Abstract
The present work deals with the biosorption performance of raw and chemically modified biomass of the brown seaweed Lobophora variegata for removal of Cd(II) and Pb(II) from aqueous solution. The biosorption capacity was significantly altered by pH of the solution delineating that the higher the pH, the higher the Cd(II) and Pb(II) removal. Kinetic and isotherm experiments were carried out at the optimal pH 5.0. The metal removal rates were conspicuously rapid wherein 90% of the total sorption occurred within 90 min. Biomass treated with CaCl2 demonstrated the highest potential for the sorption of the metal ions with the maximum uptake capacities i.e. 1.71 and 1.79 mmol g−1 for Cd(II) and Pb(II), respectively. Kinetic data were satisfactorily manifested by a pseudo-second order chemical sorption process. The process mechanism consisting of both surface adsorption and pore diffusion was found to be complex. The sorption data have been analyzed and fitted to sorption isotherm of the Freundlich, Langmuir, and Redlich–Peterson models. The regression coefficient for both Langmuir and Redlich–Peterson isotherms were higher than those secured for Freundlich isotherm implying that the biosorption system is possibly monolayer coverage of the L. variegata surface by the cadmium and lead ions. FT-IR studies revealed that Cd(II) and Pb(II) binding to L. variegata occurred primarily through biomass carboxyl groups accompanied by momentous interactions of the biomass amino and amide groups. In this study, we have observed that L. variegata had maximum biosorption capacity for Cd(II) and Pb(II) reported so far for any marine algae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global phenomenon of water contamination by toxic heavy metals has acquired serious dimensions and the researchers are engaged in combating this mammoth menace. Cadmium and lead are two of the “big three” toxic metals, the other one being mercury, are of profound concern as toxic contaminant of aqueous environment and becomes concentrated throughout the food chain to the extent of posing serious threat to health. These metals are known to cause renal dysfunction, bone degeneration, lung inefficiency, liver damage and hypertension in humans (Carson et al. 1986). Stringent environmental legislation and policies need to be framed and new technologies are quintessential to eliminate metal from contaminated/wastewater. Removal of these pollutants from aqueous effluents has conventionally been accomplished through a range of abiotic processes (Chemielewski et al. 1997; Yabe and de Oliveira 2003). These processes are expensive and at times not fully effective. Recently, a surging interest has been observed in the application of materials of biological origin for removal of heavy metals from diluted large volume solutions. Sorption with bio-materials has become an alternative method for industrial wastewater treatment such as precipitation, adsorption, coagulation etc. (Chu 1999; Matlock et al. 2001; Mehta and Gaur 2005). Biosorption is relatively inexpensive, non-hazardous, and may permit recovery of the metals from the sorbing biomass (Basha and Murthy 2007a, b; Deng et al. 2007). The potential of dead brown seaweeds in the recovery of heavy metal ions from aqueous effluents has been well demonstrated by researchers (Matheickal and Yu 1997; Davis et al. 2003a, b; Yun 2004; Basha et al. 2008).
Seaweeds are harvested or cultivated worldwide and are readily available in massive quantities for the development of highly effective biosorbent materials. Recent investigations by various groups have revealed that selected species of brown seaweeds possess excellent sorption capacities for the removal of Cd and Pb in view of their high uptake capacities and availability in enormous amounts from oceans (Matheickal and Yu 1999; Yu et al. 1999; Lodeiro et al. 2006; Sheng et al. 2007). Suarashtra-Kutchch coast of India is abounding with the seaweed Lobophora variegata which could be economically used as a potential biosorbent for heavy metal removal from aqueous solutions. The thallus of L. variegata is found to be growing in different shapes—prostrate, erect, flat, deltoid or orbicular. At grown up stage, it is 6 cm wide, 1–3 mm thick and remains attached to substratum by rhizoids. The present study is focused on the feasibility of Cadmium and Lead ion removal from aqueous solution by both raw and pretreated biomass of L. variegata. The influence of different paradigms on metal uptake such as aqueous metallic concentration, contact time, pH of solution, and biosorbent concentration was investigated in batch conditions. Various models were tested to investigate the sorption behaviour of kinetic and equilibrium. The mechanism of metal ions sorption at L. variegata—solution interface was also studied.
