Abstract
Nitrite (NO2 −) is an intermediate in a variety of soil N cycling processes. However, NO2 − dynamics are often not included in studies that explore the N cycle in soil. Within the presented study, nitrite dynamics were investigated in a Nothofagus betuloides forest on an Andisol in southern Chile. We carried out a 15N tracing study with six 15N labeling treatments, including combinations of NO3 −, NH4 + and NO2 −. Gross N transformation rates were quantified with a 15N tracing model in combination with a Markov chain Monte Carlo optimization routine. Our results indicate the occurrence of functional links between (1) NH4 + oxidation, the main process for NO2 − production (nitritation), and NO2 − reduction, and (2) oxidation of soil organic N, the dominant NO3 − production process in this soil, and dissimilatory NO3 − reduction to NH4 + (DNRA). The production of NH4 + via DNRA was approximately ten times higher than direct mineralization from recalcitrant soil organic matter. Moreover, the rate of DNRA was several magnitudes higher than the rate of other NO3 − reducing processes, indicating that DNRA is able to outcompete denitrification, which is most likely not an important process in this ecosystem. These functional links are most likely adaptations of the microbial community to the prevailing pedo-climatic conditions of this Nothofagus ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a key element for ecosystem productivity. In natural, N-limited ecosystems the amount of N available for plant and microbial uptake is dependent on the balance between inputs and outputs of N. Nothofagus forests in southern Chile are characterized by very low N depositions (Holland et al. 1999). Thus, ecosystem productivity is inversely related to the amount of N lost from the system. In moderately acid soils, leaching of NO3 − and gaseous N emissions are likely to be the most important N loss mechanisms. Both processes are dependent on the availability of nitrate (NO3 −). Therefore, soil N processes that transfer NO3 − into more stable N forms ensure long-term ecosystem sustainability. Recently, we showed that DNRA in combination with subsequent ammonium (NH4 +) immobilization is an important mechanism for mineral N retention in an N-limited undisturbed Nothofagus forest ecosystem in southern Chile (Huygens et al. 2007). As DNRA occurs under similar low-oxygen conditions as denitrification (Tiedje et al. 1982), both processes are in competition for the available NO3 − and can affect each other (Knowles 1982; Silver et al. 2001, 2005). Denitrification is next to nitrification the main pathway of nitrous oxide (N2O) production in forest ecosystems (Speir et al. 1999). Therefore a high DNRA rate has the potential to reduce gaseous N losses (Huygens et al. 2007). Undisturbed Nothofagus forests are characterized by small N2O production from denitrification and no net N2O emissions (Price et al. 2004; Speir et al. 1999). In our previous study (Huygens et al. 2007) we compared the rate of DNRA with NO3 − immobilization and concluded that gaseous N losses via denitrification are presumably low in the studied ecosystem. However, in order to show unambiguously that DNRA can successfully compete with denitrification it is important to compare directly the gross transformation rates of both processes. Our approach relies on the determination of the nitrite (NO2 −) dynamics in soil. Nitrite is an important intermediate in denitrification and both, heterotrophic and autotrophic nitrification (Müller et al. 2006). The reduction of NO3 − to NO2 − is the first step in the denitrification sequence and provides a measure of the potential for denitrification in soil (Betlach and Tiedje 1981).
Inhibition of autotrophic nitrification has been postulated as another key mechanism to prevent N losses from forest ecosystems (Vitousek et al. 1979). Several studies showed that autotrophic nitrification is lower than the heterotrophic pathway in temperate forest soils (Hart et al. 1997; Schimel et al. 1984). In old-growth forests (“climax ecosystems”) the inhibition of autotrophic nitrification is caused by the absence of Nitrobacter, a bacterial group responsible for the NO2 − oxidation to NO3 − (Rice and Pancholy 1972). Furthermore, other processes such as nitrifier-denitrification (Wrage et al. 2001), chemo-denitrification, a non-enzymatic chemical reaction of NO2 − with organic N (Paul 2007; Sprent 1987), nitrosation, a mechanism of NO2 − reaction with phenols to form organic N (Azhar et al. 1986; Paul and Clark 1996), self-decomposition to NO3 − under acidic conditions (Van Cleemput and Baert 1976) and the recently discovered anaerobic NH4 + oxidation (anammox) (Mulder et al. 1995; Paul 2007) are also involved in the soil NO2 − dynamics and in the production of gaseous N components. The arguably most important process for N2O production in soils under high moisture conditions is NO3 − reduction via NO2 − to gaseous N (Russow et al. 2000; Van Cleemput and Samater 1996; Venterea and Rolston 2000). NO2 − is sometimes considered as an intermediate in biochemical models (Betlach and Tiedje 1981; Cho and Mills 1979) but rarely in ecosystem N cycling models (Burger and Jackson 2004; Schimel and Bennett 2004).
The current “state-of-the-art” technique to quantify simultaneously occurring gross N transformation rates in soils are 15N tracing studies in combination with parameter optimization routines (Mary et al. 1998; Müller et al. 2004, 2007). However, to our knowledge no 15N tracing study so far has considered the functional role of NO2 − dynamics and related N transformations either in these ecosystems or in any other natural ecosystem. Recent progress in 15N tracing technology allows the simultaneous optimization of large number of parameters which is a prerequisite to analyze complex models in 15N tracing studies, which include NO2 − dynamics (Müller et al. 2007; Rütting and Müller 2008). The main advantage of 15N tracing models over the commonly used dilution technique (Stark 2000) is that process-specific N rates such as pool specific mineralization, autotrophic and heterotrophic pathways of nitrification or DNRA can be identified simultaneously (Rütting and Müller 2007).
Here we present results from a 15N tracing study using soil from a pristine, unpolluted Nothofagus betuloides forest in southern Chile. The aim of our study was to explore the functional role of DNRA in relation to the NO2 − dynamics and of NO2 − reduction processes in this soil. In particular we were interested in “functional links”, which we define as metabolic connections in the form of enzymatic chain reactions which are inherent in microbial networks or in single organisms. We hypothesize that DNRA is able to compete successfully with denitrification for the available NO3 − and that the oxidation of NO2 − to NO3 − in the nitrification pathway is inhibited in the old-growth forest.
Materials and methods
Study site
The study site is located at 900 m a.s.l. in the Andean mountains, Antillanca, southern Chile (40°47′ S, 72°12′ W). The local climate is characterized by average annual temperature of 4.5°C and mean annual precipitation around 7,000 mm. The forest vegetation is dominated by evergreen Nothofagus betuloides with an average tree age of 325 years (Godoy et al. 2001). Total annual bulk N deposition amounts to 11.8 kg N ha−1 in form of dissolved organic nitrogen (8.2 kg N ha−1) and dissolved inorganic nitrogen (3.6 kg N ha−1) (Oyarzún et al. 2004). The initial mineral N concentration measured in the soil samples was 441 μg NH4 +–N, 5.5 μg NO3 −–N and 0.05 μg NO2 −–N g−1 soil.
