Abstract
Changes in water pH and colour since the late 1980s were studied in 35 small boreal lakes of varying hydrological and landscape settings but similar climate and acid deposition. The data was collected during the autumnal overturn on the annual basis except in lake with weekly sampling during the ice-free period. In addition to the deposition data information about catchment soil types as well as local meteorological and hydrological conditions were used for the long-term data interpretation. The lakes are situated in a small area in southern Finland, 130 km north from Helsinki, where sulphate deposition declined by >60% in one decade since the mid-1980s. The results showed that water colour increased in most lakes while pH did not. In lakes dominated by surface runoff there was a distinct upward shift in colour, with an initial increase after the mid-1990s and a second increase in 2004. The first shift appeared when the sulphate deposition reached a level ca. 25% of that in 1988. However, the upward shift in colour also coincided with a change in hydrological conditions after several dry summers. In contrast, the second shift in colour clearly coincided with a switch in hydrology due to the abnormally wet summer of 2004 after severe drought in 2002–2003. Although the hydrological conditions indisputably had a key role in determining the annual variability in colour, a distinct negative relationship between acid deposition and water colour in 90% of the lakes strongly suggested that reduction in sulphate deposition fostered the leaching of coloured organic substances from the catchment soils. Increase in colour, in turn, strongly influenced lake water pH, and the present day higher organic matter concentrations seemingly depress pH values more than in the 1980s, before the reduction in acid deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to Vuorenmaa (2007) average sulphate deposition decreased in Finland by 45–70% from the 1980s to 2003, being strongest in southern Finland. Nitrogen deposition also decreased, but clearly less than sulphur deposition (Ruoho-Airola et al. 2004; Vuorenmaa 2004). As a consequence of the decline in sulphuric acid deposition, there has been a slight, but consistent, recovery in lake water pH in many of the most severely acidified lakes (Mannio 2001; Vuorenmaa et al. 2006; Vuorenmaa and Forsius 2008). In agreement with the elevated pH values, the fish populations of many previously acidified lakes have responded to the improved conditions by more successful reproduction compared to the 1980s (Rask et al. 1995; Nyberg et al. 2001; Tammi et al. 2005). Chemical recovery of acidified surface waters has also taken place elsewhere in Europe and North America (Stoddard et al. 1999; Evans et al. 2001, 2005; Skjelkvåle et al. 2005).
Together with decreasing acidic deposition, a rise in dissolved organic matter (DOM) concentration in lakes and rivers has been recognized as another wide spread phenomenon (e.g. Mattsson et al. 2005; Skjelkvåle et al. 2005; Roule and Moore 2006; Monteith et al. 2007). DOM concentrations have increased in lakes and rivers in the northern Hemisphere since the early 1990s, and the rise in DOM has been considered integral to the decreased sulphate deposition and recovery from acidification (Stoddard et al. 2003; Evans et al. 2001; Vuorenmaa et al. 2006; Monteith et al. 2007). Krug and Frink (1983) proposed that as a consequence of increasing pH the solubility of soil organic matter may increase, which could explain the elevated organic carbon concentrations in freshwaters. A number of studies have shown that increasing the ionic strength in soil solution reduces the rate of DOM release from the soil (Tipping and Hurley 1988; Evans et al. 1988; Vance and David 1989; Kalbitz et al. 2000). Decreasing atmospheric deposition reduces ionic strength of the soil solution and thereby should lead to an increasing DOM release from the soil. Other theories have also been proposed, including the effect of elevated temperature, atmospheric carbon dioxide concentrations and nitrogen deposition (e.g. Schindler 1998; Freeman et al. 2001, 2004; Pregitzer et al. 2004). Moreover, changes in long-term hydrology have been shown to be an important contributor in regulation of DOM fluxes from catchments (e.g. Worral and Burt 2007). For Swedish rivers Erlandsson et al. (2008) have shown that flow emerges as an important long-term driver of DOM variability (see also Tranvik and Jansson 2002), a result which is consistent with data from Finnish rivers (Arvola et al. 2004).
