Abstract
The effects of natural hydrological fluctuations on the nature and bioavailability of soil phosphorus (P) in relation to iron (Fe) and aluminum (Al) chemistry and root mass were studied along a flooded tropical forest gradient in Mapire river, Venezuela. Soil samples were collected following a complete natural hydroperiod: end of the dry season (May 2004), end of the rainy season (November 2004) and end of the subsequent dry season (May 2005), and from three zones subjected to different flooding intensities: MAX, MED and MIN zones inundated for 8, 5 and 2 months per year respectively. The results showed that flood induced the increase of resin-Pi in the MAX zone, but not in the MED and MIN zones. Flood in the soil of the MAX zone also induced the increase of the NaOH-Pi fraction, which removes inorganic P sorbed onto secondary Fe and Al minerals. Changes in this redox-sensitive P form can be considered indirect evidence that P in the MAX zone can be released from the dissolution of iron oxyhydroxide. This field study also showed that along the flooded forest gradient, fine root mass declined during the flood event. However, such decline was more pronounced in the MIN zone than in the MAX zone. In this zone fine root mass was higher than in the other zones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Floodplain forests are communities that occur on seasonally flooded land along river corridors. In such communities, periodic oscillation between inundation and drought originated by the river discharge facilitates the exchange of resources between the river channel and the connected floodplain, and has been considered the major driving force responsible for the structure, function and evolutionary history of the biota in these ecosystems (Junk et al. 1989).
Intermittent flooding and draining of soils in floodplain forests result in considerable temporal variability of soil oxygen. Under saturated soil conditions, water restricts gas diffusion and limits the soil oxygen availability (Smith and Tiedje 1979), diminishing the soil redox potential (Gambrell and Patrick 1978). When the oxygen in soils is depleted, microbes are capable of using alternative electron acceptors during the decomposition of organic matter, such as NO3-N, Fe3+, Mn4+ SO 2− 4 and CO2 (Lovley and Phillips 1986; Moore et al. 1992; McLatchey and Reddy 1998; Quantin et al. 2001). Bacterial reduction of these electron acceptors can affect the cycling of essential nutrients such as nitrogen (N) and phosphorus (P), which globally have been considered the most limiting nutrients of the net primary productivity (NPP) in forests ecosystems (Vitousek 1984; Vitousek and Sanford 1986; Schilling and Lockaby 2006).
Net primary productivity in floodplain forests requires a large nutrient supply from the river water and the soil where the forests are growing (Junk and Piedade 1997). In these forests, belowground productivity (root production) is highly sensitive to hydroperiod fluctuations. According to Megonigal and Day (1992) and Baker et al. (2001), root biomass and root production are frequently greater in periodically flooded than in continuously flooded environments. This is particularly interesting considering that root production represents a high percentage of NPP in some temperate and tropical forests ecosystems (Nadelhoffer and Raich 1992).
Tropical plant biologists have considered that productivity in the tropics is often limited by the rate of phosphorus supply (Vitousek 1984; Vitousek and Sanford 1986), due to the fact that most of the available P is being chemisorbed on amorphous and crystalline Fe and Al oxides/oxyhydroxides (Parfitt et al. 1975; Hsu 1977; López-Hernández 1977; Schwertmann and Taylor 1977; Parfitt 1978). In floodplain forests, however, P may not limit the NPP, because it can be released from the geochemical pool when the soil becomes waterlogged (Patrick and Kahlid 1974; Moore et al. 1992; Szilas et al. 1998; Young and Ross 2001).
In flooded soils several mechanisms have been connected with the release of P from the geochemical pool, including reduction and dissolution of Fe (III) phosphates, hydrolysis and dissolution of Fe and Al phosphates and release of clay-associated phosphates (Ponnamperuma 1972; Gambrell and Patrick 1978; Baldwin and Mitchell 2000; Chacón et al. 2005; Chacón et al 2006a, b). However, P solubilized by these processes can also undergo secondary chemical reactions with ferrous minerals (i.e. amorphous ferrous hydroxide gel complexes, vivianite) formed during Fe(III) reduction (Willet and Higgins 1978; Phillips and Greenway 1998) and with non-redox sensitive elements such as the aluminum (Darke and Walbridge 2000), resulting in a decrease in the soluble soil P concentration. The effect of experimentally controlled variation of soil redox potential on P mobility has been shown in several studies (Willet and Higgins 1978; Phillips and Greenway 1998; Mello and Torrent 1998; McLatchey and Reddy 1998; Mitchell and Baldwin 1998; Young and Ross 2001; Wright et al. 2001; Turner and Haygarth 2001; Corstanje and Reddy 2004; Chacón et al 2006a, b). However, substantially less research has been directed towards the understanding of how natural flooding and subsequent draining of soils change the soil P distribution and availability, especially in floodplain forests (Fabre et al. 1996; Darke and Walbridge 2000).
