Abstract
The biodegradation of waters polluted by some bisphenols, endowed with endocrine activity, has been studied by means of laccase or tyrosinase immobilized on polyacrylonitrile (PAN) beads. Bisphenol A (BPA), Bisphenol B (BPB), Bisphenol F (BPF) and Tetrachlorobisphenol A (TCBPA) have been used. The laccase-PAN beads system has been characterized as a function of pH, temperature and substrate concentration. The biochemical parameters so obtained have been compared with those of the free enzyme to evidence the modification induced by the immobilization process. Once characterized, the laccase-PAN beads have been employed in a fluidized bed reactor to determine for each of the four bisphenols the degradation rate constant (k); the τ50, i.e., the time to obtain the 50% of degradation, and the removal efficiency (RE90) after 90 min of enzyme treatment. The same parameters have been measured for each of the four pollutants with the same fluidized bed bioreactor loaded with tyrosinase-PAN beads. The internal comparison, i.e., in each of the two catalytic systems, has shown that both enzymes exhibit a removal efficiency in the following order BPF>BPA>BPB>TCBPA. The external comparison, i.e., the comparison between the two catalytic system, has shown that the catalytic power of laccase were higher than that of tyrosinase. The operational stability of both catalytic systems resulted excellent, since they maintained more than 80% of the initial activity after 30 days of work.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is an industrial raw material for polycarbonate and epoxy resins, unsaturated polyester-styrene resins and flame retardants. The final products are used as coatings on cans, powder paints, additives in thermal paper and in dental fillings, and as antioxidants in plastics. Several studies demonstrated that BPA is an Endocrine Disruptor Chemical (EDC). It mimics or interferes with the action of endogenous hormones (Ashby et al. 2000; Ashby and Tinwell 1998; Gaido et al. 1997; Kim et al. 2001; Krishnan et al. 1993; Matthews et al. 2001; Synder et al. 2003; Tinwell et al. 2000), causing adverse alterations in reproductive and development processes as well as metabolic disorders.
Like BPA, some its derivatives, such as Bisphenol B (BPB), Bisphenol F (BPF), Bisphenol AD (BPAD), Bisphenol AF (BPAF), Bisphenol S (BPS) and Tetramethylbisphenol A (TMBPA) are also used as materials for epoxy resins and polycarbonates lining large food containers and water pipes. Coatings can also be made from mixtures of BPA derivatives. Tetrabromobisphenol A (TBBPA), a halogenated derivative of BPA, is used as a flame retardant (Helleday et al. 1999; Sjodin et al. 2001; Thomsen et al. 2001). Tetrachlorobisphenol A (TCBPA) has been found in the effluent from waste-paper recycling plants (Fukazawa et al. 2001; Kuruto-Niwa et al. 2002). All these bisphenols have also been classified EDCs interfering with the biological functions of natural hormones (Kitamura et al. 2005). All have been shown to exhibit estrogenic activity, but the activities varied markedly from compound to compound. When the propane bridge of BPA was substituted with a hydrophobic group, the estrogenic activity of these compounds was increased (i.e., comparing BPAF and BPB with BPF). On the other hand, the estrogenic activity was decreased by transformation to further oxidized metabolites, or conjugates with glucuronic acid or sulfuric acid at the hydroxyl group (Elsby et al. 2001; Matthews et al. 2001; Nakagawa and Suzuki 2001; Pottenger et al. 2000; Snyder et al. 2000).
Moreover, substituents at the 3,5-positions of the A-phenyl ring markedly influenced the hormonal activity. So, TCBPA, TMBPA and DMBPA showed the higher estrogenic activity than BPA (Fukazawa et al. 2002; Kuruto-Niwa et al. 2002; Olsen et al. 2003). TCBPA, TMBPA and TBBPA showed also significant thyroid hormone activity, while BPA and other derivatives exhibited weak such activity (Kitamura et al. 2005; Sun et al. 2009).