Experimental methods
Materials
The chemicals used in this study were of analytic reagent grade and obtained from Merck India. The stock solution of Cd(II) and Pb(II) (10 mmol for each ion) was prepared by dissolving weighed quantity of the respective nitrate salts in double distilled water. The concentrations of metal solutions ranged from 0.5 to 5.5 mmol l−1. Before mixing with the biosorbent, the pH of each solution was adjusted to the appropriate value for the sorption of Cd(II) and Pb(II) ions by adding 0.1 M NaOH or 0.1 M HNO3.
Preparation of the biosorbent
The brown alga L. variegata was collected from Okha port (Latitude 22°28.580′ N, Longitude 69°04.254′ E), Arabian Sea, India. The alga was washed twice with running tap water and five times with deionized water. The washed biomass was oven-dried at 60°C for 24 h, crushed with an analytical mill, sieved (size fraction of 300–600 μm) and stored in polyethylene bottles for use. The raw biomass was chemically modified by a pre-treatment with CaCl2. A sample of 20 g of dried biomass was treated with 0.2 M CaCl2 solution (400 ml) for 24 h under slow stirring (150 rpm). The solution pH was kept constant at pH 5.0. The calcium treated biomass was repeatedly washed with deionized water to remove excess calcium from the biomass. The biomass was then heated in an oven at 60°C for 24 h and sieved for particle size of 300–600 μm (Matheickal et al. 1999).
Biosorption experiments and analytical method
All the experiments were conducted at a constant temperature of 25 ± 2°C to cater to environmentally relevant conditions. Batch equilibrium biosorption experiments were carried out in 250 ml Erlenmeyer flasks containing cadmium chloride and lead nitrate solutions (100 ml) of known concentrations which varied from 0.5 to 5.5 mmol l−1. Weighed amounts of biomass (200 mg) were added to each flask and the mixtures were agitated on the rotary shaker. The pH solution was adjusted to the required value by using HNO3 or NaOH. After 6 h of agitation, the solution was separated from the biomass by membrane filtration (Millipore 0.45 mm pore size) and the filtrates were analyzed by atomic absorption spectroscopy (AA-680, Atomic absorption/Flame emission spectrophotometer, Shimadzu) for Cd(II) and Pb(II) ions. All the instrumental conditions were optimized for maximum sensitivity as specified by the manufacturer.
Kinetic experiments were conducted on a rotary shaker with constant agitation speed of 200 rpm, containing 100 ml of solution (2 mmol of lead and cadmium) and 200 mg of biomass. The pH of the solution was kept constant at pH 5. Samples were drawn from the mixture at pre-determined time intervals for analysis.
All the biosorption experiments were repeated twice to substantiate the results. The data shown are the mean values of two replicate determinations.
Metal uptake capacity
The amount of metal sorbed at equilibrium, q e (mmol g−1) which represents the metal uptake, was calculated from the difference in metal concentration in the aqueous phase before and after biosorption, as per following equation:
where, V is the volume of metal solution (l), C i and C e are the initial and equilibrium concentration of metal in solution (mmol l−1), respectively, and W is the mass of dry seaweed (g).
Biomass characterization
The surface area, pore volume and pore size of various forms of L. variegata are measured by surface area analyzer (Micromeritics, ASAP 2010) and are given in Table 1. Infrared spectra of unloaded and metal loaded biomasses of L. variegata were obtained using a Fourier Transform Infrared Spectrometer (FT-IR GX 2000, Perkin-Elmer). Before the analysis, the wet samples were freeze-dried, and 30 mg of finely ground biomass was palleted with 300 mg of KBr (Sigma) in order to prepare translucent sample disks. The FT-IR spectra were recorded over the wave number range of 400–4,000 cm−1 with ten scans at a resolution of 4 cm−1.
