Abstract
The genes of two ring-hydroxylating dioxygenases (RHDs) of Sphingomonas sp. VKM B-2434 were cloned and expressed in Escherichia coli. The relative values of the RHD specificity constants were estimated for six polycyclic aromatic hydrocarbons (PAHs) based on the kinetics of PAH mixture conversion by the recombinant strains. The substrate specificity profiles of the enzymes were found to be very different. Dioxygenase ArhA was the most specific to acenaphthylene and showed a low specificity to fluoranthene. Dioxygenase PhnA was the most specific to anthracene and phenanthrene and showed a considerable specificity to fluoranthene. Knockout derivatives of Sphingomonas sp. VKM B-2434 lacking ArhA, PhnA, and both dioxygenases were constructed. PAH degradation by the single-knockout mutants was in agreement with the substrate specificity of the RHD remaining intact. Double-knockout mutant lacking both enzymes was unable to oxidize PAHs. A mutant form of dioxygenase ArhA with altered substrate specificity was described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerobic bacterial degradation of PAHs begins with hydroxylation of substrate catalyzed by RHDs (Gibson and Parales 2000). Many RHDs from PAH-utilizing bacterial strains have been cloned and characterized, and recently several functionally active RHDs have been cloned using metagenomic DNA (Singleton et al. 2012; Martin et al. 2013).
These enzymes show a broad substrate specificity. For example, naphthalene dioxygenase from the strain Pseudomonas sp. NCIB 9816 catalyzes conversion of more than 50 various aromatic substrates including PAHs (Resnick et al. 1996). The enzyme from the strain Sphingomonas sp. CHY-1 oxidizes various PAHs containing 2–5 aromatic rings (Jouanneau et al. 2006). Dioxygenase of the strain Sphingomonas sp. A4 capable of growth on acenaphthene and acenaphthylene catalyzes, apart from these two substrates, the oxidation of naphthalene, phenanthrene, anthracene, and fluoranthene (Pinyakong et al. 2004). RHD from the strain Sphingomonas sp. LH128 oxidizes various PAHs and heterocyclic compounds at different rates (Schuler et al. 2009). The strain Mycobacterium sp. 6PY1 has two RHDs, responsible one for phenanthrene biodegradation and the other for pyrene biodegradation, which are, however, not quite specific (Krivobok et al. 2003). Two RHDs of the strain Mycobacterium vanbaalenii PYR-1 show the highest activity to pyrene and fluoranthene, respectively; both of them also oxidize phenanthrene and other PAHs (Kweon et al. 2010). Thus, the specificity of PAH conversion by RHDs is characterized by the following properties: (1) the same RHD catalyzes oxidation of several PAHs; (2) the activity of an RHD differs with respect to different PAHs; and (3) different RHDs vary in substrate specificity.
Reliable description of RHD specificity is needed to investigate structure-specificity relationships and design of enzymes with required properties. While the selectivity of product formation by PAH-oxidizing RHDs is characterized by the ratios of products formed (Parales 2003; Kim et al. 2006), there is no reliable quantitative method to describe the RHD substrate specificity.
In this paper, we apply the earlier reported method for the analysis of PAH mixture biodegradation (Baboshin and Golovleva 2011) to recombinant E. coli strains carrying RHD genes from Sphingomonas sp. VKM B-2434, estimate the relative values of RHD specificity constants for a series of PAHs, and confirm the results by gene knockouts.
Materials and methods
Reagents
PAHs were of high purity grade (>98 %; purchased from Sigma-Aldrich, Fluka, and Merck). Restriction endonucleases were from Fermentas (Lithuania), T4 DNA ligase was from Sibenzyme (Russia), and Taq DNA polymerase was from Evrogen (Russia). All other chemicals used were of analytical reagent grade and were purchased locally. Solvents were distilled before use.
