Abstract
A composting experiment was conducted to evaluate the effect of a hyperlignocellulolytic fungal consortium and different nitrogen amendments on paddy straw composting in terms of changes in physicochemical and biological parameters. A fungal consortium comprising four lignocellulolytic mesophilic fungal cultures was used as inoculum for bioaugmentation of paddy straw in perforated pits. The comparative effect of farmyard manure (FYM), soybean trash, poultry litter and urea on the composting process was evaluated at monthly intervals in terms of physicochemical (pH, EC, available P, C:N ratio and humus content) and biological (enzymatic and microbial activity) parameters. The compost prepared from bioaugmented paddy straw composting mixture, with poultry manure as nitrogen supplement attained desirable C:N ratio in 1 month and displayed least phytotoxicity levels along with higher production of β-1,4-Exoglucanase. The combined activity of the autochthonous composting microbiota as well as the externally applied fungal inoculum accelerated the composting process of paddy straw. Supplementation of paddy straw with poultry manure in 8:1 ratio was identified as the best treatment to hasten the composting process. This study highlights the importance of application of fungal inoculum and an appropriate N-amendment such as poultry manure for preparation of compost using a substrate having high C:N ratio, such as paddy straw.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the current scenario, agricultural waste management is an important issue worldwide. Crop residues, especially cereal straw which are somewhat recalcitrant, need to be managed tactfully so that the soil organic matter resulting from their bioconversion gives maximum possible benefit to soil health. Paddy straw, one of the most recalcitrant materials among cereals, is not suitable as animal feed because of its high silica content (Juliano 1985). Also, it is burnt in various parts of the globe because of its slow degradation rate in nature. Such practices lead to wastage of large chunks of this otherwise valuable natural resource. Although direct transformation of crop wastes in fields is an alternative for their cost-effective utilization, soil application of large doses of undecomposed plant residues can lead to unfavorable effects on successive plant growth and crop yields due to production of certain phytotoxic allelochemicals (Lee et al. 1999; Chung et al. 2001; Inderjit et al. 2004). Therefore, one of the best possible alternatives to manage this resource is its bioconversion through the action of several hydrolytic enzymes produced by lignocellulolytic microorganisms (Garcia et al. 1992, 1993; Kanotra and Mathur 1994; Vuorinen 1999, 2000; Tuomela et al. 2000). The principal requirement for a compost to be safely used in soil is its degree of stability or maturity, which implies a stable organic matter content and the absence of phytotoxic compounds. C:N ratio, one of the compost maturity indices, must be less than 50:1 at the initiation stage (Madejon et al. 1998; Tuomela et al. 2000) for the composting process to proceed at a faster pace. As the C:N ratio (77:1) of paddy straw is very high, it is essential to bring down the C:N ratio by supplying an exogenous nitrogen source. In nature the bioconversion rate of paddy straw is slow and natural microbiota participate in degradation of this lignocellulosic waste. Therefore bioaugmentation with efficient lignocellulolytic microbes may improve and/or accelerate the composting process. However, extensive composting is a non-profitable practice because of severe losses of carbon and nitrogen. Therefore, for optimization of composting process of a specific crop waste, there is a definite need to understand the progressive changes in the biological and physicochemical parameters with respect to time for defining the optimum composting endpoint.
Lignocellulosic crop residues, which contain cellulose, hemicellulose and lignin, are rich sources of carbon in which the N-content is not sufficient for obtaining good quality composts. Supplementation of such residues with nitrogen-rich wastes of plant and animal origin during composting, has been investigated (Imbeah 1998; Neklyudov et al. 2006).
Keeping in view the above considerations, the present study involved the use of four highly potent lignocellulolytic fungal cultures namely Aspergillus nidulans ITCC 2011, Trichoderma viride ITCC 2211, Phanerochaete chrysosporium NCIM 1073 and Aspergillus awamori F18 which were selected on the basis of hyperlignocellulolytic activity. The selection was made on the basis of preliminary studies on the inter-compatibility of these fungi which can efficiently degrade paddy straw. This consortium was used for composting of paddy straw in perforated pits. The experiment was undertaken to study the effect of nitrogen supplements such as urea, farmyard manure (FYM), soybean trash and poultry manure on physicochemical and biochemical parameters at different time intervals to adjudge the extent of substrate degradation and to study their role in improving the composting process of paddy straw.