The main objective of this study was to determine the effects of natural hydrological fluctuations in a tropical forest ecosystem on the nature and bioavailability of soil P in relation to Fe and Al chemistry and root mass.
Materials and methods
Study area
The floodplain of the Mapire river is located in SE Venezuela, between 7°30′−8°30′N and 64°30′−65°00′W. This river is a north tributary of the lower Orinoco river, and its basin constitutes a region of low relief covered by the Pleistocene Mesa Formation, which consists of horizontally bedded alluvial sediments and to a minor extent by marsh deposits (Carbón and Schubert 1994).
The Mapire river has been classified as a black-water river due to its brown colour and its oligotrophic character in terms of nutrient, sediment load and primary productivity (Vegas-Vilarrúbia 1988). According to the climatic diagram of the region (Vegas-Vilarrúbia and Herrera 1993), the annual mean temperature is 27.4°C and the annual precipitation averages 1,333 mm, with the dry season between November and April and the rainy season from May to October.
Forests communities at the Mapire river are related to longitudinal and perpendicular gradients of flooding depth and duration, which are associated with local topography. The present study was carried out in three zones along a flooded gradient perpendicular to the course of the Mapire river: (i) a low zone near the river margin (MAX), where the flood reaches a maximum of up to 12 m and lasts up to 8 months (from May to December); (ii) an intermediate zone (MED) where the flood reaches a maximum of up to 5 m and lasts up to 5 months (from June to November); and (iii) a high zone (MIN) where the flood reaches a maximum of 1 m for 2 months (from July to September).
Soil sample collection
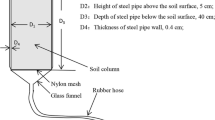
Soil samples in each zone of the flooded gradient were collected following a complete natural hydroperiod fluctuation: (1) at the end of the dry season (May 2004) when the soils were very dry, (2) at the end of the rainy season (November 2004) when the soils were still waterlogged, and (3) at the end of the subsequent dry season (May 2005). In each zone and during the three collection periods, the soil samples were collected from the surface mineral horizon (0–10 cm) at nine points arranged into three transects with three sampling points in each one (Fig. 1). At each sampling point, two soil cores of the surface mineral horizon were extracted using a circular soil corer with a 4.8 cm inside diameter (n = 18 per zone and collection date, total n = 162). At each collection date, samples were stored in plastic bags and shipped immediately to the laboratory at room temperature in order to avoid detrimental impacts to microbes (Verchot 1999). Upon arrival the soil samples to the laboratory, half of the soil samples were used to determine soil P distribution and Fe and Al fractions, while the rest of the samples were used in the determination of soil bulk density and root mass.
Soil sampling pattern in the different zones of the flooded forest gradient. T−transect; (•)—sampling point; MAX—maximum zone; MED—medium zone; MIN—minimum zone, (adapted from Rosales 1989)
P fractions
Duplicate of 0.50 g aliquots of the fresh soil were used to obtain soil P fractions according to the method of Hedley et al. (1982) as modified by Tiessen and Moir (1993). Soil aliquots were then extracted sequentially by anion exchange resin, followed by 0.5 M NaHCO3, 0.1 M NaOH, hot concentrated HCl and final complete digestion in concentrated H2SO4 and H2O2. Total P was determined in aliquots of each extract by digestion with ammonium persulphate and H2SO4 to convert all the organic P forms (Po) to inorganic P forms (Pi). Another aliquots of each extract was used to measure Pi after acidification with H2SO4 to precipitate organic matter. The pH of the final extracted solutions was adjusted, and the P concentration was determined colorimetrically by the molybdate-ascorbic acid procedure (Murphy and Riley 1962). According to Tiessen and Moir (1993), soil P fractions obtained by this procedure have been associated with the following operationally defined pools: (i) P extracted with resin, often considered the labile Pi; (ii) the bicarbonate- and hydroxide extractable P, considered as the Fe- and Al- associated Pi; and (iii) the hot concentrate HCl and digestion of the residue, defined as the highly resistant and unavailable Pi. The difference between the total P and Pi of each extract gives the Po.