The soil of the forest is classified as Umbric Vitric Andosol (IUSS Working Group WRB 2006), with typical variable charge characteristics (i.e., pH dependent) as a result of their particular clay mineral and organic matter content (Nanzyo et al. 1993; Radcliffe and Gillman 1985). Main soil characteristics are listed in Table 1. More detailed information for the study site can be found in Huygens et al. (2007) for soil characteristics and in Oyarzún et al. (2004) for water and soil chemistry.
15N tracing experiment
This study was carried out in conjunction with the study by Huygens et al. (2007). To analyze the dynamics of NO2 − we considered three additional 15N labeling treatments, where 15N enriched NO2 − was added (see below). Furthermore the concentrations and 15N excess of NO2 − were analyzed in all 15N labeling treatments.
Soil samples were taken in autumn 2004 following a stratified random procedure. In a representative forest area (25 × 20 m), 10 grids (5 × 5) were established. From each grid, three subsamples (a, b and c) were taken from the mineral A horizon (0–10 cm). The ten subsamples a, b, and c were compiled to obtain three replicates (subsamples composited per replicate). The soil was sieved (<2 mm) and dried to gravimetric water content (GWC) of about 30%, and stored for two months at 5°C. One week prior to 15N additions, the soils were pre-incubated at a water-filled-pore-space (WFPS) of 45% (GWC = 69.6% on average).
There were in total six different 15N treatments (Table 2), each with three replicates, of which either NH4 +, NO2 −, NO3 − or a combination of the various moieties were labeled with 15N at 98 atom% excess. Nitrogen was applied at a rate of 50 μg NH4Cl–N g−1, 5 μg NaNO2–N g−1 and 5 μg KNO3–N g−1 dry soil in 8 ml solutions. The analysis of 15N enrichment experiments is based on the assumption of a homogenous mixing of the isotopes (14N and 15N) in soil (Kirkham and Bartholomew 1954). Therefore, we thoroughly mixed the soil after N application, which provides a sufficient mixing (Luxhøi et al. 2003). The bulk densities were adjusted to field values (i.e., 0.51 g cm−3, resulting in 50% WFPS). Temperature (15°C) and moisture content were kept constant during the entire experiment. Soil was extracted 0.3, 2, 4, 7 and 12 days after N application with 180 ml 2 M KCl solution, shaken for 120 min.
NH4 + in the extract was determined colorimetrically by the salycilate-nitroprusside method (Mulvaney 1996) on an auto-analyzer (AA3, Brann and Luebbe, Germany). Using the same auto-analyzer, NO2 − was determined colorimetrically after a reaction with N-1-napthylethylenediamine to produce a chromophore. NO3 − was determined, as NO2 −, after on-line conversion in a Cd–Cu reductor. The NO3 − results were corrected for NO2 − present in the soil samples. The 15N contents of NH4 +, NO2 − and NO3 − were analyzed after conversion to N2O (Hauck 1982; Saghir et al. 1993; Stevens and Laughlin 1994) on a trace gas preparation unit (ANCA-TGII, PDZ Europa, UK), coupled to an Isotope Ratio Mass Spectrometer (IRMS) (20-20, SerCon, UK).
Soil organic matter fractionation
Using a modified method of Meijboom et al. (1995), we isolated five physical soil organic matter (SOM) fractions from the previously extracted soil (at each extraction time). The 2 mm sieved soil was wet-sieved over a set of three sieves (250, 150 and 50 μm). The 50–150 μm size fraction was collected and dried. The size fraction <50 μm was collected in buckets and determined after one day/night of sedimentation. The soil material on the top sieves (>150 μm) was washed into buckets and swirled with a jet of deionized water. The mineral fraction was collected from the bottom of the bucket, whereas macro-organic matter (MOM) was collected in the water level. All fractions were dried for 48 h at 45°C, and ground with a planetary ball mill (PM400, Retsch, Germany) for total N and 15N analysis with an elemental analyzer (ANCA-SL, PDZ Europa, UK), coupled to an IRMS (20-20, SerCon, UK). For the 15N tracing analysis two conceptual SOM pools, a labile SOM (Nlab) and a more recalcitrant SOM (Nrec) pool, were compiled (Huygens et al. 2007). As indicated by Hassink (1995) the macro-organic matter (MOM) fraction is an active, microbial available N pool, which we used to characterize the Nlab pool in the 15N tracing model (Fig. 1). The stable fraction of the SOM was compiled from the mineral fraction 150–2,000 μm and the size fractions 150–50 μm and <50 μm, all of them offering physical SOM protection (Huygens et al. 2005). The Nrec pool in the model is considered not to be an inert N pool, but is more resistant to N mineralization relative to the labile fraction. Both SOM pools contain organic N compounds and its associated microbial biomass N. The potential to use physically isolated SOM fractions as functional pools in modeling approaches has been demonstrated previously (Elliott et al. 1996; Skjemstad et al. 2004; Smith et al. 2002; Zimmermann et al. 2006).
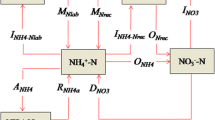
Conceptual 15N tracing model to analyze gross N transformations (Nlab = labile soil organic N, Nrec = recalcitrant soil organic N, NH4 + = ammonium, NO3 − = nitrate, NH4 + ads = adsorbed NH4 +, NO2 − = nitrite sub-pools, Nred = reduced NO2 −). The thicknesses of the arrows represent the relative importance of each transformation (for further explanation of N transformations and parameter values see Table 3)
15N tracing model
To quantify the simultaneously occurring gross N transformation rates we used the 15N tracing analysis tool by Müller et al. (2007). The 15N tracing model (Fig. 1) is based on the model by Müller et al. (2004). We added the modifications by Huygens et al. (2007), consisting of a second NH4 + immobilization and adsorption-release dynamics between free and adsorbed NH4 +, as well as a sub-model for the NO2 − dynamics (Müller et al. 2006; Rütting and Müller 2008). Similar to previous studies (e.g., Müller et al. 2004), NO3 − turnover fluxes are only linked to the more recalcitrant organic N pool (Nrec) in the model set-up. This can be explained by the fact that mineralization-immobilization turnover of NO3 − is dominated by fungi, which prefer NO3 − as their N source (Marzluf 1997) and recalcitrant organic compounds as energy source (Paul 2007). A description of the N transformations and the kinetic settings are presented in Table 3. The NO2 − sub-model considered three separate process-specific NO2 − pools, i.e., NO2 − nit (intermediate in the oxidation of NH4 + to NO3 −), NO2 − den (intermediate in the reduction of NO3 − to gaseous N) and NO2 − org (derived from the oxidation of organic N). The first two processes are expected to be the main sources of NO2 − in soils (Russow et al. 2000; Sprent 1987). However, in a previous study it was shown that NO2 − produced from organic N is an important NO2 − pool in grassland soil (Müller et al. 2006; Rütting and Müller 2008). This process was included in the sub-model because a range of micro-organisms are able to oxidize various organic N forms to NO2 − (Doxtader and Alexander 1966; Sprent 1987). The reduction of all three NO2 − sub-pools is considered to result in the formation of Nred, which is a theoretical pool in the model combining gaseous N species (e.g., N2O, NO, NO2, and N2) as well as other NO2 − consuming processes such as organic N produced via fixation of NO2 − (Azhar et al. 1986; Smith and Chalk 1979). The final model used for data analysis contains nine N pools and 16 N transformations (Fig. 1). During this study several modifications of the 15N tracing model were tested. These modifications include DNRA pathway via NO2 − den pool and heterotrophic nitrification via NO2 − org pool. Previously we also tested a version where NO2 − org was simultaneously produced from Nrec and Nlab (Rütting and Müller 2008). However, all tested modification did not result in a better fit between model and experimental data (data not shown) and were therefore not considered for final data analysis. The 15N tracing analysis tool calculates gross N transformation rates by simultaneously optimizing the model parameters of the various N transformations with a Markov chain Monte Carlo (MCMC) sampling algorithm (Müller et al. 2007) which is useful to analyze data with complex models that contain large number of parameters (Rütting and Müller 2007, 2008). The optimization of the model parameters is guided by the minimization of the difference between modeled and measured data, i.e., minimizing a misfit function f (m) in form of a quadratic weighted error (Müller et al. 2007).
