Abstract
The bioavailability and composition of dissolved organic carbon (DOC) and nitrogen (DON) were examined in 10 major sub-catchments of the Swan-Canning estuary, which bisects the city of Perth, in south-western Australia. Catchments contain a mix of forest, agriculture, and urban-dominated land-use, with the degree of development increasing near the city center. We incubated water samples from the 10 sub-catchments for 14 days at 25°C, and measured changes in DOC and DON and dissolved inorganic nitrogen (DIN). A greater proportion of DON (4–44%) was decomposed compared to DOC (1–17%). Both agricultural and urban catchments had high proportions of bioavailable DOC and DON, but overall DOC and DON losses were greatest in urban catchments. Using resin isolation techniques, we found that DOC was concentrated in the hydrophobic (humic) fraction, whereas DON had both hydrophobic and hydrophilic (non-humic) fractions. Hydrophobic DOC content was positively related to DOC decomposition. In contrast, DON decomposition was highly correlated with hydrophilic DON content and inversely related to the hydrophilic DOC/DON ratio, indicating a labile fraction of DON from non-humic sources. Taken together, these relationships suggest that bioavailable DOC may be supplied in part from terrestrial plant material, but bioavailable DON is likely to be from highly labile sources, possibly autochthonous or anthropogenic. Overall, labile DON was greater than initial DIN concentration at seven of ten sites and was even dominant in highly developed catchments. This study highlights the importance of organic N in urbanizing coastal catchments that, in addition to DIN, may serve as a readily available source of N for in-stream and estuarine production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human development has greatly modified the near-coast landscape in many regions of the world through clearing of native vegetation for agricultural, residential, and urban development. One by-product of this land-use change is a 5–15 fold increase in N export that has contributed to the occurrence of algal blooms and hypoxia in estuaries and coastal waters (Howarth et al. 1996). Inorganic N (DIN = NO3 − + NH4 +) has traditionally been the focus of increased N loading of waterways. However, recent studies have highlighted the ecological importance of dissolved organic nitrogen (DON) that can be mineralized by microbes and supply N to primary producers in streams and estuaries (Seitzinger et al. 2002).
DON is often the dominant form of nitrogen in surface waters even where there are significant anthropogenic N inputs (Scott et al. 2007), and human activity may promote DON export in populated coastal catchments (Kroeger et al. 2006). The transport and breakdown of DON is in part linked with dissolved organic carbon (DOC), but the strength of DOC and DON coupling in catchments depends on microbial processes and organic matter sources in streams. For instance, anthropogenic activity may have a greater effect on the export of DON than DOC in coastal areas (Harrison et al. 2005). Nevertheless, few studies have simultaneously examined the ecological role of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in populated catchments that drain into coastal estuaries (Pellerin et al. 2006).
The ecological condition of near-coast catchments and coastal waterbodies is under increasing pressure from land-use changes that have modified hydrologic and biogeochemical cycles. This is particularly true in Australia where many coastal catchments have been transformed from ecosystems dominated by sclerophyllous vegetation into a landscape mosaic consisting of remnant native vegetation, agricultural, residential, and urban patches with greater overall N export (Harris 2001). The Swan-Canning river and estuary system that bisects the city of Perth (population 1.5 million in 2007) typifies a catchment that is currently undergoing extensive and rapid change due to an expanding population and associated changes in land-use. N exports (kg ha−1 y−1) in the Swan-Canning catchment are greatest in areas that have been cleared for development and agriculture (Zammit et al. 2005), yet DON is often the dominant form of nitrogen in coastal rivers and streams, comprising roughly two-thirds of the total N flux to the estuary (Peters and Donohue 2001). Further, bioassays have shown that N is limiting to phytoplankton growth in the Swan-Canning estuary (Thompson and Hosja 1996), and the frequency of harmful phytoplankton blooms has increased in summer months when light and temperature are at a maximum (Robson and Hamilton 2003). Additional inorganic N supply from organic sources could play an critical ecological role, especially during summer when N retention in the estuary is high due to limited freshwater discharge to the ocean (Robson et al. 2008).
Sources of organic matter to aquatic ecosystems include natural and anthropogenic catchment sources, in-stream production, as well as atmospheric sources (Peierls and Paerl 1997; Seitzinger et al. 2002; Wiegner et al. 2006). Labile DOM sources, such as organic fertilizers, waste runoff, and microbial byproducts are generally non-humic and aliphatic with lower DOC/DON (Westerhoff and Mash 2002), and are readily decomposed by microbes (Glibert et al. 2006). Large aromatic macromolecules such as humic and fulvic acids derived from lignified plant material, which is especially prevalent in ecosystems dominated by sclerophyllous vegetation, have higher DOC/DON, and are generally considered to be more recalcitrant (Qualls and Haines 1991).