In this study we focus on the water chemistry of small forest lakes from the same small geographical area in southern Finland where sulphate deposition decreased by 60% within a decade between 1985 and 1995 (Ruoho-Airola et al. 1998; Moldan et al. 2001). In order to understand how the lakes and their catchment areas have responded to the reductions in acid deposition, we analysed the long-term changes in pH and colour in a number of lakes in relation to the hydrogen ion, nitrate and sulphate deposition as well as to the catchment soil types and lake landscape position. The water chemistry records for some of the lakes go back to the late 1970s, but from a vast majority of the lakes records cover the last +20 years. The study lakes provide a unique data set of this kind in Finland because all the lakes are close to each other and have been sampled annually exactly at the same, and no changes in the analytical methods have taken place in the course of the study period (see Järvinen et al. 2002).
The data set provides us an opportunity to analyse not only the long-term patterns in lakes’ pH and colour but also some of the key factors which may regulate and modify the recovery of small boreal lakes from acidification. Hence by using the available data we addressed the following questions: (1) Are any long-term trends in water pH and/or colour evident in the study lakes? (2) Regarding to the patterns in pH and colour are the lakes behaving in a coherent way or are there differences between the lakes in relation to their landscape settings and/or soil type? (3) Can any changes in pH and colour be explained as a result of reductions in sulphate, nitrate and hydrogen ion deposition?
Materials and methods
The 35 study lakes are situated in the Evo forest area, 130 km north of Helsinki (Fig. 1). The Evo area is sparsely populated and the only major direct human disturbance on the catchments and lakes is forestry. The lakes are small and they are inter-connected hydrologically (see Arvola et al. 1990; Järvinen et al. 2002); this means that many of the lakes contribute to a lake-chain which starts from the uppermost head-water lakes such as Lake21, Lake30 and Lake3, and which drains down to the lowermost lake within the chain, 2. Altogether the lakes and their catchments form a larger drainage area situated in the uppermost reaches of the fifth largest river basin in Finland, the River Kokemäenjoki. Most of the study lakes have one or more inflows and one outflow, and can be considered as lakes fed predominately by surface-runoff while a smaller fraction of the lakes are seepage lakes without any visible inflowing streams or outflow. Collectively these lakes are considered as ground-water lakes. One lake, Lake28, has several large springs on its bottom and this lake has been classified as a spring lake, thus a separate type among the groundwater lakes. Correspondingly, the soil types also vary between the lakes. Groundwater lakes are typically situated on the glacio-fluvial sandy deposits in the lower part of the area and surface runoff lakes on the till deposits in the upper areas. Some basic information about the study lakes and their catchments is given in Table 1.
Map of the study area. In the centre of the larger map the WGS84 coordinates are lat 61.21500, lon 25.13900. Lakes have corresponding numbers as in Table 1. Arrow point the way of water down from the larger catchment
The lakes were sampled once a year, at the end of October or during the 1st week of November (usually during week 44), just before freezing, when the lakes have their autumnal turnover (Salonen et al. 1984). This is why samples were collected only from the uppermost 1 m water layer. Sampling was organised such that all the lakes were sampled within 1 week, usually within 2–3 days. The samples were kept dark and cold and immediately after each sampling day transported to the laboratory of Lammi Biological Station, University of Helsinki. This laboratory is only 20 km from the study area, and thus the samples were fresh when the technicians started their preparation for the analyses. Analyses were carried out according to standard methods (see Arvola et al. 1996). Water colour was used as a proxy for dissolved organic matter. Colour was measured from the very beginning of the monitoring programme while dissolved organic carbon (DOC) measurements started later and only from one lake, Valkea-Kotinen, contemporary results of DOC and colour are available. The results cover, however, all seasons because of high frequency sampling in that lake, and the data set is 18 years long. Therefore the results of that lake are given as an example of the correlation between colour and DOC.
The Finnish Meteorological Institute provided the local deposition and meteorological data, and the Finnish Environment Institute provided the hydrological data. The meteorological data are based on two meteorological stations. One is in the middle of the Evo study area and the other is at Lammi Biological Station 20 km south of the Evo area. The local hydrological data come from the outlet of Valkea-Kotinen, one of the study lakes, where there has been a permanent hydrological measuring station since 1988. The local deposition data come also from Valkea-Kotinen research area. Another set of long-term hydrological data comes from the River Mustajoki, which originates from the Evo area but flows down to Lake Pääjärvi, about 10 km SW from Lammi Biological Station. The information about soil types is based on the soil maps prepared by the Geological Survey of Finland.
