Abstract
Leachate treatment is a challenging issue due to its high pollutant loads. There are several studies on feasible treatment methods of leachate. In the scope of this study, high organic content of young leachate was eliminated using an upflow anaerobic sludge blanket (UASB) and a membrane bioreactor (MBR) in sequence and effluent of the system was given to single reactor for high activity ammonia removal over nitrite (SHARON) and anaerobic ammonia oxidation (Anammox) reactors to remove nitrogen content. All reactors were set up at lab scale in order to evaluate the usage of these processes in sequencing order for leachate treatment. COD and TKN removal efficiencies were over 90 % in the combined processes which were operated during the study. The biodegradable portion of organic matter was removed with an efficiency of 99 %. BOD5 concentration decreased to 50 mg/L by UASB and MBR in sequence even the influent BOD5 concentration was over 8,000 mg/L. Although high nitrogen concentrations were observed in raw leachate, successful removal of nitrogen was accomplished by consecutive operations of SHARON and Anammox reactors. The results of this study demonstrated that with an efficient pretreatment of leachate, the combination of SHARON–Anammox processes is an effective method for the treatment of high nitrogen content in leachate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfilling, which is a widely used solid waste disposal method due to economical and easy operation, requires control and treatment of heavily polluted leachate. Due to the high pollutant loads, leachate treatment has become an important issue and several treatment configurations have been investigated by different researchers. Biological, chemical or physical treatment and novel technologies such as membrane applications, advanced oxidation techniques, single reactor for high activity ammonia removal over nitrite (SHARON) and anaerobic ammonia oxidation (Anammox) processes are being used in order to provide economical and quality treatment of organic pollutants and nitrogen compounds (Renou et al. 2008; An et al. 2008; Liang and Liu 2007; Zhang and Zhou 2006; Xu et al. 2008).
Biological systems have been applied for many years because of their cost-effectiveness and convenience in operation. Anaerobic treatment is one of the most commonly used biological methods that are effective for the treatment of young landfill leachate which contains high pollutant loads. It converts organic waste to biogas, which can be incinerated with very little generation of pollutants (Appels et al. 2011), providing heat that may be used to keep the reactor at optimum temperature. Moreover, due to the low growth rate of anaerobic bacteria, fewer biosolids and less sludge are generated in the system with respect to aerobic treatment. Several anaerobic reactor configurations for leachate treatment are possible, such as the upflow anaerobic sludge blanket (Calli et al. 2006; Kennedy and Lentz 2000), anaerobic hybrid bed reactor (Calli et al. 2006; Timur and Ozturk 1999), anaerobic upflow filter (Calli et al. 2006), sequencing batch reactor (Timur and Ozturk 1999) and anaerobic membrane bioreactor (Bohziewicz et al. 2008).
Although anaerobic treatment processes provide high organic removal efficiency, as a consequence of the high pollutant loads in leachates observed in many regions of Turkey (Akgul et al. 2011), a single stage treatment is not enough to comply with discharge regulations. Thus, an additional process is necessary to reduce effluent pollutant concentrations to below the stipulated limits. The membrane bioreactor (MBR) is an alternative aerobic or anaerobic biological technology which has been investigated for leachate treatment in many studies, either individually (Bohziewicz et al. 2008; Boonyaroj et al. 2012) or following anaerobic treatment (An et al. 2008; Xu et al. 2008). MBR is now a common technology in wastewater and leachate treatment with excellent solid–liquid separation and dissolved organic matter (DOM) removal (Patsios and Karabelas 2011). Moreover, it does not require space for sedimentation tanks; therefore it is a good option in cases of limited land area.
In recent years, alternative treatment methods have been investigated to treat wastewater with high ammonium concentrations. Anammox has been reported as a suitable treatment to remove nitrogen compounds from high nitrogen loaded wastewater with low organic matter content, and its application for nitrogen removal could lead to significantly lower (90 % reduction) operational costs (Jetten et al. 2001).