Non-linear regression analysis
All the model parameters were evaluated by non-linear regression using DATAFIT® software (Oakdale Engineering, USA). The optimization procedure required an error function to be defined in order to be able to evaluate the fit of the equation to the experimental data (Kundu and Gupta 2006). Apart from the regression coefficient (R 2), the residual or sum of square error (SSE) and the standard error (SE) of the estimate were also used to gauge the goodness-of-fit. SSE can be defined as:
SE can be defined as:
where, q i is the observation from the batch experiment i, Q i is the estimate from the isotherm for corresponding q i , m is the number of observations is in the experimental isotherm and p number of parameters in the regression model. The smaller SE and SSE values indicate the better curve fitting.
Results and discussion
Biosorption of Cd(II) and Pb(II) by L. variegata
The results exhibited that the raw biomass L. variegata was able to remove the metal ions from aqueous solutions with high efficiency (1.65 and 1.70 mmol g−1 for Cd(II) and Pb(II), respectively). A pretreatment of the biomass with CaCl2 marginally enhanced the removal capacity for both the metals (Table 2). The reported work on the removal of these metal ions by different seaweeds and other adsorbents, as given in Table 2, reveals that the biosorption capacity of L. variegata is the highest except Pseudomonas fluorescens BM07 (Noghabi et al. 2007) and Mimosa pudica for Cd(II) (Chen et al. 2008). A mixture of polysaccharides, mainly alginate, has been found to be responsible for the superior metal-sequesterability of brown seaweeds (Davis et al. 2003b). In addition to its high porosity, the algal cell constituents provide an array of chemical ligands, which provide binding functional groups for the uptake of metal ions. Many metal-binding mechanisms have been postulated to be active in biosorption processes such as ion exchange, complexation, coordination, and microprecipitation (Davis et al. 2003b). Because of the complexity of the composition of the biomaterial, it is quite possible that at least some of these mechanisms are acting simultaneously, to varying degrees, depending on the biosorbent and the solution chemistry.
Table 2 also shows that the pretreatment enhances the biosorption capacity, though moderately. The addition of calcium to alginate, whose main component is alginic acid, produced a cooperative association between chains (preferentially of α-l-guluronic acid and β-d-mannuronic acid), which gave rise to a new molecular structure with large cavities occupied by calcium ions (Matheickal et al. 1999). The rod-like shape of the poly-l-guluronic sections results in an alignment of two chain sections yielding an array of coordination sites (Fig. 1). This description is known as the “egg-box” model (Rees 1981). Also, the calcium retained by alginate played an important role during ion exchange (Figueira et al. 2000). This explains the biosorption improvement observed for Cd(II) and Pb(II). Finally, Fourier-transformed infrared (FT-IR) spectral analyses have shown that cadmium and lead biosorption to L. variegata arises from bridging complex formation with the carboxylate groups of the alginate [25], consistent with the above described “egg-box” model of Rees (1981).
Schematic representation of the calcium-induced gelation of alginate in accordance with the “egg-box” structure. After Christensen et al. (1990)
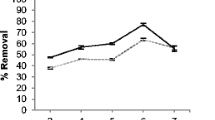
Effect of pH
The pH of the aqueous solution is a key controlling parameter in the biosorption process. Thus, the effect of hydrogen ion concentration was examined at various pH (ranging from 1 to 5). Figure 2 summarizes the uptake of Cd(II) and Pb(II) onto biomass of L. variegata at various pH. A trend of increasing metal ion binding with increasing pH was observed for raw as well as chemically-modified biomass. Maximum biosorption of the Cd(II) (0.9 mmol g−1) and Pb(II) (0.95 mmol g−1) was observed at pH 5.0.
It is well known that the sorption of metals decreases at low pH values because of competition for binding sites between cations and the products of acid hydrolysis (El-Bishtawi and Ali 2001). El-Bishtawi and Ali (2001) proposed two reactions, involving Pb, for this process, which can be adapted for other divalent metals (Me2+):
Protons and oxonium ions (H3O+) concentrations are relatively high at low pH and compete with metals for ion exchange. Results from Fig. 2 suggest that this process occurred in the Cd-, Pb-L. variegata-pH systems studied here, with the maximum biosorption of both Cd and Pb at pH of 5.
