularia in the Baltic Sea (Kanoshina et al. 2003; Mazur-Marzec et al. 2006). In the Peel Harvey Estuary, a minimum winter salinity of <10 was required for Nodularia bloom initiation, however, once the blooms commenced, they were able to grow up to salinities of 25 (Lukatelich and McComb 1986). A similar salinity range was also observed for a Nodularia bloom in Orielton Lagoon, Tasmania (Jones et al. 1994). Our observations are also broadly consistent with culture studies of Nodularia isolated from the Gippsland Lakes which have shown highest Nodularia growth rates at salinities of 5–15, with an ability to grow at >50% of their maximum growth rate at salinities of 0 and 35 (Myers et al. 2010). In the same study, akinete germination rates were found to be optimal in the salinity range of 5–25 (Myers et al. 2010). The absence of Nodularia blooms at salinities >20, despite their ability to germinate and grow under these conditions remains unclear, however, recent studies have suggested grazing pressure may exert a significant control over heterocystous cyanobacteria, including Nodularia, at elevated salinities (Chan et al. 2006; Marino et al. 2006).
N-limitation is likely to favour the growth of cyanobacteria capable of N-fixation (Moisander et al. 2003; Smith and Bennett 1999). Assuming cellular growth with a Redfield N:P ratio of 16:1, one would expect cells to become N limited when the IN:RP supply ratio is <16. Prior to all Nodularia blooms, the IN:RP ratio in the surface waters of the Gippsland Lakes was <5 and the IN concentrations were <0.4 μM, suggesting that N-limitation prevailed before a Nodularia bloom (Fig. 3). The summers of 1988–1989, 1992–1993, 1993–1994 and 2007–2008 which had favourable salinities (<20), but in which IN was slightly elevated (>0.4 μM), are notable for the absence of Nodularia blooms, suggesting that an IN concentration of ~0.4 μM may be a threshold above which Nodularia blooms do not occur in this system. Culture studies of Nodularia from the Gippsland Lakes have shown that akinete germination and cellular growth rates are not negatively affected by nitrate concentrations up to 3 mg l−1 (Myers et al. 2010). As such, competition with other phytoplankton for phosphorus is most likely to limit the development of Nodularia blooms. In the summers of 1993–1994 and 2007–2008, blooms of Microcystis and Synechococcus were recorded respectively. Heterocystous N2 fixing cyanobacteria such as Nodularia have a considerable advantage over other phytoplankton when IN concentrations are low, however, they are relatively slow growing and so they are rapidly out-competed by other species and taxa when there is an availability of IN. Cyanobacteria with a small cell size such as Synechococcus and Microcystis have both a higher affinity for IN than heterocystous species such as Nodularia, and can grow at twice the rate of heterocystous species (Flores and Herrero 1994; Reynolds 2006).
Once a Nodularia bloom commences, high rates of N2 fixation will most likely lead to a rapid transition from N to P limitation (Moisander et al. 2003). In the Gippsland Lakes, the TN:TP ratios increased from <20 (indicative of N limitation sensu Guildford and Hecky (2000)) before Nodularia blooms to >20 during blooms, with a tendency to cluster around 50 (indicative of P limitation sensu Guildford and Hecky (2000), Fig. 6a). As such, the availability of P exerted an important control over the magnitude of the bloom (Fig. 6b). Given the relatively high concentrations of RP in the bottom waters (generally >1 μM in Nodularia bloom years, Fig. 4), we surmise that this was the major source of P to the blooms.