The stoichiometry of bulk DOC and DON, the primary components of dissolved organic matter (DOM), have routinely been used to characterize its quality. However, bulk ratios represent the sum of various recalcitrant and labile compounds that may be cycled at different rates in aquatic ecosystems. Recent studies have shown that the DOC and DON content of humic and non-humic fractions may be used to compare how fast and slow turnover pools of DOC and DON supply energy and nutrients for heterotrophic production (Kaushal and Lewis 2005). Furthermore, in bioavailability experiments studies, a greater proportion of DON is often consumed compared to DOC (Kaushal and Lewis 2005; Wiegner and Seitzinger 2001, 2004; Wiegner et al. 2006). These findings suggest that DOC and DON may be concentrated in different fractions of DOM (e.g., humic and non-humic).
Our objective in this study was to assess the relationship between the chemical composition and bioavailability of dissolved organic matter entering the Swan-Canning estuary in surface flows from sub-catchments that vary in land-use. This range of catchments has a large gradient of DOC and DON concentrations which is likely due to both land-use and hydrogeologic conditions. The composition of DOM was assessed by separating it into “humic” and “non-humic” fractions (Aiken et al. 1992) and examining the stoichiometry of organic C and N in these fractions. Bioavailability experiments were used to quantify the decomposition of DOC and DON and relate it to the chemical composition of DOM. We hypothesized that overall DOM chemistry reflects catchment land-use and that its quality in turn influences the bioavailability of DOC and DON in surface waters.
Study site
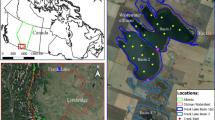
The Swan-Canning estuary bisects the city of Perth in southern Western Australia, covers 121,000 km2, and encompasses numerous sub-catchments that vary widely in drainage area, land-use, soil type, and underlying geology (Table 1). The climate is Mediterranean with two-thirds of the annual rainfall (850–1,200 mm) occurring during winter (June–September). In this study, we sampled water from major Swan-Canning sub-catchments that were flowing during the summer period (Fig. 1). Two sub-catchments (Upper Canning and Avon Rivers) drain from inland of the Swan Coastal Plain and are partially underlain by pallid clay (1–2 m) and lateritic horizons (5–10 m). Catchments on the coastal plain are underlain by highly weathered quaternary alluvial and aeolian deposits known as Bassendean sands, which are siliceous with little clay or silt content and consequently have little capacity to adsorb DOM (He et al. 1998).
Land-use and boundaries of the Swan-Canning sub-catchments. The Lower Canning site is marked (see Table 1 for catchment areas and percent land-use cover)
Once extensive lakes and wetlands on the coastal plain have been mostly drained for urban development and agriculture, but remnant permanent and ephemeral wetlands dominated by the genera in the Myrtaceae and Restionaceae are underlain with humic podzols. Previously, lakes and wetlands created a natural drainage system, which has now been replaced with impervious surfaces and deeply incised urban drains that facilitate groundwater drainage to the estuary in some catchments (Table 1: Yule Brook, Bennett Brook, Mills St Drain, Bannister Creek, South Belmont Drain and Bayswater Drain). In other catchments, natural stream/river systems are fed by urban drains (e.g., Southern River). Native vegetation dominated by a mix of sclerophyllous trees and shrubs (including Eucalyptus, Bansksia and Allcoasuarina) is found throughout catchments on well-drained soils.
While significant DOM export occurs during the rainy season, the baseflow period is also important as DOC and DON that accumulates in groundwater is gradually released in high concentration to surface waters and to the estuary during the summer months (Donohue et al. 2001; Linderfelt and Turner 2001). Additionally, developed catchments have higher runoff ratios and annual N export rates (Peters and Donohue 2001).
Methods
Sample collection
We compared the decomposition of DOC and DON during the summer baseflow period when hydrologic variation had a small effect on stream chemistry. Water was collected from 10 streams and urban drains from sub-catchments with varied land-use on the Swan Coastal Plain and Darling escarpment. Water was collected on January 10, 2007. Conductivity and temperature were measured at each site at time of sampling. Approximately 10 L of water was collected at each site into acid washed bottles. Water was filtered through pre-combusted glass fiber filters (Whatman GF/F 0.7 nominal pore size) in the field. Pre-filters (Millipore prefilter AP25) were also used where necessary to remove large particles. Samples were stored on ice during transport to the laboratory where they were refrigerated at 4°C for up to 1 week prior to inoculation. Samples for the experiment were further filtered in the laboratory through either 0.2 μm polycarbonate or 0.2 μm polyvinylidene Millipore filters to remove microbes.