Strains, plasmids and media
Bacterial strains and plasmids used in this study are listed in Table 1. Mineral medium contained (g/L): NH4NO3, 1.0; KH2PO4, 1.0; K2HPO4, 1.0; MgSO4·7H2O, 0.2; CaCl2, 0.02; FeCl3, two drops of saturated solution, pH adjusted to 7.5 by addition of 50 % KOH; the medium was filtered and sterilized by autoclaving. PAH-containing medium was prepared by addition of PAH solution (100 μL) to mineral medium (95 mL); PAH solution was obtained by dissolving acenaphthylene, acenaphthene, phenanthrene, anthracene, fluoranthene, and pyrene in methanol at a concentration of 200 µmol/L for each compound. LB medium was used as a rich medium for growth of E. coli strains and Sphingomonas sp. VKM B-2434. Cultures were cultivated on a shaker or in Petri dishes containing agarized LB medium. Appropriate antibiotics were added to the medium for clone selection and cultivation of plasmid-mediated resistant strains. Antibiotics were used at the following concentrations (mg/L): for E. coli—ampicillin, 100; tetracycline, 10; kanamycin, 20; gentamycin, 5; for Sphingomonas sp. VKM B-2434—rifampicin, 25; tetracycline, 20; kanamycin, 20; gentamycin, 5.
DNA manipulations and molecular techniques
Total DNA was isolated from the Sphingomonas sp. VKM B-2434 culture by chloroform extraction (Chen and Kuo 1993). Standard PCR, inverse PCR, and gene cloning were carried out using standard protocols (Sambrook and Russell 2001).
Cloning of RHD genes
In order to express dioxygenase ArhA, the ahdA4 reductase gene, bphA3 ferredoxin gene, and arhA1A2 dioxygenase genes were subsequently cloned into pUC18 vector using EcoRI–KpnI, KpnI–XbaI, and XbaI–PstI sites, respectively, to yield pUC-A plasmid. In order to express dioxygenase PhnA, the ahdA4 reductase gene, bphA3 ferredoxin gene, phnA1 dioxygenase large subunit gene, and phnA2 dioxygenase small subunit gene were ligated into pUC18 vector using EcoRI–KpnI, KpnI–XbaI, XbaI–PstI, and PstI–HindIII sites, respectively, to create pUC-P plasmid.
Generation of RHD knockout mutants and complementation
For the generation of an RHD knockout mutant, a DNA fragment containing the gene of the RHD catalytic subunit (arhA1 or phnA1) was amplified using a pair of primers ARH_f/ARH_r or PHN_f/PHN_r (Table 2) and cloned into pEX18Tc vector. A gentamycin resistance cassette from pSP858 or kanamycin resistance cassette from pUC4K was subsequently subcloned into the arhA1 or phnA1 gene, using the unique restriction site SacI or XhoI, to yield a plasmid pEX-A or pEX-P, respectively. The pEX18Tc derivative was then mobilized into Sphingomonas sp. VKM B-2434 RifR from E. coli S17-1. The allelic exchange was confirmed by PCR using primers flanking the site of insertion.
For complementation of knockout mutations, KpnI–PstI DNA fragments from the pUC-A and pUC-P were subcloned into pSP329 to yield pSP-A and pSP-P, respectively. The plasmids were mobilized into knockout mutants of Sphingomonas sp. VKM B-2434 RifR from E. coli S17-1.
Site-directed mutagenesis of the arhA1 gene
Site-directed mutagenesis was performed by the megaprimer method (Sambrook and Russell 2001); pUC-A plasmid was used as a template. Megaprimer (343 residues) was amplified using mutagenic primer arh_mut and a reverse primer arh_m complementary to the sequence containing unique BglII restriction site (Table 2). The generated megaprimer together with the primer bph_f (Table 2) was used in the second round of PCR. The PCR product was treated with BglII and KpnI restriction enzymes and used to replace the corresponding fragment of pUC-A plasmid. The resulting plasmid was verified by sequencing.