The statistical analyses were made using Systat 9.0, SigmaStat 3.0 and the Mann–Kendall Spreadsheet for Excel provided by the Finnish Meteorological Institute. Significance of long-term trends was tested by the non-parametric Mann–Kendall Tau-b test (MK) with Sen’s Slope estimates (Gilbert 1987; Sen 1968). It computes a coefficient representing strength and direction of a trend. The relationships between different variables were tested by the Pearson Product Moment correlation analysis. Non-parametric Mann–Whitney Rank Sum Test and an extension of that, Kruskal–Wallis One Way Analysis of Variance on Ranks, Dunn’s Method, were used for detecting differences in the data sets. Principal Components Analysis (PCA) was applied to relate the long-term trends (MK z-statistics) of water colour and pH together with the site specific key variables (lake area, catchment area, dominant soil type, hydrology of the lake). Original non-transformed data was used for the analyses.
Results and discussion
In the study area sulphate deposition decreased very rapidly and by the mid-1990s was less than 40% of that in 1988, and a few years later only 25% (Fig. 2). Nitrate N deposition also decreased but less than S deposition (Vuorenmaa 2004) since 20 years later there was still almost 50% left of the 1988 level. Decrease in hydrogen ion deposition, in turn, was somewhere between sulphur and nitrate, and thus 40% of initial level in 1988 was left 20 years later. Concurrently with the decrease in acid deposition the hydrological conditions may have changed since the autumnal runoff values decreased (Mann–Kendall; p < 0.05) during a period of 1981 till 2005, and runoff relative to precipitation clearly decreased (p < 0.1) since 1977, and at the beginning of 1990s during four consecutive summer seasons and again at the beginning of the next decade very low values were measured during the dry periods. Although the reasons for the shifts in runoff relative to precipitation are unclear, we suppose that among the key factors influencing the ratio will be the summer air temperature, which has increased statistically significantly (Mann–Kendall; p < 0.05) since 1977 (Fig. 3). This is supported by the fact that there has been no change in summer-autumn precipitation since 1977, which further implies that higher summer air temperatures may have enhanced evapotranspiration which, in turn, has changed the hydrological balance in the area. Nothing similar has been reported before in Southern Finland. However, during that period there were two wet years, one in 1996 and another in 2004, the first one between the two dry periods the second one after the two successive dry years.
In more than 80% of the 35 lakes water colour showed positive trends over the study period while water pH had statistically significant (p < 0.1) increasing trend in only one lake and negative trend in seven lakes (Table 2). In most lakes a clear upward shift in colour took place around the middle of 1990s. The colour values were generally higher in surface runoff lakes on till deposits and lower in lakes on sandy deposits and fed by groundwater, although the rate of change in colour was sometimes opposite in the long-term. This became evident when lakes which drain through the study area were analysed in detail; the two uppermost lakes (Lake27 and Lake26) are situated completely on till deposits, partially also on sandy deposits, and the lakes downstream from Lake24 only on sandy deposits, and with a continuous water flow from the uppermost lake to the lowermost one. In the uppermost three lakes the rate of change was clearly lower than in the last three lakes, although all the lakes are predominantly fed by surface runoff. The most likely explanation for this is that the downstream lakes receive higher DOC loadings from the upper parts of the drainage basin rather than from their own surroundings, and this is why their colour values have changed more relative to their initial values than in the upstream lakes with already high colour values. If groundwater lakes are compared with surface-runoff lakes, the distinction between the different lake types is even more clear (Fig. 4). In lakes fed by groundwater the rate of increase was close to zero and significantly different compared with surface runoff lakes [Kruskal–Wallis One Way Analysis of Variance on Ranks, Dunn’s Method; Q with SW(S) was 3.368 and with SW(T) 2.760)] where the slope of trend was clearly higher. Among the two surface-runoff lake groups the slopes did not differ from each other (Q = 1.234), however.