Due to their chemical characteristics and persistence in the environment, the accurate measurements of their presence in aquatic systems as well as the development of methods for their bioremediation from the wastewaters are urgently requested. Laboratory experiments have demonstrated that phenols and aromatic amines are removed from water by enzymatic degradation utilizing various phenoloxidases, such as peroxidase, laccase, or tyrosinase (Bollag 1992; Buchanan and Nicell 1997; Dec and Bollag 2000; Filazzola et al. 1999; Fukuda et al. 2001; Ikehata and Nicell 2000; Kim and Nicell 2006a; Kim and Nicell 2006b; Saito et al. 2004; Tsutsumi et al. 2001). The underlying mechanism of the removal involves enzymatic oxidation of the pollutants to free radicals or quinones that subsequently undergo polymerization and partial precipitation (Bollag 1992; Cabana et al. 2007; Dec and Bollag 1994; Uchida et al. 2001). Formation of these polymerization products is an indication of typical enzymatic activity. These precipitates are easily separable by filtration from the reaction solution.
There are many studies on the application of phenoloxidases immobilized on porous glass beads (Marin-Zamora et al. 2007), chitosan beads (D’Annibale et al. 1999; Ispas et al. 2010; Tamura et al. 2010; Zhang et al. 2008), polymeric membranes (Georgieva et al. 2008; Lante et al. 2000; Moeder et al. 2004; Rasera et al. 2009) and cross-linked as aggregates (CLEAs) or on silica nanoparticles (Cabana et al. 2009; Galliker et al. 2010). We also employed the immobilized laccase for the bioremediation of waters polluted by phenol compounds, such as cathecol (Durante et al. 2004), syringic acid (Attanasio et al. 2005), BPA (Diano et al. 2007) and phthalates (Mita et al. 2010). In any case there are few studies comparing the oxidation ability of phenoloxidases on BPA derivatives.
The aim of this paper was to investigate the ability of immobilized laccase to degrade some BPA derivatives and to evaluate how the immobilization procedure affects the kinetic properties towards the different substrates. Laccase from Trametes versicolor was immobilized on polyacrylonitrile (PAN) beads. Three bisphenols, BPB, BPF and TCBPA, were chosen as substrate models for comparison with BPA. BPA has hydrophobicity and estrogenic activity intermediate between the BPB and BPF, the values of the latter being the smallest (Kitamura et al. 2005). TCBPA was chosen because it is the BPA derivative with the highest estrogenic activity (Kitamura et al. 2005).
In order to emphasize the oxidative power of laccase in respect to other phenoloxidases, experiments have been carried out also by using, in a fluidized bed bioreactor, laccase or tyrosinase separately immobilized on PAN beads. The results are analyzed in terms of removal efficiency.
Materials and methods
Chemicals
Laccase (EC. 1.10.3.2) [23.7 U/mg of solid towards 1 μmole of catechol] from Trametes versicolor, tyrosinase from mushroom (EC. 1.14.18.1) [1,500 U/mg of solid towards tyrosine], Bisphenol A (4,4′-Isopropylidenediphenol; BPA) and Bisphenol F [Bis(4-hydroxyphenyl)-methane; BPF], were purchased from Fluka (Sigma–Aldrich Italia, Milano, Italy). Bisphenol B [2,2-Bis(4-hydroxyphenyl)butane; BPB] and Tetrachlorobisphenol A [2,2-bis-(3,5-dichloro-4-hydroxyphenyl)propane; TCBPA] were purchased from TCI Europe nv (Zwijndrecht, Belgium).
The chemical and physical characteristics, as well as the structural formula of each substrate, are summarized in Table 1. The octanol–water partition coefficient (Kow), inversely related to the solubility of the compound in water, has been reported as an indication of the compound hydrophobicity. The reported EC50 values of estrogenic activity (half maximal effective concentration) were determined (Kitamura et al. 2003) by means of the Estrogen Luciferase Reporter assay using MCF-7 cells.
All other chemicals, including PAN powder, were purchased from Sigma–Aldrich, were of analytical grade and were used without further purification.
Beads preparation and activation
PAN powder (18 g), LiNO3 (1 g) and glycerin (3 g) were dissolved in 78 ml of dimethylformamide. The homogenized mixture was pipetted and precipitated in water. The beads obtained were water-washed and immersed for 24 h in a 30% (v/v) glycerin aqueous solution. After this step the beads were dried in an oven at 70°C for a time sufficient to reach a constant weight.