Bioavailability experiment
Our methodology is similar to previous studies (Kaushal and Lewis 2005; Seitzinger et al. 2002; Wiegner et al. 2006) in that we used a common bacteria inoculum in our laboratory experiment from an estuarine site that receives water from the sub-catchments. In this way, the decomposition of DOM reflects differences in chemical composition and how estuarine microbes respond to site-specific DOM chemistry. Our inoculum was prepared by collecting a slurry (1:3 sediment to water) from the Lower Canning River 1 day prior to inoculating the water samples. The water/sediment mixture was centrifuged at 3,000 rpm for 1 min to remove particulate matter and protists (Seitzinger et al. 2002). We assessed the inoculum under a light microscope to ensure that only bacterial cells were present.
The bioavailability of DOM and supply of DIN were quantified by changes in DOC, DON, NH4 + and NO3 − over the course of the experiment. Prior to the incubation, water samples were warmed to room temperature (25°C) and 96 ml of inoculum was added to 4.8 l (1:50 inoculum to water) of 0.2 μm filtered water from each site to ensure a cell count of at least 105 cells ml−1. The inoculum was also added to 4.8 l of DI water for a control. Water from each site was split into three 1.6 l volumes and poured into triplicate 2 l conical flasks. Each flask was incubated in the dark at 25°C in a constant temperature room for 1 month. Time series samples (100 ml) were taken at the commencement of the incubation and again on days 2, 3, 5, 8, 11, and 14 and analysed for DOC, DON, NH4 + and NO3 −, and SRP. All time series samples were filtered through Whatman GF/F filters at the time of sub-sampling. Cell counts were also taken periodically on a slide with counting chambers, and we observed greater than 106 cells ml−1 throughout the course of the experiment.
Experimental results are reported as mg of C or N consumed per day, which equals the change in concentration over the course of the experiment divided by days of the incubation (14 days). Although we extended the experiment for 36 days in total, we found little change in DOC after day 14, and large variations in the NO3 − and NH4 + concentrations at day 36 suggest a turnover of the microbial community and lysis of cells. As a result, we focused on the first 14 days of the experiment when the microbes were likely to be in a growth phase. This period of incubation compares well with other studies in marine and freshwater environments (del Giorgio and Davis 2003).
DOM characterization
DOM was characterized using a modified method of rapid fractionation (Chow et al. 2004) that is based on common isolation procedures using resin column chromatography (Aiken et al. 1992; Leenheer 1981; Thurman and Malcolm 1981). We used this technique because these operational groups likely separate chemical compounds that are resistant or readily available for decomposition. Hydrophobic humic and fulvic acids tend to be of higher molecular weight and resistant to degradation (Aiken et al. 1992; Qualls and Haines 1991). Of the non-humic DOM, the transphilic fraction is thought to consist of lower molecular weight and less recalcitrant organic and aliphatic acids, and the hydrophilic fraction contains labile and N-rich compounds such as proteins, amino sugars and amino acids (Aiken et al. 1992).
Samples were acidified to pH 2 and pumped through chromatography columns of DAX-8 resin (Supelite DAX-8 Sigma Aldrich) and then XAD-4 resin (Amberlite XAD-4 Sigma Aldrich). Flow rates were set at 3 ml min−1 with a peristaltic pump and 30 ml bed volumes of resin were used to prevent DOM breakthrough. DOM that adsorbs to the DAX is operationally classified as humic and “hydrophobic”. The non-humic fraction can be further divided into “transphilic” that adsorbs to the XAD-4 resin. Lastly, DOM that passes through both resins and is not sorbed to either resin is defined as “hydrophilic” (Chow et al. 2005). We calculated the DOC and DON content of each fraction using the concentration difference between the inlet and effluent water of each column as well as the concentration of the hydrophilic fraction that was not sorbed. For example, initial sample—DAX-8 effluent = “hydrophobic”; DAX-8 effluent—XAD-4 effluent = “transphilic”, and XAD-4 effluent = “hydrophilic”. Blanks were run periodically and found to contain less than 1 mg l−1 of DOC. Due to analytical variability, the sum of final DOC and DON content of fractions were within 15 % of the initial DOC and DON concentration.
Analytical chemistry
An autoanalyzer was used to measure NH4 + by the phenol hypochlorite method and NO3 − by cadmium reduction. DOC was measured as non-purgeable organic carbon on a Shimadzu TOC-5000. TDN was measured by potassium persulfate digestion and colorometric analysis as NO3 −. DON was calculated as the difference between TDN and inorganic N (NO3 − + NH4 +). Soluble reactive phosphorus (SRP) was measured by the molybdate-antimony technique on a spectrophotometer. Specific UV absorbance (SUVA = 254 nm abs mg l−1 DOC * 100) was measured on a Shimadzu UV/VIS spectrophotometer (UV-1601).