Jones and Arvola (1984) have observed that water colour and DOC were significantly correlated in the study lakes when the values of different lakes were compared. However, the relationship between colour and DOC can be much weaker if results of one lake are considered. In Lake Valkea-Kotinen, the correlation coefficient for colour and DOC over the whole study period based on weekly samples was 0.506 (p < 0.001, n = 425). The ratio between colour and DOC, however, was statistically significantly higher (Mann–Whitney Rank Sum Test; T = 12,888.0, n > 117, p < 0.001) during the last 5 years (2000–2004) in comparison with the first 5 years (1990–1994) indicating that the relationship has changed in the course of time. There can be several reasons why the ratio has changed because it is likely to vary depending on DOC sources, organic matter solubility, concentration of iron and also due to the hydrological conditions.
Iron had positive significant long-term trends in 46% of the study lakes (Table 2) which is in accordance with the fact that in many boreal lakes, in particularly humic lakes, iron and humic substances are highly inter-connected (Pennanen and Frisk 1984). Thus, when higher colour values appear also higher iron concentrations usually can be recorded. However, the most clear trends in iron took place in seepage ground-water lakes which was opposite to the changes in colour.
In Lake Valkea-Kotinen colour values were clearly higher during wet summers than during dry summers (Fig. 5), indicating that water pathway out the catchment may importantly regulate the transport of dissolved organic substances, and thus water colour in small lakes. As has been shown by Arvola et al. (2006), high summer precipitation with complementary discharge may strongly enhance DOC load from the catchment and also increase the concentration of DOC in river water. In the case of Valkea-Kotinen, the colour values in the lake were 50% higher during the maximum discharge in comparison to the minimum in outflow. The variability in discharge was, however, several times higher compared to the variability in colour.
In spite of the remarkable decrease in acid deposition water pH had statistically significant (p < 0.1) increasing trend only in only one lake while negative trend was recorded in seven lakes (Table 2), a result in contradictory to some earlier observations (Mannio 2001; Vuorenmaa et al. 2006; Vuorenmaa and Forsius 2008). One explanation for the differences in results between this study and the previous ones might be that we focused on the late autumn results while in other studies also results from other seasons are considered. In our data the October results from Valkea-Kotinen revealed a close negative relationship between water colour and pH (r = −0.542, p < 0.00001, n = 46), a phenomenon also recorded in a majority of other study lakes (Fig. 6), while if the whole data set covering the rest of the year, except the middle winter, is taken into consideration the relationship was very weak (r = −0.0942, p = 0.0371, n = 490). This implies that especially during the summer the key biological processes such as primary production and organic matter mineralisation may also regulate water pH in many of the small lakes. In the present data the only lakes with clearly different relationship between colour and pH were those where groundwater has great influence and Lake Karhujärvi with abnormally high sulphate concentration (Rask et al. 1993). The relationship between colour and pH has not been stable, however, as the results of Valkea-Kotinen indicated; during the study period a dramatic increase (>50%) in colour took place in that lake while water pH did not change. A similar pattern in colour and pH as in Valkea-Kotinen was recorded in 65% of the study lakes.
The relationship between mean water pH and colour in study lakes. Humic lakes are indicated with black dots and lakes dominated by groundwater on sandy deposits are shown with white dots. The regression equation is given for the rest of the lakes. The numbers are in line with those in Tables 1 and 2
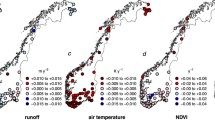
A strong relationship between sulphate deposition and water colour in 88% of the lakes strongly suggests that colour changes in the lakes can be satisfactorily explained by the reduction in sulphur deposition (Fig. 7). This relationship did not change even when the hydrological variability is accounted for using the residuals of the regression between SO4 deposition and water discharge instead of the SO4 deposition values. The only exceptions from this common rule were two seepage lakes in the lowermost part of the drainage basin without any relationships between colour and SO4 deposition as well as two other lakes on sandy deposits and with a linear relationship between colour and deposition. Thus, the results support the hypothesis that reduction in acid deposition, first of all in sulphate, has caused a dramatic upward shift in colour as has been proposed by Monteith et al. (2007). It should be kept in mind that also nitrate and hydrogen ion depositions were decreased substantially. Reduction in sulphate deposition was so pronounced however, that their role might be under-estimated. The results also demonstrate that varying hydrological conditions can strongly modify the colour values in lakes. In this regard the results are in good agreement with those of Erlandsson et al. (2008). In fact, the short-term variability in colour caused by the hydrological conditions appeared approximately in the same range as the long-term variability. This demonstrates how difficult it is to distinguish the different factors which are behind changes in colour and pH. Within our data set the PCA analysis shows that if the acid deposition is ignored and only the site specific factors are considered colour values were more closely related to the soil and hydrological type of the lakes than to the catchment and lake area. In contrast, water pH has almost opposite orientation relative to the hydrology and soil type of the lakes as well as to water colour (Fig. 8).
