Abstract
The inoculum biomass was collected from a pilot-scale (3 m3 process tank) nitritation-anaerobic ammonium oxidation (ANAMMOX) (deammonification moving bed biofilm (DeaMBBR)) reactor demonstrating the highest total nitrogen removal rate (TNRR) of 0.33 kg N m−3 day−1. This biomass was used for inoculating the anodic chamber of a microbial fuel cell (MFC) to investigate the capacity of DeaMBBR biomass to act as an exo-electrogenic consortia. Performance of MFCs inoculated with ANAMMOX-specific consortia collected from DeaMBBR (MFC-ANA) and another MFC-CON inoculated with a septic tank mixed anaerobic consortium as a control was investigated for electrochemical performance and wastewater treatment efficiency. These MFCs were operated for the total duration of 419 days during which regular feed was given and performance was monitored for first 30 cycles and last 30 cycles, with each cycle of 3 day duration. The MFC-ANA continuously generated bio-energy with higher volumetric power density (9.5 W m−3 and 6.0 W m−3) in comparison to MFC-CON (4.9 and 2.9 W m−3) during the first 30 and last 30 cycles of operational period, respectively. MFC-ANA also achieved 84 ± 2% and 80 ± 2% of COD removal efficiency and 89 ± 4% and 73 ± 2% of total nitrogen removal efficiency during first 30 and last 30 cycles of operational period, respectively. The improvement of nitrogen removal and power production in case of MFC-ANA over MFC-CON could be attributed to the ANAMMOX-denitrifiers populations and Trichococcus (14.92%) as denitrifying exo-electrogenic microbes (4.46%), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nutrient compounds (such as nitrogen) present in the wastewater can cause eutrophication, if released into the environment without treatment. Nitrification/denitrification process with enhanced phosphorus removal is typically used for municipal (mainstream) and other industrial wastewater treatment plants (Mandel et al. 2019). Anaerobic ammonium oxidation (ANAMMOX) is already a well-known process for biological nitrogen removal from organic carbon deficient and nitrogen-rich wastewaters (Strous et al. 1997) and it could also be a promising alternative for treating mainstream wastewater. ANAMMOX is a microbiological process of converting ammonium into dinitrogen gas under anoxic conditions with nitrite and NO serving as an electron acceptor. Savings on oxygen supplies (electricity) and reduced CO2 and N2O emissions are achieved, if compared to the conventional nitrification/denitrification process (Strous et al. 1997; Zekker et al. 2019).
In any bioelectrochemical system (BES), microorganisms interact with electrodes via the exchange of electrons, which are either supplied (bioelectrocatalysis) or harvested through an external electrical circuit (microbial fuel cell, MFC) (Bhowmick et al. 2018). On the other hand, the advantage of applicability for electrode–denitrifying bacteria interactions includes the production of electricity (Logan et al. 2006), wastewater treatment (Gregoire et al. 2014; Bhowmick et al. 2019a), bioremediation and production of valuable products (Tharali et al. 2016). Researchers are making efforts on nitrogen removal from the wastewater by employing BES-denitrification-ANAMMOX combined approach in order to achieve simultaneous power generation and nitrogen removal from the organic carbon containing mainstream wastewater in a single unit (Jadhav and Ghangrekar 2009). Organic content is being oxidized in anodic chamber of the MFC and electrons are accepted by the extracellular electron acceptor i.e. anode (Logan et al. 2006). Thereafter, electrons move through the external circuit towards the cathode where oxygen or other electron acceptor is reduced by the protons that are coming from the anodic chamber through the separator or proton exchange membrane (Noori et al. 2018; Bhowmick et al. 2019b). Besides, ANAMMOX is an autotrophic process and can convert ammonium to nitrogen gas without the presence of organic matter, therefore, the organic compounds in the wastewater can be fully utilized by exoelectrogenic bacteria for energy production in the MFC technology (Clauwaert et al. 2007; Zekker et al. 2020).
Moreover, in comparison to the traditional nitrification–denitrification process, ANAMMOX is an efficient alternative for nitrogen removal from the wastewater with low C/N ratio (Sheng et al. 2018; Chen et al. 2018; Jadhav and Ghangrekar 2015). As ANAMMOX bacteria are susceptible to higher organic carbon concentration and salinity level in the wastewater (Liu et al. 2009), there are two possible mechanisms that cause the lowered ANAMMOX activity. The first one is initiated by the fast growing heterotrophic bacteria competing with ANAMMOX for nutrients at higher organic carbon concentrations causing reduced ANAMMOX activity (Chamchoi et al. 2008). Whereas, the second states that the majority of ANAMMOX bacteria can also metabolize organic carbon, simultaneously with ammonium and nitrite as substrates that leads to a lower ANAMMOX activity with a lower nitrogen removal efficiency (Guven et al. 2005).
Some exoelectrogenic denitrifiers bioelectrochemically reduce NO2− under anoxic condition to generate electrons and these bacteria can be enriched with the supply of organic compounds. Electron mediators that are located outside the cells (exoelectrogens) transfer electrons to the external electron acceptor through the c-type cytochrome, which are secreted by several bacteria, such as denitrifying (Pseudomonas sp.) and sulphur reducing (G. sulfurreducens) ones (Gregoire et al. 2014). If NO2− or NO are absent, ANAMMOX consortia can perform extracellular electron transfer with Fe(III) or Mn(IV) coupled with formate oxidation. Extracellular electron transfer has been typically observed for the exoelectrogens, such as Geobacter and Shewanella through the c-type cytochromes (Shaw et al. 2019). Furthermore, ANAMMOX consortia have shown to have multi-heme cytochromes that are contributing in extracellular electron transfer (Shaw et al. 2019).
Wastewaters, like from fish processing industries (Noori et al. 2016) and municipal landfill leachate (Lee et al. 2013), have high ammonium-nitrogen and organic carbon content. The removal of total COD (up to 60%) and total Kjeldahl nitrogen (up to 40%) was observed by the activity of microbial consortia derived from digestate, endorsing the presence of denitrifying and ANAMMOX (Brocadia strains) consortia to improve the overall performance of MFC (Di Domenico et al. 2015). Within ANAMMOX denitrification-coupled process in the MFC, a continuous current density of 165 mA.m−2 was achieved along with the relationship of: NH4+ − Nconsumed/NO2− − Nconsumed/NO3− − Nproduced = 1/1.37/0.03 during the stable operation elsewhere (Li et al. 2015). Further assessment of MFCs could lead to the development of autotrophic-heterotrophic biofilm system combinations with a higher nutrient removal and electricity production.
