Abstract
Terrestrial desert ecosystems are strongly structured by the distribution of plants, which concentrate resources and create islands of fertility relative to interplant spaces. Atmospheric nitrogen (N) deposition resulting from urbanization has the potential to change those spatial patterns via resource inputs, resulting in more homogeneous soil resource availability. We sampled soils at 12 desert remnant sites around Phoenix, Arizona along a model-predicted gradient in N deposition to determine the degree to which deposition has altered spatial patterns in soil resource availability and microbial activity. Soil microbial biomass and abundance were not influenced by atmospheric N deposition. Instead, plant islands remained strong organizers of soil microbial processes. These islands of fertility exhibited elevated pools of resources, microbial abundance, and activity relative to interspaces. In both plant islands and interspaces, soil moisture and soil N concentrations predicted microbial biomass and abundance. Following experimental wetting, carbon dioxide (CO2) flux from soil of interspaces was positively correlated with N deposition, whereas in plant islands, soil CO2 flux was positively correlated with soil moisture content and soil organic matter. Soil CO2 flux in both patch types showed rapid and short-lived responses to precipitation, demonstrating the brief time scales during which soil biota may process deposited materials. Although we observed patterns consistent with N limitation of microbes in interspaces, we conclude that atmospheric N deposition likely accumulates in soils because microbes are primarily limited by water and secondarily by carbon or nitrogen. Soil microbial uptake of atmospherically deposited N likely occurs only during sparse and infrequent rainfall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
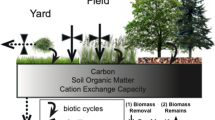
In terrestrial ecosystems, heterotrophic soil microbes influence the availability of nutrients to plants by mineralizing nutrients from organic matter, whereas plants, in turn, provide organic carbon (C) that fuels microbial growth and activity (Kaye and Hart 1997). Tight spatial coupling between autotrophs and heterotrophs is characteristic of desert ecosystems where soil microbes are concentrated beneath tree and shrub “islands” in response to strong contrasts in resources among soils beneath versus between plants (Schlesinger 1996; Kieft et al. 1998; Schade and Hobbie 2005). Despite the presence of these resource-rich islands of fertility, growth of plants in desert ecosystems is often limited by nitrogen (N) and growth of soil microbes may be limited by either N or C (Fisher et al. 1988; Schaeffer et al. 2003).
In human-dominated landscapes, emissions from agriculture and fossil-fuel combustion have significantly increased N inputs to ecosystems from the atmosphere, which may alleviate N limitation. Nitrogen deposition may increase concentrations of soil N, alter the composition of plant and microbial communities, and influence rates of nutrient cycling and retention (Aber et al. 1998; Fenn et al. 2003a; Siguenza et al. 2006; Wallenstein et al. 2006). Atmospheric deposition of N to desert ecosystems may also shift spatial patterns in soil microbial abundance and activity, resulting in greater fluxes of C and N from interplant spaces (hereafter as ‘interspaces’), which under low deposition rate show little biogeochemical activity relative to plant islands. While local spatial heterogeneity and diffuse atmospheric N deposition inputs are both known to influence the distribution and activities of soil microbes, little is known about the relative effects of these two factors. We compared the influences of atmospheric N deposition and islands of fertility on soil microbial abundance, biomass, and respiration in and around metropolitan Phoenix, Arizona.
The interactive effects of spatial heterogeneity and atmospheric N deposition may have particularly strong effects on soil microbes in arid and semi-arid ecosystems, such as the Sonoran Desert, where the distribution of soil moisture and nutrients is heterogeneous. Soil moisture, nutrients, and organic matter are higher beneath desert trees and shrubs such as mesquite (Prosopis spp.) and creosotebush (Larrea tridentata) than in plant interspaces, creating islands of fertility around shrubs (Schlesinger et al. 1996). The concentration of resources beneath plants positively influences soil microbial activity, resulting in enhanced mineralization and storage of plant-available nutrients (Gallardo and Schlesinger 1992, 1995; Schade and Hobbie 2005). Whereas rate of N mineralization is high beneath plants, soils of interspaces between plants are nutrient poor (Schlesinger and Pilmanis 1998); hence, we would expect to observe greater responses of microbes in interspace soils subject to elevated N deposition relative to those that receive minimal atmospheric N inputs.
Across ecosystem types, soil microbial responses to N deposition vary not only in the processes affected, but also in the direction and magnitude of the effects. Increased N mineralization rate has been observed in response to N deposition in forests dominated by some tree species, but not others (Lovett and Rueth 1999; Baron et al. 2000). Whereas N deposition repressed soil respiration rate in certain temperate forests (Bowden et al. 2004; Burton et al. 2004; Pregitzer et al. 2004; Wallenstein et al. 2006; Zak et al. 2006), N additions enhanced soil respiration in others (Gallardo and Schlesinger 1994; Tietema 1998; Waldrop et al. 2004). Microbial biomass carbon decreased in mixed hardwood-softwood forest plots receiving ammonium (\( {\text{NH}}^{+}_{{\text{4}}} \)) amendments, but was not affected in mixed hardwood stands receiving nitrate (\( {\text{NO}}^{-}_{{\text{3}}} \)) fertilization (Wallenstein et al. 2006; Zak et al. 2006). Such disparate results limit our ability to predict ecosystem responses to anthropogenic nutrient inputs. One explanation of these diverse patterns is that soil microbial responses to N deposition depend on the relative availabilities of C and N. Desert soils generally have low C:N (Bird et al. 2002; Saetre and Stark 2005; Stursova et al. 2006) and experimental evidence suggests soil microbial processes may be limited by C or N (Gallardo and Schlesinger 1992; Schaeffer et al. 2003). Therefore, study of desert soils may provide insight into the mechanisms regulating soil microbial responses to N inputs.