20 cm3 (12 g) of PAN beads were activated at 50°C for 60 min by treatment with 15% (w/v) NaOH aqueous solution. After washing in distilled water, the beads were treated with a 10% (v/v) aqueous solution of 1,2-diaminoethane (15 ml) at room temperature for 60 min. Then the beads were washed once more in distilled water.
Enzyme immobilization
Laccase immobilization
Laccase immobilization was carried out through a diazotization process, involving the phenolic group of tyrosine residues not present in the catalytic site. The PAN beads were treated at room temperature for 1 h with a 2.5% (v/v) glutaraldehyde (GA) aqueous solution (15 ml). GA was used as the coupling agent. After washing at room temperature with double distilled water, the PAN beads were treated, at room temperature for 90 min, with a 2% (w/v) Phenylendiamine (PDA) solution in 0.1 M sodium carbonate buffer, pH 9.0. PDA was used to obtain aminoaryl derivatives on the supports. Once the beads were water-washed, they were treated at 0°C for 40 min with an aqueous solution containing 2 M HCl and 4% NaNO2. At the end of this treatment, the beads were washed at room temperature in 0.1 M citrate buffer solution, pH 5.0, and then treated at 4°C for 16 h with the same buffer solution containing laccase at concentration of 3 mg/ml. At the end of this step, in order to remove the unbound enzymes, the beads were washed in 0.1 M citrate buffer solution, pH 5.0.
The amount of immobilized enzyme was determined by measuring through the Lowry protein assay method (Lowry et al. 1951) the initial and final concentrations of protein in the solution used for the immobilization and taking into account also the protein amount found in the washing solutions. Under the experimental conditions reported above, the amount of immobilized laccase resulted to be 3.56 ± 0.40 mg, i.e., about 0.3 mg of protein per gram of support.
When not used, the beads were stored at 4°C in 0.1 M citrate buffer pH 5.0.
Tyrosinase immobilization
Tyrosinase was immobilized by using GA in a condensation process involving its NH2-groups. For this purpose, the PAN beads were treated at room temperature for 1 h with a 2.5% (v/v) GA aqueous solution (15 ml). GA was used as coupling agent. After washing at room temperature, the beads were incubated at 4°C for 16 h in a 0.1 M phosphate buffer solution, pH 6.5, containing tyrosinase at concentration of 3 mg/ml. At the end of this step, in order to remove the unbound enzyme, the beads were washed in the phosphate buffer solution. The amount of immobilized tyrosinase, measured by Lowry protein assay method, resulted to be 3.21 ± 0.60 mg, i.e., about 0.28 mg of protein per gram of support.
When not used, the beads were stored at 4°C in 0.1 M phosphate buffer pH 6.5.
Activity assays of free and immobilized laccase
The activity of free or immobilized laccase was determined in a batch reactor by adding the enzymatic system in a vessel filled with the solution to be treated. In the case of free enzyme, 0.1 ml laccase solution (10 mg/ml) were mixed with 10 ml of buffered substrate solution and shaken in a water bath for 90 min. When immobilized laccase was used, a volume of 20 cm3 of catalytic beads were put in 40 ml of buffered substrate solution and shaken in a water bath for 90 min.
In order to determine the pH or the temperature profiles, the activity assays were carried out in a temperature range from 20 to 70°C or at pH in the range 3.0–8.0, by using 0.1 M citrate buffer for the pH from 3.0 to 6.0, or 0.1 M phosphate buffer for the pH from 6.0 to 8.0.
To determine the effect of immobilization on the kinetic parameters, the substrates concentration was varied in the range 0.1–3 mM. This range was determined on the basis of the detection limit of our apparatus and of the water solubility of used compounds. Bisphenols were dissolved separately, in the appropriate amount, in a 96% ethanol aqueous solution and then diluted with buffer to a final volume of 40 ml.
The enzymatic reaction rate was determined by monitoring, at regular time intervals, the disappearance of the substrates in the reaction solution. Once known the substrate concentration changes during the time, the initial reaction rate (expressed in μmoles/min) were obtained by multiplying the value of (d[C]/dt)t=0 by the volume of the treated solution.