Conversion of PAH mixture by a recombinant E. coli strain
The recombinant plasmids pUC-A and pUC-P were transformed into E. coli DH5α. Bacteria were grown in LB medium at 37 °C to an optical density of 0.6 at 540 nm. IPTG was added at a final concentration of 200 μM and further cultivated on a shaker at 30 °C for 4 h. After cooling the culture (24 mL) on ice, biomass was harvested by centrifugation, washed with ice-cold mineral medium, and resuspended in the mineral medium (48 mL). The biomass suspension (5 mL) was added into a flask with the PAH-containing medium (95 mL); the flask was tightly closed with a glass cap and incubated on a shaker at 30 °C for a certain period of time (0.5–4 h). Incubation was stopped by adding 1 mL of 5 N sulfuric acid solution. After the incubation, 20 µg of fluorene in 1 mL of ethyl acetate (internal standard) and 5 N potassium hydroxide solutions (1 mL) were introduced into the flask. The liquid was twice extracted with ethyl acetate, (30 + 10) mL. The extracts were evaporated in a rotor evaporator at 38 °C under vacuum (~100 mbar), with preliminary addition of 100 µL of dimethylformamide into each extract. Ethyl acetate vaporized and dimethylformamide remained. Thus, extracted hydrocarbons (including the internal standard) were dissolved in a small volume of dimethylformamide at concentrations sufficient for gas chromatography. Hydrocarbon concentrations in the extracts were determined by gas chromatography in a Kristall-2000M chromatograph (Chromatec, Russia) with the flame-ionizing detector and HP-5 column (30 m × 0.32 mm × 0.25 µm). The evaporator and detector temperatures were 300 and 330 °C, respectively. The column temperature increased from 120 to 197.5 °C during the analysis at a rate of 2.5 °C/min. Hydrocarbon mass in a sample was calculated by the ratio of the hydrocarbon peak area to the peak area of internal standard (fluorene).
PAH conversion by Sphingomonas sp. VKM B-2434 and its mutans under growth conditions
A certain volume (0.1–1 mL) of PAH solution in acetone (50 g/L) was added to 100 mL of mineral medium supplemented with vitamin B12 (1 µg/L); PAH concentrations in the medium (0.2 µmol/L) were much less than the aqueous solubility of all PAHs used. The medium was inoculated by 1 mL of a culture grown to the stationary phase in LB medium, and further cultivated on a shaker at 30 °C. After incubation, the contents of a flask were twice extracted with ethylacetate (30 + 10 mL). The volume of the extracts was brought up to 40 mL with ethylacetate, and PAH concentration in the extract was measured by gas chromatography.
Conversion of indole to indigo and acenaphthene to 1-acenaphthenol
Conversion of triptophan-derived indole to indigo was observed visually (blue coloring) during the culture growth in liquid LB medium. Oxidation of acenaphthene to 1-acenaphthenol was detected by gas chromatography by measuring the concentration of both the substrate and product.
Calculation of the relative values of specificity constants
Data on the conversion of a PAH mixture by a recombinant strain were processed as follows. For each component of the mixture, we plotted its concentration logarithm versus the logarithm of the concentration of an arbitrarily chosen component (e. g., acenaphthylene). If all PAHs are converted in the same common active site in accordance with the principles of Michaelis kinetics, the slope of each double-logarithmic plot is proportional to the specificity constant (first-order rate constant) of the respective reaction (Baboshin and Golovleva 2011). Thus, we obtain a series of relative specificity constants of the RHDs with respect to a series of PAHs. The result was expressed as proportions of the relative specificity values in the sum of such values for all PAHs in the mixture:
where k j is the relative specificity constant for substrate j, α j is the slope of the double-logarithmic plot for substrate j, ∑ n i=1 α i is the sum of the slopes of the double-logarithmic plots for all n substrates in the mixture.
Phylogenetic analysis
Search for nucleotide and amino acid sequences was performed using BLAST software. The sequences were aligned using T-Coffee (Notredame et al. 2000). The phylogenetic tree was constructed by the neighbor-joining method using TREECON (Van de Peer and De Wachter 1997).
Nucleotide sequence accession numbers
The sequences of the arhA1A2 and phnA1A2 genes of Sphingomonas sp. VKM B-2434 were deposited in GenBank under accession numbers KF734000 and KF734001, respectively.
Results
Cloning and sequencing of RHD genes
The PCR amplification of nahAc-like genes of Sphingomonas sp. VKM B-2434 was performed using the degenerate primers Nah-for and Nah-rev1 (Zhou et al. 2006). PCR products were cloned into pGEM-T Easy and sequenced. Sequencing revealed the presence of two different RHD genes in the genome. One of the RHDs shows a 99 % protein sequence identity with dioxygenase ArhA from Sphingomonas sp. A4 (Pinyakong et al. 2004). Another RHD shows a 99 % protein sequence identity with dioxygenase PhnA from Sphingomonas sp. EPA505 (accession no. CAT03469 and CAT03470) and a 98 % protein sequence identity with dioxygenase PhnA from Sphingomonas sp. CHY-1 (Jouanneau et al. 2006). Genes closely related to the genes of reductase AhdA4 and ferredoxin BphA3 from Sphingomonas sp. EPA505 were revealed using the PCR with primers corresponding to the conservative sequences of the respective genes. Based on the sequences of inverse PCR products, primers for full-size gene amplification were designed (Table 2). Genes of dioxygenases ArhA and PhnA were ligated into pUC18 vector to yield plasmids pUC-A and pUC-P, respectively. Phylogenetic analysis of deduced amino acid sequences of the ArhA and PhnA catalytic subunits was carried out (Fig. 1).