Our results showed that an upward shift in colour took place in the early of 1990s in the great majority of the study lakes, a phenomenon which coincided almost precisely with the timing of the most rapid reduction in acid deposition and particularly in sulphur. This support the hypothesis that reductions in sulphate and other acidic depositions enhanced organic matter leaching from the catchments, although the results do not indicate anything about the processes responsible for the increase in leaching.
The results also demonstrated that there was a close connection between colour and pH, indicating that organic matter (i.e. organic acids) reduces pH now more than in 1980s, before the reduction in acid deposition. In line with this Vuorenmaa and Forsius (2008) found that the recovery of acidified humic lakes has been weaker than that of clear-water acidified lakes, and the reason is the dominance of organic anions over SO4 in the humic lakes. This implies that runoff-induced surges of organic acids have suppressed recovery of the buffering capacity in many of such lakes.
Thirdly, hydrologically distinct lake types clearly behaved differently in relation to the reductions in acid deposition as well as changes in the hydrological conditions. Ground-water seepage lakes clearly form a group of lakes where the smallest changes in colour were recorded and the surface-runoff lakes on sandy deposits have experienced the most radical relative changes in this regard. However, the opposite was found for changes in iron. This emphasizes that the different elements such as humic substances and iron have their own specific processes in the catchments and also sources and pathways to the lakes. This implies that, although colour and iron can be strongly correlated particularly in humic lakes, this relationship is not necessarily valid in all circumstances.
Finally, ecological implications of the results of this study may be remarkable because increasing colour and DOC values in lakes not only affect the chemical conditions of lakes but also the physical properties such as light attenuation and its wavelength characteristics (Eloranta 1978; Jones and Arvola 1984) as well as the thermal conditions of the lakes (Arvola et al. 2010; Huotari et al. 2009). In addition to the physical and chemical responses, many metabolic processes and interactions between organisms as well as organisms and their physical and chemical environment can be influenced by changes in DOC, colour and pH (Jones 1998; Arvola et al. 1999; Kankaala et al. 2006; Huotari et al. 2009).
In conclusion, the results of this study proved that in the vast majority of the study lakes colour values have increased since the end of the 1980s, and the long-term increase in colour can be accounted for satisfactorily by the reductions in sulphate deposition although the role of nitrate and hydrogen ion can not be overlooked. Instead, the inter-annual fluctuations in colour have rather to be explained by the variation of hydrological conditions.