Statistical analysis
The data were supplied to the 15N tracing model as averages ± standard deviations of the experimental measurements (three replicates). The optimization procedure results in a probability density function (PDF) for each model parameter, which are significantly different from zero. From the PDFs, average values and standard deviations for the transformation parameters are calculated (Müller et al. 2007). Furthermore, parameters which are actually zero can be identified and consequently excluded from the model (Müller et al. 2007). Therefore the selection of N transformations and parameters is not arbitrary but based on analysis results of several model runs. For N transformations following first-order kinetics, average rates were calculated by integrating the gross N rates over the experimental period divided by the total time (Rütting and Müller 2007).
In addition a correlation matrix was calculated to find groups of N transformations that tend to be constrained together and thereby identify processes that are closely linked. Transformations with correlation coefficients r ≥ |0.8| were considered to be strongly correlated whereas |0.8| > r ≥ |0.5| indicate medium correlations (Fahrmeir et al. 2004).
Presentation of results and model set-up
The experimental values are presented as arithmetic means ± one standard deviation. The MCMC algorithm is programmed in the software MatLab (Version 7.3, The MathWorks Inc.) and calls in each iteration the 15N tracing model which is separately set up in Simulink (Version 6.5, The MathWorks Inc.), a companion software to MatLab. All six different 15N treatments (Table 2) were analyzed in one optimization run. The initial pool sizes and the 15N contents of the different model pools were obtained by extrapolating the data for 0.3 and 2 days back to time point zero (Müller et al. 2004).
Results
N pool sizes and 15N enrichments
The measured and modeled values of the soil N concentrations and their 15N enrichments are presented in Fig. 2 for mineral N forms and in Fig. 3 for the two organic N fractions.
Measured and modeled concentrations and 15N enrichments of ammonium (NH4 +), nitrite (NO2 −) and nitrate (NO3 −) of an Andisol after application of 60 μg N g−1 soil as NH4/NO2/NO3 at 98 atom% 15N excess (symbols represent average measured values ± one standard deviation; lines represent modeled values)
During the experimental period the different N pools remained more or less constant in size, indicating low net N transformation rates. At the first extraction (8 h) the measured NO2 − concentration was very low (0.027 μg N g−1 soil on average) in comparison to the added amount. A fast decline in the 15NO2 − enrichments after labeling this pool with 15N, suggests a fast inflow of unlabelled N and a high turnover of NO2 − (Fig. 2b, e). Furthermore, labeling only NO2 − did not result in a significant 15N enrichment of the measured NH4 + pool in contrast to NO3 −, which was labeled rapidly (Fig. 2d, f). This indicates a fast initial transformation of NO2 − to NO3 − in this soil. At the same time both measured fractions of SOM were slightly enriched in 15N (Fig. 3c, d) which points toward a fast incorporation of NO2 − into organic material.
The aim of the optimization algorithm is to minimize a cost function which takes into account the actual standard deviations of the observed values. Depending on the standard deviations the algorithm may not hit exactly the observed average values. In general the 15N tracing model (Fig. 1) was able to reproduce the measured N dynamics in the Andisol from a Nothofagus forest, as indicated by a close fit between the model and the experimental data (Figs. 2, 3). Only small deviations were observed for the NO3 − and NO2 − concentrations (Fig. 2b, c). However, considering the high number of measured variables (5 pools, each concentration and 15N excess) and the high variability of NO2 − concentrations in this soil the fit is satisfactory and generally within the uncertainty range of the measured data.
Gross N transformation rates
The highest N transformation rates in the Andisol were related to the exchange between free and adsorbed ammonium (NH4 + and NH4 + ads pool) (Table 3; Fig. 1). This exchange reaction was responsible for 90% of the NH4 + dynamics. The average total NO2 − production amounted to 0.71 μg N g−1 soil day−1, which was dominated by NH4 + oxidation (\( O_{{{\text{NH}}_{4} }} \)) (Table 3). This pathway contributed 93.7% to the overall NO2 − production, while the contribution of denitrification (\( R_{{{\text{NO}}_{3} }} \)) was nearly zero in this soil (contribution < 0.1%). In addition, about 6.3% of the total NO2 − production was related to the oxidation of organic N to NO2 − (Table 3). The estimated gross production of Nred amounted on average to 0.72 μg N g−1 soil day−1, of which 93.9% was related to the nitrification pathway (\( R_{{{\text{NO}}_{2} {\text{nit}}}} \)). After a peak Nred production (i.e., reduction of NO2 −) at t = 0, the subsequent production was more or less constant (Fig. 4). Besides NO2 − reduction, the oxidation of NO2 − nit to NO3 − was the only other NO2 − consuming process amounting however to less than 0.1% of total NO2 − consumption over the experimental period. Nitrate was almost exclusively produced (>99.9%) by oxidation of organic N to NO3 − (Table 3). The consumption of NO3 − was dominated by the process of DNRA (\( D_{{{\text{NO}}_{3} }} \)), which was responsible for 98.4% of the total NO3 − consumption (Table 3; Fig. 1). The other two NO3 − consuming processes, immobilization (\( I_{{{\text{NO}}_{3} }} \)) and NO3 − reduction to NO2 − den (\( R_{{{\text{NO}}_{3} }} \)) amounted only to 1.6 and <0.1% of the total NO3 − consumption, respectively.
Correlations among N transformation rates
In one sixth of all cases a significant correlation between two model parameters was observed (Table 4). The tightest correlation was found between the production and reduction of NO2 − den (\( R_{{{\text{NO}}_{3} }} \) and \( R_{{{\text{NO}}_{2} {\text{den}}}} \); R = 0.87). In the nitrification pathway, the production of NO2 − nit (\( O_{{{\text{NH}}_{4} }} \)) was positively correlated with the reduction (\( R_{{{\text{NO}}_{2} {\text{nit}}}} \); R = 0.66) but not with the oxidation of NO2 − nit to NO3 − (\( O_{{{\text{NO}}_{2} {\text{nit}}}} \)). On the other hand, no correlation existed between the production and consumption of NO2 − org (Table 4). Another significant correlation was observed between the processes related to the dynamics between NH4 + and NH4 + exchange sites (\( A_{{{\text{NH}}_{4} }} \) and \( R_{{{\text{NH}}_{4} {\text{ads}}}} \); R = 0.80) and between oxidation of recalcitrant organic N and DNRA (O Nrec and \( D_{{{\text{NO}}_{3} }} \); R = 0.78). Furthermore, both DNRA and ONrec were negatively correlated with the reduction of NO3 − (\( R_{{{\text{NO}}_{3} }} \)) and NO2 − (\( R_{{{\text{NO}}_{2} {\text{den}}}} \)) during denitrification. In contrast, the immobilization of NO3 − (\( I_{{{\text{NO}}_{3} }} \)) was positively correlated with these two transformations (Table 4).