Materials and methods
Microorganisms
Forty-two fungi, collected from different culture collection centers in India, were screened for production of extracellular lignocellulolytic enzymes under submerged fermentation of paddy straw (1%) in minimal media. Four promising fungi, A. awamori F-18, A. nidulans ITCC 2011, T. viride ITCC 2211 and P. chrysosporium NCIM 1073 were selected on the basis of their production potential of extracellular enzymes i.e., enzymes of cellulase complex (β-1,4-Exo-glucanase, β-1,4-Endo-glucanase and β-Glucosidase), xylanase, lignin peroxidase and laccase (Table 1) during submerged fermentation of paddy straw in vitro. All the fungi were maintained on potato dextrose agar (PDA) slants at 4°C and subcultured regularly at monthly intervals. A consortium of these fungi was used as inoculum for composting of nitrogen-amended paddy straw (Table 2).
Inoculum development
Boiled sorghum grains were drained, air-dried for 1 h and then coated with CaCO3 (2% w/w) and CaSO4 (4% w/w). One hundred grams of these grains were filled in screw capped bottles (Schott Duran, Germany) of 250 ml capacity and autoclaved for 15 min at 15 psi. The selected fungi were raised separately for 15 days at 30°C on sterile swollen sorghum grains. After growth, all the four individual cultures were mixed together in equal quantity to make a consortium.
Composting of paddy straw
Fresh unchopped paddy straw (40 kg) obtained after harvest of paddy crop variety, PUSA Basmati-1 was filled in above-ground perforated cemented pits (1 m3 dimensions) to make a composting pile. Nitrogen amendment was provided in the form of urea, soybean trash, farm yard manure and poultry manure. Soybean trash was collected after harvest of soybean crop variety ‘PK416’ from the research farm at Indian Agricultural Research Institute (IARI), New Delhi. Mature farm yard manure (FYM) was procured from Division of Agronomy, IARI, New Delhi. Poultry manure (consisting of poultry droppings and poultry farm litter) was collected from poultry farms located in peri-urban areas of New Delhi. All these amendments were used to bring down the C:N ratio of paddy straw in order to initiate decomposition (Table 2). Udaipur rock phosphate (32% P2O5) obtained from Rajasthan, India was incorporated in the pile as a source of insoluble phosphorous. The present investigation was carried out at the Division of Microbiology, Indian Agricultural Research Institute, New Delhi, India between November and January. The mean day temperature varied from 21 to 28°C and night temperature ranged from 7 to 13°C during the experimental period.
The fungal inoculum was added at the rate of 10 g fungal consortium per kg of substrate in one set of treatment. All the treatments were replicated thrice and for every treatment one uninoculated control was maintained (Table 2). All the substrates were mixed homogeneously and water was sprinkled at regular intervals to maintain 60% moisture level throughout composting. The composting piles were turned at fortnightly intervals to provide proper aeration. Temperature was recorded on daily basis from the middle of the compost piles to monitor different phases (mesophilic, thermophilic and cooling phases) of composting.
Sampling and chemical analyses
Samples were collected at monthly intervals from locations at different depths in the compost pit and pooled together to make a composite sample. A part of sample was air-dried for estimation of microbial activity parameters (dehydrogenase, fluorescein diacetate (FDA) hydrolysis and alkaline phosphatase). Five grams of fresh sub-sample from each pit was used for extraction of crude enzymes. The remaining amount of the collected sample was oven-dried at 60°C till constant weight. Each sample was then finely ground and used further for physicochemical analysis of compost.
Extracellular enzyme assay
Fresh compost samples were suspended in sodium phosphate buffer (pH 7.0) in Erlenmeyer flasks and shaken for 2 h on a rotary shaker for proper extraction of extracellular enzymes in the compost. Assay of various extracellular hydrolytic enzymes were based on the release of product and its quantitative determination in the reaction mixture.
Saccharifying cellulase was assayed in terms of β-1,4-exoglucanase (FPase) activity (Ghose 1987). Filter paper strips of 1 cm2 size (50 mg) were incubated in glass vials with compost extract (0.5 ml) and 0.05 M sodium citrate buffer (pH 4.8) at 50°C for 1 h.
β-1,4-endoglucanase (carboxymethyl cellulase) was estimated by incubating compost extract (0.5 ml) with equal amount of 1% carboxymethyl cellulose (low-viscosity) solution.