Given the contrasting responses of soil microbial activity and abundance to N additions observed in temperate forest ecosystems, a similarly diverse set of responses may be expected when considering arid soils. First, atmospheric N deposition may have no effect if heterotrophic microbial populations are primarily limited by C in desert ecosystems. Second, long-term N deposition may alter the composition or activity of the microbial community, increasing the efficiency of soil microbes and thus resulting in little change in microbial community respiration compared to areas of low N deposition (Hobbie 2005). Third, N deposition may result in increased microbial activity of interspaces if rates of microbially-mediated reactions are N limited (Schaeffer et al. 2003). Even in dry, desert soils, high rates of denitrification have been found following precipitation events (Peterjohn and Schlesinger 1991; Welter 2004), suggesting that increased gaseous loss may be one such result of N deposition. Atmospheric N deposition may also indirectly influence soil microbial activity in interspaces where increased soil N results in growth of annual grasses and forbs (Allen et al. 1998; Báez et al. 2007), increasing C available to soil microbes.
Here we report soil microbial responses to a model-predicted N deposition gradient that spans positions upwind, within, and downwind of metropolitan Phoenix, Arizona. Given that water is a primary limiting resource in the desert, we expected seasonality in these effects and contrasted the dry and monsoon seasons. We measured microbial abundance and biomass before and after monsoon precipitation, and soil respiration following experimental wetting. We also explored relationships between the measured rate of dry N deposition and biogeochemical assays, including microbial abundance and biomass, and contrasted creosotebush and interspace soils.
Methods
Study site
Our study included 12 desert and remnant desert sites in Maricopa County, AZ (Fig. 1). Sites were selected to span a gradient in atmospheric N deposition, occurring primarily as dry nitrogen oxides (NOx), predicted by a model based on the direction of prevailing winds (Fenn et al. 2003b). Dry deposition rates estimated by a multiscale air-quality model were 7.5 kg N ha−1 year−1 for sites upwind of the urban core, 13.5 kg N ha−1 year−1 for the urban core, and 15 kg N ha−1 year−1 downwind of the urban core (Fenn et al. 2003b). The total area was approximately 110 km (east–west) by 65 km (north–south), within which we selected 4 sites each from positions located upwind, downwind and within the Phoenix urban core. Research plots were established on alluvial fans at each site. Plant communities were dominated by species typical of lower-elevation Sonoran Desert: creosotebush (Larrea tridenata), triangle-leaf bursage (Ambrosia deltoidea), paloverde (Cercidium microphyllum and C. floridium), and saguaro (Carnegiea gigantea). Precipitation, all as rain, is distributed bimodally, with ∼65% from Pacific cyclonic storms (November–April) and ∼35% from convective monsoon storms (June–September) (Western Regional Climate Center, http://www.wrcc.dri.edu). Work was conducted between May and October 2005, contrasting the summer dry season and the summer monsoon season. Summer monsoon rainfall ranged from 2.5 to 8.5 cm across the study area.
Field sampling and laboratory procedures
Dry atmospheric deposition was collected in June 2005 on ashed glass-fiber filters (Whatman GF/A; 1.6 μm pore size, 11 cm diameter) that were attached to acid-washed vinyl screens anchored to plastic funnels (Fenn and Bytnerowicz 1993). Filters were installed at a subsample of 9 sites along the deposition gradient, 180–190 cm above the soil surface. Three replicate filters were deployed at each site for a period of 7–9 days during which time there was no recorded precipitation. Following collection, the filters were extracted with Nanopure water and filtered through pre-leached Whatman 42 ashless paper filters. The extracts were frozen until analysis for total dissolved N using persulfate-UV digestion on a Lachat Quick Chem 8000 Flow Injection autoanalyzer.
Soil moisture, texture, nutrient concentrations, and microbial processes were measured for two cover types, beneath creosotebush and in interspaces. Interspace soils were collected at maximal distances from adjacent plants, and plant-island soils were collected midway between the trunk and canopy edge. Soils were collected twice, prior to and during seasonal monsoon rains, in May–June and August 2005, respectively. At each site, triplicate samples were taken for each cover type to a depth of 2 cm using a 10-cm-diameter copper corer pounded into the soil. The samples were returned to the laboratory, sieved through a 2-mm sieve, and all analyses were performed within 48 h on the <2 mm fraction. Subsamples were dried at 105°C for 48 h to determine gravimetric soil moisture, then combusted at 550°C for 4 h to determine organic matter content as ash-free dry mass. Soil texture was determined using the hydrometer method with sand measured by mass after sieving through 53-μm mesh (Bouyoucos 1962).