The deammonifying (nitritation-ANAMMOX) biofilm systems typically involve smaller volume than suspended sludge systems due to higher specific surface area of carriers applied, which in turn leads to lower construction and operation cost (Zekker et al. 2012; Van Loosdrecht and Salem, 2006). However, due to the low growth rate of ANAMMOX consortia and the denitrifiers overgrowth of ANAMMOX rich biomass, autotrophic nitrogen removal is initiated by cultivation of the biomass in controlled low organic carbon conditions before their use in real-life systems (MFC, treatment plants etc.).
Present research aims to achieve stable and high total nitrogen (TN) removal rate and efficiency in deammonifying moving bed biofilm reactor (DeaMBBR) in continuous mode of operation for enrichment of ANAMMOX culture over the media. Thereafter, further applications of this enriched biomass in anodic chamber of MFC was foreseen. The purpose of the anodic biofilm enriched with ANAMMOX biomass was to achieve sufficient power generation and nitrogen removal in MFC compared to the conventionally used heat-treated suspended mixed inoculum. Microbiological shifts in bacterial proportions in inoculum, at the end of DeaMBBR operation and MFC were assessed further by pyrosequencing to investigate the applicability of DeaMBBR biomass as exo-electrogenic consortia.
Materials and methods
Operation of deammonification moving bed biofilm reactor
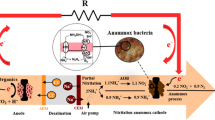
Initial biomass was cultivated on reject water-fed deammonification pilot plant (DeaMBBR) carriers with a reactor active volume of 3 m3 (Rikmann et al. 2018). Single reactor system, DeaMBBR involved aerobic nitritation stage (30 min) coupled with anoxic conditions (20–60 min) within the intermittent aeration cycle. Durations of aerobic-anoxic cycles were set based on removed NO2−–N and production of NO3−–N and their accumulations in the effluent. The DeaMBBR system’s feeding and aeration control were automated (Unitronics Vision V1040, Germany) and equipped with oxidation–reduction potential (ORP), dissolved oxygen (DO), pH, temperature, hydrostatic water level control sensor and aerator (Fig. 1). About 50% of the reactor volume was filled with the biomass carriers (specific surface area 750 m2 m−3, density 930 kg m−3). Mixing was performed by mechanical low rate stirring (< 50 rpm). The hydraulic retention time (HRT) was maintained within 3–7 days. The average operating temperature of DeaMBBR was 30.0 ± 3.5 °C. Further details on DeaMBBR operation parameters have been reported in Rikmann et al. (2018).
The biomass was taken from the mobile DeaMBBR during its commissioning at Tartu WWTP (Estonia) for sidestream treatment. The same system has successfully applied earlier and details are given in our recent work (Rikmann et al. 2018). Reactor performance in Tartu WWTP was monitored for 700 days.
Fabrication and operation of MFC
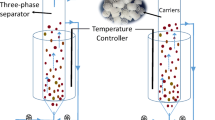
The further laboratory research was performed with the double-chambered MFCs, made of ceramic cylinders (red soil blended with 20% Montmorillonite with a wall thickness of 6 mm) with working volume of 80 ml. Carbon felt (Panex35, Zoltek Corporation, Missouri, USA) was used as anode material with a projected surface area of 90 cm2, and attached to the inner surface of the clay-ware cylinder (Ghadge et al. 2015). Bare carbon felt with projected surface area of 120 cm2 was tightly attached to the outer surface of the ceramic cylinder and served as cathode. Tap water was used as catholyte with a proper supply of bubble aeration (SOBO aerator, China). The 6 mm thick ceramic wall of cylinder served as a separator that allowed protons to migrate from anodic to cathodic chamber (Fig. 2). The potential was measured over 100 Ω of external resistance for both the MFCs and the electrodes were connected with concealed copper wire. The connections were made water-resistant with fixed proportion of the resin and glue. Before the commissioning of the reactors, the headspace was purged with nitrogen gas to remove oxygen from the system.
Two MFCs were used for this experiment. MFC-CON was inoculated with 15 ml of heat-treated (100 °C for 15 min) mixed-anaerobic sludge collected from septic tank bottom (volatile suspended solids, VSS of 19.95 g l−1 and total suspended solids, TSS of 30.22 g l−1). Heat-treatment was used for the MFC-CON biomass to reduce the activity of methanogenic bacteria as methane production could reduce the power generation in MFC. The other MFC-ANA was inoculated with ANAMMOX consortia present on biofilm carriers taken from DeaMBBR (Estonia) having VSS of 15 g l−1 and TSS of 23 g l−1. Both of the MFCs were operated at optimum temperature for ANAMMOX process (30 ± 2 °C). These MFCs were fed with synthetic wastewater consisting sucrose as a carbon source. The synthetic wastewater was prepared with chemical oxygen demand (COD) concentration of around 3 g l−1 and supplemented with microelements as given by Jadhav and Ghangrekar (2009). The ammonium ((NH4)2SO4) and nitrite (NaNO2) were used as synthetic substrates for ANAMMOX consortia to achieve ammonium-nitrogen and nitrite-nitrogen concentration of 50 mg N l−1 of each (Chatterjee et al. 2016; Zekker et al. 2012).
Wastewater treatment efficiency and power performance in MFCs were monitored and observed for first 90 days as initial stage of 30 fed-batch cycles with three days of retention time and last 30 cycles as second stage spanning last 90 days of operation period. Total operational period for these MFCs spanned for 419 days. During the rest 239 days in between, both the MFCs were regularly fed with the synthetic wastewater without regularly measuring its performance.
Bio-electrochemical monitoring and data acquisition
The potential difference and current generated by MFCs were measured using a digital multimeter with data acquisition unit (Agilent Technologies, Malaysia). Power was calculated according to the Eq. (1):
where, P is power, W; I is current, A; and V is voltage, V.