The reaction mixture prepared for assay of these enzymes was incubated at 50°C for a period of 30 min. The enzyme activity of both these enzymes was assayed spectrophotometrically at 575 nm on the basis of quantity of reducing sugar liberated taking glucose as standard (Ghose 1987). One International Unit (IU) of enzyme represents 1 μmol of glucose liberated per minute of reaction.
Xylanase activity (Bailey et al. 1992) of compost extract was measured by using oat-spelt xylan (1% w/v) as substrate and the resulting xylose concentration was quantified by Dinitrosalicylic acid method (Miller 1959). One IU of xylanase represents one μmole of xylose liberated per minute of reaction.
β-d-glucosidase (Cellobiase) was determined spectrophotometrically at 430 nm by the method of Wood and Bhat (1988) against the standard curve of p-nitrophenol. The H2O2 dependent oxidation of Azure-B (MW 305.8) was used for determination of lignin peroxidase (LiP) activity as described by Kirk et al. (1990). Laccase activity was determined by measuring change in absorbance at 436 nm with 5 mM ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid) as the substrate as described by Munoz et al. (1997). Soluble protein concentration was estimated in the compost extract by the method of Lowry et al. (1951).
Analytical methods
Electrical conductivity and pH measurement were performed with suspension of ground compost in distilled water (1:5 w/v) by electrometric determination using hand-held digital EC meter and pH meter, respectively (Jackson 1967). Organic matter was quantified by dry combustion method in muffle furnace at 550°C for 5 h. Carbon content was calculated by dividing organic matter content by 1.724 based on the assumption that organic matter contains 58% Carbon (Hesse 1971). Total N content was analyzed by Kjeldahl’s method (Jackson 1967). Humus was extracted by shaking 1 g compost sample with 20 ml of 0.1 M sodium pyrophosphate prepared in 0.1 N NaOH solutions (pH 13.0) for 1 h. The dark colored extract was filtered, dialyzed and the humus content was calculated by the method of Kononova (1966). Available phosphorous was estimated by the method of Olsen et al. (1954).
Aqueous extract of finished compost was evaluated for phytotoxicity by means of seed germination test (Zucconi et al. 1981) using cress seeds (Lepidium sativum). The germination test was carried out for 24–48 h in dark at 27°C. Seeds were placed in petridishes on sterile filter paper soaked with 30% solution of filter sterilized aqueous compost extract along with a control of distilled water.
Microbial activity
Microbial activity in fresh compost was estimated in terms of dehydrogenase activity (Casida et al. 1964), alkaline phosphatase activity (Tabatabai and Bremner 1969) and FDA hydrolysis rate (Swisher and Carroll 1980).
Dehydrogenase
Dehydrogenase activity was determined by inoculating compost sample with triphenyl tetrazolium chloride (3% w/v) in dark for 24 h at 30°C and subsequently extracting triphenyl formazan (TPF) with 20 ml methanol. The optical density of filtrate was read at 485 nm. Values of dehydrogenase are expressed as mg of TPF released per gram dry substrate (ds) per hour.
Alkaline phosphatase
Compost (1 g) was incubated with 4 ml of Modified Universal Buffer (pH 11.0) and 1 ml of p-nitrophenyl phosphate disodium salt (0.025 M). This mixture was incubated at 37°C for 1 h. Reaction was stopped by addition of 1 ml CaCl2 (0.5 M) and 4 ml NaOH (0.5 M). The mixture was centrifuged at 4,000g for 5 min and the amount of p-nitrophenol (pNP) released was determined spectrophotometrically at 400 nm. Enzyme activity was expressed as mg of pNP g−1 h−1.
Fluorescein diacetate (FDA) hydrolysis
Fresh samples (0.1 g) were suspended in 5 ml of 60 mM sodium phosphate buffer (pH 7.0) and 20 μl of FDA stock soln. (2 mg ml−1) added in a capped glass vial and shaken for 2 h at 30°C. The reaction was terminated by addition of 5% acetone (v/v). The coarse particles were removed from the solution by centrifugation at 400 g for 5 min at 15°C. Amount of fluorescein was recorded spectrophotometrically at 490 nm with reference to standard curve of fluorescein. FDA hydrolysis rate was expressed as μg of fluorescein released g−1 of compost per hour.
Statistical analysis
The triplicate sets of data for the various parameters evaluated, were subjected to ANOVA (Analysis of Variance) in accordance with the experimental design (completely randomized design) using MSTAT-C statistical package and CD (Critical Difference) values were calculated at 0.05 P-level (Gomez and Gomez 1984).