Soil inorganic N pools were measured on a subsample extracted in 2 M KCl by shaking for 1 h and filtering through pre-leached Whatman 42 ashless paper filters. All extracts were frozen until analysis for \( {\text{NO}}^{-}_{{\text{3}}} \) (cadmium reduction method) and \( {\text{NH}}^{+}_{{\text{4}}} \) (phenol-hypochlorite method) on a Lachat Quick Chem 8000 autoanalyzer.
Carbon and N pools in microbial biomass were estimated using extraction following chloroform fumigation (Brookes et al. 1985). One subsample was immediately extracted in 0.5 M K2SO4 and the second was fumigated under a chloroform atmosphere in a desiccator for 72 h, then extracted in 0.5 M K2SO4. The extracts were filtered and frozen until analysis for total dissolved N as above and for dissolved organic C on a Shimadzu TOC 5000. Microbial biomass was determined as the difference between pre- and post-fumigation concentrations of N and C.
We determined the potential for use of deposited \( {\text{NO}}^{-}_{{\text{3}}} \) by nitrate reducers and denitrifiers by enumerating these microorganisms using a most probable number (MPN) technique (Rowe et al. 1977; Staley and Griffin 1981). Whereas both denitrifying and nitrate respiring bacteria reduce nitrate to nitrite, this method assumes that denitrifying bacteria further reduce nitrite to nitrogen oxides or dinitrogen. The ability to reduce nitrate to nitrite is widespread among bacteria, however, the ability to further reduce nitrate is characteristic of fewer taxa. Subsamples of air-dried soil were homogenized and suspended in nitrate broth to form a slurry, from which 100 μl was inoculated into a 96-well micro-titer plate using serial 10-fold dilutions. Four replicate plates were prepared from each sample. Plates were incubated under an anaerobic atmosphere in a vacuum desiccator for 2 weeks, then analyzed colorimetrically using diphenylamine to test for the presence of nitrate and using N(1-napthyl)-ethylene-diamine-dihydrochloride to test for the presence of nitrite. Inoculated wells that indicated for nitrite were scored positive for nitrate reducing bacteria and wells that indicated the absence of nitrite and nitrate were scored positive for denitrifying bacteria. Scores were translated to MPN of organisms using a probability table developed by Rowe (1977).
We assessed soil respiration by measuring CO2 flux following simulated wetting events, conducted after the monsoon season in October 2005. At 9 of our sites described previously, we established three 50-cm2 plots each under plants and in interspaces, and applied Nanopure water to simulate a 1-cm precipitation event. Soil CO2 flux was measured with a PP Systems EGM-2 infrared gas analyzer. To prevent air leaks between the soil surface and respiration measurement chamber, PVC collars were installed 2 cm into the soil. Measurements were taken at least 15 min after the collars were installed to minimize effects of disturbance. CO2 flux, soil moisture, and soil temperature were measured prior to the treatment and at 0.5, 1, 2 and 24 h after the treatment.
Statistical analyses
We performed paired t-tests or, where the assumption of normality was not met, Wilcoxon signed-rank tests to compare microbial biomass and abundance, soil organic matter, soil moisture, and soil nutrient pools between plant island and interspace patches. One-way analysis of variance (ANOVA) was used to compare rate of N deposition between landscape positions relative to the urban core. These tests were performed using Systat v. 10.
Multiple linear regression was used to identify relationships among soil microbial response variables and measured predictor variables. For estimates of microbial biomass and abundance, predictor variables included soil \( {\text{NO}}^{-}_{{\text{3}}} \) and \( {\text{NH}}^{+}_{{\text{4}}} \) concentrations, soil organic matter, soil moisture, and N deposition rate. For CO2 flux, predictor variables included soil temperature, texture, moisture, and organic matter, and N deposition rate. Separate models were completed for soils of plant islands and interspaces. Models were selected using an all-subsets routine on the basis of adjusted R2 and Mallow’s Cp values using SAS v. 9. We considered models consisting of four or less predictor variables. In selecting the final model, we considered only those candidate models in which all predictor variables contributed significantly to the relationship given the other variables in the model. We evaluated multicollinearity using tolerance values and selected final models for which tolerance values were >0.5 for all predictors. We evaluated homogeneity of variance and normality using residuals. When necessary, we applied the natural logarithmic transformation to meet these assumptions.
Results
Spatial patterns in atmospheric N deposition
Mean atmospheric deposition values ranged from 0.21 to 0.60 mg N m−2 day−1 but did not vary predictably with landscape position relative to the urban core (ANOVA, F2,6 = 1.947, P = 0.223; Fig. 2). Although there was no evidence of a west–east pattern, there were individual study sites with relatively low and high rates of deposition within each of the upwind, urban core, and downwind positions. Furthermore, rate of N deposition was not correlated with soil \( {\text{NO}}^{-}_{{\text{3}}} \hbox{-}{\text{N}} \) pools prior to (R2 = 0.006, P = 0.763) or during (R2 = 0.105, P = 0.221) the monsoon rains. Similarly, there was no relationship between N deposition rate and soil \( {\text{NH}}^{+}_{{\text{4}}} \hbox{-}{\text{N}} \) during the dry season (R2 = 0.002, P = 0.866); however, a weak positive relationship was found during the monsoon season (R2 = 0.268, P = 0.040).