An Ag/AgCl reference electrode (CH Instruments, Inc., RE-5B; + 0.197 V vs. standard hydrogen electrode, SHE) was used to measure the anodic potential of MFCs. The polarization curve was obtained to determine the power density, normalized to the volume of the anodic chamber, by monitoring the voltage output at various external resistances, ranging from 10,000 Ω to 10 Ω. The relationship between the voltage and current was measured in order to define the internal resistance for both of the MFCs. The internal resistance of the MFC was estimated from the slope of the linear portion of the polarization plot (voltage vs. current). Coulombic efficiency (CE) was estimated by integrating the measured current over time relative to the maximum possible generation of coulombs during the experimentally observed removal of COD (Logan and Regan, 2006) as shown in Eq. (2):
where \({M}_{s}\) is the molecular weight of substrate (g mol−1), \(\Delta COD\) is the change in substrate concentration over a batch cycle (g l−1), \({V}_{An}\) is the anodic chamber volume (l), F is Faraday’s constant (96,485 C mole−1) and \({b}_{es}\) is the measure of generated electrons per mol of substrate oxidation (mol of e− per mol of substrate−1).
The normalized energy recovery (NER) was estimated as described by Ge et al. (2014) and expressed based on the volume of wastewater treated over the time (kWh m−3). The relation is shown in Eq. 3.
Electrochemical parameters were measured with a potentiostat (Metrohm, The Netherlands) using cyclic voltammetry (CV) analysis. In the anodic half-cell CV analysis, the anode was utilized as the working electrode and the cathode served as a counter electrode. The separator was 6 mm thick ceramic wall (made of red soil blended with 20% Montmorillonite); oxygen saturated tap water was used as catholyte and Ag/AgCl was used as reference electrode inside the anode chamber (next to the anode). The cycle was run from − 1.00 V to + 1.00 V vs. an Ag/AgCl reference electrode with a voltage scan rate of 10 mV s−1 and pH of the catholyte and anolyte at the time of measurement were 7.5 and 6.5, respectively. The CV is considered to be an intrusive technique detrimental to the performance of MFC, therefore the analysis was done at the end of operation period (Bhowmick et al. 2019c).
Total COD was analyzed for the collected samples from MFCs at a regular time interval according to Standard Methods (APHA-AWWA-WPCF 1998). Ammonium, nitrite and nitrate were measured using ion chromatograph (Metrohm, Germany) and a water quality analyzer (Orion Versastar Pro, Thermo Scientific, USA).
Next generation sequencing for bacteria & archaea community analysis
DNA extraction
Inoculated sludge and adapted biomass from DeaMBBR (taken on day 0 and day 688, respectively) and biomass after the MFC operation cycles were characterized with pyrosequencing technique. The desoxyribonucleic acid (DNA) was extracted with PowerBiofilm® DNA Isolation Kit Sample (Mo Bio Laboratories Inc., USA) according to the manufacturer’s instructions.
DNA amplification
The V3-V4 hypervariable regions of the 16S rRNA gene were polymerase chain reaction (PCR) amplified and sequenced using Illumina MiSeq 2 × 250 v2 platform (Estonian Genome Center). Archaea-specific universal primers were: ARC344F, 5′-ACGGGGYGCAGCAGGCGCGA-3′ and Arch806R, 5′-GGACTACVSGGGTATCTAAT-3′ (Takai and Horikoshi, 2000).
Taxonomic profiling by pyrosequencing
Sequence data by BION-meta was analysed according to McDonald et al. (2016). First, forward sequences were cleaned at both ends using a 99.5% minimum quality threshold for at least 18 of 20 base pairs (bp) for 5′-end and 28 of 30 bases for 3′-end. Second, shorter reads than 230 bp were removed, then cleaned from chimeras and clustered by 95% oligonucleotide similarity (k-mer length of 8 bp, step size 2 bp). Lastly, consensus reads were aligned to the SILVA reference 16S rDNA database (v123) using word length of 8 and similarity cut-off of 90%.
Next generation sequencing
The 16S Metagenomics Sequencing Library was prepared using two steps for both, PCR and purification. Initially for 1st amplicon PCR, 16S rRNA F (ACGGGGYGCAGCAGGCGCGA) and 16S rRNA R (GGACTACVSGGTATCTAAT) primer pair was used to amplify the template Amplicon out of a DNA sample using region of interest V3-V4 Specific primers (Takai and Horikoshi 2000) with attached overhang adapters followed by PCR clean up using AMPure XP beads (Ciesielski et al. 2018). The 2nd PCR (Index PCR) was followed with the presence of dual indices and Illumina sequencing adapters using the Nextera XT again cleaned up using AMPure XP beads. The Quantity and quality check (QC) of the library were analyzed in 4200 Tape Station system (Agilent Technologies) using D1000 Screen tape as per manufacturer’s instructions. Sequencing was conducted in 2 steps: 1. Cluster Generation. 2. Sequencing: Illumina SBS (Sequencing by Sequencing) according to manufacturer instructions and as stated by (Ciesielski et al. 2018).
In the initial process of the ANAMMOX-MFC data, the high quality clean reads were obtained using Trimmomatic v0.38. The method removes adaptor sequences, ambiguous reads (reads with unknown nucleotides larger than 5%), and low-quality sequences (reads with more than 10% quality threshold) along with a sliding window of 10 bp and a minimum length of 100 bp. Thereafter, the follow-up was stitching the paired end (PE) data into single end reads using FLASH (v1.2.11) (J. Hopkins University School of Medicine, U.S.A). After the stitching each operational taxonomical units (OTUs) were picked up based on their sequence similarity within the reads, and representative sequence from each OTU against Greengenes database (version 13_8). Then, the OTUs were assigned to a taxonomic identity using Greengenes reference databases and previously calculated diversity matrices. Thereafter, the OTUs pick identifies higher similar sequences across the sample and provides a comparison in community structure. All the sequences from the samples were clustered into OTUs based on their sequence similarity. These OTUs are clusters of sequences, frequently intended to represent some degree of taxonomic relatedness done using UCLUST algorithm (GigaMune, Inc., U.S.A.) at 97% sequence similarity and each resulting cluster typically represents the species. Since each OTU may consist of many sequences, a representative sequence for OTU is picked for downstream analysis. This representative sequence was used for taxonomic identification for the OTU. The taxonomies were assigned to the OTUs by UCLUST based on a threshold of 90% sequence similarity.