The Anammox process is based on autotrophic denitrification via nitrite, where Anammox bacteria combine nitrite and ammonium to form nitrogen gas (Strous et al. 1997). In the Anammox process, chemolithoautotrophic bacteria convert one mol of nitrite and one mol of ammonium directly to dinitrogen gas with hydrazine as an intermediate (Jetten et al. 1998). Since the presence of nitrite is essential for the Anammox process, a partial nitrification system producing the appropriate ratio of nitrite to ammonium nitrogen is a prerequisite for successful nitrogen removal. The SHARON process involves partial nitrification of ammonium to nitrite in a system operated at low sludge retention time (mostly in chemostat systems with a sludge retention time of 1 day) and at relatively high temperature (35 °C) and pH (7–8) (Brouwer et al. 1996; Hellinga C 1997). The SHARON process can be implemented in a simple continuous stirred tank reactor (Hellinga et al. 1998) and is ideally suited to removing nitrogen from high N-loaded wastewater (>0.5 g N/L) (Jetten et al. 1997; van Dongen et al. 2001). Lately, several partial nitrification reactor configurations have been studied to treat landfill leachate, such as a bench scale fixed bed reactor (Liang and Liu 2007), sequencing batch reactors (Ganigue et al. 2007) and the SHARON process as a preceding step to Anammox (Vilar et al. 2010). Although there are a few studies conducted on partial nitrification and Anammox systems to treat landfill leachate (Liang and Liu 2007; Liang and Liu 2008; Liu et al. 2010; Zhang and Zhou 2006), no studies on combined SHARON and Anammox systems to treat landfill leachate were found in the literature. In comparison to conventional nitrification–denitrification processes, the Anammox process would significantly decrease oxygen demand and sludge production rates without the addition of organic matter (Abma et al. 2007; van Dongen et al. 2001). Therefore, the combined use of SHARON and Anammox systems should be a good treatment option for ammonium rich wastewater such as landfill leachate.
Although several leachate treatment configurations such as membrane bioreactors (Bohziewicz et al. 2008; Boonyaroj et al. 2012), upflow anaerobic sludge blanket (UASB) (Calli et al. 2006; Kennedy and Lentz 2000), UASB combined with MBR (An et al. 2008), partial nitrification (Ganigue et al. 2007), and partial nitrification with Anammox (Liang and Liu 2007; Liang and Liu 2008) have been studied, UASB, MBR, SHARON and Anammox processes have not yet been used consecutively.
In the scope of this study, UASB, MBR, SHARON and Anammox processes were used in sequence to evaluate leachate treatment performances. High nitrogen concentration in leachate was removed by SHARON and Anammox processes in order to save the oxygen required for nitrification and decrease aeration costs. Thus, the MBR was operated at a low sludge age to avoid nitrification. Membrane bioreactors with long solid retention times were effectively used for leachate treatment in previous studies (An et al. 2008; Xu et al. 2008) and in several leachate treatment plants, however, the configuration of low sludge age MBR followed by SHARON and Anammox is a new approach. The removal of organic matter and nitrogen was quantified in each step in order to evaluate the treatment efficiencies.
Materials and methods
Landfill site and characteristics of leachate
Leachate samples were supplied from the Istanbul Kömürcüoda Landfill site which is located on the Asian side of Istanbul. The Kömürcüoda Landfill construction is 89 ha in total area and is composed of 6 different cells. Leachate generated from the active cell is collected in a lagoon and sent to a treatment plant. Raw leachate was brought from the lagoon periodically and stored at 4 °C in order to preserve its physical and chemical characteristics. It was subsequently used as a feed stream for lab-scale treatment facilities. The analytical methods used for the characterization of raw leachate and continuous observation of operational parameters were carried out according to Standard Methods (American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF): Standard Methods for the Examination of Water and Wastewater, 20th Edition 1999). Experimental analyses and their measurement methods are listed in Table 1.
Due to the low phosphorus content of leachates (Table 2), the addition of phosphorus was required in amounts proportional to cell synthesis (i.e. a COD:N:P ratio of 500:7:1 is necessary for anaerobic treatment). The required phosphorus was adjusted with ortho-phosphoric acid.
Experimental set-up
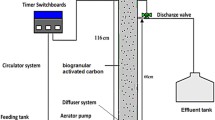
Figure 1 shows the experimental treatment system consisting of UASB, MBR, SHARON and Anammox reactors. The influent of UASB was supplied from Istanbul Kömürcüoda Landfill Site and the effluent of UASB was passed through an MBR in order to remove remaining organic content. The MBR effluent was finally treated using the SHARON and Anammox system.
Upflow anaerobic sludge blanket
The plexiglas UASB reactor used in this study had an active volume of 13.7 L. The reactor was placed in an incubator in order to adjust the temperature to 35 ± 1 °C. The flowrate of UASB was 5 L/day and the upflow velocity was increased by recycling the effluent in order to prevent settlement within the reactor. Influent was introduced at the bottom of the reactor using a peristaltic pump. At start up, the reactor was seeded with anaerobic sludge taken from PAKMAYA Yeast Industry anaerobic treatment. Organic loading rate was gradually increased to enable the seed sludge to acclimate to the leachate. It was initially adjusted to 0.75 kg COD m−3/day and raised up to 8 kg COD m−3/day during the operational period. The influent and effluent water were analyzed daily for pH, alkalinity, COD, BOD5, NH4–N and TKN. In order to observe the treatment and possible inhibition along the height of the reactor, four sampling ports were placed at uniform intervals along the reactor. Alkalinity, pH, COD, BOD5, NH4–N, TKN, TSS and VSS were measured in port samples twice a month. Free ammonia concentration was continuously monitored in effluent and port samples using the following formula (Calli et al. 2005b):
Where FAN is the concentration of free ammonia (mg/L), TAN is the total ammonia concentration (mg/L) and T is the temperature in Kelvin.