References
Arvola L, Metsälä T-R, Similä A, Rask M (1990) Phyto- and zooplankton in relation to water pH and humic content in small lakes in southern Finland. Verh Int Ver Limnol 24:688–692
Arvola L, Kankaala P, Tulonen T, Ojala A (1996) Effects of phosphorus and allochthonous humic matter enrichment on the metabolic processes and community structure of plankton in a boreal lake. Can J Fish Aquat Res 53:1646–1662
Arvola L, Salonen K, Rask M (1999) Trophic interactions. In: Eloranta P, Keskitalo J (eds) Limnology of humic waters. Backhuys Publishers, Leiden, The Netherlands, pp 265–279
Arvola L, Räike A, Kortelainen P, Järvinen M (2004) Effect of climate and land use on TOC concentrations and load in Finnish rivers. Boreal Environ Res 9:381–387
Arvola L, Järvinen M, Hakala I (2006) Nutrient export from small boreal catchments: the influence of annual and seasonal hydrology. Verh Int Ver Limnol 29:2031–2034
Arvola L, George DG, Blenckner T, Dokulil M, Jennings E, Järvinen M, Livingstone D, Nöges P, Nöges T, Weyhenmeyer G (2010) The impact of changing climate on the thermal characteristics of lakes. In: George DG (ed) The impact of climate change on European lakes. Aquatic Ecology Series 4. Springer, pp 85–101
Eloranta P (1978) Light penetration in different types of lakes in central Finland. Holarct Ecol 1:362–366
Erlandsson M, Buffam I, Fölster J, Laudon H, Temnerud J, Weyhenmeyer GA, Bishop K (2008) Thirty-five years of synchrony in the organic matter concentration of Swedish rivers explained by variation in flow and sulphate. Glob Change Biol 14:1–8
Evans A Jr, Zelazny LW, Zipper CE (1988) Solution parameters influencing dissolved organic carbon levels in three forest soils. Soil Sci Soc Am J 52:1789–1792
Evans CD, Cullen JM, Alewell C, Kopácek J, Marchetto A, Moldan F, Prechtel A, Rogora M (2001) Recovery from acidification in European surface waters. Hydrol Earth Syst Sci 5:311–325
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137:55–71
Freeman C, Evans CD, Monteith DT, Reynolds B, Fenner N (2001) Export of organic carbon from peat soils. Nature 412:785
Freeman C, Fenner N, Ostle NJ et al (2004) Export of organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198
Gilbert RO (1987) Statistical methods for environmental pollution monitoring. Van Nostrand Reinhold, New York
Huotari J, Ojala A, Peltomaa E, Pumpanen J, Hari P, Vesala T (2009) Temporal variations in surface water CO2 concentrations in a boreal lake based on high-frequency measurements. Boreal Environ Res 14:48–60
Järvinen M, Rask M, Ruuhijärvi J, Arvola L (2002) Temporal coherence in water temperature and chemistry under the ice of boreal lakes (Finland). Water Res 36:3949–3956
Jones RI (1998) Phytoplankton, primary production and nutrient cycling. In: Hessen DO, Tranvik L (eds) Aquatic humic substances: ecology and biogeochemistry. Ecological Studies 133. Springer, pp 145–175
Jones RI, Arvola L (1984) Light penetration and some related characteristics in small forest lakes in southern Finland. Verh Int Ver Limnol 22:811–816
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls of the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kankaala P, Taipale S, Grey J, Sonninen E, Arvola L, Jones R (2006) Experimental δ13C evidence for a contribution of methane to pelagic food webs in lakes. Limnol Oceanogr 51:2821–2827
Krug EC, Frink CR (1983) Acid rain and acid soil: a new perspective. Science 221:520–525
Mannio J (2001) Recovery pattern from acidification of headwater lakes in Finland. Water Air Soil Pollut 130:1427–1432
Mattsson T, Kortelainen P, Räike A (2005) Export of DOM from boreal catchments: impacts of land use cover and climate. Biogeochemistry 76:373–394
Moldan F, Wright RF, Löfgren S, Forsius M, Ruoho-Airola T, Skjelkvåle BL (2001) Long-term changes in acidification and recovery at nine calibrated catchments in Norway, Sweden and Finland. Hydrol Earth Syst Sci 5:339–349
Monteith DT, Stoddard JL, Evans CD et al (2007) Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450:537–541
Nyberg K, Vuorenmaa J, Rask M, Mannio J, Raitaniemi J (2001) Patterns in water quality and fish status of some acidified lakes in southern Finland during a decade: recovery proceeding. Water Air Soil Pollut 130:1373–1378
Pennanen V, Frisk T (1984) A statistical model for conversion of absorbance measurements with significant iron interference into organic carbon in a polyhumic lake. Aqua Fenn 14:171–178
