Abstract
Fuel and lubricating oil leaks produce an oily wastewater that creates an environmental problem for industries. Dissolved air flotation (DAF) has been successfully employed for the separation of oily contaminants. Collectors constitute an auxiliary tool in the DAF process that enhances the separation efficiency by facilitating the adhesion of the contaminant particles. The use of biosurfactants as collectors is a promising technology in flotation processes, as these biomolecules are biodegradable and non-toxic. In the present study, a biosurfactant was produced from the bacteria Pseudomonas aeruginosa UCP 0992 cultivated in 0.5% corn steep liquor and 4.0% vegetable oil residue in a bioreactor at 225 rpm for 120 h, resulting in a surface tension of 26.5 mN/m and a yield of 26 g/L. The biosurfactant demonstrated stability when exposed to different temperatures, heating times, pH values and salt and was characterised as a glycolipid with a critical micelle concentration of 600 mg/L. A central composite rotatable design was used to evaluate the effect of the crude biosurfactant added to a laboratory DAF prototype on the removal efficiency of motor oil. The isolated and formulated forms of the biosurfactant were also tested in the prototype after the optimisation of the operational conditions. The results demonstrated that all forms of the biosurfactant increased the oil separation efficiency of the DAF process by 65 to 95%. In conclusion, the use of biosurfactants is a promising alternative as an auxiliary tool in flotation processes for the treatment of oily waters generated by industrial activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drilling and petroleum extraction processes in the oil industry generates oily water, the disposal or reuse of which is only permitted after the removal of the oil and suspended solids to acceptable levels (Almeida et al. 2016). The reuse of effluents from industrial processes has become increasingly commonplace due to the economic and environmental appeal of this practice in the form of reduced production costs and aggregated value to the company in terms of sustainability (Yu and Han 2013; Rocha e Silva et al. 2015, 2018).
Gravitational separation is one of the main treatment methods for oily water and is performed by sedimenters, centrifuges, hydrocyclones, etc. However, oil removal levels only reach around 200 mg/L due mainly to the presence of emulsions, which are difficult to remove by simple gravitational methods and require auxiliary techniques, such as the addition of coagulants and surfactants (Rubio and Smith 2002; Yu and Han 2013). In this context, the flotation process has proven to be quite efficient, with the capability of removing a larger amount of oil in comparison to other methods (Albuquerque et al. 2012).
Flotation is a particle separation process based on adhesion to bubbles. The oil particle-bubble union has less density than the aqueous medium and floats to the surface of the flotation chamber, where the oil particles are removed (Bahadori et al. 2013; Rocha e Silva et al. 2018). Flotation was first used in mineral processing and has long been employed in solid/liquid separation processes that involve the use of stable foams to recover mineral particles (Peng et al. 2009). With the development of the industrial sector, the application of the flotation process was improved, leading to the emergence of dissolved air flotation (DAF), which involves the removal of a solute through adsorption, co-precipitation or occlusion in a floc transporter and subsequent release by the addition of an adequate tensioactive agent (Beneventi et al. 2009). With DAF, the water is saturated with pressurised (greater than 3 atm) air through a nozzle, forming bubbles that that reach the flotation chamber, which is at atmospheric pressure. The air becomes supersaturated and precipitates from the solution in the form of small bubbles (Babaahmadi 2010; Rocha e Silva et al. 2015).
The use of flotation as a separation method has been criticised due to the probable toxicity of the synthetic surfactants used as collectors in this process (Menezes et al. 2011; Rocha e Silva et al. 2018). Surfactants are compounds composed of amphipathic molecules with a hydrophilic portion and a hydrophobic portion that partition at the oil/water or air/water interface. The apolar portion is often a hydrocarbon chain, whereas the polar portion may be ionic (cationic or anionic), non-ionic or amphoteric. These characteristics enable surfactants to reduce surface and interfacial tension and form microemulsions, in which hydrocarbons can be solubilised in water or vice versa (Almeida et al. 2016). The development of completely biodegradable surfactants could alleviate concerns with regard to toxicity and increase the acceptance of this separation technology (Rocha e Silva et al. 2015). Thus, surfactants of a biological origin (biosurfactants) could be a viable option for increasing the use of flotation processes.
Recent studies show that microbial surfactants, which are metabolites produced by bacteria, yeasts and fungi, have the ability to solubilise and effectively mobilise adsorbed organic and inorganic compounds in contaminated soil and water (Santos et al. 2016). Biosurfactants offer excellent advantages, such as low toxicity, high solubility in the presence of organic and inorganic substances as well as stability at high temperatures, in a wide pH range, and in the presence of salt.