Bisphenols removal
A fluidized bed reactor (Fig. 1) was used for the continuous removal of the single bisphenols from the buffered solution by laccase or tyrosinase immobilized on PAN beads. The bed reactor was constituted by a polystyrene pipe (1.7 cm inner diameter, 20 cm length) filled with 12 g (20 cm3) of PAN catalytic beads. The bioreactor was fed with 40 ml of bisphenols substrate solution, at concentration 1 mM and thermostated at 25°C, recirculating at a flow rate of 140 ml/min by means of a peristaltic pump.
The amount of enzymatic degradation was calculated after 90 min of enzyme treatment considering the initial and final bisphenols concentration in the reaction solution.
Chromatografic determination of bisphenols
The bisphenols concentration in the reaction solution was measured by high performance liquid chromatography, HPLC (LC-20AT Shimadzu, Kyoto) equipped with an UV–Visible Diode Array detector (SPD-M20A). Separation of compounds was obtained with a Nucleodur Sphinx RP (25.0 cm × 4.6 mm ID, 5 μm) column (Macherey–Nagel GmbH & Co. KG, Germany) at a flow-rate of 1.3 ml/min. All analyzed samples were pre-filtered by 0.2 μm MCE (Cellulose Mixed Esters) syringe filter (Macherey–Nagel GmbH & Co. KG, Germany).
The mobile phase consisted of acetonitrile/water (30:70 v/v). The chromatographic determination was performed by using a gradient: in 2 min acetonitrile from 30 to 50%; for 8 min acetonitrile/water (50:50 v/v); in 2 min acetonitrile from 50 to 30%.
All assays were carried out in triplicate and gave standard deviations lower of 5%.
Results and discussion
Results with laccase
Biochemical characterization of free and immobilized laccase
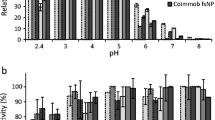
A comparative study between free and immobilized laccase was provided in terms of activity dependence on pH and temperature. The effect of pH on the activity of the free and immobilized laccase for the oxidation of each bisphenol was examined in the pH range 3.0–8.0 at 25°C. For all compounds, the initial substrate concentration was 1 mM in 30% (v/v) ethanol buffered solution.
The experimental results are shown in Fig. 2. Just to give an example, Fig. 2c, relative to BPF as substrate, shows that the oxidation reaction for free and immobilized laccase has the maximum activity at pH 5.0. The only difference is that the pH profile of immobilized laccase is broader compared to the free form, this means that the immobilization process makes the system less sensitive to the pH variations. For the immobilized enzyme, the pH range where the enzyme activity retains more than 90% of its maximum activity is 4.0–6.2; while for the free form it is 4.6–5.4. Another significant difference is that at pH 3.0 the immobilized enzyme retains more than 70% of its maximum activity, while the free form retains only 20%. Similarly, at pH 8.0, the immobilized laccase has 70% of its maximum activity, while free laccase is almost inactive. Experiments with the other bisphenols show a similar pattern.
With regard to the effect of temperature on laccase, the activity of free and immobilized enzyme was assayed with a solution 1 mM of a single substrate in 0.1 M citrate buffer at pH 5.0 in the temperature range 20–70°C. As it can be seen in Fig. 3c, relative to BPF, both free and immobilized laccase shows a maximum activity at 50°C. Moreover, the immobilized laccase has a higher activity at high temperatures (50–70°C) than that of the free counterpart. It is well known that the activity of immobilized enzyme is more resistant against high temperatures than the free form (Lante et al. 2000). Indeed it is interesting to observe that at 70°C the immobilized enzyme retains the 80% of its maximal activity, while the free enzyme has only 15% of its maximal activity.
The experimental results obtained with the other substrates are shown in Fig. 3. Also for BPA, the free and immobilized laccase presents its maximum activity at the same temperature, i.e., 40°C. In the BPB and TCBPA case, the immobilization process induces a shift of the optimum temperature from 40 to 50°C. In addition, the immobilization process involves a broadening of the temperature profile of enzyme activity for all bisphenols. This is in agreement with the results of other investigators (D’Annibale et al. 1999; Dodor et al. 2004).