Results and discussion
Changes in physicochemical parameters during composting
The C:N ratio of plant biomass is a determining factor for its degradation and a low C:N ratio during the initial decomposition phase causes manifold increase in the decomposition rate (Eiland et al. 2001). Paddy straw, the primary substrate, has a high initial C:N ratio of about 77:1 because of its low nitrogen content, whereas soybean trash having higher nitrogen content, shows a lower C:N ratio of 50:1. The initial C:N ratio in the treatments T5 and T6 was brought down to 65 by soybean trash amendments. Poultry manure and FYM, having low C:N ratio is the other two organic substrates used during composting of paddy straw to bring down the overall C:N ratio to 42–45 in different treatments (T7–T10) used during composting (Table 3).
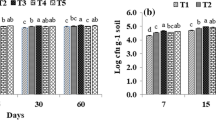
During composting the C:N ratio decreased further due to microbial activity and overall weight loss of substrate due to decomposition. Poultry manure amended straw (in the ratio of 8:1) had the least C:N ratio after composting. Such combination of composting substrates has not yet been reported elsewhere although reports are available where poultry manure has been used as composting substrate with yard trimmings (Tiquia et al. 2001) and combined agrowastes (Cuevas et al. 1988). The carbon content decreased and nitrogen content increased in most of the composting mixtures due to a concentration effect caused by degradation of labile organic carbon compounds and release of CO2 which reduced the overall weight of the composting mass. Humus content increased in all the treatments up to the second month indicating stability of the resulting compost as well as progression of composting process. Veeken et al. (2000) characterized the humic acids during composting of biowaste. They concluded that compost stability is related with microbial activity and can be measured by respiration rate/microbial numbers; and the humus content of compost can be used as index of compost stability.
The pH of a composting mixture plays a major role in governing its composting efficiency by influencing the availability of nutrients. The pH values are closely related to the microbial activity in the composting environment because most of the microorganisms grow well in neutral pH range. In our experiment the pH values (Table 4) vary from near neutral to weakly alkaline (7.50–9.10). There was a declining trend in pH from alkalinity towards neutrality at the end of composting process which is in accordance with the observations of Neklyudov et al. (2006). In another report by Guerra Rodriguez et al. (2000), co-composting of barley waste and solid poultry manure resulted in a product with final pH of 8.72 and a C:N ratio of 13. The EC value did not show any trend during the composting. The lowest value (0.6 mS cm−1) was recorded in poultry manure amended straw in conjunction with fungal consortium after 1 month of composting. The highest value of 4.4 mS cm−1 was recorded in case of soybean trash supplementation (T6) in the second month of composting. In spite of the mineralization of organic compounds, soluble salts would have been lost by leaching, resulting in lower EC value during second month of composting (Benito et al. 2003). Temperature characteristics of the composting piles at different stages of composting are shown in Fig. 1. Temperature recorded during composting process ranged from 21 to 50°C. In all the treatments, temperature of the composting piles peaked after 10 days of composting which declined to the ambient level (30–35°C) afterwards and remained stable till day 60. Cuevas et al. (1988) also observed high temperature of composting piles till 11th day of composting of combined plant wastes with chicken manure which decreased to 37°C and remained stable till end of composting.
Temperature characteristics of compost piles during composting of nitrogen amended paddy straw. T1: (♦) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (■) PS + RP (Inoculated), T3: (▲) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (●) PS + RP + Soybean trash (Inoculated), T7: (◊) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (∆) PS + RP + Urea + FYM (Inoculated), T9: (□) PS + RP + Poultry Manure (Uninoculated), T10: (○) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Soybean trash (Uninoculated), T6: (●) PS + RP + Soybean trash (Inoculated), T7: (◊) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (∆) PS + RP + Urea + FYM (Inoculated), T9: (□) PS + RP + Poultry Manure (Uninoculated), T10: (○) PS + RP + Poultry Manure (Inoculated)
The C:N ratio of all the composting mixtures decreased with the progress in incubation. After the second month of composting the values were less than 15 in all treatments except T1 which contained neither any of the N-supplements nor the fungal consortium. Inoculation with fungal strains lowered C:N ratio in a pronounced manner in all the substrates used in the study (Table 4). However, the lowest C:N ratio of 8.15 was recorded in the bioaugmented, poultry manure amended compost (T10) followed by the bioaugmented composts having soybean trash (T6) and FYM (T8) as the respective supplements (Table 4).