Measured mean rate of N deposition (n = 3, ±SE) at three positions along a modeled N-deposition gradient in central Arizona, USA (see Fig. 1)
Spatial and temporal patterns in soil and microbial characteristics
Distinct patterns in soil resource availability were strongly influenced by temporal patterns in precipitation. Soil moisture was generally low (<1%) in early summer, but increased more than 5-fold during monsoon rains (Fig. 3). Whereas there were no differences in soil moisture between cover types prior to and during summer precipitation, soil moisture was significantly greater in plant islands than in interspaces during the monsoon season (Wilcoxon signed rank test, Z11 = −2.934, P = 0.003). Soil organic matter content was low across all sites, but was significantly greater beneath plants than in interspaces both prior to (Wilcoxon signed rank test, Z12 = −3.059, P = 0.002) and following summer precipitation (Wilcoxon signed rank test, Z11 = −2.934, P = 0.003).
There was no evidence of a west–east concentration gradient of soil inorganic N pools; however, within each of the upwind, urban core, and downwind landscape positions we observed individual study sites with low and high concentrations of soil N. Inorganic soil N was heterogeneously distributed during the dry season but not during the summer monsoon. Soil \( {\text{NO}}^{-}_{{\text{3}}} \hbox{-}{\text{N}} \) concentration was greater in plant islands than in interspaces prior to monsoon rains (Wilcoxon signed rank test, Z12 = −2.981, P = 0.003) but was not significantly different between cover types during monsoon precipitation (Wilcoxon signed rank test, Z11 = −1.689, P = 0.091). Likewise, soil \( {\text{NH}}^{+}_{{\text{4}}} \hbox{-}{\text{N}} \) concentration was greater in plant islands compared to interspaces prior to summer precipitation (Wilcoxon signed rank test, Z12 = −2.589, P = 0.010) but did not vary by cover type during monsoon rains (Wilcoxon signed rank test, Z11 = 0.889, P = 0.374).
Soils beneath creosotebush had higher microbial abundance and biomass than soils in interspaces during the monsoon season (Fig. 4). Prior to seasonal precipitation there were no differences by cover type for microbial biomass C (Wilcoxon signed rank test, Z12 = 0.392, P = 0.695) and microbial biomass N (Wilcoxon signed rank test, Z12 = −0.105, P = 0.917). Microbial biomass C and N for both patch types were 2–11 times greater during the summer monsoon season compared to the dry season. During the monsoon season, microbial biomass C was significantly greater beneath plant islands (Paired t-test, t 10 = 5.95, P < 0.001), as was N of microbial biomass (paired t-test, t 10 = 3.450, P = 0.006). Density of nitrate-reducing bacteria was greater beneath creosotebush during both the dry season (Wilcoxon signed rank test, Z12 = −2.497, P = 0.013) and during the summer monsoon season (Wilcoxon signed rank test, Z11 = −2.934, P = 0.003). Most probable numbers of denitrifying bacteria did not differ by patch type in either the dry (Wilcoxon signed rank test, Z12 = −0.314, P = 0.754) or monsoon seasons (Wilcoxon signed rank test, Z11 = 0.800, P = 0.424).
Soil respiration rate prior to experimental wetting treatments was low and did not differ significantly by cover type. Average CO2 flux following wetting was greater for plant islands compared to interspaces for measurements taken at all times following wetting (Fig. 5). Thus, cumulative CO2 flux was significantly greater in plant islands than in interspaces (Paired t-test, t 7 = 5.747, P = 0.001). Respiration rate decreased appreciably after 24 h; however, the flux remained elevated compared to pre-treatment rate for both cover types. Soil moisture followed a similar temporal pattern, but moisture content was comparable between the two cover types for each time interval.
Factors influencing microbial processes
Multiple regression analyses revealed that soil microbial biomass and abundance estimates were not influenced by atmospheric N deposition. Instead, soil moisture and soil N concentrations showed positive correlations with microbial biomass and abundance in soils both beneath plant islands and in interspaces (Table 1). Most probable number of denitrifiers was unrelated to any of the measured predictor variables, and populations of nitrate reducers (most probable number) correlated positively with soil moisture, but only in interspaces.
In interspaces, soil CO2 flux correlated positively with measured N deposition rate both cumulatively (R2 = 0.509, P = 0.047) and for maximum flux (measured 30 min following wetting, on average) (R2 = 0.718, P = 0.008; Fig. 6a). Under creosotebush, cumulative soil CO2 flux was positively related to initial soil moisture content (R2 = 0.562, P = 0.032), whereas maximum flux (measured 30 min after wetting) was strongly predicted by soil organic matter (R2 = 0.869, P = 0.001; Fig. 6b). Whereas maximum and cumulative CO2 fluxes were highly correlated in interspaces (r = 0.803), the correlation between these measures was weaker beneath plants (r = 0.457).
Discussion
Soil microbial responses to atmospheric N deposition
Desert ecosystems are strongly structured by the distribution of perennial plants. Urbanization has the potential to change such spatial patterns via atmospheric N deposition, resulting in changes in soil processes that influence C and N cycles. Our hypothesis that atmospherically deposited N would increase soil respiration and microbial biomass and abundance in interspaces was partially supported, in that soil respiration rate in interspaces was correlated with rate of atmospheric N deposition. However, at the current rate of atmospheric N deposition, islands of fertility remained the prominent organizers of soil microbial processes. Inorganic N pools, microbial biomass C and N, and density of nitrate reducers were greater in soils beneath plant islands compared to interspaces, regardless of N deposition rate. After simulated precipitation events, respiration rate of soils beneath plants was at least twice the rate in interspaces. Our findings are consistent with other studies of desert soils (Schlesinger 1996; Schade et al. 2003; Schade and Hobbie 2005) and suggest that plant islands are as critical to ecosystem function in urban desert soils as they are in pristine deserts, despite elevated ambient temperatures (Hansen et al. 1999; Baker et al. 2002), changes in invertebrate fauna (Faeth et al. 2005), and altered atmospheric chemistry, including N deposition (Fernando et al. 2001).