The dependence of metal uptake on pH is related to both the surface functional groups on the cell walls of the biomass and the metal chemistry in solution. As the metals were present in their ionic state at a low pH (e.g., pH < 5; see Fig. 2), the sharp increase in metal biosorption from pH 2–5 could not be explained by the change in metal specification. This implied that the functional groups on the cell wall and its ionic state at these pHs determined the extent of biosorption. The positively charged hydrogen ions may also have competed with metal ions for binding on the ligands on the cell wall. At lower pH, the higher concentration of the hydrogen ions effectively led to fewer ligands being available for the binding of the metal ions. Increased pH (i.e., fewer H+ ions) results in more ligands were available for metal ion binding, and hence biosorption was enhanced (Matheickal et al. 1999). The typical dependence of metal uptake on pH suggested that the weak acidic carboxyl groups R–COO– (apparent pKa in the range 3.5–5.0) of algal cell wall might act as the probable biosorption sites. A good correlation between the degree of blocking of –COO– groups by esterification in Sargassum fluitans and the corresponding decrease in metal uptake were reported (Fourest et al. 1996; Davis et al. 2003a). Similar results were also obtained for the carboxyl groups in the biomass of the freshwater algae Chlorella pyrenoidosa and Cyanidium caldarium (Gardea-Torresdey et al. 1990).
In order to examine the biosorption potential of raw and treated biomass of L. variegata and to ensure that the heavy metals existed in their ionic states during biosorption, the pH in subsequent kinetic and isotherm experiments were maintained at 5.0.
Effect of solid/liquid (s/l) ratio
The effect of s/l ratio on cadmium and lead biosorption was studied at room temperature and at pH 5. Various s/l ratios including 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 g l−1 were tested, while keeping the volume (100 ml) and the initial concentration of the metal solution (2.0 mmol l−1) constant. Both raw and treated biomass showed a similar behaviour (Figure not shown). The removal rate for both Cd (II) and Pb (II) increased with increasing s/l ratio upto 2.0. However, the increase in s/l ratio above 2.0 g l−1 did not show significant increase in the removal rate. The maximum removal rate was more than 90% for both the metals when the s/l ratio is 2.0–3.0 g l−1. Thus the optimum s/l ratio was 2.0 g l−1 in terms of the cost effect.
Determination of equilibrium time
The batch experiments carried out to study the relationship between contact time and Cd and Pb uptake by seaweed biomass showed that the equilibrium time was reached in less than 120 and 200 min for Cd(II) and Pb(II), respectively (Fig. 2). It was noticed that the contact time significantly affected the metal uptake; the metal biosorption increased sharply in the first 90 min and tapered off thereafter, as equilibrium was approached. This relatively rapid uptake indicated that the biosorption process occurred mainly on the surface of the sorbent. The uptake of heavy metal ions by biosorbents occurred in two stages: the first rapid and quantitatively predominant and the second slower and quantitatively insignificant (Sag and Kutsal 1996). The rapid stage was probably due to the abundant availability of active sites on the biomass, and with the gradual occupancy of these sites, the biosorption became less efficient in the slower stage (da Costa and Leite 1991). We found that about 90% of the total metal ion biosorption was achieved within 90 min. Therefore, in subsequent equilibrium experiments, 6 h was deemed sufficient to establish equilibrium.
Dynamic modeling
The transient behavior of the batch sorption process was analyzed using the pseudo first-order (Lagergren 1898) and pseudo-second order kinetic models (Ho and McKay 1998). The rate equations are mentioned in Table 3.
The pseudo-first order considered the rate of occupation of sorption sites to be proportional to the number of unoccupied sites. The value of pseudo-first order rate constant, k 1 along with statistical parameters for both Cd(II) and Pb(II) at initial concentration of 2 mmol l−1 and pH 5.0 are given in Table 4. It was observed that the biosorption data were satisfactorily represented by the Lagergren model for both the metals only for the first 30 min and thereafter it deviated from theory (Figure not shown). In other words, the biosorption data were well represented only in the region where rapid biosorption occurred i.e. for the first 30 min. Ho and McKay (1998) reported similar observation as the biosorption data were represented well by the Lagergren first-order model only for the rapid initial phase that occurred for a contact time of 0–30 min for basic dyes onto peat particles. This confirmed that it is not appropriate to use the Lagergren kinetic model to predict the biosorption kinetics for the entire biosorption period in the present case.
