Abstract
To determine the concentration of soluble 1,4-dioxane during biodegradation, a new method using of high-performance liquid chromatography equipped with a hydrophilic interaction chromatography column was developed. The developed method enabled easy and rapid determination of 1,4-dioxane, even in saline medium. Microbes capable of degrading 1,4-dioxane were selected from the seawater samples by the seawater-charcoal perfusion apparatus. Among 32 candidate 1,4-dioxane degraders,, strain RM-31 exhibited the strongest 1,4-dioxane degradation ability. 16S rDNA sequencing and the similarity analysis of strain RM-31 suggested that this organism was most closely related to Pseudonocardia carboxydivorans. This species is similar to Pseudonocardia dioxanivorans, which has previously been reported as a 1,4-dioxane degrader. Strain RM-31 could degrade 300 mg/L within 2 days. As culture incubation times increasing, the residual 1,4-dioxane concentration was decreasing and the total protein contents extracted from growth cells were increasing. The optimum initial pH of the broth medium and incubation temperature for 1,4-dioxane degradation were pH 6–8 and 25 °C. The biodegradation rate of 1,4-dioxane by strain RM-31 at 25 °C in broth medium with 3 % NaCl was almost 20 % faster than that without NaCl. It was probably a first bacteria from the seawater that can exert a strong degrading ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater contaminant, 1,4-dioxane, is one of the most important environmental standard compounds; accordingly, its use has been restricted and limits for its levels in public and groundwater have been set. The chemical structure of 1,4-dioxane is characterized as a simple cycle ether and is very hydrophilic. This compound is generally used as an organic solvent in cosmetics, agricultural chemicals, or wax. However, it does not degrade easily once released into the environment and often dissolves in water. Therefore, the Japanese Ministry of the Environment has listed 1,4-dioxane as an environmental standard compound, and it has been limited to 0.05 mg/L in public water and groundwater since 2012.
The Japanese Ministry of the Environment defined the analytical method to determine the concentration of 1,4-dioxane using gas chromatography/mass spectrometry; however, its measurement using this method is hindered by several factors. Specifically, it requires a gas chromatography/mass spectrometry with a head space injection tool, which makes the analysis too complicated to obtain an accurate concentration result. Moreover, use of the activated carbon column for extraction of 1,4-dioxane is expensive and time consuming. Therefore, more accurate and easier methods for the measurement of 1,4-dioxane are required.
The gas chromatograph (GC) equipped with FID (flame ionization detector) was used for 1,4-dioxane monitoring during microbial growth (Sei et al. 2013; Gedalanga et al. 2014). However, using medium broth containing the high concentration of NaCl such as seawater, it might be difficult to keep determining an accurate amount of 1,4-dioxane. One possible method for the measurement of 1,4-dioxane is use of high-performance liquid chromatography (HPLC) equipped with hydrophilic interaction chromatography (HILIC). The HILIC column is generally used in the pharmaceutical field to separate hydrophobic compounds and exclude hydrophilic materials.
To select microbes that degrade 1,4-dioxane from some fields, a mixture of 1,4-dioxane and medium broth inoculated with sampling seawater were cultured in an incubation box for various lengths of times. However, the amounts of 1,4-dioxane must be determined in samples from each type of medium after each incubation period when using this method. Therefore, it is necessary to develop an easy and rapid method for determination of 1,4-dioxane (Sugiura 1997).
Environmental pollution by chemical compounds such as 1,4-dioxane has begun influencing marine systems. Several artificial chemical compounds were detected from the sea water. (U.S.EPA report 2002; Altenburger et al. 2003; Zwart and Posthuma 2005; Shindo 2013). Hence, it is important to develop methods to measure biodegradation of chemical compounds such as 1,4-dioxane in marine systems and clarify the presence of 1,4-dioxane degrading microbes in such systems so that they can be applied for bioremediation.
In this study, a novel HPLC method for determination of 1,4-dioxane and a seawater-charcoal perfusion apparatus for selection of microbes that degrade this compound from seawater was developed. Moreover, a microbe capable of degrading 1,4-dioxane was identified and characterized.