a Total nitrogen (TN) versus total phosphorus (TP) observed in surface waters during Nodularia blooms, within 2 months of a flood event, and at all other times (outside Nodularia bloom). b Surface chlorophyll a versus TP observed during Nodularia blooms and at all other times (outside Nodularia blooms) at three sites in Lake King (LKS, LKN and LKE)
Interactions between catchment inputs, internal biogeochemistry and Nodularia blooms
Catchment inflows are known to exert a strong influence over estuarine function and we now examine the dynamic of phytoplankton and Nodularia blooms with respect to river discharge and nutrient loads. In years with no Nodularia blooms, there was a strong relationship between average chlorophyll a concentration and both TN and TP loads (Fig. 5), in common with many previous studies (Boynton et al. 1996; Cloern 2001). There was, however, no clear relationship between chlorophyll a and catchment nutrient loads in Nodularia bloom years; except that there may be load thresholds below which Nodularia blooms do not occur (Fig. 5). We attribute this decoupling of biomass from external loading to two factors. Firstly, Nodularia are capable of nitrogen fixation, thus alleviating the need for an external nitrogen supply. Secondly, the depletion DO in the bottom waters and increased organic carbon loading to the sediment induces the release of P stored within the sediment (Cook et al. 2010). The supply of P to the Nodularia bloom is thus strongly controlled by physical factors, such as mixing and stratification of the water column. Under the right physical conditions, the supply of nutrients to Nodularia blooms may be virtually self-sustaining (Hearn and Robson 2000), which explains why such a large biomass of Nodularia was able to develop in the bloom year of 1987–1988 which had only moderate catchment inputs. While P inputs to the Gippsland Lakes may have no immediate influence over Nodularia blooms, it is likely that catchment inputs over the long term have led to an enrichment of P within the sediments, which is released to the water column under anoxic conditions or during high organic matter loading (Cook et al. 2010). Thus, management actions aimed at reducing P inputs to the Gippsland Lakes are only likely to yield results over long timescales, as has been observed in freshwater lakes with high internal P stores (Jeppesen et al. 1998). This contrasts with the Peel-Harvey Estuary where studies suggest that the biomass of Nodularia is strongly related to the catchment P input during the previous winter, and it was expected that bloom magnitude would respond rapidly to a reduction in catchment inputs (Lukatelich and McComb 1986; McComb and Humphries 1992).
While there was no clear relationship between in-lake chlorophyll a and catchment loads in Nodularia bloom years, short term catchment dynamics did still play a role in Nodularia blooms, with blooms only occurring in high flow and load years. We suggest that this link between the catchment and Nodularia blooms is primarily through the influence of river discharge on surface salinity. As previously discussed, Nodularia are well known to be sensitive to salinity and no Nodularia blooms commenced in the Gippsland Lakes when the salinity was above 20. The critical role of salinity in the blooms of Nodularia within estuaries is underscored by the fact that such blooms were eliminated from the Peel-Harvey Estuary after the salinity of the estuary was dramatically increased via the construction of an artificial entrance (Sellner 1997).
As previously discussed, IN concentration in the surface water of the Gippsland Lakes was another important factor that had an influence over the development of Nodularia blooms, and that elevated concentrations of IN prevented Nodularia blooms on four occasions. Elevated IN concentrations within the Gippsland Lakes were associated with large flow events, after which IN became depleted over a period of weeks (Fig. 3). High flow events in late spring and summer may have either inhibited Nodularia blooms as was the case in the summers of 1988–1989, 1992–1993, 1993–1994, or delayed the occurrence until late summer as was the case in 1996.
Extremely elevated IN concentrations were observed in the surface waters of the Gippsland Lakes during the summer of 2007–2008. This followed the June 2007 floods, which carried extremely high IN loads into the system. This unprecedented IN input almost certainly occurred as a consequence of wildfires that burned ~32% of the catchment in early 2007 (Fig. 1). This resulted in the Gippsland Lakes changing from being classed as eutrophic (TN = 21–67 μM, TP = 1.25–4 μM, Chl a = 6–20 μg l−1) prior to the 2007 flood to hyper-eutrophic (TN > 67 μM, TP > 4 μM), Chl a > 20 μg l−1) in marine systems (Hakanson et al. 2007). This finding is consistent with previous studies which have found that the dominance of N2 fixing cyanobacteria decreases at extreme eutrophication in favour of non-heterocystous cyanobacteria, including small, single-celled organisms such as the Chroococales (Smith 1990), which includes the genus Synechococcus.