Results and discussion
The biomass used for anode biofilm was cultivated in DeaMBBR on biomass carriers and it showed high total nitrogen removal rates (TNRRs) and total nitrogen removal efficiencies (TNREs) during its 700 days of operation. Thereafter, MFCs were operated for 419 days and for first and last 90 days of operation performance of these was observed in two stages, each lasting for 30 cycles in order to understand the system performance using ANAMMOX-based DeaMBBR biomass as exo-electrogenic consortia during prolong operational duration.
DeaMBBR biomass cultivation operation for MFC-ANA
The pilot-scale DeaMBBR was inoculated with reject water and was operated for around two years. The TNRRs reached to 0.33 kg N m−3 day−1 after operation of 338 days. Overall TNREs were in average 76 ± 17%. Effluent nitrite concentrations remained around 4 ± 2.5 mg N l−1, which is considered to be non-inhibitory to the ANAMMOX process (Lotti et al. 2015). During the whole operation time, the average effluent NH4+–N and NO3−–N concentrations were 213 ± 187 and 22 ± 21 mg l−1, respectively. The organic matter was also removed with an average COD removal efficiency of 72 ± 7%, and the average COD concentration in the effluent was 160 mg l−1, whereas the influent COD concentration was 768 ± 207 mg l−1. The COD/TN removal ratio being at 0.87/1 was beneficial to ANAMMOX process for DeaMBBR. Efficient TN removal rate of 0.18 ± 0.03 kg N m−3 day−1 has been achieved for pre-treated municipal wastewater with an average COD removal rate of 0.51 ± 0.11 kg COD m−3 day−1 (Lotti et al. 2015), where COD/NH4+-N ratio was higher (~ 1.9) than in current investigation. The above experimental results showed that proper operation at optimum COD/N ratio can be beneficial to improve the overall treatment efficiency of the DeaMBBR (Fig. 3a and 3b) and cultured biomass was capable of bioenergy production.
Average effluent alkalinity was observed to be 13 ± 10 mmol l−1, which turned out to be suitable for the process performance despite the fact that influent alkalinity was at high average concentration of 71 ± 11 mmol l−1, which maintained inorganic carbon required for autotrophic ammonium oxidation to nitrite. Earlier, alkalinity /N ratio < 3.45/1 in the reject water was reported to be not sufficient to achieve the theoretical nitrite to ammonium ratio of 1.32/1 (Kosari et al. 2014). However, earlier in an ANAMMOX biofilm system application without applying nitritation step, system’s optimum effluent alkalinity value of 13 ± 10 mmol l−1 at the alkalinity /N molar ratio of 5/1 has been reported to achieve the most efficient TNRR in batch assays (Zekker et al. 2012). Average effluent pH was 6.74 ± 0.63 being at the optimum range for ANAMMOX reaction, as influent pH being at the higher range (8.42 ± 0.15) due to high reject water alkalinity of 71 ± 11 mmol l−1 needed for ammonium oxidation and ANAMMOX reaction to occurr.
Power production in MFCs
In this investigation, the effect of different inoculum on power production and wastewater treatment efficiency of MFC was evaluated. Both of the MFCs started showing electricity production from the very first week of its commencement, endorsing the presence of active exo-electrogenic bacteria in heat pre-treated septic tank mix consortia as well as in cultured ANAMMOX biota from DeaMBBR biomass. After initial start-up, MFCs began giving stable performance on the basis of electrical yield as well as organic matter removal. The average operating voltage (OV) of 267 ± 17 mV was achieved under the steady-state operating condition in the very first stage (first 90 days) of observational period and 253 ± 4 mV in the second stage (last 90 days) of observational period over 100 Ω of external resistance in case of MFC-ANA, which was reasonably higher than the values obtained from MFC-CON (163 ± 18 mV and 164 ± 3 mV, respectively) (Fig. 4). These values are much higher than the reported average OV of 156 mV by incorporating COD/NH4+–N ratio of 10/1 in the feed for MFC by Jadhav and Ghangrekar 2015. The open circuit voltages (OCVs) for these two observational periods were also following the same trend (Fig. 4).
From the power density curve (Fig. 5), it can be noted that the MFC-ANA could generate a maximum power density of 9.5 W m−3, which was almost two times higher as compared to that of MFC-CON (4.9 W m−3) at the end of first 90 days of observation period (Fig. 5). Whereas, MFC-ANA demonstrated a maximum power density of 6.0 W m−3, almost two times higher than that of MFC-CON (2.9 W m−3) at the end of last 90 days of observation period. Earlier, MFC incorporating COD/NH4+-N ratio of 10/1 in the synthetic feed showed the highest maximum power density of 753 mW m−3 (Jadhav and Ghangrekar 2015) and in case of electrode made of carbon cloth and brewery wastewater as substrate the MFC showed a maximum power density of 5.1 W m−3 as reported elsewhere (Feng et al. 2008). Thus, the power density values obtained by using ANAMMOX-denitrification based biomass outperformed the similar kind of investigations for application in MFC. Moreover, by the ANAMMOX-based MFC with landfill leachate has earlier resulted in a power density of 12 mW m−2 with 94% of total nitrogen removal efficiency (Lee et al. 2013).
The total internal resistance of MFC-ANA was found to be 161 Ω and 152 Ω, respectively, from the polarization plot at the end of first and second stage of observational periods, which were much lower than the MFC-CON (386 Ω and 301 Ω, respectively). Based on the results obtained, it can be concluded that the MFC with ANAMMOX-denitrifiers based DeaMBBR consortia had better substrate utilization rate than biomass in control. This is due to the possibilities of the organic matter consumption by denitrifying bacteria to reduce the organic carbon as well as nitrogen content from the wastewater. This outcome is echoing the investigation done by Light et al. (2018), where Listeria monocytogenes, a fermentative gram-positive bacteria and host associated pathogen were able to respire through a flavin-based extracellular electron transfer (EET) process just like other exo-electrogens. More detailed investigation by Ferousi et al. (2017) demonstrated the homologs of multi-heme cytochromes in ANAMMOX consortia being responsible for EET, which is suspected to be one of the reasons that improved the power performance in the present work.