Materials and methods
Culture medium for microbes degrading 1,4-dioxane
BSM (Basal Salt Medium) was used as basal medium for microbes in seawater. This medium is one of the inorganic synthetic medium used for isolation of marine microbes, and characterized as an artificial seawater containing 3 % NaCl (Quatrano and Caldwell 1978; Gauthier et al. 1992).
The medium consisted of (NH4)2SO4 (1 g/L), NaCl (30 g/L), Mg2SO4 (0.5 g/L), KCl (0.3 g/L), K2HPO4 (1.5 g/L), CaCl2 (0.2 g/L), Vitamin 5 mixture (1 mL/L), and Trace Elements (10 mL/L), as well as 1,4-dioxane (1000 mg/L) as the sole carbon source. All reagents containing 1,4-dioxane were of the highest grade manufactured by Wako Pure Chemical Industries, Ltd (Tokyo). The vitamin 5 mixture consisted of cyanocobalamin (0.02 g/L), calcium pantothenate (0.025 g/L), p-aminobenzoic acid (0.5 g/L), nicotinic acid (0.1 g/L), and pyri1,4-dioxaneine hydrochloride (0.25 g/L). The trace elements consisted of EDTA (0.5 g/L), MgSO4·7H2O (2 g/L), FeSO4·7H2O (0.2 g/L), ZnSO4·7H2O (0.01 g/L), MnSO4·7H2O (0.005 g/L), H3BO3 (0.03 g/L), CoSO4·7H2O (0.024 g/L), CuSO4·7H2O (0.005 g/L), Na2MoO4 (0.005 g/L), and Ca(OH)2 (0.05 g/L). BSM broth medium with 1.5 % (w/v) agar was used for plate culture.
Quantitative determination of 1,4-dioxane
Amounts of 1,4-dioxane were determined by spectrophotometric detection at 200 nm using an HPLC system (Hewlett Packard Series 1100 and Hitachi Co. Ltd, Tokyo). The amounts (100 mg/L) of 1,4-dioxane in the pure-water solution were separated by HPLC equipped using an ODS column according to the method reported by Santo Scalia et al. (1990). However, when 1,4-dioxane was dissolved in BSM broth medium, the 1,4-dioxane could not be separated. Consequently, another method was necessary to identify 1,4-dioxane in BSM broth.
To determine the concentration of 1,4-dioxane during the biodegradation process, a new method using HPLC equipped with an HILIC (hydrophilic interaction chromatography) column was developed. Acetonitrile-0.1 % phosphate solvent system was applied as the eluent at 1 mL/min. Following injection of 40 µL of sample solution, 1,4-dioxane was separated by HPLC equipped with the HILIC column (TSKgel Amide-80 HR 4.6 i.d. 9 250 mm, 5 µm particle size; Tosoh Corporation, Tokyo, Japan) or ODS column (Wakopak Wakosil-II 5C18 RS, Wako, Japan) at 25 °C. BSM broth containing various amounts of 1,4-dioxane (5, 20, 50, 100, 250, 500, and 1000 mg/L) was used for calibration. All measurements were conducted in triplicate.
Use of CH3CN solution as the mobile phase during HPLC enables separation of hydrophilic compounds from mixtures of compounds containing hydrophobic groups. The hydrophobic compounds are adsorbed onto the hydrophobic support of the HILIC, while the hydrophilic compounds are eluted with solution.
Method for selection of microbes degrading 1,4-dioxane from seawater using a seawater-charcoal perfusion apparatus
To select microbes degrading 1,4-dioxane, seawater samples were obtained from four coastal areas of the Okinawa Islands, Japan. These coastal areas were not contaminated with 1,4-dioxane because the concentrations were not detected (<0.05 mg/L). The reason why the seawater of Okinawa area was selected is because the Okinawa seawater is so clean to have strong abilities to remediate pollutant materials. The sea water samples were placed in the perfusion apparatus (Fujiwara Seisakusyo Co., Japan) as shown in Fig. 1, which is a modification of the solid-charcoal perfusion method (Takagi et al. 2009; Sakakibara et al. 2011). Autoclaved Charcoal A130 (10 g, grain size 2 to 4 mm, BET specific surface area of 130 m2/g, pH 6.5; Toyo Denka Kogyo, Kochi, Japan) as a microhabitat and adsorbent of 1,4-dioxane was dipped into each seawater sample for 12 h. The dipped charcoal was set in a perfusion apparatus (Fujiwara Scientific Co., Ltd. Tokyo, Japan). The enrichment culture was carried out under dark conditions at 25 °C by circulating 250 ml of BSM containing 1000 mg/L 1,4-dioxane. The medium was circulated with air lift using an air pump through charcoal layer in the perfusion apparatus. The perfusion rate of the medium was adequately controlled by the air pump, and smooth leaching was maintained. The 1,4-dioxane concentration in culture fluids was monitored by HPLC during incubation. When the 1,4-dioxane concentration in culture fluids was decreased by almost 100 mg/L, the medium was replaced.