β-1,4-Exoglucanase (FPase) activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
β-1,4-Endoglucanase (CMCase) activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
β-Glucosidase (cellobiase) activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
Xylanase activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
Humus content increased in all the treatments up to the second month. Poultry manure treated paddy straw with bioaugmentation had substantially high humus content (2.38%) at the end of first month of composting. However, the highest value of the humus content (6.76%) was observed at the end of second month of composting in bioaugmented paddy straw compost with urea as N-supplement (treatment T4).
Available phosphorous values in most of the treatments (except T5 and T6) were higher in second month of composting (Table 4) which is contrary to the belief that during later stages of composting most of the phosphorous is immobilized in microbial cells (Coyne 1999). This trend may be due to the increased microbial activity causing release of phosphorous from rock phosphate in the available form since one of the inoculated fungi, A. awamori is an efficient P-solubilizer (Bardiya and Gaur 1974).
Enzymes produced during composting
The production of extracellular hydrolytic enzymes in the composting environment is the initial step for paddy straw degradation. Several hydrolytic enzymes are believed to control the rate at which various substrates are degraded. The hydrolysis of high molecular weight cellulose of plant residues into glucose by exoglucanases, endoglucanases and β-glucosidases is an important reaction making degradable substrates available to microorganisms. In all treatments (except the one containing poultry manure and inoculated with fungal consortium) the production of exoglucanase and β-glucosidase enzymes increased till the end of the composting. Highest activity of exoglucanase was observed after second month in the treatment T6 where paddy straw was supplemented with soybean trash containing the fungal inoculum. Soybean trash contains more lignin (40%) which might have delayed the action of cellulases (Figs. 2–5 ).
Endoglucanase is the first of the three cellulolytic enzymes to act on the lignocellulosic substrate for random cleavage of β-1,4-glucosidic bonds and hence its production in the first half of the composting process is higher as compared to the later phase of composting. Endoglucanase production showed a declining trend in most treatments from first month to second month, except for the treatments T9 and T10, which have poultry manure as supplement. Similarly, β-glucosidase activity increased in most of the treatments during second month of composting as this enzyme cleaves cellobiose moieties generated in abundance due to action of exoglucanase. T5 showed an unexpected decreasing trend of cellobiase activity. Cunha Queda (1999) and Gaind et al. (2005) reported very high level of cellulase throughout the active phase of the composting process with the total cellulase activity still intense at the end stage of composting. In our experiment exoglucanase and β-glucosidase activity increased in second month of composting but endoglucanase production was more in first month of composting. Endoglucanase is the first enzyme to act upon native cellulose which provide reactive sites for the action of exoglucanase, therefore action was more pronounced in the beginning of composting. It was noted that inoculation had a more pronounced effect in increasing exoglucanase activity during second month of composting though the treatment with poultry manure and inoculated fungal consortium had higher exoglucanase production after 1 month of composting. It may be because of the activity of resident lignocellulolytic microbiota present in poultry manure resulting in higher cellulase production. A microbial population count of poultry manure samples obtained from a poultry farm at New Delhi, India, was performed on Reese’s minimal medium containing carboxymethyl cellulose (CMC) as sole source of carbon. The resident microbiota capable of growth on CMC as sole carbon source were found to be in the tune of, 3.25 × 105 colony forming units (cfu) of bacteria per gram, 2.35 × 103 spore forming units of fungi (sfu) per gram and 3.45 × 103 cfu of actinomycetes g−1 (unpublished data).
Xylanase activity showed a marked increase in all treatments from first to second month of composting except T10. It increased substantially as the decomposition progressed and was recorded maximum in the end phase of composting. Xylanase activity in the finished compost was highest in T2 and lowest in uninoculated urea-supplemented straw (T3). The same trend was reported by Gaind et al. (2005) and Goyal et al. (2005) although the starting material was different in both of these studies. Goyal et al. (2005) had also recorded high activity of cellulase at 30 day while xylanase was found to be highest at 60 day in composting mixture of sugarcane trash, cattle dung, pressmud and poultry droppings. Since all the extracellular enzymes are proteinaceous in nature, soluble protein concentration of the composting treatments was also estimated (Fig. 6). The soluble protein concentration was found to be similar in all treatments at both the sampling intervals except for treatment T2 in the second month. Inoculation of lignocellulolytic fungi in paddy straw might have secreted more extracellular lignocellulolytic enzymes under nitrogen-starved condition (Tiquia 2002).