Al and Fe fractions estimation
Crystalline, non-crystalline and organically bound forms of Fe and Al were estimated using a selective dissolution method. Organically bound Fe and Al were extracted with sodium pyrophosphate (FeP, AlP) (McKeague 1967). Poorly crystalline forms of Fe and Al (FeO, AlO) were obtained by extracting the soil with ammonium oxalate (pH = 4 in the dark) (McKeague and Day 1966). Highly structured Fe and Al associated to crystalline Fe oxides were extracted with dithionite-citrate-bicarbonate (FeDCB, AlDCB) following the method of Mehra and Jackson (1960). It is known that the oxalate extract dissolves both non-crystalline and organically bound Fe and Al forms, while the DCB method dissolves crystalline Fe and Al associated, non-crystalline, and organically bound Fe and Al forms. Therefore estimates of the different forms of Fe and Al were obtained using the following relationship: non-crystalline Fe and Al forms (FeO, AlO) = oxalate- pyrophosphate extract; crystalline Fe and Al associated (FeDCB, AlDCB) = DCB- oxalate extract. Fe and Al concentrations were determined using atomic absorption spectroscopy.
Root mass
Soil samples collected for root mass determination were air dried and passed through a 2 mm soil sieve. Roots were sorted by size <2 mm (fine roots) and 2–9 mm (coarse roots). Live and dead fine roots were not distinguished. Fine roots were separated from the soil fraction in two steps: (i) manual extraction by 1 uninterrupted hour, and (ii) rinse the soil sample with distilled water on a nylon mesh (0.6 mm diameter mesh). The material obtained from rinsing was a mixture of fine roots and organic detritus. In order to obtain the fine root weight in this step, the mixture material was oven dried at 70°C to a constant mass. After being dried, all visible fine roots were separated by manual extraction. Total fine root weight comprised then the sum of weights obtained in the two above mentioned steps. Root mass in each diameter class was calculated by multiplying the total weight by the corresponding soil bulk density. An aliquot of fine roots obtained in the first step (0.25 g) was digested for six hours with an H2SO4−H2O2 mixture, before determining the P contents by an auto-analyser.
Statistical analysis
Statistical analysis of the data was carried out using an one-way analysis of variance (ANOVA). Data were log-transformed when necessary to meet the assumptions for ANOVA. A Tukey Honest Significant Difference (HSD) test was used as a means separation procedure. A Kruskal-Wallis non-parametric test was used when the data did not meet assumptions for ANOVA. The relationships among the different soil P fractions were analyzed using Pearson correlation coefficients and tested for significance at the 5% level. Statistical analyses were performed using STATITICA for Windows 6.0 (Statistica 2001).
Results
Seasonal changes in soil P distribution
Seasonal variation in soil P fractions along the flooded forest gradient is summarized in Table 1a. In the longer time flooded zone (MAX), the concentration of resin-Pi measured during the dry season (May 2004 and May 2005) was significantly lower than that measured at the end of the rainy season (November 2004). This seasonal pattern was not observed in the MED and MIN zones (Table 1a). The concentration of NaOH-Pi fraction, which is associated with the P strongly chemisorbed on amorphous and some crystalline Al and Fe phosphates (Tiessen et al. 1983), also increased significantly (P < 0.05) in the MAX zone during the rainy season, while in the other zones (MED, MIN) this P fraction was similar throughout the three collection periods (Table 1a). Resin-Pi and NaOH-Pi were high and positively correlated (r = 0.44, P < 0.05; n = 27) in the soils of the MAX zone, while in the other zones (MED and MIN) no significant correlations between both P fractions were obtained.
NaOH-Po was another important P fraction that was sensitive to seasonal changes in the MAX zone, but this seasonal variation was not observed in the MED and MIN zones. In the MAX zone NaOH-Po followed a similar trend to that of the NaOH-Pi fraction, with the highest value when the soil was still waterlogged (November 2004).
Unlike the previous soil P fractions, NaHCO3-Pi showed a seasonal trend in the soil of the MIN zone (Table 1a). In this zone the concentration of NaHCO3-Pi decreased significantly (P < 0.05) in November 2004, in relation to the concentrations obtained in May 2004 and May 2005. In the other zones (MAX and MED), this P fraction did not follow a defined seasonal pattern. With few exceptions, HCl-Pi and Residual-P fractions were stable over the entire study period.
Along the seasonal flooded gradient and throughout the study period, soil P fractions expressed as a percentage of total P, showed a similar trend to that of the absolute values (Table 1b).