When circulating the medium amended with 1,4-dioxane, seawater-charcoal is able to remove 1,4-dioxane by both bio-degradation and adsorption. However, because the amount of 1,4-dioxane physically absorbed with charcoal would be attain equilibrium with a certain value during incubation, the decreasing rate of 1,4-dioxane by biodegradation should be able to compare.
After 1 month of incubation, the charcoal was detached from the equipped perfusion apparatus and the enriched charcoal (1 g) was crushed and suspended in 50 mM phosphate buffer (pH 7.0); the same buffer was added to the suspension to formulate a 10−4-fold dilution. The diluted suspension was then inoculated on a BSM agar plate containing 1000 mg of 1,4-dioxane L−1. After 10 days incubation at 25 °C, the colonies formed on the agar plate were isolated. Each colony detected on the culture plate was then inoculated and aerobically pre-incubated at 25 °C by shaking with 5 mL BSM broth medium.
Degradation of 1,4-dioxane
The degradation test was performed using 20-mL aliquots of BSM broth medium (final 1,4-dioxane concentration: 1000 mg/L) after inoculation with 5 mL of pre-incubation broth as described above. Cultures were incubated statically at 25 °C in the absence of light for 5 days. At each sampling period, 0.1 mL of the culture broth was homogenized with 0.9 mL of acetonitrile and then centrifuged at 1500×g for 10 min. The residual 1,4-dioxane concentration in the supernatant was determined by the new HPLC method. All experiments in the current study were performed in triplicate. Also, to minimize the possibilities of analytical errors, evaporation, biosorption etc., killed RM31 (a selected microbe) controls were carried out by the same way except using addition with 5 mL of pre-incubation broth autoclaved at 121 °C for 20 min, instead of inoculation with 5 ml of pre-incubation broth.
To investigate 1,4-dioxane degradation by the selected microbes, the effects of BSM broth medium at different initial pHs (3–10), incubation temperatures (20–30 °C), and the presence or absence of 3 % NaCl were investigated. All experiments in the current study were performed in triplicate.
As another 1,4-dioxane degradation test, furthermore, to clarify the relationship between the 1,4-dioxane degradation and cell growth of strain RM31 the residual 1,4-dioxane concentration in broth medium and total proteins extracted from cells grown in 5 mL of incubation broth were determined. The determination of total protein content was carried out instead of the measurement of cell-growth, because the strain RM-31 visually appeared to grow very slowly, as well as to have formed hydrophobic colonies (flock) on the surface of broth medium or on the medium glass, it was difficult to measure their cell yields by simple spectrophotometry from broth samples obtained at each incubation times. Therefore, the 5-mL BSM broths with almost 800 mg/L concentration of 1,4-dioxane inoculated strain RM-31 was incubated for 2–3 days at 25 °C. After the broth tubes were centrifuged at each incubation times for 0, 2, and 3 days at 3000 rpm for 5 min respectively, each pellets obtained were washed two times by 5-mL of 100 mM phosphate buffer (pH 7.0). On the other hand, the residual 1,4-dioxane concentration in the supernatant was measured as described above. The final pellets were quickly and vigorously ground with some sea sand (particle size 300–600 µm) (Wako Pure Chemical Industries Ltd., Tokyo) and 0.5-mL of the chilled same buffer. After centrifugation at 3000 rpm, the supernatant protein contents were measured. The protein contents were determined by Lowry’s method (1951), and compared with a standard curve of bovine serum albumin. All experiments in the current study were performed in triplicate.