Biosurfactants have diverse chemical structures and are mainly classified as glycolipids and lipopeptides. These natural compounds can be produced from different substrates, especially renewable resources, such as vegetable oils and agricultural waste products (Santos et al. 2016; Soares da Silva et al. 2014). The selection of low-cost substrates is important to the overall economy of the process, as substrates account for 10 to 30% of the final cost of the product (Almeida et al. 2016). According to the literature, P. aeruginosa is one of the most widely studied microorganisms for biosurfactant production. Most biosurfactants produced by this bacterium have demonstrated the ability to reduce surface tension to around 28 mN/m. These compounds have also been applied in the remediation of water and soil contaminated with hydrocarbons due to their potential as environmental decontamination agents (Wittgens et al. 2017).
Considering the challenges described above and the need to improve currently known effluent treatment methods, the aim of the present study was to propose clean, efficient solutions for the treatment and control of oily water generated by industrial activities. For such, flotation with the use of a biosurfactant as a biodegradable collector was tested in a laboratory-scale DAF prototype.
Materials and methods
Materials
All reagents were of the highest purity available. Soybean oil waste was obtained from a restaurant in the city of Recife, Brazil, stored following the supplier’s recommendations, and used without any further processing. Corn steep liquor was obtained from Ingredion Brasil in the city of Cabo de Santo Agostinho, Brazil. A synthetic effluent was formulated with motor oil at a concentration of 15 g/L. The motor oil is commercially available for use in flex engines (gasoline, VNG and alcohol), type SAE 20W-50, with a synthetic guard (PETROBRAS) [paraffin-based lubricating oil (complex mixture of hydrocarbons) with performance enhancing additives].
Bacterial strain and preparation of seed culture
Pseudomonas aeruginosa UCP0992 was obtained from the culture collection of the Centre for Research in Environmental Sciences of the Catholic University of Pernambuco, Brazil, which is registered with the World Federation of Culture Collections. Cultures were maintained in nutrient agar slants at 4 °C. The strain from a 24-h culture was transferred to 50 ml of nutrient broth and the seed culture was prepared at a temperature of 28 °C, stirring at 150 rpm, and incubation for 10–14 h.
Fermentation media and conditions
Liquid fermentation was performed with a 3% cell suspension (optical density: 0.7) at 600 nm, corresponding to an inoculum of 107 colony-forming units/mL inoculated in a 2-L bioreactor (Tec-Bio-Plus, Tecnal Ltda., Brazil) with a working volume of 1.2 L, operating in a batch mode with controlled pH (6.8) and temperature (28 °C). The culture medium was composed of distilled water containing 4% soybean frying oil and 0.5% corn steep liquor as substrates. Fermentation was conducted at 225 rpm with aeration (1 vvm) for 120 h. Samples were collected at the end of the fermentation period to determine surface tension and the concentration of the surfactant.
Biosurfactant formulation
The broth was fermented and cells were removed through centrifugation (5000 g for 30 min). Following the addition of 0.2% potassium sorbate, the cell-free broth was stored at room temperature (28 °C) for 120 days (Freitas et al. 2016).
Surface tension and critical micelle concentration
Surface tension was determined in the cell-free broth obtained by centrifuging cultures at 5000×g for 20 min, using the ring method with a Sigma 700 Tensiometer (KSV Instruments LTD, Finland) at room temperature. The surface tension of the dilutions of isolated biosurfactant in distilled water was measured until reaching to a constant value (standard deviation less than 0.4 mN/m during ten successive measurements), which was considered the critical micelle concentration (CMC). The CMC was obtained by plotting surface tension against surfactant concentration and expressed as g/L of biosurfactant.
Isolation of biosurfactant
Cells were removed through centrifugation (5000×g) for 30 min and the biosurfactant was then extracted from the culture media. PH of the supernatant was adjusted to 2.0 using HCl (6.0 M) with the addition of an equal volume of CHCl3/CH3OH (2:1). The mixture was shaken vigorously for 15 min and allowed to set. After separation into phases, the organic phase was removed and the operation was repeated two more times. A rotary evaporator was used to concentrate the biosurfactant from pooled organic phases. A yellowish viscous product was obtained, dissolved in methanol and further concentrated by evaporation of the solvent at 45 °C (Silva et al. 2010).
Emulsifying activity with different hydrophobic compounds
The method described by Cooper and Goldenberg (1987) was used for the determination of the emulsification index (EI). An aliquot (2 mL) of a liquid hydrophobic compound (soybean oil, cotton seed oil and motor oil) was added to 2 mL of the cell-free broth in a test tube and vortexed at high speed for 2 min. The stability of the emulsion was determined after 24 h. The EI was calculated as the height of the emulsion layer divided by the total height of the mixture multiplied by 100.
Biosurfactant stability
The stability of the biosurfactant was investigated in different experiments using the cell-free broth. NaCl was added at concentrations of 2, 5 and 8% (w/v) and surface tension and emulsification activity were determined as described above. Surface tension and emulsification activity were also measured using the broth after being submitted to different temperatures (5, 70 and 120 °C) for 60 min. The effect of heating time on biosurfactant activity was investigated by maintaining the broth at 90 °C for 20, 40 and 60 min. The effect of pH (4, 8 and 10) on surface tension and emulsification was investigated by adjusting the broth with 6.0 M of either NaOH or HCl.