Conceptual models predicting responses of ecosystems to atmospheric N deposition have largely been developed for temperate forests, but may not reflect general patterns in other ecosystems. Continued input of N is thought to increase rate of nitrification and N2O flux (Aber et al. 1998). However, both experimental addition of N and study of gradients of anthropogenically deposited N have shown contrasting patterns in soil microbial responses to N inputs (Tietema 1998; Bowden et al. 2004; Burton et al. 2004). These contrasts may be due to the N limitation status of microbes, availability of added N, and timing of microbial activity relative to N deposition. In deserts, microbial respiration is typically C limited, due to C-poor soils (Schaeffer and Evans 2005; Sponseller 2007). Desert soils in and around metropolitan Phoenix have C:N ratios of ∼8 (Central Arizona Phoenix Long Term Ecological Research site (CAPLTER)), http://caplter.asu.edu/home/products/datasets.jsp) in the range at which soil microbes may be either C or N limited (Kaye and Hart 1997), whereas soils of temperate ecosystems have soil C:N ratios ranging from 17 to 33 (Tietema 1998; Wallenstein et al. 2006). Thus, microbial uptake of N in deserts may be constrained by available energy. We found that soil respiration was greater beneath plants and positively correlated with organic matter. It is important to note that although our measurements of soil CO2 flux did not distinguish between root and microbial respiration, the immediate and short-lived responses to the moisture pulse are likely attributable to increased microbial activity because plant responses occur on a timescale of days rather than hours (Fravolini et al. 2005). The temporal patterns of soil CO2 flux suggest C limitation of microbial respiration (Gallardo and Schlesinger 1992; Schaeffer et al. 2003).
In addition to finding evidence for C limitation, we also observed positive correlations between microbial abundance, biomass, and activity with metrics of N availability. If soil microbial activity responds positively to N inputs, atmospheric deposition may cause reduced reliance of soil microbes on plants for nutrients, resulting in increased microbial activity in interspaces, and effectively decoupling soil microbes from perennial shrubs. Such decoupling would occur if microbial immobilization of N is stimulated by N inputs and if N deposition causes increases in soil N pools either directly or indirectly via increases in primary productivity of annual grasses and forbs. In this study, rate of N deposition correlated only weakly with soil N pools and we observed no differences in cover of annual plants across our sites. However, our assays of inorganic N may not have detected the contribution of atmospherically deposited N to extractable soil pools due to dilution effects; we sampled soils to 2 cm depth, but the influence of deposited N may be limited to the top few millimeters of the soil profile (Johnson et al. 2007).
The only soil microbial response to correlate with rate of N deposition, soil respiration of interspaces, occurred following a simulated rain event, suggesting that the availability of deposited N may be limited by moisture. This effect was not observed beneath plants, perhaps because soil inorganic N pools there were up to two times those of interspaces. Additionally, microbial biomass and abundance reflect seasonal patterns, and thus may not correlate positively with atmospheric N deposition rate if deposited N contributes little to the total soil N pool. In contrast, respiration following a precipitation event may reflect use of substrates that are readily mobilized by the input of water.
An additional explanation for the lack of a relationship between N deposition rate and microbial abundance is the form of N deposited. Whereas we expected dry N deposition to consist mainly of NOx (Fenn et al. 2003b), recent monitoring data from metropolitan Phoenix, AZ indicate that \( {\text{NH}}^{+}_{{\text{4}}} \) is the dominant form deposited (N. Grimm, S. Hall and J. Kaye, unpublished data). Lack of correlation between N deposition rate and the abundance of nitrate-reducing and denitrifying bacteria may have resulted because of the predominance of \( {\text{NH}}^{+}_{{\text{4}}} \) in atmospheric deposition. Exogenous ammonium inputs may stimulate nitrification through the accumulation of nitrate in desert soils (Schaeffer and Evans 2005; Sirulnik et al. 2007); however, we observed no correlation between \( {\text{NO}}^{-}_{{\text{3}}} \hbox{-}{\text{N}} \) pools and atmospheric N deposition.
N inputs via atmospheric deposition may shift the activity and community composition of soil microbes, and alter microbial nutrient use efficiencies, thereby influencing whole ecosystem responses, including CO2 flux. Although we documented no changes in the abundance of nitrate reducers or denitrifiers, changes in other aspects of microbial community structure have been reported as a result of atmospheric N inputs (Gallo et al. 2004; Bradley et al. 2006; Porras-Alfaro et al. 2007). Further, distinct microbial communities may occur beneath creosotebush and in interspaces (Ewing et al. 2007), leading to different expectations about responses of these patches to N deposition. Changes in activity of extracellular enzymes, particularly oxidases, have been noted as a result of N fertilization in temperate forests (Gallo et al. 2004; Sinsabaugh et al. 2005; Waldrop and Zak 2006); however, no changes in oxidase activity were observed following long-term fertilization of desert soils (Stursova et al. 2006). A paucity of data describing structure and function of microbial communities in desert soils limits our ability to speculate on the mechanisms linking observed patterns in soil respiration to atmospheric N deposition.