Membrane bioreactor
The membrane bioreactor was operated under aerobic conditions and low sludge age to promote only carbon removal. The lab-scale MBR system used in the study had a volume of 20 L and sludge age was adjusted to 5 days. Membrane fouling was minimized by air scouring and periodical backwashing of the membranes. A polypropylene tubular membrane module with 0.1 μm pore size and area of 0.023 m2 was used in the experiment. Influent water was taken directly from the UASB reactor. Dissolved oxygen (DO), oxidation reduction potential (ORP) and pH of the reactor were monitored continuously to control the operation. The effluent water was analyzed daily for COD, BOD5, NH4–N, NO2–N, NO3–N and TKN.
SHARON reactor
The lab-scale SHARON reactor that was made from plexiglas material had a total volume of 7.5 L, and active volume of 2.5 L. The empty part of the reactor provided space for possible foaming problems of leachate. The sludge retention time (SRT) and hydraulic retention time (HRT) of the system was 1 day and average flow rate was 1,675 mL/day.
The reactor was operated at a constant temperature (35 °C) which was maintained by a water bath. High temperature and low SRT conditions in the reactor prevented the reproduction of nitrite oxidizing bacteria (NOB) and hence they were washed out of the system. Influent water was fed to the system by a peristaltic pump. A mechanical mixer was placed into the reactor to prevent sludge settlement. Continuous aeration was supplied using a fine bubble diffuser in order to provide the necessary dissolved oxygen concentration for partial nitrification. Oxygen and pH values were continuously monitored with the help of probes. The influent and effluent samples were analyzed daily for NH4 +–N, NO2–N and pH.
The seed sludge for the lab-scale reactor was taken from Paşaköy Advanced Wastewater Treatment Plant which is operated at a sludge retention time of 20 days. In order to obtain faster bacterial acclimation, the reactor was fed first with synthetic wastewater which contained K2HPO4, NaHCO3, CaCl2, MgCl2, Na2EDTA∙2H2O, FeSO4, ZnSO4, CoCl2, MnCl2, CuSO4, NiCl2, H3BO4 and ammonium nitrogen (as described by Egli et al. 2001). During the start-up period the ammonia concentration of the feed solution was kept low, but was increased gradually in response to high ammonium removal efficiencies. At the end of acclimation with synthetic wastewater, the SHARON reactor was fed with the effluent of MBR.
Anammox reactor
The seed sludge for the lab-scale reactor was from an ongoing Anammox reactor which was enriched for 160 days. The reactor was operated in upflow mode and no packing material was used. The reactor was inoculated with approximately 100 mL of Anammox culture containing approximately 200 mg MLSS/L. The reactor was operated at a constant temperature (35 °C) which was maintained by a water bath. A mixture of 90 % N2 + 10 % CO2 gas was supplied from the bottom of the column to provide anoxic conditions in the reactor. The required carbon source and pH balance was provided by CO2 from the gas mixture and HCO3 from the feed. The CO2 present in the supplied gas was sufficient to maintain the inorganic carbon requirement of the Anammox bacteria. The dissolved oxygen concentration of the feed solution was reduced by continuous sparging with nitrogen gas. The Anammox reactor was made of plexiglas material and had a volume of 2.3 L. The average flow rate of the system was 1,600 mL/day, and hydraulic retention time in the system was about 17 h. A synthetic feed solution which was prepared as described by Egli et al. (2001) was used for the Anammox system which consisted mainly of a 1:1 ratio of NH4–N and NO2–N concentrations, along with other trace elements needed for the acclimation of Anammox bacteria. The influent and effluent water were analyzed daily for NH4–N, NO2–N, NO3–N and pH parameters. 2 h composite samples were collected from the effluent for analysis.
Results and discussion
Upflow anaerobic sludge blanket reactor
Since leachate handling is a serious problem in landfills, an alternative combination of treatment processes was applied to obtain economical and efficient treatment. The UASB reactor, which was the first step of the treatment, was fed with raw leachate supplied from the Istanbul Kömürcüoda Landfill Site leachate storage lagoon. The lagoon normally receives leachate generated from active landfill cells (young leachate); however, old leachate generated in closed storage cells is sometimes introduced to the same lagoon due to operational problems. Since leachate was brought to the laboratory periodically at different time intervals, fluctuations were seen in some quality parameters. Leachate supply dates are marked on Fig. 2a and detailed characterization of raw leachate samples is represented in Table 2.