Characterisation of biosurfactant
Following re-dissolution of the extracted biosurfactant in deuterated chloroform (CDCl3), the respective 1H NMR spectra were recorded at 25 °C using an Agilent 300 Mz spectrometer operating at 300.13 MHz. Chemical shifts (δ) were given on the ppm scale relative to tetramethylsilane (TMS). Fourier transform infrared spectroscopy (FTIR) was also used to characterise the biosurfactant extract recovered from the supernatant of the P. aeruginosa UCP 0992 isolate. The FTIR spectrum (400 Perkin Elmer) with a resolution of 4 cm−1 was recorded from 400 to 4000 wavenumbers (cm−1). The hydrophobic portion was analysed using gas chromatography-mass spectrometry (Thermo Scientific Trace 1300—ISQ Single Quadrupole) in a TGMS-5 column (30 m × 0.25 mm; film thickness: 0.25 um). The column temperature was 60 °C for 3 min, ramped at 10 °C/min to 300 °C and held for 15 min. A 1-μL sample was injected. Helium was the carrier gas. The injector temperature was 300 °C and the detector temperature was 280 °C.
Dissolved air flotation system
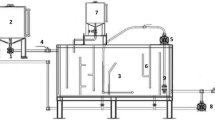
Three flotation tests were performed in an acrylic 15-L laboratory-scale DAF unit (Fig. 1). 50 g of motor oil were mixed in 10 L of water with the aid of a pump (1) in the storage tank (2) for 1 h with the aim of obtaining good oil in water saturation. An aquarium (3) was filled with 15 L of clean distilled water. After recirculation in the storage tank, the oily effluent was fed into the aquarium with the aid of the same pump (1), entering through a valve (4) [flow rate was monitored using an Arduino sensor (5)] and contaminating the clean water. The contaminated water came into contact with microbubbles formed by the injection of a controlled amount of air in the aspiration line of the pump (6). The interaction between the microbubbles and oil droplets dispersed at the base of the DAF gave rise to flocs composed of oil and air, which floated due to the lower density in comparison to the water, forming a layer of oily foam, which was collected in the collection section (7). To enhance the efficiency of the process, quantities of biosurfactant were dosed using a burette (8). A return pump (9) connected to the treated water section (10) promoted recirculation, returning the treated effluent to the storage tank, from which it could be collected (11) for subsequent analysis without coming into contact with the initial oily effluent contained in the storage tank. Each run of the DAF system lasted 5 min.
Experimental factorial design and response surface methodology
The effect of the biosurfactant on the separation efficiency of the DAF system was evaluated in experiments conducted following a central composite rotatable design (CCRD) and response surface methodology (RSM). A 24 CCRD was used to define the operating conditions of the prototype. The independent variables were the oily water flow rate, flow rate of water microbubbles, aqueous biosurfactant solution flow rate and biosurfactant concentration. The response variable was oil separation efficiency. The independent variables were coded on five levels. The complete design consisted of 28 experimental points, with four replications of the central points. Table 1 lists the coded levels of the independent variables used in the RSM design.
Separation efficiency was calculated according to Eq. (1)
in which \(\eta\) is the percentage of separation efficiency, while \(C_{I}\) and \(C_{O}\) are the oil concentrations in the inlet and outlet flow, respectively.
The experimental design was applied to the crude biosurfactant (cell-free broth obtained through centrifugation). After the determination of the optimum operating conditions, solutions of the isolated biosurfactant at concentrations established based on the CMC and solutions of the formulated biosurfactant were also tested to compare the efficiency of the different forms of the biosurfactant. Fifty-ml samples were collected from the flotation system and placed in glass flasks for the measurement of residual oil. Control experiments without the biosurfactant were conducted for the purposes of comparison.
Statistical analysis involved ANOVA, the determination of regression coefficients and the construction of graphs using the Statistica® program, version 8.0 (Statsoft Inc., USA).
Quantification of hydrophobic contaminant
Residual oil was extracted from the samples of synthetic oily effluent using the same volume of hexane (1:1, v/v). The mixture was shaken for 15 min and left at rest for the separation of the phases. The organic phase was removed and the operation was repeated two more times. The product contained in this phase was submitted to analysis in a spectrophotometer. The oil extracted from the water was analysed for its concentration after direct sampling by measuring absorbance at a wavelength of 330 nm using quartz cuvettes with a 10-mm path length in an UV–Vis spectrophotometer (SP-22- BIOSPECTRO). For the determination of the calibration curve, a standard solution of residual motor oil (5000 mg/L) was prepared in a 100-mL volumetric balloon. The solutions were diluted in n-hexane at concentrations ranging from 1 to 1000 mg/L obtained from the initial standard solution. N-hexane was used as the blank to calibrate the device. The solvent was analytical grade and adequate for the spectrophotometric equipment (Emmandi et al. 2014). All experiments were performed in triplicate at room temperature (27 °C) and mean values are reported.