Discussion
Turnover of NH4 +
The measured NH4 + concentrations (~500 μg N g−1 soil; Fig. 2a) are at the upper end of NH4 + concentrations that can be found in natural soils (Booth et al. 2005) and is possibly caused by the storage of the soil samples. Similar NH4 + concentrations are reported for other forest soils (Fitzhugh et al. 2003a, b; Hackl et al. 2004). However, there is no evidence that the high NH4 + concentrations affected the gross N transformation rates in this Andisol. In particular the rate of NH4 + oxidation (\( O_{{{\text{NH}}_{4} }} \)), which reacts very sensitively to NH4 + concentrations (Shaviv 1988), remained low and characteristic for background rather than elevated NH4 + concentrations (Table 3).
The total NH4 + production was similar to the value for an old-growth south Chilean forest on Cambisol (Perakis and Hedin 2001). Approximately 90% of the total N flow in the soil was related to the adsorption—release dynamics of NH4 + with exchange sites (NH4 + ads) (Table 3). The NH4 + adsorption removed N from the dissolved pool but kept it in a potentially available form (Tamm 1991), providing a highly effective mechanism for buffering excess NH4 + and has the advantage that stored NH4 + can become available on demand.
Low N mineralization rates have been hypothesized as a mechanism to prevent N losses from N-limited forest ecosystems (Vitousek et al. 1979). The total gross N mineralization rate (M Nlab + M Nrec) in the current study (3.7 μg N g−1 day−1; Table 3) is lower than observed previously for the same soil (Huygens et al. 2007) but is in line with the general finding that gross N mineralization in pristine southern hemisphere forests are below 10 μg N g−1 day−1 (Booth et al. 2005; Parfitt et al. 2002). Dissimilatory NO3 − reduction to NH4 + (\( D_{{{\text{NO}}_{3} }} \)) was responsible for almost 9% of total microbial NH4 + production (excluding the adsorption-release characteristic of this soil). The supply of NH4 + via this pathway was about ten times higher than direct mineralization of recalcitrant soil organic matter (Table 3). This shows that NH4 + production may not necessarily follow the classical pathway via direct mineralization of SOM. Nitrogen cycling paradigms such as the ones postulated by Schimel and Bennett (2004) should also consider the NH4 + production pathway via organic N oxidation to NO3 − and subsequent reduction to NH4 +.
The difference in total gross mineralization between the present study and our previous analysis (Huygens et al. 2007) highlights the importance of NO2 − dynamics on the estimation of simultaneously occurring soil N transformations, even those not directly related to NO2 − dynamics. Similar observations have been made in permanent grassland soils (Müller et al. 2007; Rütting and Müller 2008). This shows that the consideration of NO2 − data in 15N tracing models constrains the search for parameter constellations and thus allows a more precise quantification of gross rates for all transformations of the N cycle.
Vitousek et al. (1979) postulated inhibition of NH4 + oxidation as an effective mechanism to avoid N losses from ecosystems. Our analysis showed that \( O_{{{\text{NH}}_{4} }} \) was only responsible for a small amount of NH4 + consumption (1.5%; Table 3) which supports previous results on forest soils (Hart et al. 1997; Schimel et al. 1984). Moreover, immobilization of NH4 + into Nrec was six-times higher than into the Nlab pool (Table 3), which may provide an effective N sequestration mechanism and may support long-term ecosystem productivity (Pepper et al. 2007).
Production and consumption of NO3 −
The studied Andisol is characterized by lower NO3 − than NH4 + concentrations (Fig. 2) indicating inherently a closed N cycle (Davidson et al. 2000; Pérez et al. 1998). Moreover, the observed NO3 − turnover was lower than the NH4 + turnover (Table 3; Fig. 1), which contributes to a better N retention (Doff Sotta et al. 2008). In the 15N tracing model three microbial consumption processes for NO3 −, namely immobilization into Nrec, denitrification and DNRA, were considered (Fig. 1).
Similar to our previous study (Huygens et al. 2007), DNRA (\( D_{{{\text{NO}}_{3} }} \)) was the most important NO3 − consumption process and the main mechanism for NO3 − conversion to NH4 + showing the high potential for DNRA in the Andisol. The calculated DNRA rates amounts 0.36 ± 0.02 μg g−1 day−1, similar to values documented by Silver et al. (2001) (0.6 μg N g−1 day−1) in tropical forest soils. DNRA has also been documented in several other forest ecosystems in different climates (Bengtsson and Bergwall 2000; Doff Sotta et al. 2008; Pett-Ridge et al. 2006; Silver et al. 2003). In contrast to DNRA, denitrification (\( R_{{{\text{NO}}_{3} }} \)) was negligible in this Andisol (Table 3). This indicates that DNRA out-competes denitrification (Pett-Ridge et al. 2006; Silver et al. 2001, 2005) and at the same time is a key process for NO3 − retention. The most obvious advantage of DNRA over other NO3 − consuming processes is, that N is transferred into NH4 +, another plant available N form which is not prone to N losses under acidic conditions and therefore leads to conservation of mineral N in soils (Nijburg and Laanbroek 1997). It should be noted that our laboratory experiment was performed at a WFPS of 50%, which is lower than the favorable conditions for denitrification (Linn and Doran 1984). Therefore, our experiment might underestimate the in situ denitrification rate, where high annual precipitation gives rise to large anoxic micro-sites. DNRA might likewise be underestimated in the present laboratory study. This is supported by recent results from an in-filed study in the same forest soil where almost three times higher DNRA rates were observed (1.0 ± 0.2 μg N g−1 day−1) (Huygens et al. 2008). As proposed by Burger and Jackson (2004), a rapid conversion of NO3 − to NH4 + via NO3 − immobilization and subsequent remineralization can be an alternative pathway for NO3 − reduction to ammonium. Quick immobilization—remineralization might occur in a small SOM sub-pools and thus could be masked by larger pools (Piňeiro et al. 2006). However, as none of the five different SOM fractions we analyzed had higher 15N excess than NH4 + (data not shown), we expect this pathway to be of limited importance in this soil. Silver et al. (2001, 2005) came to similar conclusions for an upland tropical forest which is characterized by high annual precipitation.
Less than 1% of the NO3 − production was derived from oxidation of NH4 + via the NO2 − nit pool while oxidation of organic N to NO3 − (i.e., heterotrophic nitrification) was the dominant NO3 − production pathway (Fig. 1), which is in line with several 15N labeling studies on acidic forest soils (Burton et al. 2007; Grenon et al. 2004; Pedersen et al. 1999; Schimel et al. 1984; Zeller et al. 2007). Heterotrophic nitrification is predominantly carried out by fungi (Landi et al. 1993) which gain energy from compounds that belong to more recalcitrant organic N pools in soil (Paul 2007). Therefore, NO3 − production via organic N oxidation is often carried out by acid tolerant fungi in forest soils (Eylar and Schmidt 1959; Stroo et al. 1986).