In young leachate samples, COD and BOD5 concentrations were measured approximately in the range of 28,000–37,000 and 27,000–17,000 mg/L respectively. High volatile fatty acid concentrations and low pH values were observed during the sampling period. COD and BOD5 concentrations decreased below 10,000 and 5,000 mg/L respectively upon old leachate being discharged to the lagoon. At that time, the BOD5/COD ratio decreased from a maximum of 0.70 to a minimum of 0.37. The characterization results were found to be similar to the previous studies performed with Landfill Sites located in Istanbul (Akgul et al. 2011; Calli et al. 2005a; Inanc et al. 2000).
During the start-up period, the UASB reactor was seeded with sludge obtained from the anaerobic reactor of the Pakmaya yeast industry. In order to enable the microorganisms to acclimate to high organic content and ammonia nitrogen, the reactor was operated batch wise with diluted leachate. The operation of the reactor was switched to continuous flow when daily COD removal efficiency reached 70 %. Since the low organic loading rate improves the growth of biomass and formation of compact sludge (Singh et al. 1998) during start-up, initial COD loading was adjusted to 0.75 kg COD m−3/day and was gradually elevated to achieve better efficiency and granulation of sludge. Influent and effluent concentrations and removal efficiencies of COD, BOD5 and free ammonia inside the reactor are represented in Fig. 2a–c, respectively.
BOD5 removal efficiency was mostly observed to be higher than 80 % until day 270 of reactor operation; however, it dropped gradually to 60 % as the old leachate with low biodegradability and high ammonia nitrogen entered the system. Meanwhile, COD removal efficiencies dropped to the minimum values of operation (30–40 %) from day 300–420, because the leachate introduced to the UASB was old.
During young landfill leachate feeding, the biodegradable portion of organic matter is high and therefore the COD removal was maintained sufficiently. Moreover, pH and ammonia of young landfill leachate is low which minimizes the formation of free ammonia. The situation is just the opposite in old landfill leachate. The nonbiodegradable portion of organics in old landfill leachate is high and also elevated ammonia concentrations within the reactor may cause free ammonia inhibition in high pH and temperature environments. In order to avoid the inhibitory effect of free ammonia, some studies preferred to decrease the pH of raw leachate (Borzacconi et al. 1999; Calli et al. 2006). In our case, considering the excessive acid requirements of high alkalinity leachate, pH was not decreased and the system was left to acclimate by itself to high free ammonia concentrations of up to 500–600 mg/L. After day 450, although the free ammonia concentration was as high as 500 mg/L, which is higher than the inhibition threshold value reported in literature (Koster 1986), COD and BOD5 removal recovered back to 60 and 80 % respectively. During operation of the UASB, effluent COD concentrations were observed to range between 1,500 and 2,000 mg/L COD, despite high BOD removal efficiencies (90 %). This can be explained by the presence of organics which are inert to anaerobic treatment. In previous studies conducted with young leachates, the achieved COD removal was reported as 64–85 % with an anaerobic SBR system (Timur and Ozturk 1999); 80–90 % with a combined UASB, anaerobic upflow filter and hybrid bed reactor (Calli et al. 2006; Inanc et al. 2000); 77–81 % with continuous UASB and 45–86 % with an anaerobic SBR system with non-diluted leachate samples (Kennedy and Lentz 2000).
Membrane bio-reactor
The MBR was fed with the effluent of the UASB reactor in order to remove residual organic matter prior to the SHARON reactor. As mentioned in the introduction part, the MBR was operated at low sludge age to avoid nitrification. During the first months (until day 100) the sludge age of MBR could not be decreased due to insufficient growth of microorganisms. In order to prevent washout of microorganisms, the sludge age was adjusted to 10 days. During this time interval high sludge age led to the growth of nitrifiers. Ammonia was converted to nitrite and nitrate, thus, very low ammonia concentrations were observed in the effluent until day 100 due to nitrification. After day 100, sludge age was adjusted to 5 days progressively and nitrification was eliminated. In summer months, as a result of hot climate, (between days 100–150 and 510–530), nitrification was still observed despite low sludge ages. Influent and effluent concentrations and removal efficiencies of COD, BOD5 and NH4–N are represented in Fig. 3a–c, respectively.
An increasing trend in the influent COD concentration was observed during the whole operation of MBR. However, the treatment performance of MBR was not affected by this trend. In general, COD removal was observed to be around 40–85 % and decreased to its minimum values (30–65 % removal) when the leachate source was switched to old leachate from day 300 to 400. COD removal in MBR recovered and increased above 60 % after day 400. It was seen that as the concentration of recalcitrant organic matters increases in the influent, the COD removal performance decreases in both MBR and UASB reactors. However, a better toleration of worse conditions was observed in the MBR with respect to the UASB reactor. Ahmed and Lan (2012) have also mentioned that MBRs can easily adapt to the large variations in feed properties and operation conditions without affecting the effluent quality.
Despite the low COD removal performance of MBR, BOD5 removal efficiency ranged from 75 to 99 % and was mostly observed to be above 90 %. Although BOD5 in raw leachate fluctuated and adversely affected the MBR influent loads, the effluent BOD5 was found to be stable. BOD5 effluent ranged from 10 to 500 mg/L but mostly it was found to be below 50 mg/L.