The kinetic data were further analyzed using a pseudo-second order relation proposed by Ho and McKay (1998) and Fig. 3 shows the predicted kinetics from pseudo-second order model for both Cd(II) and Pb(II) onto L. variegata. The pseudo-second order rate constant k 2, the calculated h value, and the corresponding R 2, SE and SSE values are shown in Table 4. The regression coefficient (R 2) values were found to be higher and ranged from 0.9672 to 0.9970, while SE and SSE values were low and ranged from 0.0161 to 0.0801 and 0.0020 to 0.0051, respectively for both the metals. The high R 2 and low SE and SSE values confirmed that the biosorption data were well represented by pseudo-second order model for the entire biosorption period and thus supported the assumption behind the model that the biosorption was due to chemisorption, which was in consonance with the biosorption equilibrium data well represented by Langmuir isotherm equation. Also from Table 4, it was observed that the kinetic data of Cd(II) was well represented by pseudo-second order model as compared to Pb(II). Table 5 presents pseudo-second order model constants of brown marine algae obtained for metal concentrations in the liquid phase similar to those used in the present study. In spite of some differences in the experimental conditions, the initial sorption rates of Cd(II) and Pb(II) are very high while kinetic constants (k 2) are lower than those reported by others.
From a mechanistic viewpoint, to interpret the experimental data, it is necessary to identify the steps involved during sorption (Sankar et al. 1999), described by external mass transfer (boundary layer diffusion) and intraparticle diffusion (Weber and Morris 1963). The intraparticle diffusion coefficients for the biosorption of Cd(II) and Pb(II) were calculated from the slope of the plot between the amount of metal sorbed, q t (mg g−1) versus t 0.5(min0.5) after subjecting it to nonlinear regression analysis (Fig. 4). It was observed that the biosorption process of both Cd(II) and Pb(II) comprised of two phases, suggesting that the intraparticle diffusion was not the rate-limiting step for the whole reaction (Ho and Ofomaja 2005). A first linear portion ended with a smooth curve followed by a second linear portion. The double nature of the curve reflected two-stage external mass transfer followed by intraparticle diffusion of Cd(II) and Pb(II) onto L. variegata. Similar results were reported by Aguilar-Carrillo et al. (2006). The intercept of the plot provided an estimation of the thickness of the boundary layer, i.e., the larger the intercept value the greater was the boundary layer effect (Basha and Murthy 2007b). The slope of the second linear portion of the plot has been identified as the intraparticle diffusion rate constant k p (mg g−1 min−0.5). The calculated k p values for both Cd(II) and Pb(II) are given in Table 4. It was observed that the k p values for Pb(II), both raw as well as treated seaweed, were more than Cd(II). The observed higher values of k p corresponded to lower values of pseudo-second order rate constant, k 2 (Table 4), indicating that the intraparticle diffusion retarded the biosorption process for Pb(II). This also indicated that the biosorption process was rather complex and involved more than one diffusive mechanism.
Due to double nature of intraparticle diffusion (both film and pore diffusion), and in order to determine the actual rate-controlling step involved in the metal biosorption process, the kinetic data were further analyzed using the kinetic expression given by Boyd et al. (1947):
where, B b is a constant and F is the fractional attainment of equilibrium at time t given by
To compute B b t, Eq. 6 is substituted into Eq. 7 and the kinetic expression becomes
Thus, the value of B b t can be computed for each value of F, and then plotted against time (Fig. 5) to configure the so-called Boyd plots. The linearity of these plots was employed to distinguish between external-transport- (film diffusion) and intraparticle-transport-controlled rates of sorption (Wang et al. 2006). A straight line passing through the origin indicated sorption processes were governed by particle-diffusion mechanisms; or else they were governed by film diffusion (Mohan and Singh 2002). In our case, the plots were neither linear nor passed through the origin (Fig. 5). This indicated that film diffusion was the rate-limiting biosorption process for Cd(II) and Pb(II) on both raw and treated L. variegata. However, further studies are required to establish this observation. Similar type of results were reported by El-Kamash et al. (2005) and Wang et al. (2006).
