Abstract:
We compared the CO2 exchange and its controls in the plant communities of a strongly patterned aapa mire, the Kaamanen fen in northern Finland. Based on a systematic vegetation inventory and an ordination analysis, four plant community types were chosen for the study: Ericales–Pleurozium string tops, Betula–Sphagnum string margins, Trichophorum tussock flarks and Carex–Scorpidium wet flarks. We measured plant community CO2 exchange with a closed chamber technique during the growing season of 2007 and early summer of 2008. Nonlinear regression models were used for simulating the CO2 exchange over the measurement period for different mire components and for the whole mire. The CO2 exchange dynamics distinguished two functional components in the mire: an ombrotrophic component (Ericales–Pleurozium string tops) and a minerotrophic component (other plant community types). Minerotrophic plant communities responded similarly to environmental controls, the most important of these being variation in leaf area and aerobic peat volume (water level). The ombrotrophic component dynamics were more obscure; frost and possibly peat moisture played a role. The minerotrophic communities functioned as effective CO2 sinks in the simulation, while the net CO2 exchange of the ombrotrophic community was close to zero. The smaller NEE of the ombrotrophic community was due to less efficient photosynthesis per unit leaf area in combination with high ecosystem respiration resulting from a large aerobic peat volume. Our study shows that a fen/bog functional dichotomy can also exist within one mire. Wet minerotrophic communities within northern mires can act as effective CO2 sinks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The temperature increase due to human-induced global warming is expected to be greatest at high northern latitudes (IPCC 2007). Not only temperature increase as such, but also changes in water balance, snow cover, timing of snow melt and frost occurrence are likely to affect the functioning of northern ecosystems (IPCC 2007).
Northern mires play an important role in the global carbon budget. With their estimated carbon reservoir of 455 Pg (1 Pg = 1015 g) they represent ca. 30% of the total soil carbon (Gorham 1991). The mire carbon balance reacts sensitively to variations in climate; therefore, interannual variation (Alm et al. 1999) or a directional change in climate (Oechel et al. 1995) can turn northern mires from carbon sinks into carbon sources. This, in turn, would have important feedback effects on the current climate change. Especially peat moisture content and water table level are important controls of mire carbon exchange (Shurpali et al. 1995; Alm et al. 1999; Riutta et al. 2007b). The timing of snow and surface frost melt have been observed to have an important influence on the annual carbon budget of northern mires (Aurela et al. 2004).
Mires are usually mosaic structures, where communities vary in accordance with the microtopography (e.g., string, lawn and flark communities) and nutrient level. Different plant communities are exposed to different environmental conditions: water, nutrient and oxygen availability, temperature and moisture. On the other hand, they show different responses to overarching environmental factors, such as light, air temperature or increased drainage, depending on the physiological properties of the community plants. Because of the intercommunity variation both in environmental conditions and environmental responses, different plant communities in one mire can show completely different dynamics in their CO2 exchange (Waddington and Roulet 2000; Riutta et al. 2007a).
Aapa mires, or patterned fens, are principally minerotrophic, wet mires. They occur in the middle and northern boreal vegetation zones, predominantly in the maritime margins of large continental land masses: in Fennoscandia (Ruuhijärvi 1983), northwestern Russia (Botch and Masing 1983), Canada and Alaska (Zoltai and Pollet 1983). A surface pattern of wet flarks and dry hummocky strings, particularly pronounced in the north, is a characteristic of aapa mires (Ruuhijärvi 1983). There are large differences in plant community composition between the mire microforms. In the northernmost aapa mires, ice lenses remain in the strings until late summer or even longer.
Aurela et al. (1998, 2001, 2002, 2004) have measured CO2 exchange by the eddy covariance technique at a northern aapa mire at Kaamanen, Finland, for several years. The measurement series is globally outstanding in its length and, as a result, the temporal variation in the CO2 exchange is well-documented. During recent years, the mire has been a small sink of atmospheric CO2 (Aurela et al. 2004). The timing of snow melt, controlling especially the CO2 exchange in June, was found to be the most important single determinant of the annual CO2 balance (Aurela et al. 2004). Typically for micrometeorological studies, the results do not reveal the spatial variation within the sink function. Previous studies on aapa or palsa mires have shown that microsites can differ dramatically in their CO2 balance: wet microsites with sedge or shrub vegetation have been strong net sinks for CO2, whereas dry microsites, often with sparse vegetation, have been CO2 sources or weak sinks (Heikkinen et al. 2002; Nykänen et al. 2003; Bäckstrand et al. 2008). Although these studies have shown the spatial variation, they have not focused on the factors controlling plant community CO2 exchange.
In this study, we aim to quantify the relative contributions and roles of the different plant communities to the total net CO2 exchange measured at Kaamanen by the eddy covariance method. We pay special attention to the critical early growing season. Our process-based modelling approach enables us to examine the controlling factors and mechanisms behind the source/sink patterns observed both in our study and also in previous studies on aapa mires.
Materials and methods
Study site
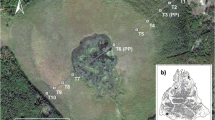
The study was carried out in an aapa mire at Kaamanen in northern Finland (69°08′N, 27°17′E, 155 m a.s.l.). The mire is situated in the northern boreal vegetation zone (Ahti et al. 1968), but the climate is already subarctic (see Aurela et al. 1998 for the climatology of the area). The mire has a pattern of wet flarks, which make up ca. 70% of the area, and dry ca. 70 cm high strings (see the aerial photograph in Aurela et al. 1998). This kind of pronounced surface pattern is typical of the northernmost aapa mires (Ruuhijärvi 1983). In addition to precipitation, the mire receives water as surface flow from the surrounding mineral ground, particularly during the spring high flow period. Due to their height, the strings are ombrotrophic in their nature, receiving water and nutrients almost solely from precipitation. Thin lenses of ice remain in the well-insulated hummocks until late summer.
The vegetation in the flarks consists of meso-eutrophic fen species (Eurola et al. 1995). The flarks are dominated by brown mosses and sedges, with occasional Trichophorum cespitosum tussocks. The uppermost parts of the strings host ombrotrophic vegetation: forest mosses and Ericales. String margins are covered by Sphagnum mosses, sedges, Betula nana and Andromeda polifolia.
Sampling
To obtain a well-grounded and objective classification for the spatial variation in vegetation, we conducted a systematic vegetation inventory over the mire in the growing season of 2006. The projection cover of each species was visually estimated in a total of 255 temporary vegetation sample plots using a circular frame 30 cm in diameter. The method is described in detail in Riutta et al. (2007a). Based on the TWINSPAN classification method (TWINSPAN for Windows 2.3; Hill 1979; Hill and Šmilauer 2005), we classified the vegetation into four plant community types: Carex–Scorpidium wet flarks, Trichophorum tussock flarks, Betula–Sphagnum string margins and Ericales–Pleurozium string tops. To test the significance of the classification obtained, we performed a canonical correspondence analysis (CCA) using Canoco for Windows 4.5 (ter Braak and Šmilauer 2002; Leps and Šmilauer 2003), treating plant community types as class variables.
The classification obtained was used as a base for the sampling design. Four study plot clusters with four 56 × 56 cm permanent gas exchange plots in each, one plot for each plant community type, were established on the site. Thus, there were four replicate measurement plots for each plant community type. The study plots were chosen to take into account the internal variation of the plant community types. The clusters were, therefore, situated 20–80 m apart from one another. In each cluster, the study plots were 0.5–6 m apart from one another. At the beginning of the study, boardwalks were installed at the site to minimize disturbance. Each study plot was surrounded by an aluminium collar inserted to a depth of 30 cm. The collar was used for anchoring the measurement chamber onto the study plot for the time of the gas exchange measurement. The collars had a flange (ca. 2 cm) with a water groove that allowed chamber placement and air-tight sealing of the measurement system during the measurement.