Microscopic observation of microbes by scanning electron microscopy (SEM)
Microscopic examination was conducted using a scanning electron microscope (JSM-5310LVB, JEOL, Japan). Colonies on the BSM-agar plate were stored in a silica-gel desiccator for 48 h prior to gold sputter coating (JFC-1200, JEOL, Japan), then observed under low vacuum. The working depth for imaging was 20 mm and the beam current was 12.0 kV. Photos were graphed by Charge Coupled Device (CCD) Camera attached with the electron microscope.
Identification of microbes degrading 1,4-dioxane
The DNA of microbes degrading 1,4-dioxane was sequenced by Techno-Suruga Laboratory Co., Ltd. (Shizuoka, Japan). The full 16S rDNA sequences were compared with reference sequences using BLAST similarity searches (Altschul et al. 1990). The sequences were aligned using MAFFT (http://mafft.cbrc.jp/alignment/server/). Evolutionary relationships were inferred using the neighbor-joining method (Saitou and Nei 1987). The phylogenetic tree was drawn using the NJplot WIN95 software (Perriere and Gouy 1996).
Statistical analysis
The data were expressed as means with standard errors (SE) and analyzed by Tukey–Kramer’s multiple comparison post hoctest. The anlysis was carried out with SSRI (Version 1.03 for Windows, SSRI Co. Ltd. Tokyo). Differences were mainly considered to be significant at P < 0.05.
Results and discussion
Novel determination of 1,4-dioxane using HPLC equipped with an HILIC column
Use of HPLC equipped with an HILIC column enabled isolation of the peak corresponding to 1,4-dioxane from the medium broth. However, the baseline gradually became higher as the retention time increased. When determination of 1,4-dioxane was conducted continuously at an interval of 15 min per sample, the decline of the baseline became worse, and it became very difficult to determine the amount of 1,4-dioxane (data not shown). To solve this problem, the solvent gradient system on HPLC was developed. During HPLC, the acetonitrile concentration in the solvent decreased from 95 to 60 % for 16 min. Then, it has been returned to initial condition back by computer program. As a result, a solvent system with acetonitrile phosphate buffer containing 95 % acetonitrile was used. This system enabled measurement of the 1,4-dioxane concentration in the medium broth within 20 min in one cycle, as shown in Fig. 2.
Use of this solvent gradient system on an HPLC equipped with an HILIC column continuously enabled certain amounts of 1,4-dioxane in the BSM broth medium to be determined. One determination cycle per sample was almost 15 min. The detection limit was 0.5 to 1 mg/L. The calibration of 1,4-dioxane in medium broth by HPLC equipped with a hydrophilic interaction column resulted in a 0.996 correlation coefficient on triplicated samples. Therefore, this method was used for subsequent analyses. The developed method enabled easy and rapid determination of 1,4-dioxane and selection of 1,4-dioxane-degrading microbes.
Isolation of 1,4-dioxane-degrading microbes from surface seawater using a seawater-charcoal perfusion method
As shown in Fig. 3, the concentration of 1,4-dioxane in BSM broth medium repeatedly changed from almost 1000 mg/L to 100 mg/L during incubation in the perfusion apparatus. The rate of 1,4-dioxane reduction during incubation depended on the individual seawater samples, with seawater from site D exhibiting the fastest reduction. Therefore, organisms were isolated from the charcoal attached to the perfusion apparatus used to culture water from site D. A total of 32 species of candidate microbes degrading 1,4-dioxane were selected as positive colonies on plates containing 1,4-dioxane and their degrading abilities were compared. Isolates from seawater that had positive colonies could be just 1,4-dioxane tolerant while growing on other residual substrates. Hence, the degradation ability was demonstrated with evidence of degradation kinetics (Nakamiya et al. 2005; Vainberg et al. 2006; Mahendra et al. 2007; Kim et al. 2009; Sun et al. 2011; Gedalanga et al. 2014).