Wastewater treatment in MFCs
Organic matter removal and energy recovery
The COD removal efficiencies of 84 ± 2% and 79 ± 2% were achieved in MFC-ANA and MFC-CON, respectively, during the first observation period (Fig. 6). Whereas, 80 ± 2% and 77 ± 2% were achieved in MFC-ANA and MFC-CON, respectively, for the second observation period (Fig. 6). The coulombic recovery in MFC-ANA was found with an average value of 27.5 ± 1.7% and 27.0 ± 0.6%, respectively for first and second stage of observation period, which was approximately 1.5 times higher compared to MFC-CON (17.7 ± 1.9% and 18.3 ± 0.5%) (Fig. 7).
The NER was also determined for both of the MFCs to understand the performance of MFC in terms of electricity generation based on the energy produced per unit volume of the wastewater treated. The maximum NER for MFC-ANA and MFC-CON was 0.685 and 0.358 kWh per m3 of treated wastewater, respectively at the first stage, and 0.433 and 0.212 kWh per m3 of treated wastewater, respectively, at the second stage of observation period (Fig. 7; Table 1). The reduction in NER values in case of second stage of observational period was suspected to be the reason of re-occurrence of methanogenic consortia as witnessed from the NGS data as discussed in later section along with slight fouling of PEM and electrode after running for more than one year might have played the major role.
Overall organic matter recovery and power production were therefore substantially enhanced by incorporating DeaMBBR consortia in the anodic chamber of MFC by recovering additional coulombs. It can be concluded that the MFC along with ANAMMOX-denitrifiers (MFC-ANA) had better substrate utilization rate than the heat-treated inoculum in control (MFC-CON), because of possibilities of the organic matter consumption by denitrifying bacteria and exo-electrogenic bacteria to reduce organic carbon as well as nitrogen content from the wastewater as discussed in subsequent sections.
Nitrogen removal
Nitrogen removal for both of the MFCs was observed for the first and last 30 cycles for investigating the applicability of ANAMMOX-denitrifing DeaMBBR biomass as exo-electrogenic consortia to improve the overall performance of MFC. After initial acclimatization stage, MFC-ANA with ANAMMOX-denitrifiers showed higher nitrogen removal efficiency in steady state period. The NH4+–N removal at first stage was found to be 89.3 ± 3.7% for MFC-ANA, around two times higher than MFC-CON (39.9 ± 4.1%). In case of MFC-ANA, NO3−-N removal efficiency was observed to be 73.8 ± 2.3%, which is almost three times higher than the MFC-CON (25.8 ± 7.1%). Total nitrogen (TN) removal efficiency of MFC-ANA was found to be 82.0 ± 3.0%, almost two times higher than the MFC-CON (36.0 ± 3%) during the first 30 cycles (Fig. 8). Highest specific nitrogen removal rate for MFC-ANA for first 30 cycles was 6.3 g N m−2 day−1. During the last 30 cycles average NH4+–N removal efficiency was found to be 78.0 ± 2.5% with a NO3−–N removal efficiency of 68.5 ± 3.0% for MFC-ANA (Fig. 8). Highest specific nitrogen removal rate for MFC-ANA for last 30 cycles was 5.7 g N m−2 day−1. Lower TN removal efficiencies, as compared to MFC-ANA, were earlier reported in ANAMMOX-denitrification MFC by Li et al. (2015), with average removal efficiencies for NH4+–N, NO2−–N and NO3−–N as 68%, 64% and − 25.2% (produced), respectively.
In general understanding, the ANAMMOX process follows the Eq. (4), where NH4+ is converted to N2 with NO2– as terminal electron acceptor. Intermediately, the NO2− is reduced to NO (Eq. 5) and then it reacts with NH4+ to produce N2H4 (Eq. 6), which finally oxidized to N2 (Eq. 7).
The N2H4 oxidation releases four low-potential electrons, which boost the Eqs. (6) and (7) and builds up the membrane potential to establish a proton motive force across the anammoxosome membrane driving the ATP synthesis in ANAMMOX consortia. However, the electrode-dependant ANAMMOX process hypothesized to form NH2OH instead of NO as per Eq. 9 (Shaw et al. 2019).
This alternate NH4+ oxidation process is coupled to EET, when working electrode is used as electron acceptor compared to NO2–. The alternative pathway suggested the presence of cytoplasmic electron carriers, such as dihydronicotinamide adenine dinucleotide (NADH) and ferredoxins, which can be oxidized at the cytoplasmic membrane using NADH dehydrogenase and/or formate dehydrogenase to directly reduce the menaquinone pool inside the cytoplasmic membrane as reported by Shaw et al. (2019). An upregulated protein similar to CymA (tetraheme c-type cytochrome) in Shewanella would then oxidize the reduced menaquinones, delivering electrons to highly upregulated periplasmic cytochromes and to a porin-cytochrome complex that spans the outer membrane of ANAMMOX consortia (Shaw et al. 2019). On or after this complex, electrons could be directly accepted by the insoluble extracellular electron acceptor (Shaw et al. 2019). This is what hypothesized to be the reason of enhanced electrochemical activity and better treatment of wastewater while using the ANAMMOX-denitrifier enriched biomass in MFC, which is further approved by using the microbiological analysis of the biomass received from the DeaMBBR and the anodic biofilm of MFC after the end of the operational period.
Cyclic voltammetry of MFCs
The MFCs were undergone electrochemical evaluations in anodic half-cell mode to understand the activity of ANAMMOX enriched DeaMBBR biomass in MFC. The CV analysis was adopted as a standard tool to investigate the redox reactions, which demonstrated well separated multiple redox current peaks with high current of the ANAMMOX attached carbon felt than the bare carbon felt (Fig. 9). The CV response of MFC with bare carbon felt (MFC-BARE) in fresh feeding solution did not show any current peaks due to its lack of bioelectrochemical activity in absence of exo-electrogenic microorganisms. For MFC-CON, the prominent oxidation peaks were found at around -0.10 V and + 0.50 V at forward scan with current response of 0.7 mA and 0.9 mA, respectively, and reduction peaks at -0.45 V and -0.80 V at reverse scan. For MFC-ANA the prominent oxidation peaks were found at + 0.10 V and + 0.60 V with current response of 1.7 mA and 2.7 mA, respectively along with a small peak at + 0.85 V. The first two peaks are characteristic to exoelectrogenic organisms’ c-type cytochromes presence as noted by Gregoire et al. (2014). It further confirms much higher electrical response of the MFC-ANA over the MFC-CON, endorsing the presence of exo-electrogenic consortia in the anodic biofilm of MFC-ANA. Hence, the CV results advocated much higher exo-electrogenic activity in the MFC-ANA followed by the MFC-CON and having the lowest activity by the MFC-BARE. The reason behind the higher energy recovery from the MFC-ANA was associated with DeaMBBR biomass based inoculum as discussed in power recovery section, which caused higher amount of organic matter and nitrogen removal from the target synthetic wastewater. Therefore, the MFC-ANA demonstrated a potential solution to higher removal efficiency of organic carbon as well as nitrogen, while providing reliable performance at low-cost of operation and maintenance to produce reusable quality treated water.