Results
Stream chemistry
The concentration of DOC and DON varied more than five-fold across stream sites. DOC ranged from 3.3 to 18.8 mg l−1 while DON ranged from 0.187 to 1.197 mg l−1 (Table 1). The Upper Canning, which drains soils with greater clay content, had the least DOC and DON of all catchments while urban-dominated sites (Mills St Drain and Bannister Creek) had the most. Nitrate concentrations were less than 0.1 mg l−1 at all sites except Southern River, Bannister Creek and Bayswater Drain. The Bayswater Drain was high in NH4 +, but otherwise NH4 + was low at all sites (<0.07 mg l−1). DOC and DON were significantly and positively correlated (R 2 = 0.80, P < 0.001), but there was no clear pattern in the DOC to DON ratio, which ranged from 12.9 to 24.9. Total dissolved nitrogen (TDN) was greater than particulate N (PN) across sites, comprising 71–100% of total N (TN). Similarly, DON comprised at least 75% of the TDN at all sites except Bayswater Drain, where DON was just over 50% of the TDN (Table 1). SUVA, an indication of sample aromaticity, was positively related to DOC concentration (R 2 = 0.60, P < 0.05).
Bioavailability experiment
In the bioavailability experiment, we observed significant loss of DOC and DON for all sites except the Upper Canning (repeated measures ANOVA; P < 0.001; Table 3). The overall loss was greater for DON (4–46%) than for DOC (1–17%). The rate of DOC loss was linear for some sites (Mills St Drain) over the 14 day period while other sites showed an initial drop (Bannister Creek and Yule Brook) or an initial increase in DOC concentration followed by a consistent decline (Avon River, Bennett Brook and Lower Canning River; Fig. 2a). The DOC concentration increased slightly in the control flasks in the last few days of the experiment, suggesting a lysis of bacterial cell walls and production of DOC. DON decomposition was more variable than DOC, but an initial DON increase followed by a decline was observed for most sites (Fig. 2b). This pattern was also apparent in the control sample, which also suggests a microbial release from the bacterial inoculum. Despite the initial influence of the inoculum, DON sharply declined at most sites between days 8 and 14 (Fig. 2c, d). Further, the greater relative decline in DON compared to DOC across site resulted in an increase in the DOC to DON ratio across the course of the experiment (i.e., between initial samples after microbial inoculation and the chemistry of flasks at the end of the experiment) (Table 3).
Inorganic N patterns suggest that NO3 − and NH4 + were initially immobilized in the first few days of the experiment; NH4 + declined on day 3 and NO3 − remained low through day 5 (Fig. 2c). However, as DON was consumed after day 5, inorganic N increased, mainly as NO3 −, at more than half of the stream sites. The notable exception was the urban-dominated Bayswater Drain, which showed an increase in NH4 + of 0.1 mg l−1 over the course of the experiment. In general, those sites with low concentrations of NO3 − and NH4 + at the beginning of the experiment exhibited no significant increase in NO3 − or NH4 +. NH4 + also decreased to near detection limits at nearly all sites except Bayswater Drain on day 14 (Fig. 2d).
DOM composition
The organic C and N content of DOM fractions differed across sites. DOC was concentrated in the hydrophobic fraction in seven of ten sites, with lesser amounts of hydrophilic DOC (Table 2). The transphilic fraction had the smallest DOC content at all sites except the Upper Canning. In contrast, DON was more concentrated in the hydrophilic fraction, and the DON content of hydrophobic and hydrophilic was evenly mixed across sites. DON content was also low in the transphilic fraction at nine of ten sites (Table 2). The DON and DOC content of fractions was related to the initial DOC and DON, but the strength of the relationships varied. For instance, the strength of initial DOC and fractions was in the order hydrophobic DOC (R 2 = 0.88, P < 0.0001) > transphilic DOC (R 2 = 0.65, P < 0.01) > hydrophilic DOC (R 2 = 0.46, P < 0.05). In contrast, initial DON was in the order; hydrophilic DON (R 2 = 0.83, P < 0.0005) > hydrophobic DON (R 2 = 0.71, P < 0.005) > transphilic DOC (R 2 = 0.43, P < 0.05).
DOC/DON of DOM fractions were highly variable across sites and ranged from 13.0 to 53.9 for hydrophobic, 10.0 to 31.6 for transphilic, and 9.9 to 20.0 for hydrophilic (Table 3). The hydrophobic fraction tended to have the highest DOC/DON, whereas the DOC/DON of the hydrophilic fraction tended to be lowest, and the transphilic fraction highly variable.