Phosphorus fractions grouped into non-occluded (resin + NaHCO3-Pi + NaOH-Pi), organic (NaHCO3 + NaOH + HCl) and occluded (concentrated HCl-extractable + residual) forms as a proportion of total P showed seasonal variation in the MAX zone. In November 2004 non-occluded and organic P increased, while occluded P forms decreased in relation to the values recorded for May 2004 and May 2005 (Table 1a).The MAX zone did not show seasonal variation in HCl-Pi and residual P fractions (Table 1a). However, when such P fractions were grouped and expressed as a proportion of the total P (occluded-P), this P form showed clear seasonal variation. From May 2004 to November 2004, occluded P decreased significantly (P < 0.05), while from November 2004 to May 2005 this P form returned towards the initial value of May 2004 (Table 1a). Seasonal variations in the grouped P forms were not detected in the MED and MIN zones, which remained stable throughout the study period (Table 1a).
Seasonal changes in Fe and Al fractions
Poorly crystalline Fe (FeO) and crystalline Fe and Al associated (FeDCB, AlDCB) were highly variable during the study period in all studied zones (Table 2). No seasonal differences in the mentioned Fe and Al fractions were found.
Organically bound Fe (FeP) was stable over the entire study period, especially in the MAX and MIN zones, while the amount of AlP in all studied zones decreased significantly after the flood event (from May 2004 to November 2004) (Table 2). The low amount obtained in this Al fraction in November 2004, stayed until the next dry season, therefore a clear seasonal pattern in the behavior of organically bound Al could not be established.
Seasonal changes in root mass and P content along the flooded gradient
Coarse and fine root mass and their variations among the zones and during the three collection dates are summarized in Table 3. Comparing the values of fine root mass during the dry season (May 2004) and after the flood period (November 2004), it can be observed that fine root mass decreased significantly by 39% in the MAX zone, 55% in the MED zone and 59% in the MIN zone. Initial values of the fine root mass in May 2004 were not recovered for the next dry season (May 2005). With few exceptions, fine root mass was significantly higher (P < 0.05) in the MAX zone than in the MED and MIN zones during the different collection dates (Table 3).
Throughout the study period, coarse root mass did not show seasonal changes in either MAX or the MIN zones. However in the MED zone, coarse root mass was significantly lower at the end of the flood event (November 2004) than during the dry seasons (May 2004 and May 2005) (Table 3). Within the same collection date, coarse root mass followed the same trend observed by the fine root mass (high values in the MAX zone in relation to the MED and MIN zones).
Phosphorus content in the fine root mass did not show seasonal variations along the flooded gradient (Table 3). Throughout the collection dates, P content in fine root mass decreased significantly (P < 0.05) from the MAX to the MIN zone (Table 3).
Discussion
Effect of seasonal variation on soil P distribution
In an earlier research along the studied flooded gradient, Chacón et al. (2005) hypothesized that in the longer time flooded zone (MAX) the combined effect of long flood period and high soil organic carbon content could make this zone a suitable site for bacterial reduction of Fe(III), which would increase the solubility of P associated to Fe oxides. Later, in a laboratory experiment with soils of the longer and shorter time flooded zones (MAX and MIN) we found that the anaerobic condition stimulated the Fe(III) reduction in these soils (Chacón et al. 2006a). Iron reduction in the last mentioned experiment did not lead to a net increase of the soluble P content. However, in both MAX and MIN zones P was high and positively correlated with the bio-produced Fe(II), implying that Fe dissolution plays an important role in the mobilization of P in these soils.
From the above mentioned research, it was clear that a long-term field study was necessary to determine if soil P distribution and Fe and Al biogeochemistry in the flooded gradient are linked to the natural hydroperiod fluctuation. The results of this study indicated that flood induced the release of P in available form (resin-Pi) in the longer time flooded zone (MAX), which was not observed in the medium and shorter time flooded zones (MED and MIN, respectively). Flood in the soil of the MAX zone also induced the increase of NaOH-Pi fraction. Comparisons of our results with other studies are difficult, because to our knowledge no one has analyzed the effects of flooding on the soil P distribution and availability in seasonal flooded forests of tropical regions. Data from a riparian forest in a temperate zone showed seasonal variations in soil P fractions (Fabre et al. 1996). The authors found that the most labile P forms (resin-Pi, NaHCO3-Pi, NaOH-Pi and NaHCO3-Po) were highly dependent on seasons with accumulation during winter and decrease during the spring. In this riparian forest, flood events were detected during the spring season and were considered, among other factors, to be the possible cause of decrease of the labile P forms in these soils. In Georgia floodplain forest, Wright et al. (2001) obtained an increase in resin-Pi when the soil was artificially flooded. However no significant changes were reported in Fe/Al phosphate fractions. The authors suggested that biological processes are a more probable explanation for flooding-induced increases in P availability than solubilization of mineral phosphates.