CO2 exchange measurements
Chamber measurements
Chamber measurements were carried out from three to five times a month during the growing season 2007 (June 6 to September 10) and twice a week during early summer 2008 (May 30 to June 26). The 2008 data was used for model validation and better examination of the crucial early summer processes.
The instantaneous net CO2 exchange in each plot was measured with a transparent plastic chamber (60 × 60 × 31 cm) equipped with a battery-operated fan, a cooling system that maintained the temperature within 2°C of the ambient temperature, and a portable infrared gas analyser (EGM-3 and EGM-4, PP Systems, UK). We followed the closed chamber method described in Alm et al. (2007). All plots where measured during one measurement day, 8 a.m. to 4 p.m., local winter time. Measurements lasting for 90–180 s were carried out first in ambient light and then under one or two shades made of thin mosquito net that reduced the amount of incoming light by 40–50% or 75–90%. Shades were placed on a rack standing approximately 50 cm above the chamber to simulate a natural, homogenous, low-light environment for the study plot. During the measurements, the CO2 concentration in the chamber headspace, the photosynthetic photon flux density (PPFD) under the chamber roof, and the chamber air temperature were recorded at 15 s intervals. After the measurements in the light, the chamber was covered with an opaque hood and the CO2 exchange in the dark was measured. The chamber was removed from the plot between these measurements to restore ambient gas concentration. Water level in a perforated tube next to each plot and peat temperatures at 5, 10 and 20 cm below the moss surface were measured simultaneously with the CO2 exchange measurements to relate the fluxes to the prevailing environmental conditions.
The net CO2 exchange was calculated from the linear change in CO2 concentration in the chamber headspace. We used the net CO2 exchange measurements in the dark to represent the ecosystem respiration (R ECO ). An estimate for the gross photosynthesis (P G ) was calculated by subtracting the CO2 exchange rate in the light conditions from the exchange rate in the subsequent dark measurement. Both P G and R ECO values are stated as positive. For this method, see Tuittila et al. (2004) and Alm et al. (2007).
Eddy covariance measurements
While the chamber measurements provide us with detailed data on different plant communities, the micrometeorological eddy covariance (EC) method gives us direct measurements of CO2 fluxes averaged on an ecosystem scale. In the EC method, the vertical CO2 flux is obtained as the covariance of the high-frequency (10 Hz) observations of vertical wind speed and the CO2 concentration (Baldocchi 2003). The eddy covariance measurement system included a USA-1 (METEK) three-axis sonic anemometer/thermometer and a closed-path LI-7000 (Li-Cor, Inc.) CO2/H2O gas analyzer. The measurement height was 5 m and the length of the heated inlet tube for the gas analyzer was 8 m. The mouth of the inlet tube was placed 15 cm below the sonic anemometer. The EC fluxes were calculated by an in-house Python program BARFLUX (Finnish Meteorological Institute) as half-hourly averages. The measurement system is presented in more detail in Aurela et al. (2002).
Describing seasonal variation in vegetation
We monitored the temporal variation in the vegetation by estimating the vascular green area (VGA) in the 16 permanent gas exchange plots every other week during the growing season of 2007, and weekly during the early summer of 2008. The method is described in detail by Wilson et al. (2007). We estimated the VGA of each species as the product of the number of green leaves in the plot and the average size of the leaf. For sedge species, the size of a leaf was measured non-destructively from marked shoots outside the study plots simultaneously with the leaf counting. For plants with evenly-sized leaves (dwarf shrubs, forbs), leaves were sampled and photographed once in the growing season of 2007. The green area was then digitally estimated from the photographs using Image Analyzer software (Martti Perämäki, Department of Forest Sciences, University of Helsinki). For Rubus chamaemorus, we used the dwarf shrub procedure in 2007 but the sedge procedure in 2008, the latter proving to be more suitable for the species. In addition, we estimated the projection cover of mosses, lichens and vascular plants once at the peak of the growing season 2007, July 20–23.
The species-specific VGAs were grouped into three functional groups: dwarf shrubs, sedges and forbs. To describe the seasonal development over the growing season of 2007, we fitted a log-normal curve to the VGA observations, separately for each functional group in each plot. Evergreen dwarf shrubs did not show a clear seasonal rhythm; therefore, most dwarf shrub VGAs were linearly interpolated between the measurement days. Linear interpolation was also used when different sedge cohorts caused two peaks in the sedge VGA. For the early summer of 2008, model building was considered unnecessary due to the frequent monitoring, and all VGAs were linearly interpolated.
Modelling CO2 exchange dynamics
To find the environmental factors affecting photosynthesis and ecosystem respiration in the different plant community types and to quantify their effect, we constructed process-based non-linear regression models for P G and R ECO , with PPFD, air temperature in the chamber, peat temperatures, water level, and VGA as independent variables.
Models were first constructed and parametrized separately for each plant community type. As the lawn and flark types, i.e., the minerotrophic plant communities—Betula–Sphagnum, Trichophorum and Carex–Scorpidium types—showed similar environmental responses, we decided to describe their CO2 exchange with collective P G (Eq. 1) and R ECO (Eq. 3) models. For the Ericales–Pleurozium type, one P G (Eq. 2) and two R ECO (Eqs. 4, 5) models were constructed: the first R ECO model (Eq. 4) has a response to water level; the other (Eq. 5) is for the early summer, when water level data were not available due to ground frost.
Model-building was based on the 2007 data. The 2008 data were used for model validation and for reconstructing the CO2 exchange for the early summer of 2008. In the minerotrophic plant communities, measured P G and R ECO values coincided well with modelled values in both years. In the Ericales–Pleurozium type, the fit was not as good; and for R ECO , the 2008 measured values did not fit the 2007 model (Eq. 5) at all. In order to better represent the data, we constructed another model for Ericales–Pleurozium R ECO for the early summer 2008 (Eq. 6).
The models are adapted from, and the response functions are discussed in detail in, Tuittila et al. (2004) and Riutta et al. (2007b).
In Eqs. 1 and 2, PPFD is the photosynthetic photon flux density, VGA is the vascular green area, T air is the air temperature and T 30 cm is peat temperature at a depth of 30 cm. Parameter P max is the maximum light-saturated photosynthesis rate and the parameter k is equal to the PPFD at which photosynthesis rate is half of its maximum. Parameter a is the initial slope of the saturating function. Parameter T opt_air denotes the temperature optimum for photosynthesis and T tol_air the temperature tolerance (deviation from the optimum at which P G is 60% of its maximum). Equation 1 describes the photosynthetic response of Carex–Scorpidium flarks, Trichophorum tussock flarks and Betula–Sphagnum lawns, as well as the photosynthetic response of Ericales–Pleurozium strings in the early summer of 2008 (E-P08). Equation 2 describes the photosynthetic response of Ericales–Pleurozium strings (E–P) in 2007. It is similar to Eq. 1 but has an additional Gaussian response to T 30 cm, T opt_30 cm and T tol_30 cm, denoting optimum and tolerance, respectively. The model parameters for the different plant community types are shown in Table 1.