PAH conversion by the recombinant E. coli strains carrying wild-type RHD genes
Both E. coli carrying pUC-A plasmid and E. coli carrying pUC-P plasmid showed an ability to oxidize indole to indigo and acenaphthene to 1-acenaphthenol. If any component of the dioxygenase system was absent, a recombinant strain was unable to produce indigo.
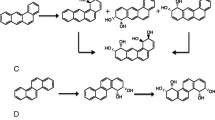
Both E. coli strains carrying pUC-A and pUC-P converted a mixture of six PAHs (Fig. 2). The conversion curves were plotted in double-logarithmic coordinates and fitted by straight lines (Fig. 3). The line slopes were taken as an estimate of the relative values of the specificity constants of dioxygenases ArhA and PhnA (Fig. 4). In a control experiment with E. coli carrying pUC18 plasmid, no PAH conversion was observed.
A double-logarithmic plot for PAH mixture conversion by E. coli carrying genes of dioxygenase ArhA (a) and by E. coli carrying genes of dioxygenase PhnA (b). For each component of the mixture, the logarithm of its concentration versus the logarithm of the concentration of acenaphthylene was plotted. The plot for acenaphthylene is not shown since its slope is trivially equal to 1. The symbols are the same as in Fig. 2
The substrate specificity profiles of dioxygenases ArhA (a) and PhnA (b). The relative values of specificity constants were estimated based on the line slopes of the double-logarithmic plot (Fig. 3). Averages are based on three independent experiments. Confidence intervals correspond to a confidence probability of 0.95
Characteristics of knockout derivatives of Sphingomonas sp. VKM B-2434 lacking RHDs
Knockout of the arhA1 gene encoding the catalytic subunit of dioxygenase ArhA resulted in the inability of Sphingomonas sp. VKM B-2434 to grow on acenaphthene, while the rate of growth on anthracene, phenanthrene, and fluoranthene remained about the same. Knockout of the phnA1 gene encoding the catalytic subunit of dioxygenase PhnA resulted in the inability of Sphingomonas sp. VKM B-2434 to grow on fluoranthene, the rate of growth on anthracene and phenanthrene decreased dramatically, while the rate of growth on acenaphthene remained about the same. Double-knockout mutant lacking both RHDs was completely unable to oxidize PAHs. PAH conversion by knockout strains under growth conditions as compared with wild-type Sphingomonas sp. VKM B-2434 is shown in Fig. 5.
Introduction of pSP-A plasmid containing wild type allele of the arhA1 gene restored the ability of the double knockout mutant to grow on acenaphthene (but not on fluoranthene); introduction of pSP-P plasmid containing wild type allele of the phnA1 gene restored the ability of the double knockout mutant to grow on fluoranthene (but not on acenaphthene); data not shown.
PAH conversion by a recombinant E. coli strain carrying the mutant arhA1 gene
In an attempt to alter the substrate specificity of dioxygenase ArhA two active site residues, Ala-202 and Ile-205, were replaced with Gly and Val residues, respectively. One more substitution (Phe73Leu) was introduced accidentally during the PCR. Thus, a triple mutant (Phe73Leu, Ala202Gly, Ile205Val) of ArhA1 was obtained. PAH mixture conversion by E. coli carrying the mutant dioxygenase ArhA was studied (Fig. 6). The specificity of the mutant dioxygenase to PAHs (Fig. 6c) was found to differ from that of the wild-type enzyme (Fig. 4a), namely the ability to convert phenanthrene and anthracene was lost. The mutant enzyme, in contrast to the wild-type dioxygenase, was unable to oxidize indole to indigo, but conversion of acenaphthene to 1-acenaphthenol remained.