Total soluble protein content during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
Microbial activity during composting
The level of dehydrogenase activity provides information about microbial growth and development as well as an indicator for monitoring compost maturation (Barrena et al. 2008). Dehydrogenase activity was chosen as an index of microbiological activity as it refers to a group of mostly intracellular enzymes in living microbial cells and linked with the biological respiratory process (Forster et al. 1993; Barrena et al. 2008). There was a marked increase in dehydrogenase activity in the second month of composting, in all the treatments (Fig. 7). The highest dehydrogenase activity was recorded in FYM + Urea treated paddy straw compost, inoculated with the fungal consortium (T8). High dehydrogenase activity during second month of composting may be because of high respiratory activity due to proliferation of both inoculated and autochthonous microbiota along with increase in nitrogen percentage which is in accordance with the observations of Benito et al. (2003). Phosphatase activity plays an essential role in the mineralization of organic phosphorous and this enzyme is generally activated when P-availability is low (Nannipieri et al. 1979). In accordance with the above report alkaline phosphatase activity in all treatments increased during second month of composting in our experimental setup. Values of alkaline phosphatase were much higher in the bioaugmented paddy straw treatments (Fig. 8) and the highest activity was recorded in soybean trash supplemented straw (T6). During co-composting of poultry manure and yard trimmings, Tiquia et al. (2001) observed a similar trend of alkaline phosphatase activity, using API-ZYM kit for enzyme assay.
Dehydrogenase activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
Fluorescein diacetate is a non-fluorescent substrate that is hydrolyzed by various enzymes (esterase, protease and lipase) found in a living cell. The hydrolyzed product, fluorescein, is a fluorescent compound which is quantified spectrophotometrically (Schnurer and Roswall 1982). Higher FDA hydrolysis rate was observed during first month of composting after thermophilic phase (Fig. 9) and then decreased in second month except for the treatments T4, T6 and T8. This indicates that bioaugmentation in N-supplemented treatment encourages microbial activity even in second month. Ryckeboer et al. (2003) also observed the same pattern of FDA hydrolysis during composting of biowaste.
Alkaline phosphatase activity during composting of nitrogen amended paddy straw. T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
Highest microbial activity was recorded in T3 at the end of first month of composting (Fig. 9). The slight decrease in microbial activity at the end of second month of composting is indicative of decline in extracellular enzyme production as well as depletion of easily available substrates (Garcia et al. 1994; Gaind et al. 2005). Thus the composting process represented the combined activity of a wide succession of environments, as one enzyme/microbial group overlapped the other and each emerged gradually due to the continual changes in temperature and progressive breakdown of complex compounds to simpler ones (Fig. 9).
Microbial activity during composting of nitrogen amended paddy straw (in terms of fluorescein diacetate hydrolysis). T1: ( ) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: (
) Paddy Straw [PS] + Rock Phosphate [RP] (Uninoculated), T2: ( ) PS + RP (Inoculated), T3: (
) PS + RP (Inoculated), T3: ( ) PS + RP + Urea (Uninoculated), T4: (
) PS + RP + Urea (Uninoculated), T4: ( ) PS + RP + Urea (Inoculated), T5: (
) PS + RP + Urea (Inoculated), T5: ( ) PS + RP + Soybean trash (Uninoculated), T6: (
) PS + RP + Soybean trash (Uninoculated), T6: ( ) PS + RP + Soybean trash (Inoculated), T7: (
) PS + RP + Soybean trash (Inoculated), T7: ( ) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: (
) PS + RP + Urea + Farm yard manure [FYM] (Uninoculated), T8: ( ) PS + RP + Urea + FYM (Inoculated), T9: (
) PS + RP + Urea + FYM (Inoculated), T9: ( ) PS + RP + Poultry Manure (Uninoculated), T10: (
) PS + RP + Poultry Manure (Uninoculated), T10: ( ) PS + RP + Poultry Manure (Inoculated)
) PS + RP + Poultry Manure (Inoculated)
The general trend of dehydrogenase production and FDA hydrolysis is different although both are microbial activity indicators. This trend may be due to participation of many intracellular microbial dehydrogenases even in the second month of composting while FDA can be hydrolyzed by various enzymes excreted out in the compost environment during active phase of microbial growth (Tiquia 2002). Germination indices of all the treatments were >60% showing maturity of finished compost at the end of second month of composting.