In Eqs. 3–6, T air is the air temperature, T 5 cm and T 10 cm are the peat temperatures at depths of 5 and 10 cm, respectively, WT is the water level and VGA is the vascular green area. In Eqs. 3, 5, and 6, the respiration response to T air or T 5 cm is described with the exponential function from Lloyd and Taylor (1994). Parameter R 10 is the respiration rate at 10°C when WT is non-limiting and VGA is zero, b is the activation energy divided by the gas constant, T ref is a reference temperature, set at 283.15 K and T 0 is the temperature minimum at which respiration reaches zero, set at 227.13 K (Lloyd and Taylor 1994). Equation 3 describes the respiration response of the Carex–Scorpidium, Trichophorum and Betula–Sphagnum types. Parameter b 2 is 50% of the maximum respiration when other factors are not limiting, b 3 denotes the slope determining the speed and direction of change in R ECO through the water-level range. Equation 4 describes the respiration response of Ericales–Pleurozium strings (E–P) when the peat was unfrozen and the water-level data were thus available. It has a Gaussian response to T 10 cm: the parameter T opt_10 cm denotes the temperature optimum for ecosystem respiration and T tol_10 cm the temperature tolerance (deviation from the optimum at which R TOT is 60% of its maximum). Parameter R WT0 is the respiration rate when temperature is not limiting and WT is zero. Parameter w denotes the initial slope of the WT function. Equation 5 describes the respiration response of E–P when the peat is still frozen: it includes both the Gaussian response to T 10 cm and the exponential Lloyd and Taylor response to T 5 cm. Equation 6 describes the respiration response of E–P in the early summer of 2008. The model parameters for the different plant community types are shown in Table 2.
Reconstructing CO2 exchange over the growing season
To derive a growing season estimate for CO2 exchange based on the chamber measurements, and to examine within-season changes in plant community CO2 dynamics, we applied the models for reconstructing P G and R ECO from early June to September 2007 with a 30-min time step.
As VGA is a crucial control of the community CO2 exchange, any difference in VGA between the study plot communities (the sample) and that community type in the mire would cause a large error in the CO2 balance of the community type (see also Riutta et al. 2007b). Indeed, we observed differences between the vascular plant cover of the community types as estimated for the study plots on July 20, 2007 and the vascular plant cover estimated in the 2006 vegetation inventory. We attribute the differences to random sampling error, to our study plot selection where sites with large shrubs were not selected for operational reasons, and to the damage caused by the metal collar installation and repeated measurements to plants with wide-ranging root systems, such as Betula nana, Salix spp., Ledum palustre and Empetrum nigrum. Regarding the observed vascular plant cover relation, we derived a correction coefficient that we applied to the VGA data in the reconstruction.
To acknowledge the differences in VGA between the study plot communities and the community types in the mire we made the reconstruction in three different ways: 1) using the original VGA and WT data from the study plot plant communities, 2) using the original WT data from the study plot plant communities but applying a correction coefficient to VGA based on the vegetation inventory data and 3) using averaged WT and (corrected) VGA for each plant community type.
To strengthen our understanding of the crucial (Aurela et al. 2004) early season CO2 exchange, we also simulated P G and R ECO for June 2008. A half-hourly reconstructed NEE was calculated as the difference between P G and R ECO . We present a net uptake of CO2 to the ecosystem as positive NEE values and a net loss of CO2 to the atmosphere as negative values.
Continuous air temperature and PPFD data were obtained from the weather station at the site. A continuous VGA was derived from the VGA models or from the interpolated VGA data. The water level in the study plots was linearly interpolated between measurement days. The peat temperature in strings at a depth of 5 cm was obtained from temperature loggers (i-buttons, Dallas Semiconductor Corp.) for the growing season of 2007. The peat temperature at a depth of 30 cm in 2007 was linearly interpolated from manual measurements, while for June 2008 the information was obtained from temperature loggers (i-buttons, Dallas Semiconductor Corp.). Those peat temperatures that were not obtained from the loggers were reconstructed from the air temperature and measured peat temperatures.
Based on the community-level CO2 exchange estimates and the area proportions of the different plant community types, derived from the vegetation inventory, we estimated the net CO2 exchange per average square metre at the Kaamanen mire for the growing season of 2007 and for the early growing season (May 29 to June 26) of 2008.
Statistical testing
We applied ANOVA to test if plant community types differ in their biotic and abiotic conditions and in their responses to controlling factors. We tested the significance of the differences in water table position and VGA between the four plant community types using one-way ANOVA of repeated measures and Tukey’s post hoc test. We tested the significance of the difference in the P G model parameters between the plant community types with a t-test. We tested the significance of the differences in P G , R ECO and NEE between the plant community types using one-way ANOVA and Tukey’s test.
Results
Plant community types in the mire
Based on the species composition, the vegetation in the Kaamanen mire was classified into Ericales–Pleurozium string tops (E–P), Betula–Sphagnum string marginal areas (B–S), Trichophorum flarks (T) and Carex–Scorpidium wet flarks (C–S) (Table 3; Fig. 1). Communities formed a compositional continuum along a moisture gradient (the first DCA axis in Fig. 2). In the moss layer, brown mosses such as Scorpidium scorpidioides dominated the wet end of the gradient. Brown mosses were replaced by Sphagnum and finally, in the dry end of the gradient, feather mosses dominated. As for vascular plants, the wet part of the gradient from Carex–Scorpidium community to Betula–Sphagnum community was dominated by different sedges, while the dry end, the Ericales–Pleurozium community, was dominated by dwarf shrubs. According to CCA, these types explained the variation in vegetation in a statistically significant way (Monte Carlo test, p = 0,002). The division to four plant community types explained 22.8% of the variation in the vegetation data.
A schematic cross-section of a string, showing the small-scale distribution of the plant community types. The presentation is based on frost and water-table measurements in late June 2008. For an aerial photograph of the study site, see Aurela et al. (1998)
DCA ordination of the plant species and communities in the Kaamanen mire. Axis 1 represents the water table gradient of the Fig. 1. The two DCA axes together explain 19% of the species distribution
Water level in different plant community types
The vegetational differences between the types were coupled with differences in environmental variables, especially water level and ground frost. Taking into account the small sample size, the water table difference between the types was clear (Fig. 3; Table 4). Most of the differences were significant (p < 0.05) (Table 5), while the differences between Trichophorum community and the other minerotrophic communities were not as evident (p ≤ 0.1) (Fig. 3; Table 5). The differences in water level reflect the morphology of the mire (Fig. 1). The strings had ice lenses inside them until late July.
Water table in the measured Ericales–Pleurozium (E–P), Betula–Sphagnum (B–S), Trichophorum (T) and Carex–Scorpidium (C–S) plant communities (and standard deviation, n = 4). Water table in the one Ericales–Pleurozium study plot where water table could be measured (no frost) during the growing season (E–P#1)
Vascular green area in different plant community types
Similarly to species composition (Fig. 2), the communities formed a continuum in respect of leaf area extent (Table 4; Fig. 4a, b) and plant functional group composition (Fig. 4c). The vascular green area decreased from the driest Ericales–Pleurozium type towards the wettest Carex–Scorpidium type (Fig. 4). The plant communities formed two groups regarding significant differences in the corrected VGA (Table 5): in the Ericales–Pleurozium and Betula–Sphagnum plant community types VGA was significantly larger than in the wet Trichophorum and Carex–Scorpidium community types (Table 5). Moss cover was denser in the dryer communities: average moss coverage was 44% (SE ± 16) in the Ericales–Pleurozium type, 54% (SE ± 26) in the Betula–Sphagnum type, 11% (SE ± 4.2) in the Trichophorum type and 5.0% (SE ± 2.0) in the Carex–Scorpidium type. The Ericales–Pleurozium type was dominated by evergreen dwarf shrubs and Rubus chamaemorus, the Betula–Sphagnum type by deciduous dwarf shrubs and sedges, and the Trichophorum and Carex–Scorpidium types by sedges (Fig. 4c).