Kinetic parameters
To know the kinetic parameters of each enzyme reaction, the initial reaction rate of each bisphenol was measured at different initial concentrations with the free and immobilized laccase. Data have been fitted by a Michaelis–Menten equation. Km and maximum reaction rate (Vmax) of the free and immobilized laccase have been calculated by using Lineweaver–Burk double reciprocal plots. The kinetic parameters are listed in Table 2. The Km values of the free laccase resulted to be 0.18 mM for BPA and BPF, and 0.50 and 0.44 mM for BPB and TCBPA, respectively. A general increase in the Km values for the immobilized laccase towards every substrate was observed. The Km absolute values resulted in the same sequence observed for the free laccase. The same behaviour has been reported by other authors (D’Annibale et al. 1999; Lante et al. 2000), indicating a decrease affinity for each substrate caused by diffusional limitations and by decreased protein flexibility after immobilization. On the contrary, a decrease in Vmax values is observed. The Vmax for immobilized laccase compared to that of the free enzyme is reduced of 2.2 times for BPA, of 3.22 times for BPB, of 2.82 times for BPF and of 2.1 times for TCBPA.
Since both Km and Vmax values varied with substrates, the values of Vmax/Km are also reported in Table 2 for easy comparison of the catalytic efficiency of each enzyme-substrate system. These values range between 4 and 41 for free enzyme, and between 2 and 11 for immobilized laccase. The BPF is the substrate towards which the free and immobilized laccase has the highest catalytic efficiency. Indeed the BPF is the BPA derivative which has the lower hydrophobicity (log Kow = 3.06). The lower value of the ratio Vmax/Km of the TCBPA is tied to a possible effect of steric hindrance due to the presence of substituents.
Evaluation of laccase biodegradation capacity
In order to determine the biodegradation capacity of the catalytic system, it has been investigated the removal efficiency towards each bisphenol at a concentration 1 mM in citrate buffer at pH 5 at T = 25°C. 1 mM was chosen considering that this concentration is higher than natural possible concentrations in wastewater (see log Kow in the Table 1). Indeed, for example, in the literature BPA measurements showed these concentrations: from 0.0005 to 0.41 mg/l in surface water, from 0.018 to 0.702 mg/l in sewage effluents, from 0.01 to 0.19 mg/kg in sediments and from 0.004 to 1.363 mg/kg dw in sewage sludge. Since, the removal efficiency decreases with the substrate concentration (Qiu and Huang 2010), the effects observed at 1 mM concentration are lower than those observable at smaller (and natural) concentrations.
In Fig. 4 the decreases of BPA, BPB, BPF and TCBPA concentrations are reported as function of time, when the immobilized laccase (●) is used.
The substrate concentrations appear to decrease with the enzyme treatment time following an exponential curve of the type \( {\frac{C\left( t \right)}{{C_{0} }}}\, = \,e^{ - kt} \), where C(t) and C0 are the BPA concentration at t and zero time, and k (min−1) is a rate constant which depend on the C0 value, or better on the ratio between substrate molecules and available enzyme active sites. The k values for each substrate are reported in Table 3. The k values relative to BPF, BPA, BPB and TCBPA have been found to decrease in this order, from 0.117 to 0.057 min−1.
From k values, two efficiency factors can be calculated: τ50 and RE90. τ50, defined by (ln 0.5)/−k, is the time required to obtain, under our experimental conditions, the 50% of substrate biodegradation. RE90 is the removal efficiency obtained after 90 min of enzymatic treatment, calculated by means of the equation:
where \( \left[ C \right]_{0} \) and \( \left[ C \right]_{90} \) are the values of the substrate concentrations at the beginning of the run and at 90 min, respectively. The τ50 and RE90 values so obtained are listed in the Table 3. 90 min of treatment with the enzyme laccase are sufficient to obtain the complete biodegradation of BPA, BPB and BPF. For TCBPA, a 96% reduction of its initial concentration has been calculated.
Laccase versus tyrosinase
By using the same methodology reported for the “laccase” based beads, similar experiments have been carried out with the bioreactors employing “tyrosinase” based beads. The experimental results have been added in Fig. 4 by using the symbol (○). The experimental conditions were the same than those employed for the laccase. Again the biodegradation curves follow the same kinetic and the new k values relative to the tyrosinase activity are reported in Table 3.
The k values for tyrosinase follow the same order than those found for laccase.