Effect of initial metal ion concentration on biosorption
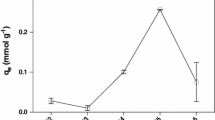
The metal uptake mechanism was particularly dependent on the initial metal concentration: at low concentrations metals were adsorbed by specific sites, while with increasing metal concentrations the specific sites were saturated and the exchange sites were filled (Lehmann and Hater 1984). The amount of Cd(II) and Pb(II) biosorbed per unit mass of the raw biomass (i.e. equilibrium biosorption capacity, q e ) increased from 0.20 to 1.65 and 0.15 to 1.70 mmol g−1, respectively, with increase in the equilibrium concentration from 0.5 to 5.5 mmol l−1. However, the treated biomass showed an increase in biosorption capacity from 0.27 to 1.71 and 0.17 to 1.79 mmol g−1 for Cd(II) and Pb(II), respectively. The increase in biosorption capacity with initial concentration might be due to higher availability of metal ions in the solution. Moreover, higher initial concentration provided increased driving force to overcome all mass transfer resistance of metal ions between the aqueous and solid phases resulting in higher probability of collision between metal ions and biosorbent.
Biosorption isotherm models
Analysis of equilibrium data was important for developing an equation that could be used to compare different biosorbents under different operational conditions and to design and optimize an operating procedure. To examine the relationship between biosorption and aqueous concentration at equilibrium, various sorption isotherm models were widely employed for fitting the data (Vijayaraghavan et al. 2006). In the present investigation the equilibrium data were analyzed using two-parameter models like Langmuir (Langmuir 1916), Freundlich (Freundlich 1906) and a three-parameter model, Redlich–Peterson (Redlich and Peterson 1959) isotherm models. Although these models did not enlighten on the mechanistic aspect of biosorption, they remain a useful and convenient tool for comparing results from different sources on a quantitative basis, providing information on sorption potential and reproducing the usual equilibrium uptake process behaviour with easily interpretable constants.
The Langmuir model served to estimate the maximum metal uptake values where they could not be reached in the experiments. The value of K L is a coefficient attributed to the affinity between the sorbent and sorbate (Langmuir 1916). The value of n, of the Freundlich model, falling in the range of 1–10 indicates favorable sorption and Redlich–Peterson isotherm has three isotherm constants, namely, K RP, a RP and β (0 < β < 1), which characterize the isotherm. The model constants of Langmuir, Freundlich and Redlich–Peterson (RP) models along with regression coefficients (R 2), standard error (SE) of the estimate and residual or sum of squares error (SSE) are given in Table 4. Figure 6 show the fitted equilibrium data of Cd(II) and Pb(II) to Langmuir and RP isotherm expressions. It was observed that the equilibrium data of Cd(II) and Pb(II) fitted both the Langmuir and RP expressions with acceptable regression coefficient values ranging from 0.9747 to 0.9907 and 0.9707 to 0.9787, respectively. However, SE and SSE values were lower in case of RP model as compared to Langmuir. The low correlation coefficient as well as high SE and SSE values for the Freundlich isotherm as compared to Langmuir and RP confirmed the non-applicability of this model for both Cd (II) and Pb(II) on raw and treated biomass. The higher regression coefficients for the Langmuir and RP isotherms predicted the monolayer coverage of metals on L. variegata biomass. The good fit of the Langmuir and RP isotherms were not the same even when the coefficient of determinations was high for both isotherms. The essential characteristics of Langmuir isotherm can be explained in terms of the dimensionless separation factor, R L (Hall et al. 1966). The R L values indicated (Table 4) that Cd(II) and Pb(II) biosorption on to L. variegata is favorable.
Table 6 presents the comparison of Langmuir parameters of L. variegata biomass for the Cd(II) and Pb(II) with those of various seaweed biomasses in literature. The maximum biosorption capacity (q m ) of L. variegata biomass for these metal ions is higher while K L values are much lower than that of other biomasses. However, the exact comparison becomes difficult because of the different experimental conditions (pH, temperature, sampling periods, and initial metal concentration).