DOM bioavailability
DOC and DON decomposition rates over the course of the experiment were significantly related to the initial concentration of DOC (R 2 = 0.79, P < 0.001) and DON (R 2 = 0.77, P < 0.001; Fig. 3). This finding is supported by DOM fractions that are related to total concentration; the hydrophobic fraction was correlated with initial DOC and the hydrophilic with initial DON. In contrast to the positive relationship between decomposition and concentration for organic C and N, we observed a significant negative relationship between DIN production (−) or consumption (+) and the initial DIN concentration with DIN production occurring when DIN concentration was above 0.15 mg l−1 (Fig. 4).
Comparisons between DOM fractions and decomposition were used to more specifically compare DOM chemistry with its bioavailability. Surprisingly, there was no improvement in the correlation between DOC decomposition and the DOC content of any specific fraction compared to the initial DOC concentration. DOC decomposition and the DOC content of the hydrophobic fraction was the only significant relationship observed, but this regression explained less variation in DOC decomposition (R 2 = 0.67, P < 0.05; Fig. 5) than the relationship between DOC decomposition and initial DOC concentration (R 2 = 0.79, P < 0.001). However, in the case of DON, the hydrophilic fraction explained more of the DON decomposition pattern. The relationship between DON decomposition and hydrophilic DON content was highly significant and explained much of the DON loss (R 2 = 0.81, P < 0.0005; Fig. 6). Furthermore, DON loss during the experiment was inversely related to the DOC/DON of the hydrophilic fraction (R 2 = 0.65, P < 0.01; Fig. 7).
Revised DON pools
We distinguished the biologically reactive pools of organic N from DON loss observed in the experiment. The labile pool is defined as the proportion of DON that was consumed, and the recalcitrant pool is the proportion that remained. The recalcitrant pool was greater than the labile pool at all sites (Fig. 8). Although labile DON was the smaller proportion of the total DON pool, its importance was highlighted when compared to total inorganic N concentration at each site. In eight of ten sites, the labile DON pool was up to four times greater than the concentration of DIN. Further, estimates of DON lability in this experiment are likely to be conservative since we measured net DON mineralization rather than gross N mineralization which includes internal recycling of microbial metabolism through uptake of mineralized or leaked microbial N during the experiment.
Discussion
DOM fractions and bioavailability
This study highlights the compositional differences between DOC and DON that influence their fate in aquatic ecosystems. We found that DOC decomposition was related to the hydrophobic DOC, composed of aromatic and humic compounds, that are typically considered to be more recalcitrant (Qualls and Haines 1992). While DON was found in both hydrophobic and hydrophilic fractions, DON bioavailability was only related to the hydrophilic (non-humic) fraction. These findings suggest that a plant-derived catchment source of DOC contributes in part to bioavailable DOC, but the relatively weak relationship between hydrophobic DOC and DOC consumption indicates that DOC may be supplied from numerous compounds, including humic and non-humic sources. In contrast, DON decomposition was only related to the hydrophilic fraction, suggesting that N-rich compounds such as proteins and amino acids supply N demand for heterotrophic production. Nitrogen contained within humic compound structures is generally less available for uptake than N-rich functional groups such as amino acids, amino sugars, and proteins (Bronk 2002). These relationships between DOC and DON fractions and DOC and DON degradation support the growing body of evidence for slow and fast turnover pools of DOC and DON in lotic ecosystems (Brookshire et al. 2005; Kaushal and Lewis 2005).
We found that DOC/DON increased between initial and final samples in all sites over the course of the experiment, indicating a greater microbial demand for DON than DOC. Selective uptake of DON may occur by enzymatic cleavage of N-rich functional groups or preferential uptake of low C/N molecules within DOM (Qualls and Haines 1992). Greater microbial utilization of DON from the hydrophilic fraction (low DOC/DON) than from the hydrophobic fraction (high DOC/DON) would elevate the overall DOC/DON ratio remaining in solution. The ratio of DOC and DON loss during the experiment was low at most sites (<7) and we observed a negative relationship between the DON decomposition and the DOC/DON ratio where the greatest DON consumption at DOC/DON of around 10. These several lines of supporting evidence suggest that N-rich compounds may be preferentially utilized by microbes.
DON has proven to be an important source of N when the N concentration is low in montane ecosystems (Kaushal and Lewis 2005), but in wetlands inorganic N may have little effect on DOC and DON consumption (Wiegner and Seitzinger 2004). We did not explicitly test the effects of inorganic nutrients on DOM decomposition by nutrient addition. However, our results suggest that although DIN had little effect on DON consumption, initial DIN was proportional to overall DIN production. Greater DIN availability may lower microbial need for inorganic N immobilization and thereby allow DIN concentrations to remain elevated during the experiment.