Conclusion
Recurring blooms of Nodularia Spumigena within the Gippsland Lakes are caused by the favourable physicochemical conditions of low salinity (<20), low inorganic nitrogen (<0.4 μM), low inorganic nitrogen: reactive phosphorus ratios (<5) and warm conditions. Nodularia biomass is strongly related to total phosphorus concentrations in the surface waters of the Gippsland lakes and the major source of this is most likely internal recycling. As a consequence of this, average annual phytoplankton biomass becomes decoupled from catchment nutrient loads in Nodularia bloom years. Changes to these physicochemical conditions such as de-stratification and elevated surface water inorganic nitrogen concentrations may lead to no summer bloom, or to the dominance of other species of cyanobacteria such as Synechococcus.
References
APHA (1992) Standard methods for the examination of water and wastewater. APHA, AWWA & WPCF, Washington
Ayers GP, Gillett RW, Selleck PW, Bentley ST (1995) Rainwater composition and acid deposition in the vicinity of the fossil fuel-fired power plants in southern Australia. Water Air Soil Pollut 85:2313–2318
Boynton WR, Hagy JD, Murray L, Stokes C, Kemp WM (1996) A comparative analysis of eutrophication patterns in a temperate coastal lagoon. Estuaries 19:408–421
Chan F, Marino RL, Howarth RW, Pace ML (2006) Ecological constraints on planktonic nitrogen fixation in saline estuaries. II. Grazing controls on cyanobacterial population dynamics. Mar Ecol Prog Ser 309:41–53
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Cook PLM, Holland DP, Longmore AR (2010) Effect of a flood event on the dynamics of phytoplankton and biogeochemistry in a large temperate Australian lagoon. Limnol Oceanogr 55:1123–1133
Cooper DM, Watts CD (2002) A comparison of river load estimation techniques: application to dissolved organic carbon. Environmetrics 13:733–750
Flores E, Herrero A (1994) Assimilatory nitrogen metabolism and its regulation. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer, Dordrecht, pp 487–517
Francis G (1878) Poisonous Australian lake. Nature 2:11–12
Grasshoff K (1983) Methods of seawater analysis. Verlag Chemie, Weinheim
Grayson RB, Tan KS, Western A (2001) Estimation of sediment and nutrient loads into the Gippsland Lakes. Centre for Environmental Applied Hydrology, Department of Civil and Environmental Engineering, University of Melbourne. Available at http://www.gippslandlakestaskforce.vic.gov.au/publications/inlakeresearch/Load.pdf
Guildford SJ, Hecky RE (2000) Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnol Oceanogr 45:1213–1223
Hakanson L, Bryhn AC, Hytteborn JK (2007) On the issue of limiting nutrient and predictions of cyanobacteria in aquatic systems. Sci Total Environ 379:89–108
Harris GP, Batley G, Webster IT, Molloy R, Fox D (1998) Review of water quality and status of the aquatic ecosystems of the Gippsland Lakes. CSIRO, Melbourne, p 34
Hearn CJ, Robson BJ (2000) Modelling a bottom diurnal boundary layer and its control of massive alga blooms in an estuary. Appl Math Model 24:843–859
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Jeppesen E, Sondergaard M, Jensen JP, Mortensen E, Hansen AM, Jorgensen T (1998) Cascading trophic interactions from fish to bacteria and nutrients after reduced sewage loading: an 18-year study of a shallow hypertrophic lake. Ecosystems 1:250–267
Jones GJ, Blackburn SI, Parker NS (1994) A toxic bloom of Nodularia spumigena Mertens in Orielton Lagoon, Tasmania. Aust J Mar Freshw Res 45:787–800
Kanoshina I, Lips U, Leppanen JM (2003) The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2:29–41
Kendall A, Stuart A, Ord JK (1983) Advanced theory of statistics. Griffin, London
Lukatelich RJ, McComb AJ (1986) Nutrient levels and the development of diatom and blue-green-algal blooms in a shallow Australian estuary. J Plankton Res 8:597–618