The majority of residual organic matter in MBR effluent was the inert part, which is resistant to biodegradation. The percentage of soluble inert COD to total COD obtained in our case was 10–20 % (data not shown) however, it was mentioned as 30 % (Bilgili et al. 2008) and from 3 to 10 % (Hasar et al. 2009) in past studies.
MBRs have been mostly applied for biologically or chemically pretreated leachate (Ahn et al. 2002; Hasar et al. 2009) or old landfill leachate (Jakopović et al. 2008; Tsilogeorgis et al. 2008). Ahn et al. (2002) applied MBR to the effluent of the chemical precipitation process and it provided only 38 % COD removal. However Hasar et al. (2009) achieved 60–90 % COD removal with leachate which was pretreated with stripping and flocculation processes. In mature (old) landfill leachates, the organic removal performance in MBRs is lower. The COD removal in previous studies has been reported to be between 23 % (Jakopović et al. 2008) and 40–60 % (Tsilogeorgis et al. 2008).
BOD5 and COD treatment efficiencies of the combined UASB and MBR system were found to be above 99 and 80 % respectively, which correlates to the findings of the study conducted by Xu et al. (2008) with a similar anaerobic-MBR configuration. Although UASB-MBR combination achieves good organic removal performance, there are some disadvantages of using low sludge age MBR. For instance, because of low sludge age, the MLSS concentration could not be increased and therefore the required optimum HRT was high. Moreover, low sludge age promotes the formation of extracellular polymeric substances (EPS) (Le-Clech et al. 2006) and leads to fouling in MBRs.
SHARON Reactor
The SHARON reactor, where partial nitrification was carried out, operated over 440 days. In the first period, from days 0 to 74, in order to provide bacterial enrichment and acclimation, the reactor was fed with synthetic wastewater. Influent and effluent ammonium and nitrite concentrations in the SHARON reactor with respect to time are shown in Fig. 4a, b respectively. The influent ammonium concentration was gradually increased up to 70 mg/L, and subsequently effluent of the MBR system was fed as influent to the SHARON system from day 75 (the second period). During the times when ammonium concentration was low in the MBR effluent, ammonium nitrogen was added to the influent of the SHARON reactor: the NH4–N concentration in the feed was gradually increased in proportion to the removal efficiency of the system. During the first 35 days of the second period, approximately 60 % of influent ammonium was converted to nitrite nitrogen. Effluent of the SHARON reactor began to be given as feed to the Anammox system on day 90 of the SHARON reactor operation.
The removal efficiency of the system started to decrease due to low alkalinity between days 112 and 200. In order to increase removal efficiency, the required alkalinity was provided by addition of HCO3. In addition, to promote nitrite accumulation sludge age in the system was decreased to less than 1 day. The removal efficiency and nitrite accumulation rose after day 215. Approximately 40 % of total influent ammonium nitrogen was converted to nitrite nitrogen from day 215 to 320. The influent ammonium concentration was gradually increased up to 1,200 mg/L (0.804 kg N/m3 d) after that point (day 322), hence partial nitritation percentages were observed to decrease depending on free ammonia concentration. It is thought that the gradual increase of influent ammonia concentration caused an increase in pH (>8.7) and a corresponding increase in free ammonia concentration which could have favored the free ammonia inhibition of ammonium oxidizing bacteria (AOB). Free ammonia concentration reached up to around 550 mg/L in the reactor (data not shown). In the literature it has been shown that ammonia oxidizers are inhibited in the range of 10–150 mg/L (Park and Bae 2009). After this high influent period, to overcome the free ammonia inhibition problem in the system, manual pH control was started and the influent ammonium nitrogen concentration decreased to 200 mg/L. Subsequently, ammonium removal efficiency had an increasing trend and correspondingly influent concentration was gradually raised up to 700 mg/L (0.47 kgN/m3 d). Average ammonium removal efficiency was observed to be 74 %, and approximately 65 % of the removed ammonium nitrogen was converted to nitrite nitrogen in the system. The main aim of this work is to investigate the treatment effect of combined Anammox and SHARON processes. The removal efficiencies that were reached in the SHARON system were sufficient to obtain a suitable feed for the Anammox process.