Our analysis shows a significant correlation between the gross rates of ONrec and \( D_{{{\text{NO}}_{3} }} \) (R = 0.78; Table 4). This further supports a functional link between oxidation of recalcitrant organic N to NO3 − (i.e., heterotrophic nitrification) and subsequent reduction via DNRA to NH4 +. This means that established ecosystems are characterized by microbial communities with energetically favorable pathways for NH4 + production and pathways that prevent N losses via leaching and/or gaseous N production. To our knowledge no other study so far had reported a similar functional link between heterotrophic NO3 − production and DNRA.
Dynamics of NO2 −
In the current study we present a detailed process-based analysis of NO2 − dynamics in a natural ecosystem. 15NO2 − has previously been used to investigate the fate of NO2 − in soils (Burns et al. 1995; Fitzhugh et al. 2003a, b; Nelson and Bremner 1969) including production of gaseous N (Russow et al. 2000; Venterea 2007). However, to our knowledge no study so far has performed a detailed tracing experiment to quantify the various gross N transformations related to NO2 − production and consumption in soil. The 15N tracing model (Fig. 1) developed in the present study separates total NO2 − into process-specific sub-pools (Müller et al. 2006) which are most likely associated with different soil micro-sites (Van Cleemput and Samater 1996). Despite this additional complexity, the model was able to reproduce the measured concentration and 15N enrichment of NO2 − appropriately (Fig. 2b, e). We increased the NO2 − pool by 90 times its background concentration (addition of 5 μg N g−1 soil to 0.055 μg NO2 −–N g−1 soil) to demonstrate the potential for NO2 − production and consumption in this soil. This increase is similar to other experiments (Fitzhugh et al. 2003a). However, the measured NO2 − concentrations at the first soil sampling (8 h after labeling) were in the range of background concentrations. Similar observations have been made by Islam et al. (2008) in acidic soils, where significant NO2 − losses occurred directly after additions of NO2 −. Possible mechanisms, which are responsible for this phenomenon, are chemical fixation of NO2 − by SOM or self-decomposition to gaseous N forms (Fitzhugh et al. 2003b; Islam et al. 2008). The latter pathway may has been responsible for the large amount of NO2 − that was reduced to the theoretical Nred pool shortly after N application (Fig. 4). Furthermore, the large amounts of extractable Fe (Table 1) in the Andisol may also have promoted NO2 − self-decomposition to NO3 − immediately after N addition (Van Cleemput and Baert 1984; Van Cleemput and Samater 1996). Evidence for this process comes from 15N enrichment of NO3 − 8 h after NO2 − labeling at the first extraction (Fig. 2f). As we made no extraction prior to 8 h, we cannot clarify the exact mechanisms for this rapid conversion of NO2 − to NO3 −. The suggested rapid abiotic transformations of NO2 − to NO3 − and SOM are taken care of by the model set-up, as the initial model pools are interpolated from the first two measurements (see above). The effect of NO2 − additions on potential stimulation of microbial activity is still unclear (Fitzhugh et al. 2003a). However, we did not find any evidence that the NO2 − application caused an unusual behavior of the N transformations in the system during the rest of the experimental period where NO2 − concentrations were at background levels (Fig. 2b).
Production of NO2 −
The fast dilution of applied 15NO2 − indicated a rapid production of unlabeled NO2 − entering the overall NO2 − pool (Fig. 2e). Almost 94% of the NO2 − produced during the 12 day experimental period was related to the process of NH4 + oxidation, which is in line with previous findings that nitrification is the dominant NO2 − producing process in a variety of soils (Burns et al. 1996; Russow et al. 2000). As only a small amount of this produced NO2 − is further oxidized to NO3 − (Table 3) it seems possible that Nitroso- but not Nitro-Bacteria are active in this Andisol. Support for this hypotheses comes from investigations in old-growth forests, where autotrophic nitrification was inhibited by the absence of Nitrobacter (Rice and Pancholy 1972). Furthermore, Archaea could also be partly responsible for NH4 + oxidization in this ecosystem (Leininger et al. 2006). Denitrification had, in contrast to other findings (Burns et al. 1996; Russow et al. 2000), only a negligible contribution to the total NO2 − production in this Andisol (Table 3) which is in line with findings by Smith et al. (1997). Instead, oxidation of organic N to NO2 − was responsible for more than 6% of the total NO2 − production. This organic pathway of NO2 − production has been documented before (Doxtader and Alexander 1966) but usually ignored in studies investigating the soil NO2 − dynamics. However, recent findings indicate that this organic NO2 − production process is likely to be more important than previously believed (Rütting and Müller 2008). The Andisol soil is iron-rich (Table 1) and therefore NO2 − production could also have been catalyzed by Fe-ions as proposed by the “ferrous wheel hypothesis” (Davidson et al. 2003, 2008). Any NO2 − production via this process is derived from abiotic reduction of NO3 − and therefore would have been part of the NO3 − reduction rate (\( R_{{{\text{NO}}_{3} }} \)), which may therefore be a combined rate of biotic (denitrification) and abiotic (“ferrous wheel hypothesis”) reactions. Since \( R_{{{\text{NO}}_{3} }} \) is only a tiny rate in this Andisol we can exclude both processes from having a major contribution in this study.
Consumption of NO2 −
The highest consumption of NO2 − in our study was related to the reduction of nitrification-related NO2 − (NO2 − nit) to Nred (Table 3). Moreover, our results indicate that a functional link exists between NH4 + oxidation and NO2 − nit reduction (R = 0.66; Table 4) rather than oxidation to NO3 −. The kinetic parameters for NO2 − nit reduction (\( R_{{{\text{NO}}_{2} {\text{nit}}}} \)) and NO2 − nit oxidation (\( O_{{{\text{NO}}_{2} {\text{nit}}}} \)) differed by four orders of magnitude (i.e., 34.8 and 0.003 h−1, respectively, Table 3) indicating that \( R_{{{\text{NO}}_{2} {\text{nit}}}} \) will easily out-compete \( O_{{{\text{NO}}_{2} {\text{nit}}}} \) for the available NO2 −. The exact nature of \( R_{{{\text{NO}}_{2} {\text{nit}}}} \) could not be identified within the scope of this study. However, processes such as nitrifier-denitrification (Wrage et al. 2001), nitrosation with phenolic compounds (Azhar et al. 1986), chemo-denitrification (Chalk and Smith 1983) and the anaerobic ammonium oxidation (anammox) (Mulder et al. 1995) could all be partly responsible. More detailed studies regarding the function of phenols and detailed analyses of process-based gaseous N dynamics in this soil are required to unravel the N transformations related to the production of Nred. The rapid NO3 − enriched with 15N after addition of 15N labeled NO2 − (Fig. 2f) cannot be explained by the low rate of \( O_{{{\text{NO}}_{2} {\text{nit}}}} \). Perhaps, more than one transformation process is responsible for \( O_{{{\text{NO}}_{2} {\text{nit}}}} \) whereas one may be a fast abiotic (i.e., self-decomposition) and the second a slower biotic (i.e., autotrophic nitrification) process. Similar findings were observed for NO3 − immobilization in temperate forest soils (Berntson and Aber 2000). Further studies are required to investigate the exact nature of processes, which are responsible for these findings.