Vascular green area in the plant community types. a Modelled VGA for the study plots (line). Average measured study plot VGA (scatterplots) and standard deviation (error bars, n = 4). b Modelled VGA corrected to represent the average community type VGA in the mire. Standard deviation for each plant community type derived from the vegetation inventory (error bars). c Measured VGA 11th July, 2007, divided into functional groups
The dwarf shrub VGA showed little temporal variation during the growing season. The sedge VGA showed pronounced seasonal dynamics: it followed the same kind of loglinear curve in all plant community types with sedges, reaching its peak on average on July 21, 2007.
CO2 exchange in different plant community types
Measured net CO2 exchange
The measured net carbon dioxide exchange in the study plots varied between −1200 and 900 mg CO2 m−2 h−1. The largest instantaneous CO2 uptake fluxes were measured in the Betula–Sphagnum and Ericales–Pleurozium types, while the net CO2 exchange in the Carex–Scorpidium type was always close to zero (Fig. 5). The most negative instantaneous net CO2 exchange was measured in the Ericales–Pleurozium type. Measured chamber fluxes were within the same range or smaller than the measured eddy covariance fluxes, which is for a large part explained by the fact that VGA was smaller in the study plots than in the mire in general (Table 4).
Measured carbon dioxide exchange (NEE) in 2007 in Ericales–Pleurozium, Betula–Sphagnum, Trichophorum and Carex–Scorpidium plant community types. Positive values denote carbon uptake, negative values carbon release from the ecosystem. Daytime (local winter time 8 a.m.–4 p.m.) eddy covariance flux is presented as a reference
Modelled CO2 exchange responses
The plant community photosynthesis (P G ) in the Kaamanen mire was controlled by the photosynthetic photon flux density (PPFD), the vascular green area (VGA) and the air temperature (Eqs. 1, 2); Ericales–Pleurozium P G was also affected by peat temperature at a depth of 30 cm (Eq. 2). The ecosystem respiration (R ECO ) was controlled by temperature, water level, and VGA (Eqs. 3–6).
Although the actual controlling factors for photosynthesis and ecosystem respiration were approximately the same in all the plant communities, the communities could be divided into two groups based on the relative importance of the controlling factors and their response shapes. By response shapes we refer to model equations and parameter values presented in Eqs. 1–6 and Tables 1 and 2. We distinguished two functional components in the mire: an ombrotrophic component (Ericales–Pleurozium strings) and a minerotrophic component (other plant community types). The two most important spatially variable factors controlling CO2 exchange at the Kaamanen mire were WT and VGA. These controlled CO2 exchange differently in the Ericales–Pleurozium community type than in the three other plant community types. Also temperature responses differed.
R ECO response to WT was of different shape in the Ericales–Pleurozium community type than in the other three plant community types (Fig. 6). WT also explained a smaller part of the data variation in the Ericales–Pleurozium plant community type (Fig. 6). When examined separately, the R ECO water level responses for the minerotrophic communities differed, but when combined, a linear (Betula–Sphagnum), a nonexistent (Trichophorum) and an exponential (Carex–Scorpidium) water response merged into one sigmoidal water response (Fig. 6). For the minerotrophic component, R ECO has the often-used shape (Tuittila et al. 2004; Riutta et al. 2007a, b) with an exponential temperature response from Lloyd and Taylor (1994), and a sigmoidal water response. The Ericales–Pleurozium model responses are somewhat more unorthodox, with obscure optimum-shaped temperature responses, possibly reflecting peat moisture or some other factors, rather than temperature.
P G per unit VGA was substantially lower in the Ericales–Pleurozium plant community type than in the other plant communities (Table 4). For the other plant community types the variation in VGA was, apart from PPFD, the main control for PG, while for the Ericales–Pleurozium community type temperature and possibly moisture factors were more important.
In P G , we could fit models of the same shape separately to all the plant community types and then test the differences in the parameter values between the community types statistically. The models followed the form of Eq. 1 except that we used a linear, not a saturating VGA response. We tested the ecophysiological model parameters: the shape of the PPFD response (k), the air temperature optimum (T opt_air ) and tolerance (T tol_air ). All differences between the model parameters were clearly insignificant (p ≥ 0.83), which we consider as evidence for the use of the combined model for the three minerotrophic plant community types.
We also tested the difference in the model parameters between the collective minerotrophic plant community P G model (Eq. 1; Table 1) and the ombrotrophic P G model (Eq. 2; Table 1). The model parameter P max was significantly lower in the Ericales–Pleurozium model compared to the model for the other plant communities (p = 0.036). The air temperature optimum (T opt_air ) was lower (p = 0.060) in the Ericales–Pleurozium model than in the minerotrophic model, only 19°C, and tolerance (T tol_air ) larger (p = 0.039). The impact of ground frost was included in the ombrotrophic P G model through the peat temperature at a depth of 30 cm. Low temperatures in the deep peat were found in connection with low photosynthesis.
The measured values in 2007 and the data from June 2008 that we used for model validation coincided well with the modelled values (Fig. 7). The photosynthesis and ecosystem respiration models were based on data from one growing season only, but the explaining power (R 2) of the models, especially for the minerotrophic types, was good (Tables 1, 2) and validation with the early growing season 2008 data showed a good fit. In the Ericales–Pleurozium model the explaining power (R 2) was lower and there was more unexplained variation (Tables 1, 2).
Comparison of the observed and modelled values. Gross photosynthesis (P G ) (Eqs. 1, 2; Table 1) and ecosystem respiration (R ECO ) (Eqs. 3, 4–6; Table 2) of the ombrotrophic Ericales–Pleurozium (E–P) plant community and the minerotrophic Betula–Sphagnum (B–S), Trichophorum (T) and Carex–Scorpidium (C–S) plant communities. Note the different scales in the R ECO figures
CO2 exchange reconstructed over the growing season
The most important factors controlling CO2 exchange in the minerotrophic component, i.e. peat aerobic volume (a control of ecosystem respiration) and vascular green area (a control of photosynthesis), increased from the wet Carex–Scorpidium plant community type towards the driest Betula–Sphagnum type. When the VGA was corrected to the level of the mire in general, two significantly different groups of plant communities appear regarding environmental factors and gross CO2 exchange (Tables 4, 5). In Ericales–Pleurozium and Betula–Sphagnum communities peat aerobic volume, VGA, P G and R ECO were significantly larger than in the wet Trichophorum and Carex–Scorpidium communities (Figs. 3, 4; Table 5). The resulting NEE, however, was the same for all three minerotrophic communities (Table 4), whereas the ombrotrophic Ericales–Pleurozium NEE differed significantly (Table 5).
The large R ECO in the ombrotrophic Ericales–Pleurozium plant community is the result of the large aerobic peat volume (Table 4). The VGA in the Ericales–Pleurozium community was also the largest of the plant community types. However, as the Ericales–Pleurozium P G per unit leaf area was considerably less efficient than for the minerotrophic types (Table 4), the resulting NEE was not as high as that in the minerotrophic communities (Fig. 8b).