Conclusions
Based on the results obtained in the present investigation (changes in C:N ratio and available phosphorous in particular) it can be inferred that poultry manure is the best suited N-supplement out of the four N-amendments for rapid composting of paddy straw. For evaluating the stability and maturity of compost, C:N ratio, humus content, microbial biomass and phytotoxicity level are important parameters. Fungal inoculation improved the decomposition rate by causing increase in the cellulolytic and xylanolytic enzyme activities and thereby accelerating the decomposition process.
References
Bailey MJ, Bailey P, Poutanen K (1992) Inter laboratory testing method for assay of xylanase activity. J Biotechnol 23:257–270. doi:10.1016/0168-1656(92)90074-J
Bardiya MC, Gaur AC (1974) Isolation and screening of microorganisms dissolving low grade rock phosphate. Folia Microbiol (Praha) 19:386–389. doi:10.1007/BF02872824
Barrena R, Vasquez F, Sanchez A (2008) Dehydrogenase activity as a method for monitoring the composting process. Bioresour Technol 99:905–908. doi:10.1016/j.biortech.2007.01.027
Benito M, Masaguer A, Moliner A, Arrigo N, Palma RM (2003) Chemical and microbiological parameters for the characterisation of the stability and maturity of pruning waste compost. Biol Fertil Soils 37(3):184–189
Casida LE Jr, Klein DA, Santaro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376. doi:10.1097/00010694-196412000-00004
Chung IM, Ahn JK, Yun SJ (2001) Identification of allelopathic compounds from rice (Oryza sativa L.) straw and their biological activity. Can J Plant Sci 81:815–819
Coyne MS (1999) Soil microbiology: an exploratory approach. Delmar Publisher, London
Cuevas VC, Samulde SM, Pajaro PG (1988) Trichoderma harzanium Rifai as activator for rapid composting of agricultural wastes. Philipp Agric 71(4):461–469
Cunha Queda AC (1999) Dinamica do azoto durante a compostagem de materiais biologicos (nitrogrn dynamics during putrescible buiomass composting). Ph.D. Dissertation, instituto superior de agronomia universidade tecnica de lisboa, lisbon, Portugal
Eiland F, Klamer M, Lind AM, Leth M, Baath E (2001) Influence of initial C:N ratio on chemical and microbial composition during long term composting of straw. Microb Ecol 41:272–280
Forster JC, Zech W, Wiirdinger E (1993) Comparison of chemical and microbiological methods for the characterization of the maturity of composts from contrasting sources. Biol Fertil Soils 16:93–99. doi:10.1007/BF00369409
Gaind S, Pandey AK, Lata N (2005) Biodegradation study of crop residues as affected by exogenous inorganic nitrogen and fungal inoculants. J Basic Microbiol 45(4):301–311. doi:10.1002/jobm.200410483
Garcia C, Hernandez T, Costa F, Ceccanti B, Ciardi C (1992) Changes in ATP content, enzyme activity and inorganic nitrogen species during composting of organic wastes. Can J Soil Sci 72:243–253
Garcia C, Hernandez T, Costa F (1993) Hydrolases in the organic matter fractions of sewage sludge: changes with composting. Bioresour Technol 45:47–52. doi:10.1016/0960-8524(93)90142-X
Garcia C, Hernandez T, Costa F, Ceccanti B (1994) Biochemical parameters in soil regenerated by addition of organic waste. Waste Manag Res 12:457–466
Ghose TK (1987) Measurements of cellulase activities. Pure Appl Chem 59:257–268. doi:10.1351/pac198759020257
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Goyal S, Dhull SK, Kapoor KK (2005) Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresour Technol 96:1584–1591. doi:10.1016/j.biortech.2004.12.012
Guerra Rodriguez E, Vasquez M, Diaz-Ravina M (2000) Co-composting of barley wastes and solid poultry manure. Bioresour Technol 75:223–225. doi:10.1016/S0960-8524(00)00069-9
Hesse PR (1971) Soil chemical analysis. John Murray, London
Imbeah M (1998) Composting piggery waste: a review. Bioresour Technol 63:197–203. doi:10.1016/S0960-8524(97)00165-X
Inderjit S, Rawat D, Foy CL (2004) Multifaceted approach to determine rice straw phytotoxicity. Can J Bot 82:168–176. doi:10.1139/b03-137