Discussion
The existence of two RHDs with different substrate specificities in Sphingomonas sp. VKM B-2434 strain predicted earlier based on the kinetics of PAH mixture conversion (Baboshin and Golovleva 2011) was confirmed in this work by molecular methods. Substrate specificities of dioxygenases ArhA and PhnA were found to be broad but distinctly different (Fig. 4). Of the compounds tested, ArhA is the most specific to acenaphthylene. PhnA is the most specific to anthracene and phenanthrene, and, unlike ArhA, has a considerable specificity to fluoranthene. While Sphingomonas sp. VKM B-2434 is able to oxidize a variety of PAHs, the metabolism of acenaphthylene/acenaphthene and fluoranthene is the most efficient in terms of energy utilization (Baboshin and Golovleva 2011a). Thus, the strain can be assumed to specialize in acenaphthylene/acenaphthene and fluoranthene consumption, which appears to be natural since the common metabolic pathway via naphthalene-1,8-dicarboxylic acid is used for the degradation of these substrates. The fluoranthene molecule is much larger than the acenaphthylene or acenaphthene molecule, and this is why these molecules are unlikely to be efficiently converted by the same enzyme. Dioxygenases ArhA and PhnA presumably serve to oxidize acenaphthylene/acenaphthene and fluoranthene, respectively. These considerations are supported by the results of gene knockouts.
At the same time, both RHDs are active against medium-molecular-weight PAHs, phenanthrene and anthracene. The strain is incapable of complete mineralization of these PAHs but is able to cleave one aromatic ring in them and to use them as a sole source of energy (Baboshin et al. 2008); the ability is due to the relaxed specificity of the RHDs.
Inability of the double-knockout mutant lacking both investigated enzymes to convert PAHs is evidence that dioxygenases ArhA and PhnA represent all PAH-oxidizing RHDs of Sphingomonas sp. VKM B-2434. Although a number of RHD genes are present in sphingomonad genome (Pinyakong et al. 2003), a single enzyme is usually involved in the initial oxidation of PAH molecules (Pinyakong et al. 2004; Demaneche et al. 2004; Yu et al. 2007). To our knowledge, Sphingomonas sp. VKM B-2434 is the first characterized sphingomonad strain carrying two such enzymes.
The used method of the double-logarithmic plot enables simple and accurate comparison of RHD specificities to PAHs. The traditional approach of substrate specificity estimation is of little value since the data on the kinetic properties of RHDs are very scarce. To our knowledge, steady-state-kinetic parameters for an RHD with respect to naphthalene were reported in the only paper by Jouanneau et al. (2006), and there are no data on the kinetics of high-molecular-weight PAH conversion; this is probably due to the difficulty of obtaining RHD enzyme preparations and performing the kinetic studies. RHD activities with respect to different substrates were usually assessed by comparing the rates of individual PAH conversion by a recombinant strain or (rarely) by a purified enzyme (Kasai et al. 2003; Krivobok et al. 2003; Jouanneau et al. 2006; Schuler et al. 2009; Kweon et al. 2010; Martin et al. 2013; Singleton et al. 2012). Such an analysis allows only tentative conclusions concerning the specificities of the RHDs due to the differences in Michaelis constants for various PAHs and possibly different impacts of various PAHs and products of their conversion on RHD activities. The system of competing reactions occurring in a common active site is free from these drawbacks since both the catalytic activity and substrate affinity are taken into account and all the competing reactions are equally influenced by any factor.
A series of papers were dedicated to the estimation of the kinetic parameters of PAH conversion by pure bacterial strains (Stringfellow and Aitken 1995; Dimitriou-Christidis et al. 2007; Desai et al. 2008). Surprisingly, similar experiments with RHD-carrying recombinant strains were not conducted. The kinetics of PAH conversion by a recombinant strain E. coli carrying an RHD can be interpreted more reliably than the kinetics related to a PAH-degrading strain because E. coli does not contain any other enzymes of PAH metabolism. Particularly, the ability of the recombinant strain to oxidize PAHs shows that PAHs are transferred into bacterial cells by way of simple diffusion. Furthermore, the rate of PAH conversion by bacteria is likely to be determined by the reaction in the active site of the RHD and does not depend on the stage of substrate diffusion into the cell. This follows from the fact that PAH mixture conversion by a microbial culture usually satisfies the multisubstrate model, according to which all components of the mixture compete for a single active site in accordance with the principles of Michaelis kinetics (Stringfellow and Aitken 1995; Guha et al. 1999; Lotfabad and Gray 2002; Knightes and Peters 2006; Dimitriou-Christidis and Autenrieth 2007; Desai et al. 2008). The linearity of the double-logarithmic plot in our experiments with the recombinant strains indicates that PAH conversion satisfies the multisubstrate model (Baboshin and Golovleva 2011). Therefore, the kinetic parameters of PAH conversion by a recombinant strain are likely to correspond to the recombinant RHD present in the strain.