In the literature, various studies reported landfill leachate treatment by partial ammonium oxidation to nitrite, but there are few studies about use of the SHARON process in leachate treatment. Van Dongen et al. (2001) operated a combination of SHARON and a granular sludge sequencing batch Anammox reactor for the treatment of sludge recycle liquor from the WWTP Rotterdam. The authors observed 53 % nitrite accumulation in the SHARON system after applying an ammonium load of 1.2 kg N/m3 d, and more than 80 % of the ammonia was converted into dinitrogen. Liang and Liu (2007) studied the partial nitrification process for landfill leachate treatment using a bench scale fixed bed reactor (ammonium loads between 0.2 and 1.0 kg N/m3 d). They reported 60–74 % NH4–N removal efficiency, 56–70 % partial nitritation efficiency, and obtained a nitrite to ammonium ratio between 1.0 and 1.4. Ganigue et al. (2007) examined partial nitrification in a sequencing batch reactor treating urban landfill leachate. By applying high ammonium loads from 1 to 1.5 kg N/m3 d, stable partial nitritation was achieved, demonstrating the feasibility of this technology as a preceding step to the Anammox process. In another study, Vilar et al. (2010) carried out partial nitrification using the SHARON system for anaerobically pretreated leachate and raw leachate which had been diluted by a 1:5 ratio. The authors reported that in the case of anaerobically pretreated leachate, influent ammonium was converted to nitrite with 60 % efficiency, and when raw leachate was used as feed for SHARON, partial nitrification efficiency diminished to 31 %. Free ammonia concentrations were reported to be between 9.98 and 42.35 mg NH3/L.
Anammox Reactor
The continuous flow Anammox system was operated for 510 days under gradually increased ammonia and nitrite loadings. In the first period, from days 0 to 160, the Anammox system was fed with synthetic wastewater. During the start-up period, low ammonium nitrogen and nitrite nitrogen concentrations were provided and subsequently the concentrations of both in the feed were gradually increased from 100 to 400 mg/L in proportion to the removal efficiency of the system. Effluent of the SHARON reactor was fed to the Anammox system on day 163 of the reactor operation (day 90 of the SHARON reactor). To obtain suitable feed for the Anammox system, ammonium and nitrite were added in a 1:1 ratio (NH4–N/NO2–N) when necessary (until day 398). During the first period, nitrite and ammonium removal efficiencies of the system were observed to be approximately 90 and 81 % respectively. Influent and effluent feed concentrations in the Anammox reactor with respect to time are shown in Fig. 5. After the reactor was fed with SHARON effluent, average nitrite and ammonium average removal efficiencies reached 89 and 77 % respectively. Feed concentrations were gradually increased from 100 to 400 mg/L. Then, SHARON reactor effluent was fed directly to the Anammox reactor as an influent without the addition of nitrite and ammonium nitrogen. Hence, average removal efficiencies of ammonium nitrogen dropped to 43 %, although nitrite nitrogen removal efficiency remained at approximately the same level. Influent ammonium concentrations reached around 1,000 mg/L and nitrite nitrogen concentrations reached around 800 mg/L. The concentrations of both NH4–N and NO2–N in the feed declined to around 250 mg/L because of inhibition in the SHARON reactor. Influent NH4–N and NO2–N concentration of the system was gradually raised up to 800 mg/L (1.13 kg N/m3 d) in parallel with removal efficiencies. Average nitrite nitrogen and ammonia nitrogen removal efficiencies were respectively 92 and 78 % for the entire process after initiating the Anammox system with effluent from the SHARON reactor.
In the literature, there are few studies focused on landfill leachate treatment using partial nitrification and Anammox systems. Liang and Liu (2007) used partial nitrification and Anammox processes followed by two underground soil infiltration systems to treat landfill leachate (NH4–N load 0.27–1.2 kg/m3 d for partial nitrification and 0.06–0.11 kg/m3 d for the Anammox process). The authors reported that the removal efficiencies for NH4–N and NO2–N were 60 and 64 % respectively in the Anammox reactor, and the overall treatment efficiencies for NH4–N and total nitrogen removal were 97 and 87 % respectively after the soil infiltration process. In another study Zhang and Zhou (2006) investigated the treatment effect of the Anammox process on a mixture of landfill leachate and found that ammonium nitrogen and nitrite nitrogen removal efficiency was 87.51 and 74.95 % respectively. Liu et al. (2010) used a combined process consisting of a short-cut nitrification reactor (maximum inorganic N load 1.47 kg/m3 d) and an upflow Anammox reactor (maximum inorganic N load 0.91 kg/m3 d) to treat the diluted effluent of a UASB reactor used for treating high ammonium municipal landfill leachate. They reported an ammonium removal efficiency of over 80 % in the shortcut nitrification reactor, and removal efficiencies of both ammonium and nitrite were 93 % for the Anammox reactor.
In our study, maximum inorganic nitrogen load reached up to 1.13 kg N/m3 d and the ammonium and nitrite removal efficiency reached over 84 and 99 % respectively. The overall removal efficiency provided was on average 92 % for nitrite nitrogen and 78 % for ammonium nitrogen. Comparing our obtained results with the literature, in this study the Anammox reactor that was fed with SHARON effluent resulted in higher removal efficiencies at high nitrogen loads. However, the operational problems that occur in the SHARON reactor directly affect the performance of Anammox. For instance, when the influent ammonia nitrogen could not be converted to 50 % nitrite in the SHARON reactor, ammonia nitrogen removal in the Anammox reactor decreased.