Changes in concentration of 1,4-dioxane during culture in the perfusion apparatus with seawater from four sites (a–d). Site A (diamonds): seawater from the northern west shore of Okinawa Main Island.Site B (triangles): seawater from the northwestern shore of Okinawa Main Island. Site C (circles): seawater from the southern west shore of Miyako Island. Site D (squares): seawater from the west central shore of Okinawa Main Island
Three strains that showed obvious degradation of 1,4-dioxane (Nos. 17, 24, and 31) were selected (Fig. 4). Three microbes that possessed 1,4-dioxane-degrading abilities were isolated from surface seawater using a seawater-charcoal perfusion method, among which strain RM-31 exhibited the strongest 1,4-dioxane-degrading ability.
Comparison of degrading ability of 1,4-dioxane by selected microbe strains Concentration of 1,4-dioxane after 7 days of incubation by each strain. The control shows the 1,4-dioxane concentration in sterilized BSM broth medium. Strain No. 12 was used as a negative control. Strain No. 17, 24, and 31 exhibited positive 1,4-dioxane degradation. Error bars show the standard deviations of triplicate experiments. Different superscript letters indicate significant differences at P < 0.05
Identification of strain RM-31
The SEM image of strain RM-31 is shown in Fig. 5. Based on the aerial mycelia of this microbe, it was characterized as an Actinomycete. Figure 6 shows a phylogenetic tree comparing the sequence of strain RM-31 with similar sequences. Similarity analysis using BLAST suggested that strain RM-31 was Pseudonocardia carboxydivorans Y8 (homology 100 %). This organism is very closely related to Pseudonocardia dioxanivorans, which was previously reported to have a high ability to degrade 1,4-dioxane (Parales et al. 1994; Mahendra and Cohen 2005; Grostern et al. 2012; Grostern and Cohen 2013; Sales et al. 2013; Sei et al. 2013; Mahendra et al. 2013).
Phylogenetic tree based on analysis of full 16S rRNA sequences of Pseudonocardia sp. RM-31 and their closest related sequences within Pseudonocardia sp. The tree is based on 1323 unambiguous nucleotide positions present in all sequences. Bootstrap percentages based on 100 resamplings are listed at the nodes. The scale bar represents 0.01 changes per nucleotide
Effects of incubation conditions of Pseudonocardia sp. strain RM-31 on 1,4-dioxane degradation characteristics
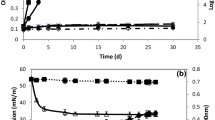
As shown in Fig. 7, when the changes in the concentration of 1,4-dioxane were determined during incubation the level of 1,4-dioxane decreased 300 mg/L from the initial concentration of about 1000 mg/L within 2 days, after which it decreased slowly. On the other hand, for the control which was inoculated with killed RM-31 the 1,4-dioxane concentration was hardly changed.
Degradation profile of 1,4-dioxane by Pseudonocardia sp. RM-31. The 1,4-dioxane concentration is shown as the average value from triplicate experiments during 25-ml culture incubation. The closed circles are shown as the residual 1,4-dioxane concentration resulted by alive RM-31. The open circles are shown as control 1,4-dioxane concentration resulted by killed RM-31. The line shows the 1,4-dioxane degradation profile calculated from triplicate experiments. Error bars show the standard deviations of triplicate experiments
The effects of initial pH, incubation temperature, and concentration of NaCl of BSM broth medium on the degradation rate of 1,4-dioxane are compared in Figs. 8 and 9. The optimal initial pH and temperature were 6–8 and 25 °C, respectively. The 1,4-dioxane degradation rate by strain RM-31 in broth medium with 3 % NaCl was almost 20 % faster than that without NaCl.