Jackson ML (1967) Soil chemical analysis. Prentice Hall of India Ltd, New Delhi
Juliano BO (1985) Rice hull and rice straw. In: Chemistry and Technology, 2nd edn. American Association of Cereal Chemists, Minnesota, pp 689–755
Kanotra S, Mathur RS (1994) Biodegradation of paddy straw with cellulolytic fungi and its application on wheat crop. Bioresour Technol 47:185–188. doi:10.1016/0960-8524(94)90120-1
Kirk TK, Higuchi T, Chang HM (1990) Lignin biodegradation, microbiology, chemistry and potential applications, vols 1 & 2. CRC, Boca Raton
Kononova MM (1966) Soil organic matter. Pergamon, Oxford
Lee CW, Yoneyama K, Takeuchi Y, Konnai M, Tamogami S, Kodama O (1999) Momilactones A and B in rice straw harvested at different growth stages. Biosci Biotechnol Biochem 63:1318–1320. doi:10.1271/bbb.63.1318
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–277
Madejon E, Galli E, Tomati U (1998) Composting of wastes produced by low water consuming olive mill technology. Agrochimica 42:135–146
Miller GL (1959) Use of DNSA reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Munoz C, Guillen F, Martinez AT, Martinez MJ (1997) Induction and characterization of Laccase in the lignocellulolytic fungus Pleurotus erygnii. Curr Microbiol 34:1–5. doi:10.1007/s002849900134
Nannipieri P, Pedrazzini F, Arcara PG, Piovanelli C (1979) Changes in amino acids, enzyme activities, and biomasses during soil microbial growth. Soil Sci 127:26–34. doi:10.1097/00010694-197901000-00004
Neklyudov AD, Fedotov GN, Ivankin AN (2006) Aerobic processing of organic waste into composts. Appl Biochem Microbiol 42(4):341–353. doi:10.1134/S0003683806040016
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorous in soil by extraction with sodium bicarbonate. USDA circular No. 939. Govt. printing office, Washington, pp 1–9
Ryckeboer J, Mergaert J, Coosemans J, Deprins K, Swings J (2003) Microbiological aspects of biowaste during composting in a monitored compost bin. J Appl Microbiol 94:127–137. doi:10.1046/j.1365-2672.2003.01800.x
Schnurer J, Roswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Swisher R, Carroll GC (1980) Fluroscein diacetate as an estimator of microbial biomass on coniferous needle surface. Microb Ecol 6:217–226. doi:10.1007/BF02010387
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. doi:10.1016/0038-0717(69)90012-1
Tiquia SM (2002) Evolution of extracellular enzyme activities during manure composting. J Appl Microbiol 92:764–775. doi:10.1046/j.1365-2672.2002.01582.x
Tiquia SM, Wan JHC, Tam NFY (2001) Extracellular enzyme profile during co-composting of poultry manure and yard trimmings. Process Biochem 36:813–820. doi:10.1016/S0032-9592(00)00281-8
Tuomela M, Vikman M, Hatakka A, Itavaava M (2000) Biodegradation of lignin in a compost environment: a review. Bioresour Technol 72:169–183. doi:10.1016/S0960-8524(99)00104-2
Veeken A, Nierop K, Wilde Vd, Hamelers B (2000) Characterisation of NaOH-extracted humic acids during composting of a biowaste. Bioresour Technol 72:33–41. doi:10.1016/S0960-8524(99)90096-2
Vuorinen AH (1999) Phosphatases in horse and chicken manure composts. Compost Sci Util 7:47–54
Vuorinen AH (2000) Effect of bulking agent on acid and alkaline phosphomonoesterase and β-d-glucosidase activities during manure composting. Bioresour Technol 75:113–138. doi:10.1016/S0960-8524(00)00042-0
Wood TM, Bhat KM (1988) Methods of measurement of cellulase activity. Methods Enzymol 160:87–112. doi:10.1016/0076-6879(88)60109-1
Zucconi F, Forte M, Monaco ADE, Bertoldi M (1981) Biological evaluation of compost maturity. Biocycle 22:27–29
Acknowledgements
Authors are thankful to the National Agricultural Technology Project scheme of Indian Council of Agricultural Research for funding the present research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, A.K., Gaind, S., Ali, A. et al. Effect of bioaugmentation and nitrogen supplementation on composting of paddy straw. Biodegradation 20, 293–306 (2009). https://doi.org/10.1007/s10532-008-9221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-008-9221-3