Our results contrast with the above mentioned studies and suggest that during the flood phase occurred changes at the mineralogical level, which allowed the observed increase in the size of NaOH-Pi, which remove inorganic P sorbed onto secondary Fe and Al minerals (Tiessen et al. 1983). The direct relationship between resin-Pi and NaOH-Pi in the soil of the MAX zone, suggests also that the available P pool is partly controlled by the dynamics of P chemisorbed on Fe and Al oxides. It is likely that phosphorus released during the flood event is used quickly by microbial and plant uptake in the period between the end of the rainy season and the beginning of the dry season, which leads to the significant decrease in the concentration of resin-Pi and NaOH-Pi fractions, when the soil of the MAX zone is completely dry.
In wetlands soils, the release of organic P during the rewetting of the dry soil has been attributable to the lysis of microbial cells (Qiu and McComb 1995, Turner and Haygarth 2001). However, during periods of anoxic soil conditions in upland non-flooded tropical forest soils, it was determined that Fe(III) reduction lead to the release of organic P (Peretyazhko and Sposito 2005). Our results in the MAX zone are in agreement with the last mentioned study, because flood induced the increase of NaOH-Po fraction (Hedley et al. 1982). Organic P released from Fe(III) dissolution during the flood event could play an important role as a source of P for plants growing on these soils, particularly under reduced conditions. It is well known that extracellular phosphatase can mineralize organic phosphorus (Tarfdar and Jungk 1987) with the production of inorganic PO 3−4 , which is the only P taken up by plants and microorganisms (Stevenson 1986). In the MAX zone of this study, Chacón et al. (2006a) reported that anoxic soil conditions lead to an increase of acid phosphatase activity (APA). A similar behavior in APA was observed when the soil of the a temperate wetland was flooded (Corstanaje and Reddy 2004).
In the shorter time flooded zone (MIN) it was hypothesized that the longer dry period can produce a high accumulation of microbial biomass, which is subsequently released at the end of the dry season (Chacón et al. 2005). In this study we hypothesize that the large pool of P immobilized by the microbial biomass during the dry season could be released and used to cover the plant and microbial demand during the rainy season. That P consumption, probably leads to the observed decrease of NaHCO3-Pi in the MIN zone.
The partitioning of total soil P into non-occluded (resin + NaHCO3- + NaOH-Pi), organic (NaHCO3- + NaOH- + HCl-Po) and occluded (concentrated HCl-extractable + residual) forms was initially used by Walker and Syers (1976) to develop a conceptual model of soil development, which was later confirmed by Crews et al. (1995). This model has also been used to predict the effect of tropical forest disturbance on the size of the different P pools (García-Montiel et al. 2000; Chacón and Dezzeo 2004). We think that natural hydroperiod fluctuation could also affect the distribution of the three different P pools by altering the mineral stability and organic matter turnover. From the data of this study it was clear that the long flood period in the MAX zone lead to an increase of total P partitioned into non-occluded and organic P forms, and a decrease in occluded or non-available P forms. In contrast, in the zones with medium (MED) and short (MIN) flood periods, such P pools were not sensitive to seasonal variation.
From the above discussion it is important to emphasize that to our knowledge no study has thus far determined the small-scale time variability of these P pools in tropical flooded forests. Therefore, if the labile P pools are indeed changing by effect of the natural hydroperiod fluctuation, as it was discussed in this study, more experimental work is clearly required in order to determine the effect of such P conversion on the overall rate of P acquisition in these ecosystems.
Seasonal variation in Fe and Al biogeochemistry and their relationship with soil P dynamics
Our results of poorly and crystalline Fe oxy-hydroxide did not show changes associated with natural hydroperiod fluctuation. There are two possible explanations why we did not detect changes in the Fe-oxyhydroxides content. The first one is that the mineral dissolution during the flood event was probably too small as to produce any detectable change on Fe pools. The other possible explanation is that Fe analysis was performed in a unsuitable form, due to the fact that soil extraction was carried out under oxic conditions and that we used an undisturbed (not sieved) soil instead of the fine soil fraction, where probably are concentrated most of the Fe minerals. The proposed first explanation is supported by the results obtained by Shenker et al. (2005), who suggested that the processes of mineral dissolution that take place upon re-flooding of the soils do not reflect a short-term effect, but might contribute with the P release for decades.