Results and discussion
Biosurfactant production
Considering the potential of Pseudomonas aeruginosa as a biosurfactant producer when cultivated in soluble and/or insoluble substrates, the bacterium was initially cultivated in a bioreactor containing a medium formulated with 4% soybean oil residue and 0.5% corn steep liquor as the carbon and nitrogen sources, respectively. The biosurfactant produced was able to reduce the surface tension of water from 70 mN/m to around 26.5 mN/m and the yield was 26 g/L.
Silva et al. (2010) produced a biosurfactant from P. aeruginosa cultivated in a medium containing 3% glycerol that lowered surface tension to 27.6 mN/m and a yielded 6.5 g/L. In subsequent studies, Silva et al. (2013) found that P. cepacia cultivated in a medium with 2% waste frying oil and 3% corn steep liquor produced a biosurfactant that lowered surface tension to 26 mN/m and yielded 8 g/L. Aparna et al. (2012) produced a biosurfactant from Pseudomonas sp. 2B cultivated in a medium containing 1% molasses and found a surface tension of 30.14 mN/m and a yield of 4.97 g/L. Oliveira et al. (2009) produced a biopolymer from P. alcaligenes PCL cultivated in a medium supplemented with mineral salts and palm oil, which lowered surface tension to 28 mN/m and yielded 2.3 g/L. Monteiro et al. (2007) produced a biosurfactant from P. aeruginosa DAUPE 614 cultivated in a medium with glycerol and ammonium nitrate that lowered surface tension to 27.3 mN/m and yielded 3.9 g/L. Deepika et al. (2015) report the production of a biosurfactant in a salt medium supplemented with molasses that lowered surface tension to 33.03 mN/m and had a yield of 5.26 g/L. In another study, Deepika et al. (2016) produced a rhamnolipid from P. aeruginosa KVD-HR42 cultivated in a medium containing karanja oil and sodium nitrate that lowered surface tension to 30.14 mN/m, with a yield of 5.9 g/L. Nicolò et al. (2017) report a biosurfactant produced by P. aeruginosa L05 cultivated in a medium supplemented with mineral salts and 0.4% B. carinata oil that had a yield of 5.0 g/L. In comparison to these studies, the yield in the present investigation was substantially greater, demonstrating that the biopolymer obtained under the conditions specified herein is a promising agent for use on an industrial scale.
Biosurfactant stability
The use of biosurfactants as coadjutants in the treatment of oily waters requires products that remain stable under the necessary operational and environmental conditions (Almeida et al. 2016). Moreover, due to economic considerations in the oil industry, most biosurfactants require either whole-cell culture broths or crude preparations (Almeida et al. 2016). Therefore, the application of the biosurfactant from P. aeruginosa UCP0992 in its crude form without costly extraction and purification steps was investigated in stability studies as well as the subsequent flotation experiments. Table 2 displays the results of the stability tests using the cell-free broth containing the crude biosurfactant produced from P. aeruginosa when exposed to variations in temperature, pH, salinity and heating time.
Satisfactory surface tension values were found independently of the pH, temperature or salinity to which the biosurfactant was submitted. Lower surface tension values were found under alkaline conditions, with a slight increase in tension when the biosurfactant was submitted to acidic conditions. Similarly, emulsification indices were higher in alkaline media. Glycolipids exhibit optimum aqueous solubility at neutral to alkaline pH, which is attributed to their acidic nature (pKa = 5.6). The single free carboxylic acid group corresponding to the β-hydroxy fatty acid moiety makes glycolipids anionic. When the pH is increased from 5 to 8, the negative charge of the polar head increases, which is reflected greater aqueous solubility. If high concentrations of surface-active molecules are found, an increase of pH can change the morphology of the micelle structure formed above the CMC from lamellar to vesicular and finally to micelles (Abdel-Mawgoud et al. 2009).
Despite the relationship between surface tension and the emulsification index, the ability of a molecule to form a stable emulsion is not always associated with surface tension activity. Thus, a good biosurfactant is not necessarily a good emulsifier (Santos et al. 2016). Among the different oils tested, the best emulsification results were achieved with motor oil.
Emulsification indices ranged from 50 to 60% in the temperature and heating time tests, with a slight reduction in emulsifying capacity at higher temperatures and with a longer heating time. Lower temperatures and a shorter heating time also favored the reduction in surface tension.
With regard to the salt concentration, the biosurfactant maintained its surface tension reducing capacity in media with up to 8% NaCl, with a tendency toward a reduction in surface tension with the increase in salt concentration, whereas emulsification capacity decreased in the presence of salt. According to Helvac et al. (2004), electrolytes have a direct effect on carboxylate groups in glycolipids. The solution/air interface is negatively charged due to the ionised carboxylic acid groups in an alkaline medium, with strong repulsive forces between the molecules of the glycolipid. This negative charge is shielded by Na+ ions, leading to the formation of a close-packed monolayer and consequent reduction in surface tension.
The emulsification results are promising with regard to the application of the biosurfactant from P. aeruginosa in the treatment of oily waters, as lubricating oil is normally present in the composition of the industrial effluents to be treated.