Finally, the weak influence of atmospheric N inputs on soil microbes is commensurate with the dry deposition rate that we measured. Our observed rate of dry deposition was lower than other measured rates of dry deposition using similar passive sampling techniques (Saxena et al. 1997; Lovett et al. 2000). For example, in a xeric region of southern California, USA, atmospheric N deposition rate was an order of magnitude greater than we observed (Fenn and Bytnerowicz 1993) and in a mesic region in Maryland, USA, dry nitrate-deposition rate was 2–4 times greater than our total N dry deposition rate (Raymond et al. 2004). Whereas our observed rate of dry N deposition was lower than the model-predicted rate for Phoenix, Arizona (Fernando et al. 2001), it was comparable to long-term dry N deposition rate measured with passive collectors in the CAPLTER. Our estimates were within the range of 6-year average rate of dry N deposition for positions upwind of, within, and downwind of the urban core (Lohse et al. in review). Given that we measured N deposition only once, during the summer dry season, potential explanations for the difference between modeled and observed rates of dry N deposition include temporal variability in prevailing wind directions or in emission sources. In addition, the deposited materials may be transformed or volatilized and returned to the atmosphere by high summer temperatures and photochemical reactions rather than incorporated into soils (Peterjohn and Schlesinger 1990; Andersen and Hovmand 1999).
Spatial and temporal patterns in soil resources and microbes
Plants stimulate N mineralization processes by contributing organic matter to the soil, which results in higher inorganic N availability beneath plants. Plants may also concentrate N deposited from the atmosphere by increasing surface area on which deposited materials are collected (Fenn and Bytnerowicz 1993). However, we did not observe a correlation between measured rate of N deposition and the soil inorganic N pool beneath plants, suggesting that observed differences in soil N between plants and interspaces were due solely to plant-mediated processes. Increased N availability associated with shrub islands is localized beneath the canopy, whereas interspace soils are characterized by lower nutrient concentrations and little biotic activity (Schlesinger and Pilmanis 1998).
Microbial population sizes were influenced by similar factors both under plants and in interspaces whereas correlates of soil respiration showed spatial patterns. Biomass and abundance of soil microbes beneath plant islands and in interspaces were both correlated with availability of water and \( {\text{NO}}^{-}_{{\text{3}}}, \) an expected result for dry, N-poor desert soils. Beneath plants, moisture and organic matter influenced flux of CO2, which corresponds with previous work that showed water limitation of soil respiration in urban Phoenix (Green and Oleksyszyn 2002) and C limitation in desert soils (Schaeffer et al. 2003; Sponseller 2007). Soil respiration in interspaces did not correlate with these predictors and instead, after wetting, was correlated only with rate of atmospheric N deposition.
Temporally, we measured large shifts in soil resource availability and microbial activity in response to precipitation both on a seasonal and event basis. Only during the monsoon rains, when the microbial pool is likely at an annual maximum, were significant effects of plant islands on microbial biomass and abundance observed. Activities of microbes in deserts are strongly linked to available water, and dry seasons are characterized by long periods of inactivity (Austin et al. 2004; Sponseller 2007). Thus, similar values beneath plants and in interspaces during these dry periods likely reflect a lack of biological activity.
Spatial patterns in soil resources and microbial attributes that were established by vegetation were apparent at characteristic time points. We observed significant differences between plant islands and interspaces for inorganic N concentration only during the dry season. In contrast, soil moisture and inorganic N were spatially homogeneous during the monsoon season. A redistribution of resources likely results from physical processes including transport across the soil surface in overland flow or enhanced diffusion within the soil matrix (Belnap et al. 2005; Welter et al. 2005). Additionally, changes in relative nitrogen concentrations may have been due to microbial mineralization and nitrification or plant and microbial uptake of N following rains (Gebauer and Ehleringer 2000; Austin et al. 2004).
Temporal variability in microbial activity may influence responses to N inputs. Soil microbes both beneath plants and in interspaces showed rapid and short-lived responses to precipitation, demonstrating the limited potential for soil biota to process deposited materials. Using our estimates of soil CO2 flux following simulated storms and a microbial biomass C:N value of 13 (this study), we calculated the fraction of atmospherically deposited N that could be incorporated into microbial biomass for assimilation efficiencies ranging from 20% to 60%. On a daily timescale following a precipitation event, if microbes used solely N deposited from atmosphere, interspace microbes would use only 18–53% of deposited N whereas microbes beneath plants could use 40–121%. Despite potential for microbes to exploit a potentially large new pool of N, anthropogenic nutrient additions are not sufficient to overcome the limitation of sparse and infrequent rainfall, and deposited N is likely to accumulate, rather than be sequestered in microbial biomass.