In this study we measured Fe fractions in order to associate the flood induced changes on Fe oxyhydroxide with the behavior of the redox sensitive P fractions (i.e. NaOH- and occluded-P). Unfortunately, we do not have direct evidence that the flood event induced Fe dissolution. However, in the MAX zone NaOH- and occluded-P were highly affected by the oscillation of soil oxygenation derived from the natural cycle of drawdown-reflood. This can be considered as indirect evidence that P is released from the reduction of Fe-hydroxide or Fe-P minerals.
Organically-bound Fe and Al fractions (AlP, FeP) followed different patterns. While FeP was stable over the entire study period, AlP declined during the flooding phase. The loss of organic- matter aluminum complexes (OM-Al) by the action of floodwaters is an expected result in black water rivers (Darke and Walbridge 2000). These organic complexes have important implications for P retention (Gerke and Jungk 1991; Darke and Walbridge 2000; Chacón and Dezzeo 2004).
Coarse and fine roots mass and their relationship with soil P dynamics
It is well documented that root biomass and production in forested floodplains respond to the magnitude, duration and timing of flood events (i.e. Day 1987; Powell and Day 1991; Megonigal and Day 1992; Baker et al. 2001). Continuous flooding reduces the belowground carbon allocation in relation to periodic and not flooded soil conditions.
In this study total fine root mass declined during the flood event along the three zones of the flooding gradient. Such decline was more pronounced in the MIN zone than in the MAX zone, in spite of the fact that flooding period in the MAX zone is 8 months, while in the MIN zone is only 2 months. Additionally it is important to emphasize that the highest fine root mass in all collection dates was detected in the MAX zone. Our data contrast, in part, with previous mentioned works, because in the zone with the longer flood period (MAX), fine root mass should be smaller than that of the zone with the shorter flood period (MIN) and also, flood event should lead to a greater decrease in the fine root mass in the zone with the longer flood period. It is possible that in the MAX zone, P provided under anaerobic soil condition is being used quickly to produce new fine roots, which can on the one hand compensates the losses induced by flood, and on the other help to maintain P within the ecosystem. In fact, our data of P content in the fine root mass of the MAX zone were significantly higher than the values obtained in the MED and MIN zones in all collection dates. In experiments of P fertilization it was reported that waterlogged roots showed morphology more favorable to nutrient uptake (finer roots) and increased both the affinity for P (lower K m ) and the velocity of P uptake (V m ) (Rubio et al. 1997).
Contrary to our expectations, fine root mass along the forest gradient was not recovered to the pre-flooded levels in the subsequent dry season. The results in this study contribute with a good experimental evidence that the fine root mass is modulated by the annual flood event. However, from this data we are not able to explain the dynamics of the fine root mass rebuild, which may not be an annual process. A better understanding of the root dynamics in this floodplain forests will require long-term studies in the field that establish the patterns of root production and decomposition.
With the exception of the MED zone, coarse roots were not affected by the hydroperiod fluctuation. There are two possible explanations why we did not detect any change in the coarse root mass in the Max and MIN zones. One explanation is that turnover rate of the coarse roots is too slow to be able to detect changes associated with seasonality. Another explanation is that coarse roots are much heavier than fine roots and therefore are less likely to be exported from the ecosystem during the flood event. According to Bloomfield et al. (1996) coarse roots have a much lower senescence and turnover rates than fine roots. Fine roots are considered to be ephemeral, with a lifespan not longer than one growing season. Factors regulating the dynamics of coarse roots in the MED zone can not be identified from our results.
Conclusions
Our field study showed that 8 months of flooding increased the bioavailability of soil P compared with the shorter flood periods (2 and 5 months). This P increase seems to be tightly linked to mineral dissolution processes. This study also showed that finer roots were affected by the flood event. However, this effect was less pronounced in the longer term flood zone.