Effects of initial pH on the degradation of 1,4-dioxane by Pseudonocardia sp. RM-31. The profiles show the residual ratio (%, mean values of triplicate analyses) of 1,4-dioxane at each incubation time on broth medium with an initial pH of 3 (open diamonds), 4 (open squares), 5 (open triangles), 6 (open circles), 7 (closed diamonds), 8 (closed squares), 9 (closed triangles), and 10 (closed circles). All triplicates were within 5 % of the mean
Effects of incubation temperature and NaCl concentration on degradation of 1,4-dioxane by Pseudonocardia sp. RM-31. The profiles are the residual ratio (%) of 1,4-dioxane at each incubation time on broth medium with 3 % NaCl at 20 °C (closed triangles), 25 °C (closed squares), 30 °C (closed diamonds), and without NaCl at 25 °C (open squares). Error bars show the standard deviations of triplicate experiments
The effects of initial pH, incubation temperature, and concentration of NaCl of BSM broth medium on the degradation rate of 1,4-dioxane were found to be similar to those on other microbes (Sei et al. 2013), with the optimal initial pH and temperature being 6–8 and 25 °C, respectively. The biodegradation rate of 1,4-dioxane by strain RM-31 at 25 °C in broth medium with 3 % NaCl was almost 20 % faster than that without NaCl. The biodegradation performance of 1,4-dioxane by strain RM-31 differed from that of other microbes (Sei et al. 2013) in that 1,4-dioxane degradation was significantly inhibited by 3 % NaCl. These findings suggest that strain RM-31 would exhibit a high ability to degrade 1,4-dioxane at high NaCl sites.
Cell yields of the Pseudonocardia sp. have been reported to be low when grown on 1,4-dioxane (White et al. 1996; Li et al. 2010; Sei et al. 2013). Similarly, strain RM-31 grew very slowly. In addition, the cells formed hydrophobic colonies (flock) on the surface of the broth medium or on the glass of the tube containing the medium. Instead of the direct determination of cell growth, the protein production from microbes was measured. Using 5-mL culture broth with 800 mg/L of 1,4-dioxane concentration, the total protein extracted from the grown cell and residual 1,4-dioxane concentration were determined (Fig. 10). As culture incubation times increasing, the residual 1,4-dioxane concentration was decreasing and the total protein contents were increasing. It was suggested that RM-31strain could make degradation of 1,4-dioxane, following to the protein production by cell growth.
Changes in the 1,4-dioxane concentration and the amounts of protein contents during incubation of 5-ml BSM broth inoculated strain RM-31. Black bar concentration of 1,4-dioxane at each 0, 2, 3 incubation days. Black circle total amounts of protein extracted from growth cells per tube. Error bars show the standard deviations of triplicate experiments. Different superscript letters indicate significant differences at P < 0.05
Strain RM-31 is the first bacteria from seawater shown to have a strong ability to degrade 1,4-dioxane in seawater. The degradation rate by strain RM-31 was almost 31.6 mg L−1/h (Fig. 7), while the rate of 1,4-dioxane degradation calculated in a previous report (Sei et al. 2013) was about 5.0 mg L−1/h. Even though the culture conditions and media differed between these studies, Pseudonocardia dioxanivorans strain RM-31 exhibited faster degradation of 1,4-dioxane than other microbes that have been investigated to date. Although strain RM-31 could degrade 300 mg/L of 1,4-dioxane from 1000 mg/L to 700 mg/L within 2 days, then it did not keep the rapid degradation rate. This might have been caused by feedback inhibition of cell growth or degrading enzymes.
These findings confirm the existence of 1,4-dioxane degraders in seawater and indicate that they have the potential for application to bioremediate this compound.