Microbiological analysis
Comparing initial inoculum and final DeaMBBR biomass, the ANAMMOX consortia proportion increased from 0 to 4% during DeaMBBR cultivation (Fig. S1). The family level distribution is also presented, which further revealed that the shifting of ANAMMOX consortia with percentages of abundances in DeaMBBR of 4 to 0.14% in MFC-ANA (Fig. S2). However, Pseudomonas strains abundance increased from 0 to 4.46% in MFC-ANA due to the presence of easily biodegradable organic carbon in MFC. Pseudomonas strains have been shown to achieve electricity production with 17.2% of bacterial proportion in earlier investigation (Park et al. 2014). The biomass of the DeaMBBR contained 1014 operational taxonomic units (OTUs), being decreased over time to about 246 OTUs (data not shown) in MFC-ANA.
The next generation sequencing (NGS) was performed to reveal the changes in microbial consortia before and after the long-term adaption of DeaMBBR biomass in MFC. Most attained sequences could not be attributed to certain species. Therefore, as species remained unknown for some of the sequences, the pyrosequencing results are given at genus level (Fig. S2). Furthermore, sequences that could not even be classified into a known genus were assigned into “unclassified” bacteria (Fig. S2).
The NGS was performed for anodic biofilm to get a very clear overview to identify the shifting of bacterial-archaea members beyond 0.1% abundance with maximum accuracy. The Nitrosomonas europaea and Pseudomonas caeni were absent in the enriched anodic biofilm in MFC-ANA, which were previously appeared in the DeaMBBR biomass. Notably, the anodic microbes in MFC-ANA are predominantly enriched with Trichococcus (14.92%), Methnomassiliicoccaceae (12.09%), Methanosarcina mazei (11.54%), Synergistaceae vadinCA02 (6.88%), Peptostreptococcaceae (6.24%) with below ~ 5% abundance including major exoelectrogens such as Pseudomonas (4.46%), Clostridium sensu stricto (3.71%). Important methylotrophic methanogens of the order Methanosarcinales and Methanosarcina mazei in large proportions could be developed due to the presence of CO2, acetate, or methylotrophic substrates as sole carbon and energy sources. In a recent investigation, it was shown that M. mazei was able to use molecular nitrogen as sole nitrogen source when growing on methanol and characterized with a single nitrogen fixation (nif) gene cluster (Veit et al. 2005).
Large proportion of Trichococcus genus (14.92%) is also agreed with the previous investigation showing the Trichococcus genus found in the anodic zone in constructed wetland with high ammonia concentration (Liu et al. 2009). The NGS study revealed that in the MFC-ANA, the microbial shifting occurred with g_Pirellulaceae_ Unclassified (0.01%), g_Flavobacteriaceae_Unclassified (0.03%), Candidatus Brocadia (0.1%), Acinetobacter Johnsonii (0.5%), Planctomycetacea (0.08%), Pseudomonas (4.46%), Pirellulaceae (0.001%), Flavobacterium (0.004%), Acinetobacter (2.3%), Planctomycetacea g unclassified (0.08%), Synergistaceae (6.8%), Rhodocyclales (0.1%), Pseudomonadacea (4.7%), Brocadiales (0.1%), Chitinophagaceae (0.06%), Methanospirillacea (2.2%), Moraxellaceae (2.3%), Nitrososphaeracea (0.02%), Planctomycetes (0.1%), Anaerolinaceae (0.02%), Desulfobulbaceae (0.001%), and Shewanellaceae (0.001%). Interestingly, low abundance of Geobacter (0.1%) and Shewanellaceae (0.001%), well known exoelectrogens (Logan et al. 2006) was found in MFC-ANA biofilm, probably due to Pseudomonadacea (4.7%) taking their role as exoelectrogen in the specific nitrogen-rich synthetic wastewater treated be the MFC-ANA. Therefore, the improvement of power production in case of MFC-ANA than MFC-CON could be attributed to the ANAMMOX population dominated by Trichococcus (14.92%) as denitrifying exo-electrogenic microbes that again supported higher nitrogen removal in case of MFC-ANA than MFC-CON. To conclude, the DeaMBBR biomass enriched in ANAMMOX-denitrifiers as exo-electrogenic inoculum in MFC demonstrated potential to achieve a high removal efficiency of both, organic carbon and nitrogen and provided appreciable performance for the treatment of nitrogen rich wastewater.
Conclusion
The applicability of ANAMMOX-denitrifier rich DeaMBBR biomass as inoculum in MFC was demonstrated to have exo-electrogenic behaviour for better performance of MFC. The MFC with this biomass (MFC-ANA) showed almost two times higher volumetric power density for first and last 30 cycles of operational period (9.5 and 6.0 W m−3, respectively) compared to MFC-CON (4.9 and 2.9 W m−3, respectively). The coulombic efficiency was also found to be higher in case of MFC-ANA (27.5 ± 1.7% and 27.0 ± 0.6%) compared to MFC-CON (17.7 ± 1.9% and 18.3 ± 0.5%) for the first 30 and last 30 cycles, respectively. MFC-ANA also achieved 84 ± 2% and 80 ± 2% of COD removal efficiency and 89 ± 4% and 73 ± 2% of total nitrogen removal efficiency during first 30 and last 30 cycles of operational period, respectively. Microbiological shifts in inoculum by the next generation sequencing showed the abundance of ANAMMOX bacteria was highest at the starting phase of MFC-ANA with 4% decreasing to 0.14% at the end of operation period of 469 days without significant drop in the nitrogen removal rate. This is because of the presence of effective electroactive consortia dominated by Trichococcus (14.92%) and Pseudomonas (4.46%) microorganisms that supports the higher exo-electrogenic activity and nitrogen removal efficiency in case of MFC-ANA than MFC-CON for treating nitrogen rich wastewater with bioenergy recovery.