Conclusion
This study suggests that soil respiration in deserts experiencing urbanization is influenced by anthropogenic N inputs from urban activities, but that plant islands remain the predominant organizers of microbial processes. One challenge of investigating the influence of ambient atmospheric deposition on soil microbes is reconciling the temporal and spatial scales of these processes. Whereas microbial activity can be characterized as a brief pulse that follows precipitation events, deposition is influenced by diel and weekly patterns of fossil-fuel consumption, and seasonal and interannual patterns in weather and climate (Shen et al. 2005). Microbes are concentrated beneath plant islands (m2) while deposition is relatively diffuse and influences entire airsheds (km2). Given the correlation between rate of N deposition and growth of human populations in arid and semi-arid regions (Fenn et al. 2003b), however, it is critical to understand the responses of soil processes to this increased N loading.
References
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in generate forests; hypotheses revised. BioScience 48:921–934
Allen EB, Padgett PE, Bytnerowicz A, Minnich R (1998) Nitrogen deposition effects on coastal sage vegetation of southern California. In: Bytnerowicz A, Arbaugh MJ, Schilling SL (eds) Proceedings of the international symposium on air pollution and climate change effects on forest ecosystems. U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Albany, CA, pp 131–139
Andersen HV, Hovmand MF (1999) Review of dry deposition measurements of ammonia and nitric acid to forest. For Ecol Manage 114:5–18
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235
Báez S, Fargione J, Moore DI, Collins SL, Gosz JR (2007) Atmospheric nitrogen deposition in the northern Chihuahuan desert: temporal trends and potential consequences. J Arid Environ 68:640–651
Baker LA, Brazel AJ, Selover N, Martin C, Steiner F, Nelson A, Musacchio L (2002) Urbanization and warming of Phoenix (Arizona, USA): impacts, feedbacks and mitigation. Urban Ecosyst 6:183–203
Baron JS, Rueth HM, Wolfe AM, Nydick KR, Allstott EJ, Minear JT, Moraska B (2000) Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems 3:352–368
Belnap J, Welter JR, Grimm NB, Barger N, Ludwig JA (2005) Linkages between microbial and hydrologic processes in arid and semiarid watersheds. Ecology 86:298–307
Bird SB, Herrick JE, Wander MM, Wright SF (2002) Spatial heterogeneity of aggregate stability and soil carbon in semi-arid rangeland. Environ Pollut 116:445–455
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analysis of soils. Agron J 54:464–465
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manage 196:43–56
Bradley K, Drijber RA, Knops J (2006) Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biol Biochem 38:1583–1595
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform publication and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO3− deposition reduces soil respiration in northern hardwood forests. Global Chang Biol 10:1080–1091
Ewing SA, Southard RJ, Macalady JL, Hartshorn AS, Johnson MJ (2007) Soil microbial fingerprints, carbon, and nitrogen in a Mojave Desert creosote-bush ecosystem. Soil Sci Soc Am J 71:469–475
Faeth SH, Warren PS, Shochat E, Marussich WA (2005) Trophic dynamics in urban communities. BioScience 55:399–407
Fenn ME, Bytnerowicz A (1993) Dry deposition of nitrogen and sulfur to ponderosa and Jeffrey pine in the San Bernardino National Forest in southern California. Environ Pollut 81:277–285
Fenn ME, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L, Bowman WD, Sickman JO, Meixner T, Johnson DW, Neitlich P (2003a) Ecological effects of nitrogen deposition in the western United States. BioScience 53:404–420
Fenn ME, Haeuber R, Tonnesen GS, Baron JS, Grossman-Clarke S, Hope D, Jaffe DA, Copeland S, Geiser L, Rueth HM, Sickman JO (2003b) Nitrogen emissions, deposition, and monitoring in the western United States. BioScience 53:391–403
Fernando HJS, Lee SM, Anderson J, Princevac M, Pardyjak E, Grossman-Clarke S (2001) Urban fluid mechanics: air circulation and contaminant dispersion in cities. Environ Fluid Mech 1:107–164
Fisher FM, Zak DR, Cunningham GL, Whitford WG (1988) Water and nitrogen effects on growth and allocation patterns of creosotebush in the northern Chihuahuan Desert. J Range Manage 41:387–391
Fravolini A, Hultine KR, Brugnoli E, Gazal R, English NB, Williams DG (2005) Precipitation pulse use by an invasive woody legume: the role of soil texture and pulse size. Oecologia 144:618–627
Gallardo A, Schlesinger WH (1992) Carbon and nitrogen limitation of soil microbial biomass in desert ecosystems. Biogeochemistry 18:1–17
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26:1409–1415
Gallardo A, Schlesinger WH (1995) Factors determining soil microbial biomass and nutrient immobilization in desert soils. Biogeochemistry 28:55–68
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol 48:218–229
Gebauer RLE, Ehleringer JR (2000) Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81:1415–1424
Green DM, Oleksyszyn M (2002) Enzyme activities and carbon dioxide flux in a Sonoran desert urban ecosystem. Soil Sci Soc Am J 66:2002–2008
Hansen J, Ruedy R, Glascoe J, Sato M (1999) GISS analysis of surface temperature change. J Geophys Res Atmospheres 104:30997–31022
Hobbie SE (2005) Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–656