Reconstructed a daily net carbon dioxide exchange and b growing season 2007 net and gross carbon dioxide exchange in Ericales–Pleurozium (E–P), Betula–Sphagnum (B–S), Trichophorum (T) and Carex–Scorpidium (C–S) plant community types and in the mire on average (ch for chamber measurement reconstruction, ec for eddy covariance results). Positive values denote CO2 uptake, negative values CO2 release from the ecosystem into the atmosphere
All plant community types functioned as CO2 sinks in the reconstruction of the growing season of 2007. The minerotrophic plant community types were efficient sinks, while the CO2 balance for the ombrotrophic Ericales–Pleurozium type was close to zero.
CO2 exchange in the whole mire
The extrapolated CO2 exchange for the whole mire ecosystem followed approximately the same dynamics as the CO2 exchange measured with the eddy covariance method (Fig. 8a). Both the P G and R ECO rates were lower, however, than the eddy covariance results, except for the early growing season. The largest differences in NEE between the two estimates take place at peaks of high NEE (Fig. 8a). As a result of these differences, the total growing season CO2 balance extrapolated from the chamber measurements is lower than the balance given by the eddy covariance measurements (Fig. 8b).
When daily NEE measured with the eddy covariance technique and daily NEE extrapolated from the chamber results were plotted against average PPFD (Fig. 9), it appeared that PPFD response, as measured by the eddy covariance technique, varied within the growing season. Most days the eddy covariance results match with the chamber results, but on 17–20 days in the peak growing season the response followed a different, yet consistent, pattern (Fig. 9). On the 17 differing days marked in Fig. 9, 117 g of CO2 is fixed according to eddy covariance result, which is 49% of the total CO2 uptake in the growing season 2007. In the chamber extrapolation the uptake during those 17 days is only 50 g.
Discussion
Minerotrophic communities appear to be stronger sinks than the ombrotrophic community
The pattern that emerged, in which the minerotrophic plant communities (Carex–Scorpidium, Trichophorum and Betula–Sphagnum) in the Kaamanen mire were efficient sinks for CO2 whereas the ombrotrophic Ericales–Pleurozium community CO2 balance was close to zero, coincides with the results from previous studies in northern Fennoscandia. Similarly to our study, Heikkinen et al. (2002) found flarks and strings with Betula nana to be CO2 sinks, while strings without Betula nana were CO2 sources. Likewise, in a study by Nykänen et al. (2003) at a palsa mire not far from Kaamanen, palsa surfaces with sparse vegetation were CO2 sources, whereas flarks and palsa surfaces with shrub vegetation were CO2 sinks. Bäckstrand et al. (2008) found a similar pattern at a palsa mire in subarctic Sweden: Eriophorum sites took up considerably more CO2 than palsa sites (Bäckstrand et al. 2008; Fig. 8).
The minerotrophic component: functionally similar communities replacing each other along a water-table gradient
The differences in photosynthesis and ecosystem respiration between the minerotrophic plant community types were solely due to the environmental gradients in water level and leaf area. The pattern is similar to that observed by Laine et al. (2007) in an oceanic blanket bog. Our results showed that a range of different minerotrophic plant community types, although fairly different in their species composition, water level and vascular green area, can be similar in their photosynthetic and respiration responses, and show similar dynamics in their CO2 exchange. Furthermore: when combined, some apparently differing responses may merge into a logical, collective pattern. This was the case for the water-level responses in the Kaamanen fen, just as it was for the water-level responses in the blanket bog studied by Laine et al. (2007, Table 6b).
The spatial variation in mire plant community composition is linked to variations in photosynthetic and respiration responses (Bubier et al. 2003; Riutta et al. 2007b). Using tools such as TWINSPAN, we can divide the ecosystem into qualitatively and quantitatively distinct plant communities. But how many divisions should we make, how many different components do we have to study separately to understand the system, and how many different models or parameterizations must we construct to describe the ecosystem processes reliably? Williams et al. (2006) found, in their study of an arctic catchment in Alaska, that tussock tundra sites situated at different points of a topographical gradient could be described with the same photosynthesis and respiration models, whereas shrub and sedge sites had different temperature and light responses. Laine et al. (2009) found that in a northern boreal mire called Kiposuo, models parameterized for the entire mire produced growing season estimates that were within the uncertainty range of the results from models parameterized by taking into account different levels of spatial variation. The Kiposuo mire is situated a mere 6 km north-east of the Kaamanen fen, but differs from it in having uniform surface topography and exclusively minerotrophic plant communities.
The ombrotrophic component: cut off from the fen system
There has been a lot of research on the spatial variability of CO2 exchange at more southern peatlands in the boreal and temperate zones, some also in aapa mires. The height of the Kaamanen strings makes their CO2 exchange different from the hummocks of these studies. Previously studied hummocks on aapa mires (Moore 1989; Bubier et al. 1998; Riutta et al. 2007b) have usually had a water level of from −20 to −30 cm and been Sphagnum-dominated, resembling the Betula–Sphagnum community type in this study.
Ericales–Pleurozium strings are, due to their height, cut off from contact with flowing mire water (Fig. 1). They are not part of the same water-level gradient: the water level does matter for ecosystem respiration, but the response is different (Eqs. 3, 4; Fig. 6). In studies on peatland carbon exchange, the usual procedure for including soil moisture in the models is by measuring the water level relative to the moss surface. This approach has produced plausible models with good R 2s (Bubier et al. 2003; Tuittila et al. 2004; Riutta et al. 2007a). However, Lafleur et al. (2005) found only a weak relationship between ecosystem respiration and water-table depth in the temperate Mer Bleue bog.
The peatlands in the studies by Bubier et al. (2003), Tuittila et al. (2004) and Riutta et al. (2007a) are fens with a water table varying between −35 and +5 cm (Burrows et al. 2005 for the peatland in Bubier et al. (2003), −48 and +10 cm (Tuittila et al. 2004) and −45 and 0 cm (Riutta et al. 2007a) below moss surface. In the Mer Bleue bog, water table varied between 30 and 75 cm below the hummock tops (Lafleur et al. 2005), a range similar to that of Kaamanen strings (Fig. 3). Both in the Mer Bleue bog and Kaamanen fen, there was only a weak control of water level on ecosystem respiration. In Mer Bleue and probably also in Kaamanen, the connection of water level to surface peat moisture was weak (Lafleur et al. 2005), due to hummock/string height. Silvola et al. (1996) found in their study on soil CO2 emission from 26 peatland sites that the relative increase in ecosystem respiration was reduced when the water table fell below 30 cm. In simulations of the carbon exchanges of Mer Bleue, the Peatland Carbon Simulator (Frolking et al. 2002) predicted that there is very little contribution to R ECO from the peat layer below −35 cm, even when the water table is quite deep. Our observation on R ECO response to water level in the Kaamanen mire (Fig. 6) coincides with these results. Surface peat temperature played a central role in the Ericales–Pleurozium R ECO models (Eqs. 4–6). This is again similar to the results from Mer Bleue (Lafleur et al. 2005) where peat temperatures at depths of −5 and −10 cm were observed to follow a similar temporal trend as R ECO .
Soil moisture is a control for both ecosystem respiration and photosynthesis (e.g. Illeris et al. 2004). The unusually low temperature optimums in all ombrotrophic models and the existence of a temperature optimum in the respiration models could relate to evapotranspiration and peat moisture. Ecosystem respiration as such increases with temperature increase in all known soils (Lloyd and Taylor 1994; Bekku et al. 2003).
The linkage between the vascular green area and P G did not work in the same way as for the minerotrophic component, due to the differences in the functional traits of the plants (Table 3; Chapin and Shaver 1989, Table 3, calculated from Chapin et al. 1980): minerotrophic communities are dominated by sedges and deciduous shrubs with a high photosynthetic capacity per unit leaf area, while Ericales–Pleurozium communities consist mainly of evergreen shrubs with a low photosynthetic capacity. In the Kaamanen string community, we observed large variations in both photosynthesis and respiration within the growing season, some of which remains unexplained.