The specificity profile of dioxygenase PhnA (Fig. 4b) is similar to the result of kinetic investigation of PAH conversion by Sphingomonas sp. EPA505, the strain carrying almost the same dioxygenase (Fig. 7). This observation could be an argument that RHD specificity is the same in the sphingomonad and E. coli cell. On the other hand, there is a discrepancy between our present results and our results on the kinetics of PAH conversion by Sphingomonas sp. VKM B-2434 reported earlier (Baboshin and Golovleva 2011). In the previous experiments with the biomass of Sphingomonas sp. VKM B-2434 the specificity to fluoranthene was 3.6 times higher than that to phenanthrene; in contrast, in the present experiments with the recombinant strains, the specificity to phenanthrene was much higher than that to fluoranthene. Consequently, we can assume that some factors in the sphingomonad cell modify the RHD specificity.
Specific affinities for PAH conversion by Sphingomonas sp. EPA505, a strain carrying an RHD with 99 % identity to dioxygenase PhnA. Confidence intervals correspond to a confidence probability of 0.95. The kinetic parameters were estimated by Dimitriou-Christidis et al. (2007) from the curves of individual PAH conversion
One can hope that the method of describing the RHD substrate specificity in combination with molecular modelling and site-directed mutagenesis will allow obtaining RHDs with desired substrate specificity.
References
Baboshin MA, Golovleva LA (2011a) Multisubstrate kinetics of PAH mixture biodegradation: analysis in the double-logarithmic plot. Biodegradation 22:13–23
Baboshin MA, Golovleva LA (2011b) Characterization of hydrophobic organic contaminant biodegradation by COD analysis. Int Biodeterior Biodegr 65:883–889
Baboshin MA, Akimov VN, Baskunov BP, Born TL, Khan SU, Golovleva LA (2008) Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation 19:567–576
Chen W, Kuo T (1993) A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acid Res 21:2260
Demaneche S, Meyer C, Micoud J, Louwagie M, Willison JC, Jouanneau Y (2004) Identification and functional analysis of two aromatic-ring-hydroxylating dioxygenases from a Sphingomonas strain that degrades various polycyclic aromatic hydrocarbons. Appl Environ Microbiol 70:6714–6725
Desai AM, Autenrieth RL, Dimitriou-Christidis P, McDonald TJ (2008) Biodegradation kinetics of select polycyclic aromatic hydrocarbon (PAH) mixtures by Sphingomonas paucimobilis EPA505. Biodegradation 19:223–233
Dimitriou-Christidis P, Autenrieth RL (2007) Kinetics of biodegradation of binary and ternary mixtures of PAHs. Biotechnol Bioeng 97:788–800
Dimitriou-Christidis P, Autenrieth RL, McDonald TJ, Desai AM (2007) Measurement of biodegradability parameters for single unsubstituted and methylated polycyclic aromatic hydrocarbons in liquid bacterial suspensions. Biotechnol Bioeng 97:922–932
Gibson DT, Parales RE (2000) Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol 11:236–243
Guha S, Peters CA, Jaffé PR (1999) Multisubstrate biodegradation kinetics of naphthalene, phenanthrene, and pyrene mixtures. Biotechnol Bioeng 65:491–499
Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP (1998) A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86
Ivashina TV, Fedorova EE, Ashina NP, Kalinchuk NA, Druzhinina TN, Shashkov AS, Shibaev VN, Ksenzenko VN (2010) Mutation in the pssM gene encoding ketal pyruvate transferase leads to disruption of Rhizobium leguminosarum bv. viciae–Pisum sativum symbiosis. J Appl Microbiol 109:731–742
Jouanneau Y, Meyer C, Jakoncic J, Stojanoff V, Gaillard J (2006) Characterization of a naphthalene dioxygenase endowed with an exceptionally broad substrate specificity toward polycyclic aromatic hydrocarbons. Biochemistry 45:12380–12391
Kasai Y, Shindo K, Harayama S, Misawa N (2003) Molecular characterization and substrate preference of a polycyclic aromatic hydrocarbon dioxygenase from Cycloclasticus sp. strain A5. Appl Environ Microbiol 69:6688–6697