Over the experimental period (12 days) we estimated a total NO2 − reduction of 8.6 μg N g−1 soil (Table 3), which amounts to 0.14% of the total N present prior to the experiment (average total N = 6,342 μg N g−1 soil). The product of the NO2 − reduction processes is a theoretical pool (Nred), which may contain in addition to gaseous N species (N2O, NO, NO2, N2) also NO2 − consumed by other biotic and abiotic processes as discussed above. Consequently Nred is not equal to gaseous N losses from the soil. Furthermore, based on the mass balance we did not find any evidence for N loss from the ecosystem. The strong competition for NO3 − in the soil may explain why gaseous N losses from these Nothofagus forests are negligible (Perakis and Hedin 2001). Further studies are needed to clarify the exact mechanisms of gaseous N production and emission in these Nothofagus ecosystems.
Conclusions
With the present study we confirmed our previous findings (Huygens et al. 2007) that pristine Nothofagus forests are characterized by negligible N losses due to a tight N cycle with high turnover rates and NO3 − consumption dominated by DNRA. Here, we show that DNRA has the potential to out-compete denitrification for the available NO3 −. However, further direct measurements of denitrification are required to confirm our results. With the present study we provide evidence that a functional link exists between (1) heterotrophic nitrification and DNRA as well as (2) between NH4 + oxidation (nitritation) and subsequent NO2 − reduction. To the best of our knowledge no other study has reported similar findings in any ecosystem. Therefore, more studies in various ecosystems are required to unambiguously prove if theses functional links are a general pattern in terrestrial soil. The preference of NO2 − nit reduction over oxidation to NO3 − is most likely related to the development of functionally linked microbial community structures which have adapted to the temporally prevailing anoxic conditions in this soil. This is in line with our observation that DNRA successfully out-competes immobilization and denitrification for the available NO3 −, i.e., processes requiring reducing conditions are strongly supported in this Andisol.
Nitrite is an important intermediate in several N transformation processes (Russow et al. 2000; Sprent 1987; Van Cleemput and Samater 1996; Venterea and Rolston 2000) and we showed that ignoring NO2 − dynamics may lead to erroneous estimates of gross N rates. Including NO2 − dynamics in 15N tracing studies provides us with more detailed and arguably more realistic N cycle models (Rütting and Müller 2008). Additionally, NO2 − data constrain the search for parameter constellations and thus allows a more precise quantification of gross N transformation rates even for rates which are not directly involved in the NO2 − dynamics.
Abbreviations
- Anammox:
-
Anaerobic ammonium oxidation
- DNRA:
-
Dissimilatory nitrate reduction to ammonium
- GWC:
-
Gravimetric water content
- MCMC:
-
Markov chain Monte Carlo
- MOM:
-
Macro organic matter
- PDF:
-
Parameter density function
- SOM:
-
Soil organic matter
- WFPS:
-
Water filled pore space
References
Azhar ES, Verhe R, Proot M, Sandra P, Verstraete E (1986) Binding of nitrite-N on polyphenols during nitrification. Plant Soil 94:369–382. doi:10.1007/BF02374331
Bengtsson G, Bergwall C (2000) Fate of 15N labelled nitrate and ammonium in a fertilized forest soil. Soil Biol Biochem 32:545–557. doi:10.1016/S0038-0717(99)00183-2
Berntson GM, Aber JD (2000) Fast nitrate immobilization in N saturated temperate forest soils. Soil Biol Biochem 32:151–156. doi:10.1016/S0038-0717(99)00132-7
Betlach MR, Tiedje JM (1981) Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol 42:1074–1084
Booth MS, Stark JM, Rastetter EB (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157. doi:10.1890/04-0988
Burger M, Jackson LE (2004) Plant and microbial use and turnover: rapid conversion of nitrate to ammonium in soil with roots. Plant Soil 266:289–301. doi:10.1007/s11104-005-1362-0
Burns LC, Stevens RJ, Laughlin RJ (1995) Determination of the simultaneous production and consumption of soil nitrite using 15N. Soil Biol Biochem 27:839–844. doi:10.1016/0038-0717(94)00219-Q
Burns LC, Stevens RJ, Laughlin RJ (1996) Production of nitrite in soil by simultaneous nitrification and denitrification. Soil Biol Biochem 28:609–616. doi:10.1016/0038-0717(95)00175-1
Burton J, Chen C, Xu Z, Ghadiri H (2007) Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol Biochem 39:426–433. doi:10.1016/j.soilbio.2006.08.011
Chalk PM, Smith CJ (1983) Chemodenitrification. In: Freney JR, Simpson JR (eds) Gaseous loss of nitrogen from plant soil systems. Martinus Nijhoff and Dr. W. Junk, Dordrecht, pp 65–89
Cho CM, Mills JG (1979) Kinetic formulation of the denitrification process in soil. Can J Soil Sci 59:249–257
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50:667–680
Davidson EA, Chorover J, Dail DB (2003) A mechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Chang Biol 9:228–236. doi:10.1046/j.1365-2486.2003.00592.x
Davidson EA, Dail DB, Chorover J (2008) Iron interference in the quantification of nitrate in soil extracts and its effect on hypothesized abiotic immobilization of nitrate. Biogeochemistry 90:65–73. doi:10.1007/s10533-10008-19231-10536
Doff Sotta E, Corre MD, Veldkamp E (2008) Differing N status and N retention processes of soils under old-growth lowland forest in Eastern Amazonia, Caxiuanã, Brazil. Soil Biol Biochem 40:740–750. doi:10.1016/j.soilbio.2007.10.009
Doxtader KG, Alexander M (1966) Nitrification by heterotrophic soil microorganisms. Soil Sci Soc Am Proc 30:351–355
Elliott ET, Paustian K, Frey SD (1996) Modeling the measurable or measuring the modelable: a hierarchical approach to isolating meaningful soil organic matter fractionations. In: Powlson DS, Smith P, Smith JU (eds) Evaluation of soil organic matter models using existing long-term datasets. Springer, Berlin, pp 161–179
Eylar OR, Schmidt EL (1959) A survey of heterotrophic micro-organisms from soil for ability to from nitrite and nitrate. J Gen Microbiol 20:473–481
Fahrmeir l Künstler R, Pigeot I, Tutz G (2004) Statistik—Der Weg zur Datenanalyse. Springer, Berlin
Fitzhugh RD, Christenson LM, Lovett GM (2003a) The fate of 15NO2 − tracer in soils under different tree species of the Catskill mountains, New York. Soil Sci Soc Am J 67:1257–1265