This could relate to moss and dwarf shrub photosynthesis. Mosses have a clear, species-specific moisture optimum for photosynthetic capacity (Dilks and Proctor 1979): moss photosynthesis in mires is largely controlled by surface peat moisture. The photosynthetic capacity of cold climate evergreen dwarf shrubs has been observed to show large variations, even during the growing season (Karlsson 1985; Lundell et al. 2008), which could be a result of water stress during summer (Karlsson 1985) or of some other, yet unidentified, limiting factor overriding temperature during the summer months (Lundell et al. 2008). Our results are, however, contrasting to several previous studies where mire communities dominated by evergreen plants have been more stable in their CO2 dynamics (Leppälä et al. 2008), as well as more resistant to drought (Bubier et al. 2003; Riutta et al. 2007a) than communities dominated by graminoids.
Rather than to more southern fen hummocks, the Kaamanen strings have a functional resemblance to palsas. Ground frost remains in the surface peat until late July, and the subarctic climate with its low temperatures and continuous radiative forcing poses challenges to the plants. The vegetation zonation of palsas studied by Tsuyuzaki et al. (2008) in their preliminary study in northern Alaska was similar to that of the Kaamanen mire: Vaccinium vitis-idaea on the top areas, Sphagnum spp. on intermediate locations and Carex aquatilis on the bottom areas. The top areas showed extremely low water content at the time of measurement in early August (Tsuyuzaki et al. 2008). Tsuyuzaki et al. (2008) concluded that the vegetation zonation of palsas was determined by the water content of the peat and duff layers, rather than by the water level. The same could apply to CO2 exchange, as in Mer Bleue (Lafleur et al. 2005). On mineral soil in the high Arctic, the spatial variability in soil moisture has been proposed as the primary driver of NEE (Sjögersten et al. 2006). We postulate that the string CO2 uptake, both P G and R ECO , were partly controlled by a factor or factors we did not measure, such as surface peat moisture.
The discrepancy between eddy covariance and chamber results
The eddy covariance (EC) method gives us continuous direct measurements of CO2 fluxes averaged on an ecosystem scale, whereas the whole-mire reconstruction based on chamber measurements is derived from measurement data that has a very limited coverage in space and time. In addition, the main focus of interest, NEE, is in the chamber method a small difference between two very large fluxes. This means that relatively small errors or deviations between plant communities in reconstructed P G and R ECO can become very large in reconstructed NEE. Despite the potentially large uncertainties, good match between eddy covariance and chamber results has been reached in several previous studies on mire CO2 exchange (e.g. Laine et al. 2006; Riutta et al. 2007a).
The difference between the Kaamanen EC results and chamber reconstruction results originates from days with unusually high NEE relative to average PPFD (Fig. 9). During the early growing season both in 2007 and 2008 the EC results and the chamber reconstruction match very well (Fig. 8a) but in the peak growing season large discrepancies occur. The 17 days marked in Fig. 9 comprise one fifth of the measurement period (94 days) but are responsible for half of all EC measured CO2 uptake in the growing season of 2007. The procedure of reconstructing CO2 exchange over time is based on the assumptions that all the factors controlling CO2 exchange are included in the model and that the model responses do not change during the growing season. These assumptions do not seem to be true for Kaamanen in the growing season of 2007.
The largest uncertainties in our models occur in the models for the ombrotrophic string component, the Ericales–Pleurozium plant community type. The CO2 exchange responses are more complicated in the Ericales–Pleurozium plant community than in the minerotrophic plant communities where P G is straightforwardly determined by VGA and by water table position (Lafleur et al. 2005). The large VGA and aerobic peat volume (low WT) can potentially bring about large CO2 fluxes, either uptake or release, should the conditions such as moisture or photosynthetic capacity of the plant leaves be favorable. The sites of the studies where good match between eddy covariance results and chamber reconstruction has been found (Laine et al. 2006; Riutta et al. 2007a) lack the kind of high microforms (WT < −40) present in Kaamanen. It would therefore be tempting to attribute the discrepancy between eddy covariance results and chamber reconstruction to the ombrotrophic component of the mire. In the lack of further studies, however, the source of the discrepancy between eddy covariance and chamber results remains unidentified.
Kaamanen strings: bog islands in the minerotrophic fen
Minerotrophy–ombrotrophy, or the fen-bog functional dichotomy, is the key division in peatland ecology (Rydin and Jeglum 2006). Minerotrophic systems, or fens, are dominated by species with a high nutrient demand, a short leaf life span and high leaf photosynthetic capacity. By contrast, ombrotrophic bogs are dominated by species with a low nutrient demand, a long leaf life span and low leaf photosynthetic capacity (Rydin and Jeglum 2006). Generally, in the long term course of development, fens are gradually transformed into bogs when invading Sphagnum mosses form thick peat that isolates the vegetation from minerogenic waters (Rydin and Jeglum 2006). This successional gradient of mires can be observed by paleoecological methods (e.g. Tuittila et al. 2007) or from chronosequences of different-aged mires (Klinger and Short 1996; Leppälä et al. 2008). In the within-mire scale, the succession does not proceed in a uniform way in different surfaces. Aapa mires are combinations of wet, nutrient-rich, fen-like flarks and dry, nutrient-poor, bog-like strings. Strings in the Kaamanen mire can be perceived, judged by their evergreen vegetation and peat thickness, as bog islands in a minerotrophic fen. Bogs and fens show important differences in their CO2 exchange dynamics. Leppälä et al. (2008) showed, in a mire chronosequence in Finland, that the compositional differences in the vegetation of bogs and fens were linked to differences in CO2 dynamics. This was also true on the miniature scale for the Kaamanen mire.
Ward et al. (2009) found, in their plant removal experiment, that the presence of ericoid dwarf shrubs had a suppressing effect on short-term carbon cycling at a peatland site. Graminoids assimilate CO2 at a greater rate than dwarf shrubs or bryophytes (Ward et al. 2009) because of their acquisitive evolutive strategy compared to the conservative strategy of ericoid dwarf shrubs (Lavorel and Garnier 2002). Indeed, in a successional gradient on the Finnish coast of the Gulf of Bothnia (Leppälä et al. 2008), young graminoid-dominated sites assimilated more CO2 than old sites with a larger proportion of evergreen vegetation. In Russia, too, marshes and fens have been found to have higher C accumulation than bogs (Botch et al. 1995), and in Canada, Bubier et al. (1999) found that sedge-dominated fens sequestered more CO2 than mires dominated by shrubs.
Several studies on the spatial variation in mire CO2 exchange have revealed the importance of the functional differences between plant communities. Our results suggest that the robust division between ombrotrophic and minerotrophic ecosystems, or ecosystem components might be sufficient in describing the functional patterns.
Early growing season processes
Aurela et al. (2004) observed the timing of snow melt to be the most important single determinant of the annual CO2 balance in the Kaamanen fen, affecting through the June CO2 balance. Snow melts in Kaamanen in the period Apr 20–May 23 (Aurela et al. 2004), so the mechanism mediating the effect has to involve a time lag.