Fourier transform infrared (FT-IR) analysis
FT-IR spectroscopy has been frequently used to detect vibrational frequency changes in seaweeds (Sheng et al. 2004). It offers excellent information on the nature of the bonds present and allows identification of different functional groups on the cell surface. FT-IR analysis of the L. variegata was carried out using KBr method by incorporating the sample into a KBr pellet.
The FT-IR spectra of raw and treated seaweed, when loaded with metals shows shifting wave number. Shift in wavelength showed that a metal binding process takes place on the surface of alga (Matheickal and Yu 1997). The extent of band shifting also gives an indication of the degree of interaction of functional groups with metal cations.
The carboxylic group contained the minor groups as O–H stretching, C–O stretching and O–H bending (Sheng et al. 2004). The O–H bending group was seen to shift clearly at a wave number of 1,415 cm−1 for all metal loaded seaweed except raw seaweed loaded with Pb(II). This result suggested that the O–H bending group of carboxylic acid was involved in the metal binding process.
For amine group there were changes in wave number for N–H stretching in raw seaweed and raw seaweed loaded with Cd(II) and Pb(II) with respect to biosorption. The change in wave number for N–H stretching was observed from 3,440 cm−1 to 3,438 cm−1 and 3,406 cm−1 for treated seaweed with Cd(II) and Pb(II), respectively. For N–H bending group, the wave number was 1,633 cm−1 and 1,632 cm−1 for raw seaweed and treated seaweed, respectively. The change in the intensity of the bands suggested change in the amino groups present in the biomass. Shifting was observed in all metal loaded biomass except treated seaweed with Cd(II), indicating a clear role of amine group in the metal uptake.
The FT-IR spectroscopic analysis indicated shift in wave number from 3,430 to 3,406 cm−1 and 3,440 to 3,406 cm−1 representing N–H stretching of amide group for raw and treated seaweed loaded with metals, respectively. This indicated that N–H stretching of amide group was also involved in the sorption of Cd(II) and Pb(II).
Conclusions
Biosorption performance of both raw and treated biomass of locally derived macroalgae, L. variegata was investigated for the removal of cadmium and lead from aqueous solutions. The comparison of metal biosorption performance was based on expressing metal uptake against the key equilibrium biosorption parameters such as solution pH, biomass dosage, initial concentration of metal ions and time required for sorption equilibrium. Results demonstrated that higher pH (5.0) favored metal ion removal. Kinetic studies showed that about 90% of the total metal ions biosorption occurred within 90 min and the data were described by a pseudo-second order model obtaining the kinetic rate constant and the equilibrium biosorption capacity of the alga. Analysis of mechanistic steps involved in the biosorption process indicated that the biosorption process is particle-diffusion-controlled. Experimental results were well modeled according to the Langmuir as well as Redlich–Peterson sorption isotherms. The maximum uptake capacities for cadmium and lead were 1.65 and 1.71 mmol g−1 for raw biomass and 1.71 and 1.79 mmol g−1 for treated biomass. The presence and participation of carboxylic as well as amino and amide groups in metal uptake was confirmed by FT-IR analysis. The results of this study demonstrated that the brown seaweed L. variegata constituted a promising material for the development of a low cost biosorption technology for the removal of Cd(II) and Pb(II) from wastewaters.