Marino R, Chan F, Howarth RW, Pace ML, Likens GE (2006) Ecological constraints on planktonic nitrogen fixation in saline estuaries. I. Nutrient and trophic controls. Mar Ecol Prog Ser 309:25–39
Mazur-Marzec H, Krezel A, Kobos J, Plinski M (2006) Toxic Nodularia spumigena blooms in the coastal waters of the Gulf of Gdansk: a ten-year survey. Oceanologia 48:255–273
McComb AJ, Humphries R (1992) Loss of nutrients from catchments and their ecological impacts in the Peel-Harvey estuarine system, Western Australia. Estuaries 15:529–537
Moisander PH, Paerl HW (2000) Growth, primary productivity, and nitrogen fixation potential of Nodularia spp. (Cyanophyceae) in water from a subtropical estuary in the United States. J Phycol 36:645–658
Moisander PH, Hench JL, Kononen K, Paerl HW (2002) Small-scale shear effects on heterocystous cyanobacteria. Limnol Oceanogr 47:108–119
Moisander PH, Steppe TF, Hall NS, Kuparinen J, Paerl HW (2003) Variability in nitrogen and phosphorus limitation for Baltic Sea phytoplankton during nitrogen-fixing cyanobacterial blooms. Mar Ecol Prog Ser 262:81–95
Myers JH, Beardall J, Allinson G, Salzman S, Gunthorpe L (2010) Environmental influences on akinete germination and development in Nodularia spumigena (Cyanobacteriaceae), isolated from the Gippsland Lakes, Victoria, Australia. Hydrobiologia 649:239–247
Pooley G (1978) Gippsland Regional Environmental Study: the origin and fate of nutrients from natural sources in the Latrobe Valley Victoria—final report. Ministry for Conservation, Victoria, p 43
Reynolds CS (2006) Ecology of phytoplankton. Ecology, biodiversity, and conservation. Cambridge University Press, Cambridge
Sellner KG (1997) Physiology, ecology, and toxic properties of marine cyanobacteria blooms. Limnol Oceanogr 42:1089–1104
Smith VH (1990) Nitrogen, phosphorus, and nitrogen-fixation in lacustrine and estuarine ecosystems. Limnol Oceanogr 35:1852–1859
Smith VH, Bennett SJ (1999) Nitrogen: phosphorus supply ratios and phytoplankton community structure in lakes. Arch Hydrobiol 146:37–53
Solly WW (1966) A bloom of the alga Nodularia in Lake Wellington, Victoria, June-July 1965. Aust Soc Limnol Newsl 5:28–29
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa
Tan KS, Fox D, Etchells T (2005) Generator for uncertainty measures and load estimates using alternative formulae. Australian Centre for Environmetrics, The University of Melbourne, Melbourne, p 36
Webster IT, Parslow JS, Grayson RB, Molloy RP, Andrewartha J, Sakov P, Tan KS, Walker SJ, Wallace BB (2001) Gippsland Lakes environmental study assessing options for improving water quality and ecological function. Commonwealth Scientific and Industrial Research Organisation Glen Osmond, p 97. Available at http://www.gcb.vic.gov.au/documents/Gipps26Nov01.pdf
Acknowledgments
We thank Andy Stephens and Guillaume Martinez from the Victorian Environmental Protection Authority, for providing data, interpretation and comments on this manuscript. David Fox kindly gave us permission to use the loads calculation tool ‘Gumleaf’. We also thank Barry Hart, Chris Barry, Simon Roberts, Andy Longmore and Mike Grace for sharing their knowledge of the biogeochemistry and functioning of the Gippsland Lakes, and for comments on early versions of this manuscript. Discussions with Will Shenton helped crystallise our conceptual understanding of Nodularia bloom development. This project was supported by a grant from the Gippsland Lakes and Catchment Taskforce.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cook, P.L.M., Holland, D.P. Long term nutrient loads and chlorophyll dynamics in a large temperate Australian lagoon system affected by recurring blooms of cyanobacteria. Biogeochemistry 107, 261–274 (2012). https://doi.org/10.1007/s10533-010-9551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9551-1