Also in this case from data obtained with the tyrosinase beads we have calculated the τ50 and RE90 values reported in Table 3. 90 min of treatment with the enzyme tyrosinase are sufficient to obtain a biodegradation of all substrates more than 90%.
By comparing the time to obtain the 50% of initial concentration reduction, it is interesting to observe that by using the tyrosinase that time is quite doubled compared to that calculated for the laccase.
Additionally, for both enzymes, the BPF is the substrate towards which the enzymes have the greatest biodegradation capacity. In any case the biodegradation capacity of both enzymes is interesting for practical application.
Operational stability of catalytic systems
In order to evaluate the industrial exploitation of catalytic systems, the knowledge of their operational stability is an important parameter. The latter has been estimated by reusing every day the same PAN catalyst beads for 30 successive days. Each run had duration of 90 min. The time stability of the free enzyme was tested by preparing a stock of enzyme solution and by assaying every day the activity of the same enzyme amount. All daily runs were performed in standard experimental conditions: 1 mM of BPA in buffered solution pH 5.0, at 25°C.
The operational stability of free and immobilized laccase are shown in Fig. 5. It can be seen that the immobilized laccase retains over 85% of its original activity after 30 days. After the same time, the free laccase retains only 5% of its initial activity. The results indicate that the laccase immobilized on PAN beads exhibits a good operational stability.
Similar results (data not reported) were obtained with the free and immobilized tyrosinase.
Conclusions
In this study we have investigated the possible application of laccase or tyrosinase immobilized on PAN beads in fluidized bed reactors for the bioremediation of water polluted by EDCs, such as some BPA derivatives. The industrial value of a biocatalyst system depends on its operational stability and on its ability to process a wide range of substrates. The obtained results have shown that both the laccase from Trametes versicolor and tyrosinase from mushrooms are able to oxidize different bisphenols. In particular, the BPF is the substrate towards which the immobilized enzymes have the highest bioremediation capacity.
For all substrates, the immobilized enzyme is less sensitive to the pH and temperature changes compared to the free form. The improved stability and reusability of catalytic PAN beads represent a potential advantage in wastewater treatment.
When the PAN beads with immobilized laccase were used in the fluidized bed reactor, the higher removal efficiencies (≈100%) were obtained for all bisphenols. The immobilized tyrosinase, under the same experimental conditions, showed a less removal efficiency (~90%), not withstanding the specific activity of this enzyme results 1,500 U/mg, about 65 time that of laccase.
References
Ashby J, Tinwell H (1998) Uterotrophic activity of bisphenol A in the immature rat. Environ Health Persp 106:716–720
Ashby J, Odum J, Paton D, Lefevre PA, Beresford N, Sumpter JP (2000) Re-evaluation of the first synthetic estrogen, 1-keto-1,2,3,4-tetrahydrophenanthrene, and bisphenol A, using both the ovariectomised rat model used in 1933 and additional assays. Toxicol Lett 115:231–238
Attanasio A, Diano N, Grano V, Sicuranza S, Rossi S, Bencivenga U, Fraconte L, Di Martino S, Canciglia P, Mita DG (2005) Nonisothermal bioreactors in the treatment of vegetation waters from olive oil: laccase versus syringic acid as bioremediation model. Biotechnol Progr 21:806–815
Bollag JM (1992) Decontaminating soil with enzymes. Environ Sci Technol 26:1876–1881
Buchanan ID, Nicell JA (1997) Model development for horseradish peroxidase catalyzed removal of aqueous phenol. Biotechnol Bioeng 54:251–261
Cabana H, Jiwan JLH, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, Jones JP (2007) Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsis polyzona. Chemosphere 67:770–778
Cabana H, Jones JP, Agathos SN (2009) Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol Bioeng 102:1582–1592