Fitzhugh RD, Lovett GM, Venterea RT (2003b) Biotic and abiotic immobilization of ammonium, nitrite, and nitrate in soils developed under different tree species in the Catskill Mountains, New York, USA. Glob Chang Biol 9:1591–1601. doi:10.1046/j.1365-2486.2003.00694.x
Godoy R, Oyarzún CE, Gerding V (2001) Precipitation chemistry in deciduous and evergreen Nothofagus forests of southern Chile under a low-deposition climate. Basic Appl Ecol 2:65–72. doi:10.1078/1439-1791-00037
Grenon F, Bradley RL, Titus BD (2004) Temperature sensitivity of mineral N transformation rates, and heterotrophic nitrification: possible factors controlling the post-disturbance mineral N flush in forest floors. Soil Biol Biochem 36:1465–1474. doi:10.1016/j.soilbio.2004.04.021
Hackl E, Bachmann G, Zechmeister-Boltenstern S (2004) Microbial nitrogen turnover in soils under different types of natural forest. For Ecol Manage 188:101–112. doi:10.1016/j.foreco.2003.07.014
Hart SC, Binkley D, Perry DA (1997) Influence of red alder on soil nitrogen transformations in two conifer forests of contrasting productivity. Soil Biol Biochem 29:1111–1123. doi:10.1016/S0038-0717(97)00004-7
Hassink J (1995) Density fractions of soil macroorganic matter and microbial biomass as predictors of C and N mineralization. Soil Biol Biochem 27:1099–1108. doi:10.1016/0038-0717(95)00027-C
Hauck RD (1982) Nitrogen isotope ratio analysis. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Soil Science Society of America, American Society of Agronomy, Madison, WI, pp 735–779
Holland EA, Dentener FJ, Braswell BH, Sulzman JM (1999) Contemporary and pre-industrial global reactive nitrogen budgets. Biogeochemistry 46:7–43
Huygens D, Boeckx P, Van Cleemput O, Oyarzún CE, Godoy R (2005) Aggregate and soil organic carbon dynamics in South Chilean Andisols. Biogeosciences 2:159–174
Huygens D, Rütting T, Boeckx P, Van Cleemput O, Godoy R, Müller C (2007) Soil nitrogen conservation mechanisms in a pristine south Chilean Nothofagus ecosystem. Soil Biol Biochem 39:2448–2458. doi:10.1016/j.soilbio.2007.04.013
Huygens D, Boeckx P, Templer PH, Paulino L, Van Cleemput O, Oyarzún CE, Müller C, Godoy R (2008) Mechanisms for retention of bioavailable nitrogen in volcanic rainforest soil. Nat Geosci 1:543–548. doi:10.1038/ngeo252
Islam A, Chen D, White RE, Weatherley AJ (2008) Chemical decomposition and fixation of nitrite in acidic pasture soils and implications for measurement of nitrification. Soil Biol Biochem 40:262–265. doi:10.1016/j.soilbio.2007.07.008
IUSS Working Group WRB (2006) World reference base for soil resources. World soil resources report no. 103. FAO, Rome
Kirkham D, Bartholomew WV (1954) Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci Soc Am Proc 18:33–34
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Landi L, Badalucco L, Pomarè F, Nannipieri P (1993) Effectiveness of antibiotics to distinguish the contributions of fungi and bacteria net nitrogen mineralization, nitrification and respiration. Soil Biol Biochem 25:1771–1778. doi:10.1016/0038-0717(93)90182-B
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. doi:10.1038/nature04983
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci Soc Am J 48:1267–1272
Luxhøi J, Nielsen NE, Jensen LS (2003) Influence of 15NH4 +-application on gross N turnover rates in soil. Soil Biol Biochem 35:603–606. doi:10.1016/S0038-0717(03)00002-6
Mary B, Recous S, Robin D (1998) A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol Biochem 30:1963–1979. doi:10.1016/S0038-0717(98)00068-6
Marzluf GA (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61:17–32
Meijboom FW, Hassink J, von Noordwijk M (1995) Density fractionation of soil macroorganic matter using silica suspensions. Soil Biol Biochem 27:1109–1111. doi:10.1016/0038-0717(95)00028-D
Mulder A, Van de Graaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol Ecol 16:177–184. doi:10.1111/j.1574-6941.1995.tb00281.x
Müller C, Stevens RJ, Laughlin RJ (2004) A 15N tracing model to analyse N transformations in old grassland soil. Soil Biol Biochem 36:619–632. doi:10.1016/j.soilbio.2003.12.006
Müller C, Stevens RJ, Laughlin RJ (2006) Sources of nitrite in a permanent grassland soil. Eur J Soil Sci 57:337–343. doi:10.1111/j.1365-2389.2005.00769.x (Erratum. Eur J Soil Sci 357:279)
Müller C, Rütting T, Kattge J, Laughlin RJ, Stevens RJ (2007) Estimation of parameters in complex 15N tracing models via Monte Carlo sampling. Soil Biol Biochem 39:715–726. doi:10.1016/j.soilbio.2006.09.021
Mulvaney RL (1996) Nitrogen-inorganic forms. In: Sparks DL (ed) Methods of soil analysis. Soil Science Society of America, American Society of Agronomy, Madison, WI, pp 1123–1184
Nanzyo M, Dahlgren RA, Shoji S (1993) Chemical characteristics of volcanic ash soils. In: Shoji S, Nanzyo M, Dahlgren RA (eds) Volcanic ash soils: genesis, properties and utilization. Elsevier, Amsterdam, The Netherlands, pp 147–187
Nelson DW, Bremner JM (1969) Factors affecting chemical transformations of nitrite in soils. Soil Biol Biochem 1:229–239. doi:10.1016/0038-0717(69)90023-6
Nijburg JW, Laanbroek HJ (1997) The influence of Glyceria maxima and nitrate input on the composition and nitrate metabolism of the dissimilatory nitrate-reducing bacterial community. FEMS Microbiol Ecol 22:57–63. doi:10.1111/j.1574-6941.1997.tb00356.x
Oyarzún CE, Godoy R, de Schrijver A, Staelens J, Lust N (2004) Water chemistry and nutrient budgets in an undisturbed evergreen rainforest of southern Chile. Biogeochemistry 71:107–123. doi:10.1007/s10533-004-4107-x
Parfitt RL, Scott NA, Ross DJ, Salt GJ, Tate KR (2002) Land-use change effects on soil C and N transformations in soils of high N status: comparisons under indigenous forest, pasture and pine plantation. Biogeochemistry 66:203–221. doi:10.1023/B:BIOG.0000005324.37711.63
Paul EA (2007) Soil microbiology, ecology, and biochemistry. Academic Press, Amsterdam
Paul EA, Clark FE (1996) Soil microbiology and biochemistry. Academic Press, San Diego
Pedersen H, Dunkin KA, Firestone MK (1999) The relative importance of autotrophic and heterotrophic nitrification in a conifer forest soil as measured by 15N tracer and pool dilution techniques. Biogeochemistry 44:135–150
Pepper DA, Eliasson PE, McMurtrie RE, Corbeels M, Ågren GI, Strömgren M, Linder S (2007) Simulated mechanisms of soil N feedback on the forest CO2 response. Glob Chang Biol 13:1265–1281. doi:10.1111/j.1365-2486.2007.01342.x