Johnson SL, Neuer S, Garcia-Pichel F (2007) Export of nitrogenous compounds due to incomplete cycling within biological soil crusts of arid lands. Environ Microbiol 9:680–689
Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. TREE 12:139–143
Kieft TL, White CS, Loftin SR, Aguilar R, Craig JA, Skaar DA (1998) Temporal dynamics in soil carbon and nitrogen resources at a grassland-shrubland ecotone. Ecology 79:671–683
Lohse KA, Hope D, Sponseller RA, Allen JO, Grimm NB Atmospheric deposition of nutrients across a desert city. Sci Total Environ (in review)
Lovett GM, Rueth HM (1999) Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecol Appl 9:1330–1344
Lovett GM, Traynor MM, Pouyat RV, Carreiro MM, Zhu WX, Baxter JW (2000) Atmospheric deposition to oak forests along an urban-rural gradient. Environ Sci Technol 34:4294–4300
Peterjohn WT, Schlesinger WH (1990) Nitrogen loss from deserts in the Southwestern United-States. Biogeochemistry 10:67–79
Peterjohn WT, Schlesinger WH (1991) Factors controlling denitrification in a Chihuahuan Desert ecosystem. Soil Sci Soc Am J 55:1694–1701
Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2007) Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296:65–75
Pregitzer KS, Zak DR, Burton AJ, Ashby JA, MacDonald NW (2004) Chronic nitrate additions dramatically increase the export of carbon and nitrogen from another hardwood ecosystems. Biogeochemistry 68:179–197
Raymond HA, Yi S-M, Moumen N, Han Y, Holsen TM (2004) Quantifying the dry deposition of reactive nitrogen and sulfur containing species in remote areas using a surrogate surface analysis approach. Atmos Environ 38:2687–2697
Rowe R, Todd R, Waide J (1977) Microtechnique for most-probable-number analysis. Appl Environ Microbiol 33:675–680
Saetre P, Stark JM (2005) Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species. Oecologia 142:247–260
Saxena A, Kulshrestha UC, Kumar N, Kumari KM, Prakash S, Srivastava SS (1997) Dry deposition of sulphate and nitrate to polypropylene surfaces in a semi-arid area of India. Atmos Environ 31:2361–2366
Schade JD, Hobbie SE (2005) Spatial and temporal variation in islands of fertility in the Sonoran Desert. Biogeochemistry 73:541–553
Schade JD, Sponseller R, Collins SL, Stiles A (2003) The influence of Prosopis canopies on understorey vegetation: effects of landscape position. J Veg Sci 14:743–750
Schaeffer SM, Evans RD (2005) Pulse additions of soil carbon and nitrogen affect soil nitrogen dynamics in an arid Colorado Plateau shrubland. Oecologia 145:425–433
Schaeffer SM, Billings SA, Evans RD (2003) Responses of soil nitrogen dynamics in a Mojave Desert ecosystems to manipulations in soil carbon and nitrogen availability. Oecologia 134:547–553
Schlesinger WH (1996) On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–374
Schlesinger WH, Pilmanis AM (1998) Plant-soil interactions in deserts. Biogeochemistry 42:169–187
Schlesinger WH, Raikes JA, Hartley AE, Cross AF (1996) On the spatial patterns of soil nutrients in desert ecosystems. Ecology 77:364–374
Shen WJ, Wu J, Kemp PR, Reynolds JF, Grimm NB (2005) Simulating the dynamics of a Sonoran ecosystem: model parameterization and validation. Ecol Model 189:1–24
Siguenza C, Crowley DE, Allen EB (2006) Soil microorganisms of a native shrub and exotic grasses along a nitrogen deposition gradient in southern California. Appl Soil Ecol 32:13–26
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Sirulnik AG, Allen EB, Meixner T, Fenn ME, Allen MF (2007) Changes in N cycling and microbial N with elevated N in exotic annual grasslands of southern California. Appl Soil Ecol 36:1–9
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Chang Biol 13:426–436
Staley TE, Griffin JB (1981) Simultaneous enumeration of denitrifying and nitrate reducing bacteria by a microtiter most-probable-number (MPN) procedure. Soil Biol Biochem 13:385–388
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Tietema A (1998) Microbial carbon and nitrogen dynamics in coniferous forest floor material collected along a European nitrogen deposition gradient. For Ecol Manage 101:29–36
Waldrop MP, Zak DR (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9:921–933
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manage 222:459–468
Welter JR (2004) Nitrogen transport and processing in the intermittent drainage network: linking terrestrial and aquatic ecosystems Arizona State University, Tempe
Welter JR, Fisher SG, Grimm NB (2005) Nitrogen transport and retention in an arid land watershed: influence of storm characteristics on terrestrial-aquatic linkages. Biogeochemistry 76:421–440
Zak DR, Holmes WE, Tomlinson MJ, Pregitzer KS, Burton AJ (2006) Microbial cycling of C and N in northern hardwood forests receiving chronic atmospheric NO −3 deposition. Ecosystems 9:242–253
Acknowledgements
Support for this research was provided in part by grants from the Arizona State University Office of the Provost through the School of Life Sciences Enrichment Program and by NSF grants DEB-0423704 and DEB-0514382. We thank Rebecca Martin, Craig McCrackin, and Nicole Styles for assistance in the field and Shannon Johnson for expertise with microbial abundance methods. Ryan Sponseller and two anonymous reviewers provided valuable comments on previous versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCrackin, M.L., Harms, T.K., Grimm, N.B. et al. Responses of soil microorganisms to resource availability in urban, desert soils. Biogeochemistry 87, 143–155 (2008). https://doi.org/10.1007/s10533-007-9173-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-007-9173-4