References
APHA-AWWA-WPCF (1998) Standard Methods for the Examination of Water and Wastewater, 20th edn. American Public HealthAssociation American Water Works Association and WaterPollution Control Federation.
Bhowmick GD, Noori MT, Das I, Neethu B, Ghangrekar MM, Mitra A (2018) Bismuth doped TiO2 as an excellent photocathode catalyst to enhance the performance of microbial fuel cell. Int J Hydrogen Energy 43(15):7501–7510. https://doi.org/10.1016/j.ijhydene.2018.02.188
Bhowmick GD, Kibena-Põldsepp E, Matisen L, Merisalu M, Kook M, Käärik M, Leis J, Sammelselg V, Ghangrekar MM, Tammeveski K (2019a) Multi-walled carbon nanotube and carbide-derived carbon supported metal phthalocyanines as cathode catalysts for microbial fuel cell application. Sustain Energy Fuels 3:3525–3537. https://doi.org/10.1039/C9SE00574A
Bhowmick GD, Das S, Ghangrekar MM, Mitra A, Banerjee R (2019b) Improved wastewater treatment by combined system of microbial fuel cell with activated carbon/TiO2 cathode catalyst and membrane bioreactor. J Inst Eng Ser A 100:675–682. https://doi.org/10.1007/s40030-019-00406-7
Bhowmick GD, Das S, Verma HK, Neethu B, Ghangrekar MM (2019c) Improved performance of microbial fuel cell by using conductive ink printed cathode containing Co3O4 or Fe3O4. Electrochim Acta 310:173–183. https://doi.org/10.1016/j.electacta.2019.04.127
Chamchoi N, Nitisoravut S, Schmidt JE (2008) Inactivation of ANAMMOX communities under concurrent operation of anaerobic ammonium oxidation (ANAMMOX) and denitrification. Biores Technol 99:3331–3336. https://doi.org/10.1016/j.biortech.2007
Chatterjee P, Ghangrekar MM, Rao S (2016) Development of anammox process for removal of nitrogen from wastewater in a novel self-sustainable biofilm reactor. Biores Technol 218:723–730. https://doi.org/10.1016/j.biortech.2016.07.002
Chen CJ, Zhang M, Yu XL, Mei J, Jiang Y, Wang YQ, Zhang T, Tian C (2018) Effect of C/N ratios on nitrogen removal and microbial communities in the anaerobic baffled reactor (ABR) with an anammox-coupling-denitrification process. Wat Sci Technol 70:2338–2348. https://doi.org/10.2166/wst.2018.516
Ciesielski S, Czerwionka K, Sobotka D, Dulski T, Makinia J (2018) The metagenomic approach to characterization of the microbial community shift during the long-term cultivation of anammox-enriched granular sludge. J Appl Gen 59:109–117
Clauwaert P, Rabaey K, Aelterman P, De Schamphelaire L, Pham TH, Boeckx P, Boon N, Verstraete W (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360. https://doi.org/10.1021/es062580r
Di Domenico EG, Petroni G, Mancini D, Geri A, Palma LD, Ascenzioni F (2015) Development of electroactive and anaerobic ammonium-oxidizing (Anammox) biofilms from digestate in microbial fuel cells. Biomed Res Int 351014:1–10. https://doi.org/10.1155/2015/351014
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880. https://doi.org/10.1007/s00253-008-1360-2
Ferousi C, Lindhoud S, Baymann F, Kartal B, Jetten MSM, Reimann J (2017) Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr Opin Chem Biol 37:129–136. https://doi.org/10.1016/j.cbpa.2017.03.009
Ghadge AN, Jadhav DA, Pradhan H, Ghangrekar MM (2015) Enhancing waste activated sludge digestion and power production using hypochlorite as catholyte in clayware microbial fuel cell. Bioresour Technol 182:225–231
Gregoire KP, Glaven SM, Hervey J, Lin B, Tender LM (2014) Enrichment of a High-Current Density Denitrifying Microbial Biocathode. J Electrochem Soc 161:3049–3057
Guven D, Dapena A, Kartal B, Schmid MC, Maas B, van de Pas-Schoonen K, Sozen S, Mendez R, Op den Camp HJM, Jetten MSM, Strous M, Schmidt I (2005) Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl Environ Microbiol 71:1066–1071. https://doi.org/10.1128/AEM.71.2.1066-1071.2005
Jadhav DA, Ghangrekar MM (2015) Effective ammonium removal by anaerobic oxidation in microbial fuel cells. Environ Technol 36:767–775. https://doi.org/10.1080/09593330.2014.960481
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100:717–723. https://doi.org/10.1016/j.biortech.2008.07.041
Lee Y, Martin L, Grasel P, Tawfiq K, Chen G (2013) Power generation and nitrogen removal of landfill leachate using microbial fuel cell technology. Environ Technol 34:2727–2736. https://doi.org/10.1080/09593330.2013.788040
Li C, Ren H, Xu M, Cao J (2015) Study on anaerobic ammonium oxidation process coupled with denitrification microbial fuel cells (MFCs) and its microbial community analysis. Bioresour Technol 175:545–552. https://doi.org/10.1016/j.biortech.2014.10.156
Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, Iavarone AT, Ajo-Franklin CM, Portnoy DA (2018) A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562:140–144. https://doi.org/10.1038/s41586-018-0498-z
Liu C, Yamamoto T, Nishiyama T, Fujii T, Furukawa K (2009) Effect of salt concentration in anammox treatment using non-woven biomass carrier. Jour Biosci Bioeng 107:519–523. https://doi.org/10.1016/j.jbiosc.2009.01.020
Lotti T, Kleerebezem R, Hu Z, Kartal B, de Kreuk MK, van Erp Taalman C, Kruit Kip J, Hendrickx TLG, van Loosdrecht MCM (2015) Pilot-scale evaluation of anammox based mainstream nitrogen removal from municipal wastewater. Environ Technol 36(9):1167–1177. https://doi.org/10.1080/09593330.2014.982722
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. https://doi.org/10.1021/es0605016