References
Altenburger R, Nendza M, Schuurmann G (2003) Mixture toxicity and its modeling by quantitative Structure-activity relationships. Environ Toxicol Chem 22:1900–1915
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Gauthier MJ, Lafay MJ, Christen R, Fernandez L, Acquaviva M, Bonin P, Bertrand JC (1992) Marinobacter hydrocabonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydorocarbon-degrading marine bacterium. Int J Sys Bacteriol 42:568–576
Gedalanga PB, Pornwongthong P, Mora R, Chiang SYD, Baldwin B, Ogles D, Mahendra S (2014) Identification of biomarker genes to predict biodegradation of 1,4-dioxane. Appl Environ Microbiol 80:3209–3218
Grostern A, Cohen LA (2013) Rubisco-based CO2 fixation and C1 metabolism in the actinobacterium Pseudonocardia dioxanivorans CB1190. Environ Microbiol 15:3040–3053
Grostern A, Sales CM, Zhuang WQ, Erbilgin O, Cohen LA (2012) Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by Pseudonocardia dioxanivorans strain CB1190. Appl Environ Microbiol 78:3298–3308
Kim YM, Jeon JR, Murugesan K, Kim EJ, Chang YS (2009) Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 20:511–519
Li M, Fiorenza S, Chatham JR, Mahendra S, Alvarez PJJ (2010) 1,4-Dioxane biodegradation at low temperature in Arctic groundwater samples. Water Res 44:2894–2900
Lowry OH, Rosenbrough NJ, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–269
Mahendra S, Cohen LA (2005) Pseudonocardia dioxanivorans sp. Nov., a novel actinomycete that grows on 1,4-dioxane. Int J Syst Evol Microbiol 55:593–598
Mahendra S, Petzold CJ, Baidoo EE, Keasling JD, Cohen LA (2007) Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria. Environ Sci Technol 41:7330–7336
Mahendra S, Grostern A, Cohen LA (2013) The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane. Chemosphere 91:88–92
Nakamiya K, Hashimoto S, Ito H, Edmonds JS, Morita M (2005) Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl Environ Microbiol 71:1254–1258
Parales RE, Adamus JE, White N, May HD (1994) Degradation of 1,4-dioxane by an actinomycete in pure culture. Appl Environ Microbiol 60:4527–4530
Perriere G, Gouy M (1996) WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369
Quatrano RS, Caldwell BA (1978) Isolation of a unique marine bacterium capable of growth on a wide variety of polysaccharides from Macroalgae. Appl Environ Microbiol 36:979–981
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakakibara F, Takagi K, Kataoka R, Kiyota H, Sato Y, Okada S (2011) Isolation and identification of dieldrin-degrading Pseudonocardia sp. strain KSF27 using a soil-charcoal perfusion method with aldrin trans-diol as a structural analog of dieldrin. Biochem Biophys Res Commun 411:76–81
Sales CM, Grostern A, Parales JV, Parales RE, Cohen LA (2013) Oxidation of the cyclic ethers 1,4-dioxane and tetrahydrofuran by a monooxygenase in two Pseudonocardia species. Appl Environ Microbiol. doi:10.1128/AEM.02418-13
Scalia S, Guarneri M, Menegatti E (1990) Determination of 1,4-dioxane in cosmetic products by high-performance liquid chromatography. Analyst 115:929–931
Sei K, Miyagaki K, Kakinoki T, Fukugasako K, Inoue D, Ike M (2013) Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 24:665–674
Shindo K (2013) The present status of prediction of ecotoxicological risk from chemicals in the aquatic environment and future development of the marine environmental protection for the fisheries activity. Rep Mar Ecol Res Inst 16:29–50
Sugiura K (1997) Physicochemical properties and biodegradability of crude oil. Environ Sci Technol 31:45–51
Sun B, Ko K, Ramsay JA (2011) Bioregradation of 1,4-dioxane by a Flavobacterium. Biodegradation 22:651–659
Takagi K, Iwasaki A, Kamei I, Satsuma K, Yoshioka Y, Harada N (2009) Aerobic mineralization of hexachlorobenzene by newly isolated pentachloronitrobenzene-degrading Nocardioides sp. strain PD653. Appl Environ Microbiol 75:4452–4458
U.S.EPA report (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to marine and estuarine organisms. 3rd Edition. http://water.epa.gov/scitech/methods/cwa/wet/disk1_index.cfm
Vainberg S, McClay K, Masuda H, Root D, Condee C, Zylstra GJ, Steffan RJ (2006) Bopgradation of ether pollutants by Psedonocardia sp. Strain ENV478. Appl Environ Microbiol 72:5218–5224
White GF, Russell NJ, Tidswell EC (1996) Bacterial scieeion of ether bonds. Microbiol Rev 60:216–232
Zwart D, Posthuma L (2005) Complex mixture toxicity for single and multiple species: proposed methodologies. Environ Toxicol Chem 24:2665–2676
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsui, R., Takagi, K., Sakakibara, F. et al. Identification and characterization of 1,4-dioxane-degrading microbe separated from surface seawater by the seawater-charcoal perfusion apparatus. Biodegradation 27, 155–163 (2016). https://doi.org/10.1007/s10532-016-9763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-016-9763-8