Kim S-J, Kweon O, Freeman JP, Jones RC, Adjei MD, Jhoo J-W, Edmondson RD, Cerniglia CE (2006) Molecular cloning and expression of genes encoding a novel dioxygenase involved in low- and high-molecular-weight polycyclic aromatic hydrocarbon degradation in Mycobacterium vanbaalenii PYR-1. Appl Environ Microbiol 72:1045–1054
Knightes CD, Peters CA (2006) Multisubstrate biodegradation kinetics for binary and complex mixtures of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 25:1746–1756
Krivobok S, Kuony S, Meyer C, Louwagie M, Willison JC, Jouanneau Y (2003) Identification of pyrene-induced proteins in Mycobacterium sp. strain 6PY1: evidence for two ring-hydroxylating dioxygenases. J Bacteriol 185:3828–3841
Kweon O, Kim S-J, Freeman JP, Song J, Baek S, Cerniglia CE (2010) Substrate specificity and structural characteristics of the novel Rieske nonheme iron aromatic ring-hydroxylating oxygenases NidAB and NidA3B3 from Mycobacterium vanbaalenii PYR-1. mBio 1:e00135-10
Lotfabad SK, Gray MR (2002) Kinetics of biodegradation mixtures of polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol 60:361–366
Martin F, Malagnoux L, Violet F, Jakoncic J, Jouanneau Y (2013) Diversity and catalytic potential of PAH-specific ring-hydroxylating dioxygenases from a hydrocarbon-contaminated soil. Appl Microbiol Biotechnol 97:5125–5135
Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217
Parales R (2003) The role of active-site residues in naphthalene dioxygenase. J Ind Microbiol Biotechnol 30:271–278
Pinyakong O, Habe H, Omori T (2003) The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). J Gen Appl Microbiol 49:1–19
Pinyakong O, Habe H, Kouzuma A, Nojiri H, Yamane H, Omori T (2004) Isolation and characterization of genes encoding polycyclic aromatic hydrocarbon dioxygenase from acenaphthene and acenaphthylene degrading Sphingomonas sp. strain A4. FEMS Microbiol Lett 238:297–305
Resnick SM, Lee K, Gibson DT (1996) Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol 17:438–457
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schuler L, Jouanneau Y, Chadhain SMN, Meyer C, Pouli M, Zylstra GJ, Hols P, Agathos SN (2009) Characterization of a ring-hydroxylating dioxygenase from phenanthrene-degrading Sphingomonas sp. strain LH128 able to oxidize benz[a]anthracene. Appl Microbiol Biotechnol 83:465–475
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784–791
Singleton DR, Hu J, Aitken MD (2012) Heterologous expression of polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenase genes from a novel pyrene-degrading betaproteobacterium. Appl Environ Microbiol 78:3552–3559
Stringfellow WT, Aitken MD (1995) Competitive metabolism of naphthalene, methylnaphthalene and fluorene by phenanthrene-degrading pseudomonads. Appl Environ Microbiol 61:357–362
Van de Peer Y, De Wachter R (1997) Construction of evolutionary distance trees with TREECON. Comput Applic Biosci 13:227–230
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Yu CL, Liu W, Ferraro DJ, Brown EN, Parales JV, Ramaswamy S, Zylstra GJ, Gibson DT, Parales RE (2007) Purification, characterization, and crystallization of the components of a biphenyl dioxygenase system from Sphingobium yanoikuyae B1. J Ind Microbiol Biotechnol 34:311–324
Zhou HW, Guo CL, Wong YS, Tam NFY (2006) Genetic diversity of dioxygenase genes in polycyclic aromatic hydrocarbon-degrading bacteria isolated from mangrove sediments. FEMS Microbiol Lett 262:148–157
Acknowledgments
The reported study was partially supported by the Russian Foundation for Basic Research (RFBR), Research Project No. 11-04-00831-a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baboshin, M., Ivashina, T., Chernykh, A. et al. Comparison of the substrate specificity of two ring-hydroxylating dioxygenases from Sphingomonas sp. VKM B-2434 to polycyclic aromatic hydrocarbons. Biodegradation 25, 693–703 (2014). https://doi.org/10.1007/s10532-014-9692-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-014-9692-3