Conclusions
Sequential use of UASB and MBR systems with low sludge age provided over 99 % BOD5 and 90 % COD removal in young leachate samples. Average NO2–N and NH4–N removal efficiencies reached over 92 and 78 % respectively for the entire process after feeding the Anammox reactor with the effluent from the SHARON process. The results presented in this study show that a combined UASB-MBR-SHARON and Anammox configuration allowed reliable discharge effluent quality by improving the removal efficiency of COD and ammonia. Over 90 % COD, TKN and 99 % BOD5 removal efficiencies were obtained in the whole system.
References
Abma WR, Schultz CE, Mulder JW, van der Star WRL, Strous M, Tokutomi T, van Loosdrecht MCM (2007) Full-scale granular sludge Anammox process. Water Sci Technol 55(8–9):27–33. doi:10.2166/Wst.2007.238
Ahmed FN, Lan CQ (2012) Treatment of landfill leachate using membrane bioreactors: a review. Desalination 287:41–54. doi:10.1016/j.desal.2011.12.012
Ahn W, Kang M, Yim S, Choi K (2002) Advanced landfill leachate treatment using an integrated membrane process. Desalination 149(1–3):109–114
Akgul D, Mertoglu B, Yuzer B (2011) Characterization of landfill leachates in Turkey. Fresenius Environ Bull 20(10):2638–2642
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF) (1999) Standard Methods for the examination of water and wastewater, 20th Edn
An YY, Yang FL, Chua HC, Wong FS, Wu B (2008) The integration of methanogenesis with shortcut nitrification and denitrification in a combined UASB with MBR. Bioresour Technol 99(9):3714–3720. doi:10.1016/j.biortech.2007.07.020
Appels L, Lauwers J, Degreve J, Helsen L, Lievens B, Willems K, Van Impe J, Dewil R (2011) Anaerobic digestion in global bio-energy production: potential and research challenges. Renew Sust Energy Rev 15(9):4295–4301. doi:10.1016/j.rser.2011.07.121
Bilgili MS, Demir A, Akkaya E, Ozkaya B (2008) COD fractions of leachate from aerobic and anaerobic pilot scale landfill reactors. J Hazard Mater 158(1):157–163. doi:10.1016/j.jhazmat.2008.01.055
Bohziewicz J, Neczaj E, Kwarciak A (2008) Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 221:559–565
Boonyaroj V, Chiemchaisri C, Chiemchaisri W, Theepharaksapan S, Yamamoto K (2012) Toxic organic micro-pollutants removal mechanisms in long-term operated membrane bioreactor treating municipal solid waste leachate. Bioresource Technol 113(2012):174–180
Borzacconi L, Lopez I, Ohanian M, Vinas M (1999) Anaerobic–aerobic treatment of municipal solid waste leachate. Environ Technol 20(2):211–217
Brouwer M, Van Loosdrecht MCM, Heijnen JJ (1996) One reactor system for ammonium removal via nitrite. Paper presented at the STOWA Report, 96-01. STOWA, Utrecht, The Netherlands
Calli B, Mertoglu B, Inanc B (2005a) Landfill leachate management in Istanbul: applications and alternatives. Chemosphere 59(6):819–829. doi:10.1016/j.chemosphere.2004.10.064
Calli B, Mertoglu B, Inanc B, Yenigun O (2005b) Effects of high free ammonia concentrations on the performances of anaerobic bioreactors. Process Biochem 40(3–4):1285–1292. doi:10.1016/j.procbio.2004.05.008
Calli B, Mertoglu B, Roest K, Inanc B (2006) Comparison of long-term performances and final microbial compositions of anaerobic reactors treating landfill leachate. Bioresour Technol 97(4):641–647. doi:10.1016/j.biortech.2005.03.021
Egli K, Fanger U, Alvarez PJJ, Siegrist H, van der Meer JR, Zehnder AJB (2001) Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch Microbiol 175(3):198–207
Ganigue R, Lopez H, Balaguer MD, Colprim J (2007) Partial ammonium oxidation to nitrite of high ammonium content urban land fill leachates. Water Res 41(15):3317–3326. doi:10.1016/j.watres.2007.04.027
Hasar H, Unsal SA, Ipek U, Karatas S, Cınar O, Yaman C, Kınacı C (2009) Stripping/flocculation/membrane bioreactor/reverse osmosis treatment of municipal landfill leachate. J Hazard Mater 171:309–317. doi:10.1016/j.jhazmat.2009.06.003
Hellinga C, van Loosdrecht MCM., Heijnen JJ (1997) Model based design of a novel process for ammonia removal from concentrated flows. Proceedings of 2nd Mathmod Symposium 865 (70)
Hellinga C, Schellen AAJC, Mulder JW, van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37(9):135–142