D’Annibale A, Rita Stazi S, Vinciguerra V, Di Mattia E, Giovannozzi Sermanni G (1999) Characterization of immobilized laccase from Lentinula edodes and its use in olive-mill wastewater treatment. Process Biochem 34:697–706
Dec J, Bollag JM (1994) Dehalogenation of chlorinated phenols during oxidative coupling. Environ Sci Technol 28:484–490
Dec J, Bollag JM (2000) Phenoloxidase-mediated interactions of phenols and anilines with humic materials. J Environ Qual 29:665–667
Diano N, Grano V, Fraconte L, Caputo P, Ricupito A, Attanasio A, Bianco M, Bencivenga U, Rossi S, Manco I, Mita L, Del Pozzo G, Mita DG (2007) Nonisothermal bioreactors in enzymatic remediation of waters polluted by endocrine disruptors: the BPA as model of pollutant. Appl Catal B Environ 69:252–261
Dodor D, Hwang H, Sin E (2004) Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzyme Microb Technol 35:210–217
Durante D, Casadio R, Martelli L, Tasco G, Portaccio M, De Luca P, Bencivenga U, Rossi S, Di Martino S, Grano V, Diano N, Mita DG (2004) Isothermal and non-isothermal bioreactors in the detoxification of waste waters polluted by aromatic compounds by means of immobilised laccase from Rhus veinicifera. J Mol Catal B Enzym 27:191–206
Elsby R, Maggs JL, Ashby J, Park BK (2001) Comparison of the modulatory effects of human and rat liver microsomal metabolism on the estrogenicity of bisphenol A: implications for extrapolation to humans. J Pharmacol Exp Ther 297:103–113
Filazzola M, Sannino F, Rao M, Gianfreda L (1999) Effect of various pollutants and soil-like constituents on laccase from Cerrena unicolor. J Environ Qual 28:1929–1938
Fukazawa H, Hoshino K, Shiozawa T, Matsushita H, Terao Y (2001) Identification and quantification of chlorinated bisphenol A in wastewater from wastepaper recycling plants. Chemosphere 44:973–979
Fukazawa H, Watanabw M, Shiraishi F, Shiraishi H, Shiozawa T, Matsushita H, Terao Y (2002) Formation of chlorinated derivatives of bisphenol A in waste paper recycling plants and their estrogenic activities. J Health Sci 48:242–249
Fukuda T, Uchida H, Takashima Y, Uwajima T, Kawabata T, Suzuki M (2001) Degradation of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun 284:704–706
Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP (1997) Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharm 143:205–212
Galliker P, Hommes G, Schlosser D, Corvini PFX, Shahgaldian P (2010) Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J Colloid Int Sci 349:98–105
Georgieva S, Godjevargova T, Portaccio M, Lepore M, Mita DG (2008) Advantages in using non-isotermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera. J Molecular Catal B Enzym 55:177–184
Helleday T, Tuominen KL, Bergman A, Jenssen D (1999) Brominated flame retardants induce intragenic recombination in mammalian cells. Mutat Res 439:137–147
Ikehata K, Nicell JA (2000) Characterization of tyrosinase for the treatment of aqueous phenols. Biores Technol 74:191–199
Ispas CR, Ravalli MT, Steere A, Andreescu S (2010) Multifunctional biomagnetic capsules for easy removal of phenol and bisphenol A. Water Res 44:1961–1969
Kim YJ, Nicell JA (2006a) Laccase-catalyzed oxidation of bisphenol A with the aid of additives. Process Biochem 41:1029–1037
Kim YJ, Nicell JA (2006b) Impact of reaction conditions on the laccase-catalyzed conversion of bisphenol A. Biores Technol 97:1431–1442
Kim HS, Han SY, Yoo SD, Lee BM, Park KL (2001) Potential estrogenic effects of bisphenol-A estimated by in vitro and in vivo combination assays. J Toxicol Sci 26:111–118
Kitamura S, Ohmegi M, Sanoh S, Sugihara K, Yoshihara S, Fujimoto N, Ohta S (2003) Estrogenic activity of styrene oligomers after metabolic activation by rat liver microsomes. Environ Health Persp 111:329–334
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84:249–259
Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D (1993) Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132:2279–2286
Kuruto-Niwa R, Terao Y, Nozawa R (2002) Identification of estrogenic activity of chlorinated bisphenol A using a GFP expression system. Environ Toxicol Pharm 12:27–35
Lante A, Crapisi A, Krastanov A, Spettoli P (2000) Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem 36:51–58
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marin-Zamora ME, Rojas-Melgarejo F, Garcia-Canovas F, Garcia-Ruiz PA (2007) Effects of the immobilization supports on the catalytic properties of immobilized mushroom tyrosinase: a comparative study using several substrates. J Biotechnology 131:388–396