Critical micelle concentration of biosurfactant
The CMC is defined the minimum concentration of biosurfactant required for the maximum reduction in surface tension and the formation of micelles. Thus, a low CMC indicates an efficient biosurfactant (Campos et al. 2013). In the present study, an increase in biosurfactant concentration led to a reduction in the surface tension of water from 70 to 26 mN/m, which was achieved at a concentration of 600 mg/l, with no further reduction occurring thereafter. This CMC differs from that reported for other glycolipids produced by P aeruginosa (Santos et al. 2016). Different CMC values may be due to differences in the purity and composition of the glycolipid as well as differences with regard to the bacterial strain, medium and cultivation conditions (Silva et al. 2014).
Characterisation of biosurfactant
Figure 2 displays the FTIR spectrum of the biosurfactant isolated from P. aeruginosa UCP 0992. The vibration extending from 3300 to 3500 cm−1 is characteristic of O–H stretching. The peak at 3000 to 2800 cm−1 is characteristic of aliphatic chains. The C=O group is evidenced around 1710 cm−1. The peaks at 1550 to 1400 cm−1 may be due to a C double bond and the peak at ~ 1260 cm −1 corresponds to the ketone group.
Figure 3 displays the 1H NMR spectrum. The signals of the biosurfactant from P. aeruginosa UCP 0992 between δ 0.60 and 1.6 ppm suggest aliphatic and methyl groups. The signals between δ 2.0 and 2.2 ppm indicate the aldehyde group. The signals at δ 3.5 ppm and between δ 4.6 and 4.8 ppm are attributed to hydroxyl groups and those between δ 5.0 and 5.4 ppm correspond to double bounds.
The GC–MS analysis of the biosurfactant was compared to data from the library. The chromatogram (Fig. 4) displays two evident peaks. The first (45.53%) indicates a structure related to the carbonyl group and the second (28.21%) indicates to a structure containing a hydroxyl group. The molar mass of the structure was between 150 and 200 (m/z).
The literature describes the characterisation of biosurfactants produced by species of Pseudomonas sp. using 1H NMR, FTIR spectroscopy and GC–MS analysis (Charles Oluwaseun et al. 2017; Varjani and Upasani 2017; Moussa et al. 2014). Soares da Silva et al. (2017) obtained similar results to those of the present study in the characterisation of a biosurfactant obtained from P. cepacia, with peaks in the 1H NMR spectrum between δ0.75 and 2.5 ppm indicating aliphatic and methyl groups and peaks between δ2.0 and 4.8 indicating carbonyl and hydroxyl groups, respectively. In the FTIR spectrum, the same authors report absorption bands between 2966 and 2863 cm−1 for aliphatic groups and a band at 1700 cm−1for C=O groups. In the present study, the 1H NMR, FTIR spectroscopy and GC–MS analyses of the biosurfactant produced by P. aeruginosa UCP 0992 indicate a glycolipid.
Use of biosurfactant in DAF system for treatment of oily water
Collectors/coagulants are normally used to enhance separation efficiency in flotation systems. These collectors may be surfactants. Tensioactive agents are added to the pressurised water in DAF to reduce the air/water surface tension in the saturator, which enhances the separation process (Menezes et al. 2011). Some microbial biosurfactants have been successfully used in the removal of heavy metals from an acidic mine effluent using flotation columns. Biosurfactants from species of Candida were able to remove more than 90% of metallic cations (Albuquerque et al. 2012; Menezes et al. 2011; Sarubbo et al. 2015). Another study demonstrated the potential of a biosurfactant produced from Candida sp in the removal of oil in a semi-industrial scale DAF prototype, which increased the separation efficiency from 80 to 98% (Rocha e Silva et al. 2015). Based on these promising results, the present study tested a new bacterial biosurfactant as a replacement for synthetic surfactants in a laboratory-scale DAF system using a factorial design.
Table 3 displays the removal rates achieved with the application of the CCRD matrix using the crude biosurfactant from P. aeruginosa UCP 0992 as a collector. Removal efficiency ranged from 82 to 95%. Multiple regression analysis using RSM was performed to adjust the response function to the experimental data and investigate the simultaneous influence of the four variables studied. The following quadratic polynomial equation best fit the data:
in which Y is separation efficiency (%) and X1, X2, X3 and X4 are coded values for oily water flow rate (mL/min), water + microbubble flow rate (mL/min), biosurfactant solution flow rate (L/min) and biosurfactant concentration (g/L), respectively. The optimal values from the CCRD were obtained by solving the regression equation and analysing the response surface contour plots. An oily water flow rate of 5.00 L/min, water + microbubble flow rate of 6.50 L/min, biosurfactant solution flow rate of 2.00 L/min and biosurfactant concentration of 0.35 g/L were the most favourable for the oil removal process using the biosurfactant, achieving a 94.88% separation rate (Run 8) (Table 3).