References
Baker TT, Conner WH, Lockaby BG, Stanturf JA, Burke MK (2001) Fine root productivity and dynamics on a forested floodplain in south Carolina. Soil Sci Soc Am J 65:545–556
Baldwin DS, Mitchell AM (2000) The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: a synthesis. Regul Rivers Res Manag 16:457–467
Bloomfield J, Vogt K, Wargo P (1996) Tree root turnover and senescence. In: Waisle Y, Eshel A, Kafkafi U (eds) Marcel Dekker Inc. 270 Madison Avenue, NY, pp 363–381
Carbón J, Schubert C (1994) Late Cenozoic history of the eastern Llanos of Venezuela: geomorphology and stratigraphy of the mesa formation. Quatern Int 21:91–100
Chacón N, Dezzeo N (2004) Phosphorus fractions and sorption processes in soil samples taken in a forest-savanna sequence of the Gran Sabana in southern Venezuela. Biol Fertil Soils 40:14–19
Chacón N, Dezzeo N, Muñoz B, Rodríguez JM (2005) Implications of soil organic carbon and the biogeochemistry of iron and aluminum on soil phosphorus distribution in flooded forests of the lower Orinoco River, Venezuela. Biogeochemistry 73:555–566
Chacón N, Flores S, Gonzalez A (2006a) Implications of iron solubilization on soil phosphorus release in seasonally flooded forests of the lower Orinoco river, Venezuela. Soil Biol Biochem 38:1494–1499
Chacón N, Silver WL, Dubinsky EA, Cusack DF (2006b) Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84
Corstanaje R, Reddy KR (2004) Response of biogeochemical indicators to a drawdown and subsequent reflood. J Environ Qual 33:2357–2366
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Darke AK, Walbridge MR (2000) Al and Fe biogeochemistry in a floodplain forest: Implications for P retention. Biogeochemistry 51:1–32
Day FP (1987) Effects of flooding and nutrient enrichment on biomass allocation in Acer rubrum seedlings. Amer J Bot 74:1541–1554
Fabre A, Pinay G, Ruffinoni C (1996) Seasonal changes in inorganic and organic phosphorus in the soil of a ripatian forest. Biogeochemistry 35:419–432
Gambrell RP, Patrick WH (1978) Chemical and microbiological properties of anaerobic soils and sediments. In: Hook DD, Crawford RMM (eds) Plant life in anaerobic environments. Ann Arbor Science Publ Inc., Ann Arbor, pp 233–247
Garcia-Montiel DC, Nelly CH, Melillo J, Thomas S, Steudler PA, Cerri CC (2000) Soil phosphorus transformations following forest clearing for pasture in the Bazilian Amazon. Soil Sci Soc Am J 64:1792–1804
Gerke J, Jungk A (1991) Separation of phosphorus bound to organic matrices from inorganic phosphorus in alkaline soil extracts by ultrafiltration. Commun Soil Sci Plant Anal 22:1621–1630
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Hsu PH (1977) Aluminum oxides and oxyhidroxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Science Society America Madison, WI, pp 99–143
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the international Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Sciences 106, pp 110–127
Junk WJ, Piedade MT (1997) Plan life in the floodplain with special reference to herbaceous plants. In: Junk WJ (ed) The Central Amazon floodplain ecology of a pulsing system. Springer-Verlag, Berlin Heidelberg, pp 147–181
López-Hernández D (1977) La química del fósforo en suelos ácidos. Universidad Central de Venezuela, Ediciones de la Biblioteca. Caracas
Lovley DR, Phillips EJP (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–689
Mckeague JA (1967) An evaluation of 0,1 M pyrophosphate and pyrophosphate-dithionate in comparison with oxalate as extractants of the accumulation products in podzols and some other soils. Can J Soil Sci 47:95–99
McKeague JA, Day JH (1966) Dithionite and oxalate extractable Fe and Al as aids differentiating various classes of soils. Can J Soil Sci 46:13–22
McLatchey GP, Reddy KR (1998) Regulation of organic matter decomposition and nutrient release in a wetland soil. J Environ Qual 27:1268–1274
Megonigal JP, Day FP (1992) Effects of flooding on root and shoot production of bald cypress in large experimental enclosures. Ecology 73:1182–1193
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionate-citrate system buffered with sodium carbonate. Clay Clay Miner 7:317–327
Mello JWV, Torrent VB (1998) Phosphorus and iron mobilization in flooded soils from Brazil. Soil Sci 163:122–132
Mitchell A, Baldwin DS (1998) Effects of desiccation/oxidation on the potential for bacterially mediated P release from sediments. Limnol Oceanogr 43:481–487
Moore PA, Reddy KR, Graetz DA (1992) Nutrient transformations in sediments as influenced by oxygen supply. J Environ Qual 21:387–393
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