Matthews JB, Twomey K, Zacharewski TR (2001) In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol 14:149–157
Mita L, Sica V, Guida M, Nicolucci T, Grrimaldi T, Caputo L, Bianco M, Rossi S, Bencivenga U, Mohy Eldin MS, Tufano MA, Mita DG, Diano N (2010) Employment of immobilised lipase from Candida rugosa for the bioremediation of waters polluted by dimethylphthalate, as a model of endocrine disruptors. J Mol Catal B Enzym 62:133–141
Moeder M, Martin C, Koeller G (2004) Degradation of hydroxylated compounds using laccase and horseradish peroxidase immobilized on microporous polypropylene hollow fiber membranes. J Membrane Sci 245:183–190
Nakagawa Y, Suzuki T (2001) Metabolism of bisphenol A in isolated rat hepatocytes and oestrogenic activity of a hydroxylated metabolite in MCF-7 human breast cancer cells. Xenobiotica 31:113–123
Olsen CM, Meussen-Elholm ET, Samuelsen M, Holme JA, Hongslo JK (2003) Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4, 4’-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7. Pharmacol Toxicol 92:180–188
Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM Jr (2000) The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci 54:3–18
Qiu L, Huang Z (2010) The treatment of chlorophenols with laccase immobilized on sol-gel-derived silica. World J Microbiol Biotechnol 26:775–781
Rasera K, Ferla J, Dillon AJP, Riveiros R, Zeni M (2009) Immobilization of laccase from Pleurotus sajor-caju in polyamide membranes. Desalination 245:657–661
Saito T, Kato K, Yokogawa Y, Nishida M, Yamashita N (2004) Detoxification of bisphenol A and nonylphenol by purified extracellular laccase from a fungus isolated from soil. J Biosci Bioeng 98:64–66
Sjodin A, Carlsson H, Thuresson K, Sjolin S, Bergman A, Ostman C (2001) Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol 35:448–454
Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SC, Fennell TR (2000) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168:225–234
Sun H, Shen OX, Wang XR, Zhou L, Zhen SQ, Chen XD (2009) Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol In Vitro 23(5):950–954
Synder SA, Westerhoff P, Yoon Y, Sedlak DL (2003) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168:225–234
Tamura A, Satoh E, Kashiwada A, Matsuda K, Yamada K (2010) Removal of alkylphenols by the combined use of tyrosinase immobilized on ion-exchange resins and chitosan beads. J Appl Polym Sci 115(1):137–145
Thomsen C, Janak K, Lundanes E, Becher G (2001) Determination of phenolic flame-retardants in human plasma using solid-phase extraction and gas chromatography-electron-capture mass spectrometry. J Chromatogr B Biomed Sci Appl 750:1–11
Tinwell H, Joiner R, Pate I, Soames A, Foster J, Ashby J (2000) Uterotrophic activity of bisphenol A in the immature mouse. Regul Toxicol Pharmacol 32:118–126
Tsutsumi Y, Haneda T, Nishida T (2001) Removal of estrogenic activities of bisphenol A and nonylphenol by oxidative enzymes from lignin-degrading basidiomycetes. Chemosphere 42:271–276
Uchida H, Fukuda T, Miyamoto H, Kawabata T, Suzuki M, Uwajima T (2001) Polymerization of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun 287:355–358
Zhang J, Liu X, Xu Z, Chen H, Yang Y (2008) Degradation of chlorophenols catalyzed by laccase. Int Biodeter Biodegr 61:351–356
Acknowledgments
This work has been carried out under the cooperation agreement between the University “Prof. Dr. Assen Zlatarov” of Burgas (Bulgaria) and the Italian Interuniversity Consortium “National Institute for Biostructures and Biosystems” (INBB). This work was also supported by scientific research sector of University “Prof. Dr Assen Zlatarov” of Burgas, by the Italian Ministry of Health/ISPESL under the National Strategic Project “Salute della donna”, by the Italian Ministry of Health/ISZM (Portici-Italy) and by the MIUR through a PRIN project (Funds 2008—Diano).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolucci, C., Rossi, S., Menale, C. et al. Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation 22, 673–683 (2011). https://doi.org/10.1007/s10532-010-9440-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-010-9440-2