Ecosystem implications in urbanizing coastal catchments
An understanding of the flux and biological activity of N supplied to estuaries has become more important as eutrophication, hypoxia and the occurrence of harmful algal blooms increases in estuaries and coastal zones. On a catchment scale, multiple factors such as wetland coverage, soil type, disturbance, hydrologic flowpath, and riparian sources may affect the relative flux of DOC, DON and DIN (Petrone et al. 2007a, b). Variation in DOC and DON concentrations and DOC/DON ratios across the Swan-Canning sub-catchments reflects multiple DOM sources and complex linkages between catchment soils and surface waters. Moreover, the dominance of DON over DIN, and low contribution of PN, is indicative of low gradient catchments with sandy soils that have little adsorption capacity (Peters and Donohue 2001).
The proportion of DOC (1–17%) and DON (4–44%) consumed in this study supports the results of the few previous studies that have examined concurrent DOC and DON decomposition in freshwater ecosystems (Kaushal and Lewis 2005; Wiegner and Seitzinger 2001, 2004; Wiegner et al. 2006). In all cases, DON proved to be more bioavailable than DOC in streams, rivers, and wetlands studied. This trend reflects the general nature of DOM compounds in freshwater ecosystems - labile DON may be mineralized and cycled back into organic N more quickly than C-rich humic compounds, which may be gradually lost through respiration along hydrologic flowpaths unless supplied by autochthonous sources in the stream. In fact, in some cases, uptake of DON may exceed DIN uptake (Brookshire et al. 2005). Further, differential cycling of DOC and DON may be exacerbated in microcosm experiments where DOC is lost as CO2 during incubations, but DON is re-mineralized. In this way, our laboratory estimates of bioavailability are likely to underestimate in situ measurements since our experiment was limited to the growth phase of microbes and addition sources of DOM and inorganic nutrients were not supplied. Nevertheless, the high proportion of DON loss estimated in this study for some catchments is in the upper range of reported DON decomposition (see review by Wiegner and Seitzinger 2004).
Urbanization may influence stream ecosystems by enhancing overland flow on impervious surfaces and increasing area-specific runoff and the delivery of nitrogen (Lewis and Grimm 2007). Reduced interaction of surface and groundwater flowpaths with riparian zones may also increase inorganic N by reducing plant uptake and denitrification. At the same time, DOC and DON dissolution and delivery may also be reduced by limiting interactions with organic-rich surface soils (Walsh et al. 2005), which can provide a source of DOM in native forest catchments (Petrone et al. 2007a). Catchment dynamics in coastal zones underlain with sandy soils may be unique with respect to these views of urbanization and controls of DOM in surface waters. The least developed catchment in this study, Upper Canning, had the lowest concentrations of DOC and DON. The greatest factor governing DOM mobility and delivery to Swan-Canning streams may be low adsorption soils that allow DOM transport to sub-surface soils, groundwater and, ultimately, to surface streams and incised urban drains.
The dynamics of DOM in urbanizing coastal catchments has received little attention, but recent studies have shown that urban and sub-urban runoff sources may be highly labile (Seitzinger et al. 2002). Anthropogenic sources such as wastewater runoff may be of low C/N (Westerhoff and Mash 2002), and increased use of organic fertilizers is a concern where heterotrophic and autotrophic production in coastal areas may be limited by natural inputs (Glibert et al. 2006). Further, it has been suggested that the flux of bioavailable DON from catchments may increase with N saturation of soils due to N deposition (Brookshire et al. 2007). Nutrient saturation in the Swan-Canning sub-catchments may occur through fertilizer usage in agricultural areas, public parks and domestic gardens as well as animal and waste runoff from agricultural fields and urban surfaces (Peters and Donohue 2001). In fact, a recent survey of fertilizer usage in the Perth metropolitan area found that organic fertilizers (e.g. manures, mulches and composts) comprise the majority of added TN and TP in the suburban zone, and median application rates for residential lots were 138 and 43 kg ha−1 y−1, respectively (Kitsios and Kelsey 2008). Long-term application of organic fertilizers may be a factor in the quality and export of DON from urbanised coastal catchments which has not been explicitly examined to date.