Pregitzer KS, Zak DR, Burton Aj, Ashby JA, MacDonald NW (2004) Chronic nitrate additions dramatically increase the export of carbon and nitrogen. Biogeochemistry 68:179–197
Rask M, Arvola L, Salonen K (1993) Effects of catchment deforestation and burning on the limnology of a small forest lake in southern Finland. Verh Int Ver Limnol 25:525–528
Rask M, Raitaniemi J, Mannio J, Vuorenmaa J, Nyberg K (1995) Losses and recoveries of fish populations in acidified lakes of southern Finland in the last decade. Water Air Soil Pollut 85:315–320
Roule N, Moore TR (2006) Environmental chemistry—browning the waters. Nature 444:283–284
Ruoho-Airola T, Syri S, Nordlund G (1998) Acid deposition trends at the Finnish integrated monitoring catchments in relation to emission reductions. Boreal Environ Res 3:205–219
Ruoho-Airola T, Anttila P, Salmi T (2004) Airborne sulphur and nitrogen in Finland—trends and exposure in relation to air transport sector. J Environ Monit 6:1–11
Salonen K, Arvola L, Rask M (1984) Autumnal and vernal circulation of small forest lakes in southern Finland. Verh Int Ver Limnol 22:103–107
Schindler DW (1998) A dim future for boreal waters and landscapes. Bioscience 48:157–164
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s tau. J Am Stat Assoc 63:1379–1389
Skjelkvåle BL, Stoddard JL, Jeffries DS, Tørseth K, Høgåsen T, Bowman J, Mannio J, Monteith DT, Mosello R, Rogora M, Rzychon D, Veselý J, Wieting J, Wilander A, Worsztynowicz A (2005) Regional scale evidence for improvements in surface water chemistry 1990–2001. Environ Pollut 137:165–176
Stoddard JL, Jeffries DS, Lükewille A, Clair T, Dillon PJ, Driscoll CT, Forsius M, Johannessen M, Kahl JS, Kellogg JH, Kemp A, Mannio J, Monteith D, Murdoch P, Patrick S, Rebsdorf A, Skjelkvåle B-L, Stainton MP, Traaen T, van Dam H, Webster K, Wieting J, Wilander A (1999) Regional trends in aquatic recovery from acidification in North America and Europe. Nature 401:575–578
Stoddard JL, Karl JS, Deviney FA, DeWalle DR, Driscoll CT, Herlihy AT, Kellogg JH, Murdoch PS, Webb JR, Webster KE (2003) Response of surface water chemistry to the Clean Air Act Amendments of 1990. Report EPA 620/R-03/001. United States Environmental Protection Agency, North Carolina
Tammi J, Rask M, Vuorenmaa J, Lappalainen A, Vesala S (2005) Population responses of perch (Perca fluviatilis) and roach (Tutilus rutilus) to recovery from acidification in small Finnish lakes. Hydrolobiologia 528:107–122
Tipping E, Hurley MA (1988) A model of solid-solution interactions in acid organic soils, based on the complexation properties of humic substances. J Soil Sci 39:505–519
Tranvik IL, Jansson M (2002) Terrestrial export of organic carbon. Nature 415:861–862
Vance GF, David MB (1989) Effect of acid treatment on the leachate chemistry of a New England spodosol: importance of the B horizon on dissolved organic carbon retention. J Soil Sci Soc Am 53:1242–1247
Vuorenmaa J (2004) Long-term changes of acidifying deposition in Finland (1973–2000). Environ Pollut 128:351–362
Vuorenmaa J (2007) Recovery responses of acidified Finnish lakes under declining acid deposition. Helsinki, Finnish Environment Institute. 50 s. Monogr Boreal Environ Res 30:1–50
Vuorenmaa J, Forsius M (2008) Recovery of acidified Finnish lakes: trends, patterns and dependence of catchment characteristics. Hydrol Earth Syst Sci 12:465–478
Vuorenmaa J, Forsius M, Mannio J (2006) Increasing trends of total organic carbon concentrations in small forest lakes in Finland from 1987 to 2003. Sci Total Environ 365:47–65
Worral T, Burt TP (2007) Flux of dissolved organic carbon from UK rivers. Glob Biogeochem Cycl 21:GB1013. doi:10.1029/2006GB002709
Acknowledgments
The authors thank Riitta Ilola and Jaakko Vainionpää for the chemical analyses made at the lab of the Lammi Biological Station and all individuals and institutes who have made it possible to carry out the field work, technical support as well as the analyses. Also we want to thank Roger I. Jones for his comments and the English corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arvola, L., Rask, M., Ruuhijärvi, J. et al. Long-term patterns in pH and colour in small acidic boreal lakes of varying hydrological and landscape settings. Biogeochemistry 101, 269–279 (2010). https://doi.org/10.1007/s10533-010-9473-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9473-y