Inanc B, Calli B, Saatci A (2000) Characterization and anaerobic treatment of the sanitary landfill leachate in Istanbul. Water Sci Technol 41(3):223–230
Jakopović HK, Matošić M, Muftić M, Čurlin M, Mijatović I (2008) Treatment of landfill leachate by ozonation, ultrafiltration, nanofiltration and membrane bioreactor. Fresenius Environ Bull 17:687–695
Jetten MSM, Horn SJ, vanLoosdrecht MCM (1997) Towards a more sustainable municipal wastewater treatment system. Water Sci Technol 35(9):171–180
Jetten MSM, Strous M, van de Pas-Schoonen KT, Schalk J, van Dongen UGJM, van de Graaf AA, Logemann S, Muyzer G, van Loosdrecht MCM, Kuenen JG (1998) The anaerobic oxidation of ammonium. FEMS Microbiol Rev 22(5):421–437
Jetten MSM, Wagner M, Fuerst J, van Loosdrecht M, Kuenen G, Strous M (2001) Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr Opin Biotechnol 12(3):283–288
Kennedy EJ, Lentz EM (2000) Treatment of landfill leachate using sequencing batch and continuous flow upflow anaerobic sludge blanket (UASB) reactors. Water Res 34(14):3640–3656
Koster IW (1986) Characteristics of the pH-influenced adaptation of methanogenic sludge to ammonium toxicity. J Chem Technol Biotechnol 36(10):445–455
Le-Clech P, Chen V, Fane TAG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Membr Sci 284(1–2):17–53
Liang Z, Liu HX (2007) Control factors of partial nitritation for landfill leachate treatment. J Environ Sci (China) 19(5):523–529
Liang Z, Liu JX (2008) Landfill leachate treatment with a novel process: anaerobic ammonium oxidation (Anammox) combined with soil infiltration system. J Hazard Mater 151(1):202–212. doi:10.1016/j.jhazmat.2007.05.068
Liu J, Zuo JE, Yang Y, Zhu SQ, Kuang SL, Wang KJ (2010) An autotrophic nitrogen removal process: short-cut nitrification combined with ANAMMOX for treating diluted effluent from an UASB reactor fed by landfill leachatee. J Environ Sci (China) 22(5):777–783. doi:10.1016/S1001-0742(09)60176-5
Park S, Bae W (2009) Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem 44(6):631–640. doi:10.1016/j.procbio.2009.02.002
Patsios SI, Karabelas AJ (2011) An investigation of the long-term filtration performance of a membrane bioreactor (MBR): the role of specific organic fractions. J Membr Sci 372(1–2):102–115. doi:10.1016/j.memsci.2011.01.055
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150(3):468–493. doi:10.1016/j.jhazmat.2007.09.077
Singh RP, Kumar S, Ojha CSP (1998) A critique on operational strategies for start-up of UASB reactors: effects of sludge loading rate and seed/biomass concentration. Biochem Eng J 1(2):107–119
Strous M, VanGerven E, Zheng P, Kuenen JG, Jetten MSM (1997) Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (anammox) process in different reactor configurations. Water Res 31(8):1955–1962
Timur H, Ozturk I (1999) Anaerobic sequencing batch reactor treatment of landfill leachate. Water Res 33(15):3225–3230
Tsilogeorgis J, Zouboulis A, Samaras P, Zamboulis D (2008) Application of a membrane sequencing batch reactor for landfill leachate treatment. Desalination 221(1–3):483–493
van Dongen U, Jetten MSM, van Loosdrecht MCM (2001) The SHARON–Anammox process for treatment of ammonium rich wastewater. Water Sci Technol 44(1):153–160
Vilar A, Eiroa M, Kennes C, Veiga MC (2010) The SHARON process in the treatment of landfill leachate. Water Sci Technol 61(1):47–52. doi:10.2166/Wst.2010.786
Xu YP, Zhou YQ, Wang DH, Chen SH, Liu JX, Wang ZJ (2008) Occurrence and removal of organic micropollutants in the treatment of landfill leachate by combined anaerobic-membrane bioreactor technology. J Environ Sci (China) 20(11):1281–1287
Zhang HG, Zhou SQ (2006) Treating leachate mixture with anaerobic ammonium oxidation technology. J Cent South Univ Technol 13(6):663–667
Acknowledgments
The authors thank Marmara University Scientific Research Committee (Project No. BAPKO FEN-C-DRP-060510-0145) and The Scientific and Technological Research Council of Turkey (TUBITAK) Project No: 108Y269 for funding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akgul, D., Aktan, C.K., Yapsakli, K. et al. Treatment of landfill leachate using UASB-MBR-SHARON–Anammox configuration. Biodegradation 24, 399–412 (2013). https://doi.org/10.1007/s10532-012-9597-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-012-9597-y