Based on our results here, the time-lag mechanism that causes this pattern is different in the minerotrophic and ombrotrophic mire components. After snowmelt, two processes start at the Kaamanen fen. First, in the minerotrophic mire component, sedge and deciduous shrub leaf growth begins when the snow and ice melts, but it takes some time for the VGA to grow. Second, the removal of the snow insulation allows the sun’s radiative warming to start to melt the ice cores inside the strings. Where frost or other lack of water does not impede, evergreen plants can start photosynthetizing immediately following snow melt (Bubier et al. 1998; Moore et al. 2006), or even before that (Lundell et al. 2008). Kingsbury and Moore (1987) proposed that string plants suffer from transpiration stress in the early growing season frost conditions. Our results support this view: ground frost, included in the model through the peat temperature at a depth of 30 cm, had a negative impact on photosynthesis in the ombrotrophic (string) component. It is common for evergreen plants to down-regulate their photosynthetic capacity in times of low temperature and ground frost (Öquist and Huner 2003; Harris et al. 2006; Lundell et al. 2008).
Implications
There has been an ongoing debate among plant community ecologists around the framework referred to as the ‘Holy Grail’ in ecology: whether it is justified to use plant functional traits, rather than species identities, to generalize complex community dynamics and predict the effects of environmental changes (Lavorel and Garnier 2002; Dorrepaal 2007; Suding and Goldstein 2008). Our results imply that, at least for the CO2 dynamics of the Kaamanen subarctic minerotrophic mire, functional classification serves its purpose well. Communities dominated by graminoids and deciduous shrubs respond to environmental factors in a uniform way, which is different from the response of communities dominated by dwarf shrubs. Essentially, the community functional type composition of the Kaamanen mire reflects the within-mire variation in hydrological conditions and nutrient status.
When estimating CO2 fluxes on a landscape scale, plant functional types are simple to observe and widely used in land surface models (LSMs) (Williams et al. 2009). If minerotrophic mire types were observed to behave in a consistent way also on a wider scale, good ecophysiological, process-based CO2 flux estimations could be built for them using remote sensing data of plant functional types, temperature, water level and vascular green area. The results could be applicable to large, remote areas in Siberia and high-latitude North America with similar vegetation (Tsuyuzaki et al. 2008). The area of wet minerotrophic plant community types is currently increasing in the region of discontinuous permafrost in North America, as the melting of string permafrost creates wet depressions in the terrain (Halsey et al. 1995). Due to the large production by Sphagnum mosses and sedges, the CO2 absorption of these wet surfaces is substantially more efficient than that of the permafrost formations, which are dominated by forest mosses and dwarf shrubs (Turetsky et al. 2007). Our study confirms that wet minerotrophic mire types can be considerable CO2 sinks. This can be of importance to the world carbon balance in the altered conditions, under which ombrotrophic permafrost ecosystems melt into wet minerotrophic ecosystems.
Abbreviations
- VGA:
-
Vascular green area
- TWINSPAN:
-
Two Way INdicator SPecies ANalysis
References
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5:169–211
Alm J, Schulman L, Walden J, Nykänen H, Martikainen PJ, Silvola J (1999) Carbon balance of a boreal bog during a year with an exceptionally dry summer. Ecology 80:161–174
Alm J, Shurpali NJ, Tuittila E-S, Laurila T, Maljanen M, Saarnio S, Minkkinen K (2007) Methods for determining emission factors for the use of peat and peatlands—flux measurements and modelling. Boreal Environ Res 12:85–100
Aurela M, Tuovinen JP, Laurila T (1998) Carbon dioxide exchange in a subarctic peatland ecosystem in northern Europe measured by the eddy covariance technique. J Geophys Res D: Atmos 103:11289–11301
Aurela M, Laurila T, Tuovinen JP (2001) Seasonal CO2 balances of a subarctic mire. J Geophys Res D: Atmos 106:1623–1637
Aurela M, Laurila T, Tuovinen JP (2002) Annual CO2 balance of a subarctic fen in northern Europe: importance of the wintertime efflux. J Geophys Res D: Atmos 107:4607
Aurela M, Laurila T, Tuovinen JP (2004) The timing of snow melt controls the annual CO2 balance in a subarctic fen. Geophys Res Lett 31:L16119
Bäckstrand K, Crill PM, Mastepanov M, Christensen TR, Bastviken D (2008) Total hydrocarbon flux dynamics at a subarctic mire in northern Sweden. J Geophys Res G: Biogeosci 113:G03026
Baldocchi DD (2003) Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: past, present and future. Global Change Biol 9:479–492
Bekku YST, Nakatsubo A, Kume M, Adachi M, Koizumi H (2003) Effect of warming on the temperature dependence of soil respiration rate in arctic, temperate and tropical soils. Appl Soil Ecol 22:205–210
Botch MS, Masing VV (1983) Mire ecosystems in the USSR. In: Gore AJP (ed) Ecosystems of the World 4B. Mires: swamp, bog, fen and moor. Elsevier, Amsterdam
Botch MS, Kobak KI, Vinson TS, Kolchugina TP (1995) Carbon pools and accumulation in peatlands of the former Soviet-Union. Global Biogeochem Cycles 9:37–46
Bubier JL, Crill PM, Moore TR, Savage K, Varner RK (1998) Seasonal patterns and controls on net ecosystem CO2 exchange in a boreal peatland complex. Global Biogeochem Cycles 12:703–714
Bubier JL, Frolking S, Crill PM, Linder E (1999) Net ecosystem productivity and its uncertainty in a diverse boreal peatland. J Geophys Res D: Atmos 104:27683–27692
Bubier JL, Bhatia G, Moore TR, Roulet NT, Lafleur PM (2003) Spatial and temporal variability in growing-season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems 6:353–367
Burrows EH, Bubier JL, Mosedale A, Cobb GW, Crill PM (2005) Net ecosystem exchange of carbon dioxide in a temperate poor fen: a comparison of automated and manual chamber techniques. Biogeochemistry 76:21–45
Chapin FS, Shaver GR (1989) Differences in growth and nutrient use among arctic plant-growth forms. Funct Ecol 3:73–80
Chapin FS, Johnson DA, Mckendrick JD (1980) Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem—implications for herbivory. J Ecol 68:189–209
Dilks TJ K, Proctor MCF (1979) Photosynthesis, respiration and water content in bryophytes. New Phytol 82:97–114
Dorrepaal E (2007) Are plant growth-form based classifications useful in predicting northern ecosystem carbon cycling feedbacks to climate change? J Ecol 95:1167–1180
Eurola S, Huttunen A, Kukko-oja K (1995) Suokasvillisuusopas. Oulun yliopisto, Oulangan biologinen asema, Oulu
Frolking S, Roulet N T, Moore T R, Lafleur P M, Bubier J L, Crill P M (2002) Modeling the seasonal to annual carbon balance of Mer Bleue bog, Ontario, Canada. Global Biogeochem Cycles 16. doi:10.1029/2001GB1457
Gorham E (1991) Northern peatlands—role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Halsey LA, Vitt DH, Zoltai SC (1995) Disequilibrium response of permafrost in boreal continental western Canada to climate-change. Clim Change 30:57–73