In general, it may be easier to attribute elevated inorganic N to anthropogenic inputs than organic N, which is a heterogeneous mixture of organic compounds. While we did not attempt to distinguish between autochthonous or natural/anthropogenic catchment sources of DOM in this study, we have shown that DOC and DON components may be concentrated in different fractions of DOM that could be from unique catchment sources. Further, we found that labile DON may be greater than DIN during the summer season. Given that the residence time of freshwater in the Swan estuary during low flow period is on the order of several weeks (Chan and Hamilton 2001), much of this added N would become available for production in the upper estuary. In future studies, specific examination of DOC and DON from likely catchment sources such as native vegetation, in-stream algae and macrophytes, and anthropogenic sources would enable us to identify important catchment sources that may contribute to organic N loads. A greater understanding of DOM bioavailability and its sources is especially important for catchment managers concerned with energy and nutrients that regulate aquatic primary and secondary production. In particular, the proportion of bioavailable DON in streams and rivers draining into estuaries may be a critical but overlooked component of nitrogen cycle in urbanising coastal environments.
References
Aiken GR, Mcknight DM, Thorn KA, Thurman EM (1992) Isolation of hydrophilic organic-acids from water using nonionic macroporous resins. Org Geochem 18:567–573. doi:10.1016/0146-6380(92)90119-I
Bronk DA (2002) Dynamics of DON. In: Hansell DA, Carlson CA (eds) Biogeochemistry of marine dissolved organic matter. Elsevier, London, pp 153–247
Brookshire ENJ, Valett HM, Thomas SA, Webster JR (2005) Coupled cycling of dissolved organic nitrogen and carbon in a forest stream. Ecology 86:2487–2496. doi:10.1890/04-1184
Brookshire ENJ, Valett HM, Thomas SA, Webster JR (2007) Atmospheric N deposition increases organic N loss from temperate forests. Ecosystems (NY Print) 10:252–262. doi:10.1007/s10021-007-9019-x
Chan TU, Hamilton P (2001) Effect of freshwater flow on the succession and biomass of phytoplankton in a seasonal estuary. Mar Freshw Res 52:869–884. doi:10.1071/MF00088
Chow CWK, Fabris R, Drikas M (2004) A rapid fractionation technique to characterise natural organic matter for the optimisation of water treatment processes. J Water Supply 53:85–92
Chow AT, Gao S, Dahlgren RA (2005) Physical and chemical fractionation of dissolved organic matter and trihalomethane precursors: a review. J Water Supply 54:475–507
del Giorgio PA, Davis J (2003) Patterns in dissolved organic matter lability and consumption across aquatic ecosystems. In: Findlay SEG, Sinsabaugh RL (eds) Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press, London, pp 399–424
Donohue R, Davidson WA, Peters NE, Nelson S, Jakowyna B (2001) Trends in total phosphorus and total nitrogen concentrations of tributaries to the Swan-Canning Estuary, 1987 to 1998. Hydrol Process 15:2411–2434. doi:10.1002/hyp.300
Glibert PM, Harrison J, Heil C, Seitzinger S (2006) Escalating worldwide use of urea—a global change contributing to coastal eutrophication. Biogeochemistry 77:441–463. doi:10.1007/s10533-005-3070-5
Harris GP (2001) Biogeochemistry of nitrogen and phosphorus in Australian catchments, rivers and estuaries: effects of land use and flow regulation and comparisons with global patterns. Mar Freshw Res 52:139–149. doi:10.1071/MF00031
Harrison JA, Caraco N, Seitzinger SP (2005) Global patterns and sources of dissolved organic matter export to the coastal zone: results from a spatially explicit, global model. Global Biogeochem Cycles 19:GB4S04. doi:10.1029/2005GB002480
He JZ, Gilkes RJ, Dimmock GM (1998) Mineralogical properties of sandy podzols on the Swan Coastal Plain, south-west Australia, and the effects of drying on their phosphate sorption characteristics. Aust J Soil Res 36:395–409. doi:10.1071/S97061
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K et al (1996) Regional nitrogen budgets and riverine N&P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35:75–139. doi:10.1007/BF02179825
Kaushal SS, Lewis WM (2005) Fate and transport of organic nitrogen in minimally disturbed montane streams of Colorado, USA. Biogeochemistry 74:303–321. doi:10.1007/s10533-004-4723-5
Kitsios A, Kelsey P (2008) Nutrient survey of urban areas in coastal catchments of Western Australia. Western Australia Department of Water Internal Report
Kroeger KD, Cole ML, Valiela I (2006) Groundwater-transported dissolved organic nitrogen exports from coastal watersheds. Limnol Oceanogr 51:2248–2261