ANOVA was performed to test the significance and acceptability of the quadratic model (Table 4). The p-values and F-values (with 95% confidence interval) indicate that all terms were statistically significant (p < 0.05; F > 4) with the exception of the interactions between the variables oily water flow rate (X1) and biosurfactant concentration (X4) and between biosurfactant solution flow rate (X3) and biosurfactant concentration (X4). The low pure error (0.36) indicates good reproducibility of the experimental data.
The explained variance (R2 = 0.99595) ensured adequate fit (R = 0.9916) and the predicted versus actual values for separation efficiency determined by the model were very close to the straight line (Fig. 5), which indicates that the model is suitable for predicting separation efficiency under the experimental conditions.
The effects and statistical significance of the forecasting model variables were graphically illustrated using Pareto charts (Fig. 6). The linear term of factor X4 contributed significantly, i.e., an increase in biosurfactant concentration implies an increase in separation efficiency. The linear term of factor X2 also contributed positively to separation efficiency. However, the quadratic term of X2 led to a reduction in efficiency. The linear term of factor X1 contributed negatively to separation efficiency, but the quadratic term of factor X1 contributed positively to separation efficiency. Both linear and quadratic terms of X3 contributed positively to the increase in separation efficiency.
Figure 7 displays the fitted response surface plot for separation efficiency obtained by the model of Eq. (2). Graphic representation enables the visualisation of the relationship between the response and experimental levels of each variable and type of interactions between test variables in order to deduce the optimum conditions. Better separation efficiency was found when the oily water flow rate was maintained at its minimum level and interacted with the water + microbubble flow rate (Fig. 7a), biosurfactant solution flow rate (Fig. 7b), and biosurfactant concentration (Fig. 7c). However, these interactions were weak and did not produce well-defined regions in the graphs. The elliptic curve of the graph in Fig. 7d indicates a high degree of interaction between water + microbubble flow rate and biosurfactant flow rate, with greater separation efficiency at the level just above the central region of the response surface. The combination of greater microbubble flow and greater biosurfactant concentration led to maximum separation efficiency (Fig. 7e) and the parallelism found in the graph of the interaction demonstrates that it is possible to predict separation efficiency by varying only one of these variables. Figure 7f shows that high separation efficiency was also achieved when both biosurfactant solution flow and biosurfactant concentration were maintained at their maximum levels.
Response surface plots and contour plots for maximum separation efficiency generated using data in Table 4; Inputs = 28 experimental runs carried out under conditions established by CCRD; separation efficiency as function of a oily water flow rate and microbubble water flow rate; b biosurfactant solution flow rate and oily water flow rate; c biosurfactant concentration and oily water flow rate; d microbubble water flow rate and biosurfactant solution flow rate; e biosurfactant concentration and microbubble water flow rate; f biosurfactant concentration and biosurfactant solution flow rate
Flotation experiments following optimisation of operational conditions
After the optimisation of the operational conditions, new experiments were conducted replacing the crude biosurfactant with the isolated and formulated forms to determine the influence of the extraction process and addition of the chemical conservative potassium sorbate on the efficiency of the biomolecule. The results demonstrate that the isolated and formulated biosurfactants were capable of removing 95.89% and 94.75% of the oil, respectively. Control experiments (without the biosurfactant) conducted with the optimised operational conditions resulted in an oil removal rate of about 75% using the microbubble process alone, demonstrating the importance of adding the biosurfactant from P. aeruginosa UCP 0992 as a collector in the separation process.
Considering the 94.88% removal rate with the crude biosurfactant, the formulated product (addition of the conservative) could be advantageous, as this form of the biosurfactant maintains stable tensioactive properties when stored for a period of 120 days, which demonstrates its potential for commercial use. On the other hand, isolated biosurfactant may be more interesting in terms of demand and logistics, when it is not possible to store large volumes of formulated biosurfactant. In this case, the isolated biosurfactant becomes more attractive, although it represents an additional cost, as the final price of the biosurfactant will be higher due to the inclusion of an extraction step.
Figure 8 shows the difference in the quality of the emulsion formed with and without the biosurfactant, demonstrating that the emulsification and consequent removal of the oil is substantially improved in the presence of the biosurfactant (Fig. 8b).
The efficiency of the flotation-biosurfactant system is also evidenced when a concentration of oil of 15 g/L is used, which is much higher than the maximum limit permitted by Brazilian law (20 ppm) (CONAMA 2011). This suggests that the DAF-biosurfactant combination will ensure adequate treatment of effluents under actual conditions of the treatment of industrial oily effluents and enable the reuse of the clean water. In a study conducted by Watcharasing et al. (2009), a 60% oil removal rate was found in a flotation system using synthetic surfactants. Thus, the biosurfactant tested herein achieved greater separation efficiency.
It is important to stress that the biosurfactant from P. aeruginosa UCP 0992, besides being used in its crude form, was produced in a culture medium prepared only with industrial waste products, which further reduces the costs. As substrates used in the production of biosurfactants account for 20 to 30% of the production cost, the final price of the biosurfactant from P. aeruginosa was calculated based on the price of pure biosurfactants available on the market, with a final price estimated to be around 0.6 to 1.5 €/kg (Hazra et al. 2012).