Harris GC, Antoine V, Chan M, Nevidomskyte D, Koniger M (2006) Seasonal changes in photosynthesis, protein composition and mineral content in Rhododendron leaves. Plant Sci 170:314–325
Heikkinen JEP, Maijanen M, Aurela M, Hargreaves KJ, Martikainen PJ (2002) Carbon dioxide and methane dynamics in a sub-Arctic peatland in northern Finland. Polar Res 21:49–62
Hill MO (1979) TWINSPAN—A FORTRAN program for arranging multivariate data in an ordered two-way table by classification of the individuals and attributes. Ithaca, NY: Cornell University, Department of Ecology and Systematics
Hill MO, Šmilauer P (2005) TWINSPAN for Windows, version 2.3. Centre for Ecology and Hydrology & University of South Bohemia, Huntingdon & Ceske Budejovice
Illeris L, Christensen TR, Mastepanov M (2004) Moisture effects on temperature sensitivity of CO2 exchange in a subarctic heath ecosystem. Biogeochemistry 70:315–330
IPCC (International Panel on Climate Change) (2007) Climate Change 2007: fourth assessment report. Valencia, Espanja: IPCC
Karlsson PS (1985) Photosynthetic characteristics and leaf carbon economy of a deciduous and an evergreen dwarf shrub—Vaccinium-Uliginosum L and V-Vitis-Idaea L. Holarct Ecol 8:9–17
Kingsbury CM, Moore TR (1987) The freeze-thaw cycle of a sub-Arctic fen, northern Quebec, Canada. Arct Alp Res 19:289–295
Klinger LF, Short SK (1996) Succession in the Hudson Bay lowland, northern Ontario, Canada. Arct Alp Res 28:172–183
Lafleur PM, Moore TR, Roulet NT, Frolking S (2005) Ecosystem respiration in a cool temperate bog depends on peat temperature but not water table. Ecosystems 8:619–629
Laine A, Sottocornola M, Kiely G, Byrne KA, Wilson D, Tuittila E-S (2006) Estimating net ecosystem exchange in a patterned ecosystem: example from a blanket bog. Agric For Meteorol 138:231–243
Laine A, Byrne KA, Kiely G, Tuittila ES (2007) Patterns in vegetation and CO2 dynamics along a water level gradient in a lowland blanket bog. Ecosystems 10:890–905
Laine A, Riutta T, Juutinen S, Väliranta M, Tuittila E-S (2009) Acknowledging the spatial heterogeneity in modeling/reconstructing carbon dioxide exchange in a northern aapa mire. Ecol Modell 220:2646–2655
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Leppälä M, Kukko-Oja K, Laine J, Tuittila E-S (2008) Seasonal dynamics of CO2 exchange during primary succession of boreal mires as controlled by phenology of plants. Ecoscience 15:460–471
Leps J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Lloyd J, Taylor JA (1994) On the temperature-dependence of soil respiration. Funct Ecol 8:315–323
Lundell R, Saarinen T, Åstrom H, Hänninen H (2008) The boreal dwarf shrub Vaccinium vitis-idaea retains its capacity for photosynthesis through the winter. Botany-Botanique 86:491–500
Moore TR (1989) Plant-production, decomposition, and carbon efflux in a subarctic patterned fen. Arct Alp Res 21:156–162
Moore TR, Lafleur PM, Poon DMI, Heumann BW, Seaquist JW, Roulet NT (2006) Spring photosynthesis in a cool temperate bog. Global Change Biol 12:2323–2335
Nykänen H, Heikkinen JEP, Pirinen L, Tiilikainen K, Martikainen PJ (2003) Annual CO2 exchange and CH4 fluxes on a subarctic palsa mire during climatically different years. Global Biogeochem Cycles 17:1018
Oechel WC, Vourlitis GL, Hastings SJ, Bochkarev SA (1995) Change in arctic CO2 flux over 2 decades—effects of climate-change at Barrow, Alaska. Ecol Appl 5:846–855
Öquist G, Huner NPA (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Riutta T, Laine J, Tuittila ES (2007a) Sensitivity of CO2 exchange of fen ecosystem components to water level variation. Ecosystems 10:718–733
Riutta T, Laine J, Aurela M, Rinne J, Vesala T, Laurila T, Haapanala S, Pihlatie M, Tuittila E-S (2007b) Spatial variation in plant community functions regulates carbon gas dynamics in a boreal fen ecosystem. Tellus 59B:838–852
Ruuhijärvi R (1983) The Finnish mire types and their regional distribution. In: Gore AJP (ed) Ecosystems of the World 4B. Mires: swamp, bog, fen and moor. Regional studies. Elsevier, Amsterdam
Rydin H, Jeglum J (2006) The biology of peatlands. Oxford University Press, Oxford
Shurpali NJ, Verma SB, Kim J, Arkebauer TJ (1995) Carbon-dioxide exchange in a peatland ecosystem. J Geophys Res D: Atmos 100:14319–14326
Silvola J, Alm J, Ahlholm U, Nykänen H, Martikainen PJ (1996) CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J Ecol 84:219–228
Sjögersten S, van der Wal R, Woodin SJ (2006) Small-scale hydrological variation determines landscape CO2 fluxes in the high Arctic. Biogeochemistry 80:205–216
Suding KN, Goldstein LJ (2008) Testing the Holy Grail framework: using functional traits to predict ecosystem change. New Phytol 180:559–562
ter Braak CJF, Šmilauer P (2002) CANOCO Reference manual and CanoDraw for Windows User’s guide: software for Canonical Community Ordination, (version 4.5), Microcomputer Power, Ithaca, 500 pp
Tsuyuzaki S, Sawada Y, Kushida K, Fukuda M (2008) A preliminary report on the vegetation zonation of palsas in the Arctic National Wildlife Refuge, northern Alaska, USA. Ecol Res 23:787–793
Tuittila E-S, Vasander H, Laine J (2004) Sensitivity of C sequestration in reintroduced sphagnum to water-level variation in a cutaway peatland. Restor Ecol 12:483–493
Tuittila E-S, Väliranta M, Laine J, Korhola A (2007) Quantifying patterns and controls of mire vegetation succession in a southern boreal bog in Finland using partial ordinations. J Veg Sci 18:891–902
Turetsky MR, Wieder RK, Vitt DH, Evans RJ, Scott KD (2007) The disappearance of relict permafrost in boreal north America: effects on peatland carbon storage and fluxes. Global Change Biol 13:1922–1934
Waddington JM, Roulet NT (2000) Carbon balance of a boreal patterned peatland. Global Change Biol 6:87–97
Ward SE, Bardgett RD, McNamara NP, Ostle NJ (2009) Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct Ecol 23:454–462
Williams M, Street LE, van Wijk MT, Shaver GR (2006) Identifying differences in carbon exchange among arctic ecosystem types. Ecosystems 9:288–304
Williams M, Richardson AD, Reichstein M, Stoy PC, Peylin P, Verbeeck H, Carvalhais N, Jung M, Hollinger DY, Kattge J, Leuning R, Luo Y, Tomelleri E, Trudinger CM, Wang Y (2009) Improving land surface models with FLUXNET data. Biogeosciences 6:1341–1359
Wilson D, Alm J, Riutta T, Laine J, Byrne KA (2007) A high resolution green area index for modelling the seasonal dynamics of CO2 exchange in peatland vascular plant communities. Plant Ecol 190:37–51
Zoltai SC, Pollet FC (1983) Wetlands in Canada: their classification, distribution and use. In: Gore AJP (ed) Ecosystems of the World 4B. Mires: swamp, bog, fen and moor. Elsevier, Amsterdam, pp 245–268
Acknowledgements
We thank Antti Miettinen and Sanna Ehonen for assistance in the field and Kauko Pistemaa for technical support. The help of Sari Juutinen in realizing the experimental design is highly appreciated. Financial support to E.-S. Tuittila from the Academy of Finland (project code 118493) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maanavilja, L., Riutta, T., Aurela, M. et al. Spatial variation in CO2 exchange at a northern aapa mire. Biogeochemistry 104, 325–345 (2011). https://doi.org/10.1007/s10533-010-9505-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9505-7