Perakis SS, Hedin LO (2001) Fluxes and fates of nitrogen in soil of an unpolluted old-growth temperate forest, southern Chile. Ecology 82:2245–2260
Pérez CA, Hedin LO, Armesto JJ (1998) Nitrogen mineralization in two unpolluted old-growth forests of contrasting biodiversity and dynamics. Ecosystems 1:361–374. doi:10.1007/s100219900030
Pett-Ridge J, Silver WL, Firestone MK (2006) Redox fluctuations frame microbial community impacts on N-cycling rates in humid tropical forest soil. Biogeochemistry 81:95–110. doi:10.1007/s10533-006-9032-8
Piňeiro G, Oesterheld M, Batista WB (2006) Opposite changes of whole-soil vs. pools C:N ratios: a case of Simpson’s paradox with implications on nitrogen cycling. Glob Chang Biol 12:804–809. doi:10.1111/j.1365-2486.2006.01139.x
Price SJ, Sherlock RR, Kelliher FM, McSeveny TM, Tate KR, Condron LM (2004) Pristine New Zealand forest soil is a strong methane sink. Glob Chang Biol 10:16–26. doi:10.1046/j.1529-8817.2003.00710x
Radcliffe DJ, Gillman GP (1985) Surface charge characteristics of volcanic ash soils from the southern highlands of Papua New Guinea. In: Cadas EF, Yaalon DH (eds) Volcanic soil. Catena, Braunschweig, pp 35–46
Rice EL, Pancholy SK (1972) Inhibition of nitrification by climax ecosystems. Am J Bot 59:1033–1040. doi:10.2307/2441488
Russow R, Sich I, Neue H-U (2000) The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: results of kinetic 15N tracer investigations. Chemosphere Glob Chang Sci 2:359–366. doi:10.1016/S1465-9972(00)00012-X
Rütting T, Müller C (2007) 15N tracing models with a Monte Carlo optimization procedure provide new insights on gross N transformations in soil. Soil Biol Biochem 39:2351–2361. doi:10.1016/j.soilbio.2007.04.006
Rütting T, Müller C (2008) Process-specific analysis of nitrite dynamics in a permanent grassland soil by using a Monte Carlo sampling technique. Eur J Soil Sci 59:208–215. doi:10.1111/j.1365-2389.2007.00976.x
Saghir NS, Mulvaney RL, Azam F (1993) Determination of nitrogen by microdiffusion in mason jars. 1. Inorganic nitrogen in soil extracts. Commun Soil Sci Plant Anal 24:1745–1762
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. doi:10.1890/03-8002
Schimel JP, Firestone MK, Killham KS (1984) Identification of heterotrophic nitrification in a Sierran forest soil. Appl Environ Microbiol 48:802–806
Shaviv A (1988) Control of nitrification rate by increasing ammonium concentration. Fert Res 17:177–188. doi:10.1007/BF01050278
Silver WL, Herman DJ, Firestone MK (2001) Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 82:2410–2416
Silver WL, Thompson AW, Bradbury D, Chapin FS III, Ewel JJ, Firestone MK (2003) Global patterns in dissimilatory nitrate reduction: a latitudinal gradient in nitrogen retention and loss. American Geophysical Union, Fall Meeting abstract B32B-0389
Silver WL, Thompson AW, Reich A, Ewel JJ, Firestone MK (2005) Nitrogen cycling in tropical plantation forests: potential controls on nitrogen retention. Ecol Appl 15:1604–1614. doi:10.1890/04-1322
Skjemstad JO, Spouncer LR, Cowie B, Swift RS (2004) Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Aust J Soil Res 42:79–88. doi:10.1071/SR03013
Smith CJ, Chalk PM (1979) Mineralization of nitrite fixed by soil organic matter. Soil Biol Biochem 11:515–519. doi:10.1016/0038-0717(79)90011-7
Smith RV, Doyle RM, Burns LC, Stevens RJ (1997) A model for nitrite accumulation in soils. Soil Biol Biochem 29:1241–1247. doi:10.1016/S0038-0717(97)00028-X
Smith JU, Smith P, Monaghan R, Macdonald AJ (2002) When is a measured soil organic matter fraction equivalent to a model pool? Eur J Soil Sci 53:405–416. doi:10.1046/j.1365-2389.2002.00458.x
Speir TW, Townsend JA, More RD, Hill LF (1999) Short-lived isotopic method to measure nitrous oxide emissions from a soil under four low-fertility management systems. Soil Biol Biochem 31:1413–1421. doi:10.1016/S0038-0717(99)00061-9
Sprent JI (1987) The ecology of the nitrogen cycle. Cambridge University Press, Cambridge
Stark JM (2000) Nutrient transformations. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 215–234
Stevens RJ, Laughlin RJ (1994) Determining nitrogen-15 nitrite or nitrate by producing nitrous oxide. Soil Sci Soc Am J 58:1108–1116
Stroo HF, Klein TM, Alexander M (1986) Heterotrophic nitrification in an acid forest soil and by an acid-tolerant fungus. Appl Environ Microbiol 52:1107–1111
Tamm CO (1991) Nitrogen in terrestrial ecosystems (Ecological Studies 81). Springer, Berlin
Tiedje JM, Sexstone AJ, Myrold DD, Robinson JA (1982) Denitrification: ecological niches, competition and survival. Antonie van Leeuwenhoek 48:569–583. doi:10.1007/BF00399542
Van Cleemput O, Baert L (1976) Theoretical considerations on nitrite self-decomposition reactions in soils. Soil Sci Soc Am J 40:322–324
Van Cleemput O, Baert L (1984) Nitrite: a key compound in N loss processes under acid conditions? Plant Soil 76:233–241. doi:10.1007/BF02205583
Van Cleemput O, Samater AH (1996) Nitrite in soils: accumulation and role in the formation of gaseous N compounds. Fert Res 45:81–89. doi:10.1007/BF00749884
Venterea RT (2007) Nitrite-driven nitrous oxide production under aerobic soil conditions: kinetics and biochemical controls. Glob Chang Biol 13:1798–1809. doi:10.1111/j.1365-2486.2007.01389.x
Venterea RT, Rolston DE (2000) Mechanisms and kinetics of nitric oxide and nitrous oxide production during nitrification in agricultural soils. Glob Chang Biol 6:303–316. doi:10.1046/j.1365-2486.2000.00309.x
Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA, Todd RL (1979) Nitrate losses from disturbed ecosystems. Science 204:469–474. doi:10.1126/science.204.4392.469
Wrage N, Velthof GL, Van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. doi:10.1016/S0038-0717(01)00096-7
Zeller B, Recous S, Kunze M, Moukoumi J, Colin-Belgrand M, Bienaimé S, Ranger J, Dambrine E (2007) Imfluence of tree species on gross and net N transformations in forest soil. Ann For Sci 64:151–158. doi:10.1051/forest:2006099
Zimmermann M, Leifeld J, Schmidt MWI, Smith P, Fuhrer J (2006) Measured soil organic matter fractions can be related to pools in the RothC model. Eur J Soil Sci 58:658–667. doi:10.1111/j.1365-2389.2006.00855.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rütting, T., Huygens, D., Müller, C. et al. Functional role of DNRA and nitrite reduction in a pristine south Chilean Nothofagus forest. Biogeochemistry 90, 243–258 (2008). https://doi.org/10.1007/s10533-008-9250-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-008-9250-3