The low cost of the proposed DAF process is evident by the small amount of biosurfactant (350 ppm) required to achieve maximum efficiency (run number 8 in Table 3). Moreover, the DAF system is highly efficient, simple to operate and requires a low investment, as the implantation cost is approximately tenfold lower that that required for other methods, such as separation by continuous centrifugation. As many industries generate huge amounts of oily waters that require adequate treatment before being discarded or reused, the benefits of treating oily waters with the system developed herein demonstrates its considerable market potential.
Conclusion
The results obtained in the present study indicate that the efficiency of the DAF process for the treatment of oily water can be enhanced with the use of the biosurfactant produced by P. aeruginosa, as demonstrated by the significant increase in the oil removal rate. Thus, the system presented herein is a favourable strategy for decontaminating oily industrial effluents. Besides increasing the separation efficiency of the DAF system, the use of biosurfactants is a sustainable practice from the environmental standpoint, as it enables the use of industrial waste products, which are sometimes available from a single industrial complex. Moreover, the bench-scale prototype enables an analysis of characteristics that are fundamental to the establishment of parameter levels (set points) and equipment control strategies.
References
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NAH (2009) Characterization of rhamnolipid produced by Pseudomonas aeruginosa isolate Bs20. Appl Biochem Biotechnol 157:329–345. https://doi.org/10.1007/s12010-008-8285-1
Albuquerque CF, Luna-Finkler CL, Rufino RD et al (2012) Evaluation of biosurfactants for removal of heavy metal ions from aqueous effluent using flotation techniques. Int Rev Chem Eng 4:156–161
Almeida DG, Soares da Silva R, Luna JM et al (2016) Biosurfactants: promising molecules for petroleum biotechnology advances. Front Microbiol 7:1718. https://doi.org/10.3389/fmicb.2016.01718
Aparna A, Srinikethan G, Smitha H (2012) Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B 95:23–29. https://doi.org/10.1016/j.colsurfb.2012.01.043
Babaahmadi A (2010) Dissolved air flotation: numerical investigation of the contact zone on geometry, multiphase flow and needle valves. Master’s thesis, Dept. Civil and Environm. Eng., Chalmers University of Technology, Göteborg, Sweden
Bahadori A, Clark M, Boyd B (2013) Essentials of water systems design in the oil, gas, and chemical processing industries. Springer, New York
Beneventi D, Allix J, Zeno E et al (2009) Simulation of surfactant contribution to ink removal selectivity in flotation deinking lines. Sep Purif Technol 64:357–367. https://doi.org/10.1016/j.seppur.2008.10.033
Campos JM, Montenegro Stamford TL, Sarubbo LA et al (2013) Microbial biosurfactants as additives for food industries. Biotechnol Prog 29:1097–1108. https://doi.org/10.1002/btpr.1796
Charles Oluwaseun AC, Julius Kola O, Mishra P et al (2017) Characterization and optimization of a rhamnolipid from Pseudomonas aeruginosa C1501 with novel biosurfactant activities. Sustain Chem Pharm 6:26–36. https://doi.org/10.1016/j.scp.2017.07.001
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Conselho Nacional Do Meio Ambiente—CONAMA. Dispõe sobre as condições e padrões de lançamento de efluentes. Resolução n. 430. de 13 de Maio de 2011, Brasil
de Silva CFS, Rufino RD, Luna JM et al (2013) Enhancement of biosurfactant production from Pseudomonas cepacia CCT6659 through optimisation of nutritional parameters using response surface methodology. Tenside Surfactants Deterg 50:137–142. https://doi.org/10.3139/113.110241
de Silva R, Almeida DG, Rufino RD et al (2014) Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci 15:12523–12542. https://doi.org/10.3390/ijms150712523
Deepika KV, Ramu Sridhar P, Bramhachari PV (2015) Characterization and antifungal properties of rhamnolipids produced by mangrove sediment bacterium Pseudomonas aeruginosa strain KVD-HM52. Biocatal Agric Biotechnol 4:608–615. https://doi.org/10.1016/j.bcab.2015.09.009
Deepika KV, Kalam S, Ramu Sridhar P et al (2016) Optimization of rhamnolipid biosurfactant production by mangrove sediment bacterium Pseudomonas aeruginosa KVD-HR42 using response surface methodology. Biocatal Agric Biotechnol 5:38–47. https://doi.org/10.1016/j.bcab.2015.11.006
Emmandi R, Sastry MIS, Patel MB (2014) Low level detection of benzene in food grade hexane by ultraviolet spectrophotometry. Food Chem 161:181–184. https://doi.org/10.1016/j.foodchem.2014.04.031
Freitas BG, Brito JGM, Brasileiro PPF et al (2016) Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Front Microbiol 7:1646. https://doi.org/10.3389/fmicb.2016.01646