Leenheer JA (1981) Comprehensive approach to preparative isolation and fractionation of dissolved organic-carbon from natural-waters and wastewaters. Environ Sci Technol 15:578–587. doi:10.1021/es00087a010
Lewis DB, Grimm NB (2007) Hierarchical regulation of nitrogen export from urban catchments: interactions of storms and landscapes. Ecol Appl 17:2347–2364. doi:10.1890/06-0031.1
Linderfelt WR, Turner JV (2001) Interaction between shallow groundwater, saline surface water and nutrient discharge in a seasonal estuary: the Swan-Canning system. Hydrol Process 15:2631–2653. doi:10.1002/hyp.302
Peierls BL, Paerl HW (1997) Bioavailability of atmospheric organic nitrogen deposition to coastal phytoplankton. Limnol Oceanogr 42:1819–1823
Pellerin BA, Kaushal SS, McDowell WH (2006) Does anthropogenic nitrogen enrichment increase organic nitrogen concentrations in runoff from forested and human-dominated watersheds? Ecosystems (NY Print) 9:852–864. doi:10.1007/s10021-006-0076-3
Peters NE, Donohue R (2001) Nutrient transport to the Swan-Canning Estuary, Western Australia. Hydrol Process 15:2555–2577. doi:10.1002/hyp.304
Petrone K, Buffam I, Laudon H (2007a) Hydrologic and biotic control of nitrogen export during snowmelt: a combined conservative and reactive tracer approach. Water Resour Res 43:W06420. doi:10.1029/2006WR005286
Petrone KC, Hinzman LD, Shibata H, Jones JB, Boone RD (2007b) The influence of fire and permafrost on sub-arctic stream chemistry during storms. Hydrol Process 21:423–434. doi:10.1002/hyp.6247
Qualls RG, Haines BL (1991) Geochemistry of dissolved organic nutrients in water percolating through a forest ecosystem. Soil Sci Soc Am J 55:1112–1123
Qualls RG, Haines BL (1992) Biodegradability of dissolved organic-matter in forest throughfall, soil solution, and stream water. Soil Sci Soc Am J 56:578–586
Robson BJ, Hamilton DP (2003) Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Mar Freshw Res 54:139–151. doi:10.1071/MF02090
Robson BJ, Bukaveckas PA, Hamilton DP (2008) Modelling and mass balance assessments of nutrient retention in a seasonally-flowing estuary (Swan River Estuary, Western Australia). Estuar Coast Shelf Sci 76:282–292. doi:10.1016/j.ecss.2007.07.009
Scott D, Harvey J, Alexander R, Schwarz G (2007) Dominance of organic nitrogen from headwater streams to large rivers across the conterminous United States. Global Biogeochem Cycles 21:GB1003. doi:10.1029/2006GB002730
Seitzinger SP, Sanders RW, Styles R (2002) Bioavailability of DON from natural and anthropogenic sources to estuarine plankton. Limnol Oceanogr 47:353–366
Thompson PA, Hosja W (1996) Nutrient limitation of phytoplankton in the upper Swan River Estuary, Western Australia. Mar Freshw Res 47:659–667. doi:10.1071/MF9960659
Thurman EM, Malcolm RL (1981) Preparative isolation of aquatic humic substances. Environ Sci Technol 15:463–466. doi:10.1021/es00086a012
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24:706–723
Westerhoff P, Mash H (2002) Dissolved organic nitrogen in drinking water supplies: a review. J Water Supply 51:415–448
Wiegner TN, Seitzinger SP (2001) Photochemical and microbial degradation of external dissolved organic matter inputs to rivers. Aquat Microb Ecol 24:27–40. doi:10.3354/ame024027
Wiegner TN, Seitzinger SP (2004) Seasonal bioavailability of dissolved organic carbon and nitrogen from pristine and polluted freshwater wetlands. Limnol Oceanogr 49:1703–1712
Wiegner TN, Seitzinger SP, Gilbert PM, Bronk DA (2006) Bioavailability of dissolved organic nitrogen and carbon from nine rivers in the eastern United States. Aquat Microb Ecol 43:277–287. doi:10.3354/ame043277
Zammit C, Sivapalan M, Kelsey P, Viney NR (2005) Modelling the effects of land-use modifications to control nutrient loads from an agricultural catchment in Western Australia. Ecol Modell 187:60–70
Acknowledgements
This research was supported by the Ernest Hodgkin Trust, the Department of Water and Water Corporation of Western Australia, and the CSIRO Water for Healthy Country Flagship program. We thank Kate Bowler and Gary Ogden for assistance with laboratory analyses. A special thanks to Malcolm Robb, Emma Van Looij, and Luke Riley from the Department of Water for facilitating access to chemical data and providing land-use figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrone, K.C., Richards, J.S. & Grierson, P.F. Bioavailability and composition of dissolved organic carbon and nitrogen in a near coastal catchment of south-western Australia. Biogeochemistry 92, 27–40 (2009). https://doi.org/10.1007/s10533-008-9238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-008-9238-z