Parfitt RL, Atkinson RJ, Smart R St C (1975) The mechanism of phosphate fixation by iron oxides. Soil Sci Soc Amer Proc 39:837–841
Parfitt RL (1978) Anion adsorption by soils and soil materials. Adv Agron 30:1–50
Patrick WK, Khalid RA (1974) Phosphate release and sorption by soils and sediments: effect of aerobic and anaerobic conditions. Science 186:53–55
Peretyazhko T, Sposito G (2005) Iron(III) reduction and phosphorus solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652
Phillips IR, Greenway M (1998) Changes in water-soluble exchangeable ions, cation exchange capacity, and phosphorusmax in soils under alternanting waterlogged and drying conditions. Commun Soil Sci Plant Anal 29:51–65
Ponnamperuma FN (1972) The chemistry of submerged soils. Advan Agron 26:29–96
Powell SW, Day FP (1991) Root production in four communities in the Great Dismal Swamp. Am J Bot 78:288–297
Qiu S, McComb AJ (1995) Planktonic and microbial contributions to phosphorus release from fresh and air-dried sediments. Mar Freshw Res 46:1039–1045
Quantin C, Becquer T, Rouiller JH, Berthelin J (2001) Oxide weathering and trace metal release by bacterial reduction in a New Caledonia Ferralsol. Biogeochemistry 53: 323–340
Rosales J (1989) Análisis florístico-estructural y algunas relaciones ecológicas en un bosque inundable en la boca del río Mapire (estado Anzoétgui). Msc Thesis, Instituto Venezolano de Investigaciones Científicas, Caracas, p 233
Rubio G, Martín O, Alvarez CA, Lavado RS (1997) Mechanisms for the increase in phosphorus uptake of waterlogged plants: soil phosphorus availability, root morphology and uptake kinetics. Oecologia 112:150–155
Shenker M, Seitelbach S, Brand S, Haim A, Litaor MI (2005) Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur J Soil Sci 56:515–525
Schilling EB, Lockaby BG (2006) Relationships between productivity and nutrient circulation within two contrasting southeastern U.S. floodplain forests. Wetlands 26:181–192
Schwertmann U, Taylor RM (1977) Iron oxides. In: Dixon JB, Weed SB (eds) Minerals in Soil Environments. Soil Sci Soc Am Madison, WI, pp 145–180
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem 11:261–267
Statistica (2001) Statistica for windows. StatSoft Inc., Tulsa OK
Stevenson JJ (1986) Cycles of soil: C, N, P, S, micronutrients. John Wiley, New York
Szilas CP, Borgaard OK, Hansen HCB (1998) Potential iron and phosphate mobilization during flooding of soil material. Water Air Soil Pollut 106:97–109
Tarfdar JC, Jungk A (1987) Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol Fertil Soils 3:199–204
Tiessen H, Stewart JWB, Moir JO (1983) Changes in organic and Pi composition of two grassland soils and their particle size fractions during 60–90 years of cultivation. J Soil Sci 34:815–823
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis (special publication of the Canadian Society of Soil Science). Lewis, Boca Raton, pp 75–86
Turner BL, Haygarth PM (2001) phosphorus solubilization in rewetted soils. Nature 411:258
Vegas-Vilarrúbia T (1988) Aproximación a una clasificación de los ríos de aguas negras venezolanos atendiendo a las características de sus sustancias húmicas y de sus variables físico-químicas. M.Sc. Thesis, Instituto Venezolano de Investigaciones Científicas, Caracas, DC
Vegas-Vilarrúbia T, Herrera R (1993) Effects of periodic flooding on the water chemistry and primary production of the Mapire systems (Venezuela). Hydrobiologia 262:31–42
Verchot LV (1999) Cold storage of a tropical soil decreases nitrification potential. Soil Sci Soc Am J 63:1942–1944
Vitousek PM (1984) Literfall, nutrient cycling, and nutrient limitation in tropical forest. Ecology 65:285–298
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Willet IR, Higgins ML (1978) Phosphate sorption by reduced and reoxidized rice soils. Aust J Soil Res 16:319–326
Wright RB, Lockaby BG, Walbridge MR (2001) Phosphorus availability in an artificially flooded southeastern floodplain forest soil. Soil Sci Soc Am J 65:1293–1302
Young EO, Ross DS (2001) Phosphate release from seasonally flooded soils: a laboratory microcosm study. J Environ Qual 30:91–101
Acknowledgements
The authors thank two anonymous reviewers for helpful comments on this manuscript. We would like thank Edgardo Pérez who assisted most of the root extraction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chacón, N., Dezzeo, N., Rangel, M. et al. Seasonal changes in soil phosphorus dynamics and root mass along a flooded tropical forest gradient in the lower Orinoco river, Venezuela. Biogeochemistry 87, 157–168 (2008). https://doi.org/10.1007/s10533-007-9174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-007-9174-3