Hazra C, Kundu D, Chaudhari A (2012) Biosurfactant Assisted bioaugmentation in bioremediation. In: Satyanarayana T, Johri BN, Prakash A (eds) Microorganisms in environmental management: microbes and environment. Springer, New York, pp 631–664
Helvac SS, Peker S, Özdemir G (2004) Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloids Surf B 35:225–233. https://doi.org/10.1016/j.colsurfb.2004.01.001
Menezes CTB, Barros EC, Rufino RD et al (2011) Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage, using the dissolved air flotation technique. Appl Biochem Biotechnol 163:540–546. https://doi.org/10.1007/s12010-010-9060-7
Monteiro SA, Sassaki GL, de Souza LM et al (2007) Molecular and structural characterization of the biosurfactant produced by Pseudomonas aeruginosa DAUPE 614. Chem Phys Lipids 147:1–13. https://doi.org/10.1016/j.chemphyslip.2007.02.001
Moussa TAA, Mohamed MS, Samak N (2014) Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz J Chem Eng 31:867–880. https://doi.org/10.1590/0104-6632.20140314s00002473
Nicolò MS, Cambria MG, Impallomeni G et al (2017) Carbon source effects on the mono/dirhamnolipid ratio produced by Pseudomonas aeruginosa L05, a new human respiratory isolate. N Biotechnol 39:36–41. https://doi.org/10.1016/j.nbt.2017.05.013
Oliveira FJS, Vazquez L, de Campos NP, de Franca FP (2009) Production of rhamnolipids by a Pseudomonas alcaligenes strain. Process Biochem 44:383–389. https://doi.org/10.1016/j.procbio.2008.11.014
Peng JF, Song YH, Yuan P et al (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640. https://doi.org/10.1016/j.jhazmat.2008.04.061
Rocha e Silva FCP, Rocha e Silva NMP, de Moura AE et al (2015) Effect of biosurfactant addition in a pilot scale dissolved air flotation system. Sep Sci Technol 50:618–625. https://doi.org/10.1080/01496395.2014.957319
Rubio J, Smith RW (2002) Overview of flotation as a wastewater treatment technique. Miner Eng 15:139–155. https://doi.org/10.1016/S0892-6875(01)00216-3
Santos DKF, Rufino RD, Luna JM et al (2016) Biosurfactants: multifunctional biomolecules of the 21st Century. Int J Mol Sci 17:401. https://doi.org/10.3390/ijms17030401
Sarubbo LA, Rocha RB Jr, Luna JM et al (2015) Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem Ecol 31:707–723. https://doi.org/10.1080/02757540.2015.1095293
Silva SNRL, Farias CBB, Rufino RD et al (2010) Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf B 79:174–183. https://doi.org/10.1016/j.colsurfb.2010.03.050
Silva EJ, Chaprão MJ, Silva IA et al (2018) Biosurfactant application as alternative collectors in dissolved air flotation system. Chem Eng Trans 64:547–552. https://doi.org/10.3303/CET1864092
Soares da Silva de CF R, Rufino RD, Luna JM et al (2013) Enhancement of biosurfactant production from Pseudomonas cepacia CCT6659 through optimisation of nutritional parameters using response surface methology. Tenside Surfactants Deterg 50:137–142. https://doi.org/10.3139/113.110241
Soares da Silva de CF R, Almeida DG, Meira HM et al (2017) Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal Agric Biotechnol 1:2. https://doi.org/10.1016/j.bcab.2017.09.004
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol 232:389–397. https://doi.org/10.1016/j.biortech.2017.02.047
Watcharasing S, Kongkowit W, Chavedej S (2009) Motor oil removal from water by continuous froth flotation using extended surfactant: effects of air bubble parameters and surfactant concentration. Sep Pur Technol 70:179–189. https://doi.org/10.1016/j.seppur.2009.09.014
Wittgens A, Kovacic F, Müller MM et al (2017) Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl Microbiol Biotechnol 101:2865–2878. https://doi.org/10.1007/s00253-016-8041-3
Yu L, Han M (2013) A review of treating oily wastewater. Arab J Chem 10:S1913–S1922. https://doi.org/10.1016/j.arabjc.2013.07.020
Acknowledgements
This study was funded by the following Brazilian fostering agencies: State of Pernambuco Foundation for the Support of Science and Technology (FACEPE), the Research and Development Program of the National Agency of Electrical Energy (ANEEL), the Candeias Energy Company (Global Group), the Research Project (code PD-06961-0005/2016), the National Council for Scientific and Technological Development (CNPq) and the Coordination for the Advancement of Higher Level Education Personnel (CAPES). The authors are grateful to the laboratories of the Centre for Sciences and Technology of the Catholic University of Pernambuco (UNICAP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, E.J., Silva, I.A., Brasileiro, P.P.F. et al. Treatment of oily effluent using a low-cost biosurfactant in a flotation system. Biodegradation 30, 335–350 (2019). https://doi.org/10.1007/s10532-019-09881-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-019-09881-y