Logan BE, Regan JM (2006) Microbial fuel cells—challenges and applications. Environ Sci Technol 40:5172–5180. https://doi.org/10.1021/es0627592
Mandel A, Zekker I, Jaagura M, Tenno T (2019) Enhancement of anoxic phosphorus uptake of denitrifying phosphorus removal process by biomass adaption. Int J Env Sci Technol. https://doi.org/10.1007/s13762-018-021
McDonald JE, Larsen N, Pennington A, Connolly J, Wallis C, Rooks DJ, Hall N, McCarthy AJ, Allison HE (2016) Characterising the canine oral microbiome by direct sequencing of reverse-transcribed rRNA molecules. PLoS ONE 11:e0157046
Noori MT, Bhowmick GD, Tiwari BR, Ghangrekar MM, Mukhrejee CK (2018) Application of low-cost Cu–Sn bimetal alloy as oxygen reduction reaction catalyst for improving performance of the microbial fuel cell. MRS Adv Mater Res Soc 3:663–668. https://doi.org/10.1557/adv.2018
Noori MT, Ghangrekar MM, Mukherjee CK (2016) V2O5 microflower decorated cathode for enhancing power generation in air-cathode microbial fuel cell treating fish market wastewater. Int J Hydrogen Energy 41:3638–3645. https://doi.org/10.1016/j.ijhydene.2015.12.163
Park T-J, Ding W, Cheng S, Brar MS, Ma APY, Tun HM, Leung FC (2014) Microbial community in microbial fuel cell (MFC) medium and effluent enriched with purple photosynthetic bacterium (Rhodopseudomonas sp.). AMB Express 4:1–8
Rikmann E, Zekker I, Tenno T, Saluste A, Tenno T (2018) Inoculum-free start-up of biofilm- and sludge-based deammonification systems in pilot scale. Int J Environ Sci Technol 15:133–148. https://doi.org/10.1007/s13762-017-1374-3
Shaw, D.R., Ali, M., Katuri, K. P., Gralnick, J.A., Reimann, J., Mesman, R., van Niftrik, L., Jetten, M.S.M., Saikaly, P.E., 2019. Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria. https://www.biorxiv.org/content/10.1101/855817v1. https://doi.org/10.1101/855817
Sheng S, Liu B, Hou XY, Liang Z, Sun XB, Du LF, Wang DP (2018) Effects of different carbon sources and C/N ratios on the simultaneous anammox and denitrification process. Int Biodeter Biodegradation 127:26–34. https://doi.org/10.1016/j.ibiod.2017.11.002
Strous M, Van Gerven E, Zheng P, Kuenen JG, Jetten MSM (1997) Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (anammox) process in different reactor configurations. Water Res 31:1955–1962. https://doi.org/10.1016/S0043-1354(97)00055-9
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66:5066–5072. https://doi.org/10.1128/aem.66.11.5066-5072.2000
Tharali AD, Sain N, Osborne WJ (2016) Microbial fuel cells in bioelectricity production. Front Life Sci 9:252–266
Zekker I, Rikmann E, Tenno T, Lemmiksoo V, Menert A, Loorits L, Vabamäe P, Tominga SM, Tenno T (2012) Anammox Bacteria enrichment and phylogenic analysis in moving bed biofilm reactors. Environ Eng Sci 29:946–950
Zekker I, Kivirüüt A, Rikmann E, Mandel A, Jaagura M, Tenno T, Artemchuk O, Rubin S, Tenno T (2019) Enhanced efficiency of nitritating-anammox SBR achieved at low decrease rates of oxidation-reduction potential. Env Eng Sci. https://doi.org/10.1089/ees.2018.0225
Zekker I, Raudkivi M, Artemchuk O, Rikmann E, Priks H, Jaagura M, Tenno T (2020) Mainstream-sidestream wastewater switching promotes anammox nitrogen removal rate in organic-rich, low-temperature streams. Environ Technol. https://doi.org/10.1080/09593330.2020.1721566
Zhang C, Liang P, Yang X, Jiang Y, Bian Y, Chen C (2016) Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens Bioelectron 81:32–53
Zhao H, Zhao J, Li F, Li X (2016) Performance of denitrifying microbial fuel cell with biocathode over nitrite. Front Microbiol 7:1–7. https://doi.org/10.3389/fmicb.2016.00344
Kosari SF, Rezania B, Lo KV, Mavinic DS (2014) Operational strategy for nitrogen removal from centrate in a two-stage partial nitrification–Anammox process. Environ Technol 35:1110–1120. https://doi.org/10.1080/09593330.2013.861872
van Loosdrecht MC, Salem S (2006) Biological treatment of sludge digester liquids. Water Sci Technol 53:11–20
Veit K, Ehlers C, Schmitz RA (2005) Effects of nitrogen and carbon sources on transcription of soluble methyltransferases in Methanosarcina mazei Strain Go¨1. J Bacteriol 187:6147–6154. https://doi.org/10.1128/JB.187.17.6147-6154.2005
Acknowledgements
This work was financially supported by the EU-India research co-funding mechanism INNO-INDIGO, by the Department of Biotechnology, Government of India (BT/IN/INNO-INDIGO/28/MMG/2015-16) for IIT Kharagpur, India. This research was financially supported by IUT20-16, BT/IN/INNO-INDIGO/28/MMG/2015-16) and Saraswati 2.0 for University of Tartu, Estonia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10532_2020_9907_MOESM1_ESM.pdf
Supplementary file1 (PDF 9 kb)—Figure S1. Autotrophic and heterotrophic N-removing culture variation in initial inoculum and DeaMBBRbiofilm samples.
10532_2020_9907_MOESM2_ESM.pdf
Supplementary file2 (PDF 28 kb)—Figure S2. Distribution of total strains in initial inoculum, DeaMBBR biomass, and anodic biofilm of MFCANA.
Rights and permissions
About this article
Cite this article
Zekker, I., Bhowmick, G.D., Priks, H. et al. ANAMMOX-denitrification biomass in microbial fuel cell to enhance the electricity generation and nitrogen removal efficiency. Biodegradation 31, 249–264 (2020). https://doi.org/10.1007/s10532-020-09907-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-020-09907-w