References
Aguilar-Carrillo J, Garrido F, Barrios L, Garcia-Gonzalez MT (2006) Sorption of As, Cd and Tl as influenced by industrial by-products applied to an acidic soil: equilibrium and kinetic experiments. Chemosphere 65:2377–2387
Basha S, Murthy ZVP (2007a) Seaweeds for engineering metal biosorption: a review. In: Mason LG (ed) Focus on hazardous materials research. Nova Science Publishers, New York, pp 165–209
Basha S, Murthy ZVP (2007b) Kinetic and equilibrium models for biosorption of Cr(VI) on chemically modified seaweed, Cystoseira indica. Process Biochem 42:1521–1529
Basha S, Murthy ZVP, Jha B (2008) Biosorption of hexavalent chromium by chemically modified seaweed, Cystoseira indica. Chem Eng J 137:480–488
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites 2. J Am Chem Soc 69:2836–2848
Carson BL, Ellis HV, McCann JL (1986) Toxicology and biological monitoring of metals in humans. Lewis Publishers, Michigan, pp 71–133
Castaldi P, Santona L, Enzo S, Melis P (2008) Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J Hazard Mater. doi:10.1016/j.jhazmat.2007.12.040
Chemielewski MJS, Urbanski TS, Migdal W (1997) Separation technologies for metal recovery from industrial wastes. Hydrometallurgy 45:333–334
Chen WM, Wu CH, James EK, Chang JS (2008) Metal biosorption capability of Cupriavidus taiwanensis and its effects on heavy metal removal by nodulated Mimosa pudica. J Hazard Mater 151:364–371
Christensen BE, Indergaard M, Smidsrød O (1990) Polysaccharide research in Trondheim. Carbohyd Poly 13:239–255
Chu W (1999) Lead metal removal by recycled alum sludge. Water Res 33:3019–3025
da Costa ACA, Leite SGF (1991) Metals biosorption by sodium alginate immobilized Chlorella homphaera cells. Biotechnol Lett 13:559–562
Davis TA, Lanes F, Volesky B, Mucci A (2003a) Metal selectiviry of Sargassum sp. and their alginates in relation to their α-l-guluronic acid content and conformation. Environ Sci Technol 37:261–267
Davis TA, Volesky B, Mucci A (2003b) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Deng L, Zhu X, Wang X, Su Y, Su H (2007) Biosorption of copper(II) from aqueous solutions by green alga Cladophora fascicularis. Biodegradation 18:393–402
El-Bishtawi RF, Ali AA-H (2001) Sorption kinetics of lead ions by zeolite tuff. J Environ Sci Health A36:1055–1072
El-Kamash AM, Zaki AA, Abed-El-Geleel M (2005) Modeling batch kinetics and thermodynamics of zinc and cadmium removal from waste solutions using synthetic zeolite A. J Hazard Mater 127:211–220
Figueira MM, Volesky B, Ciminelli VST, Roddick FA (2000) Biosorption of metals in brown seaweed biomass. Water Res 34:196–204
Fourest E, Serre A, Roux JC (1996) Contribution of carboxyl groups to heavy metal binding sites in fungal wall. Toxicol Environ Chem 54:1–4
Freitas OMM, Martins RJE, Delerue-Matos CM, Boaventura RAR (2008) Removal of Cd (II), Zn(II) and Pb (II) from aqueous solutions by brown marine macro algae: kinetic modeling. J Hazard Mater. doi:10.1016/j.jhazmat.2007.08.081
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem (Leipzig) 57A:385–470
Gardea-Torresdey JL, Becker-Hapak MK, Hosea JM, Darnall DW (1990) Effect of chemical modification of algal carboxyl groups on metal ion binding. Environ Sci Technol 24:1372–1378
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fund 5:212–223
Hashim MA, Chu KH (2004) Biosorption of cadmium by brown, green and red seaweeds. Chem Eng J 97:249–255
Ho YS, McKay G (1988) The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng 76:822–827
Ho YS, Ofomaja AE (2005) Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem 40:3455–3461
Kundu S, Gupta AK (2006) Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chem Eng J 122:93–106
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe, K Sven. Vetenskapsakad Handl 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. I. solids. J Am Chem Soc 38:2221–2295
Lehmann RG, Hater RD (1984) Assessment of copper-soil bond strength by desorption kinetics. Soil Sci Soc Am J 48:769–772
Lodeiro P, Barriada JL, Herrero R, Sastre de Vicente ME (2006) The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environ Pollut 142:264–273
Matheickal JT, Yu Q (1997) Biosorption of heavy metals from wastewater using Australian marine algae biomass. Dev Chem Mineral Proc 5:5–20
Matheickal JT, Yu Q (1999) Biosorption of lead (II) and copper (II) from aqueous solutions by pre-treated biomass of Australian marine algae. Bioresour Technol 69:223–229
Matheickal JT, Yu Q, Woodburn GM (1999) Biosorption of cadmium (II) from aqueous solutions by pre-treated biomass of marine alga Durvillaea potatorum. Water Res 33:335–342
Matlock MM, Howerton BS, Atwood DA (2001) Irreversible precipitation of mercury and lead. J Hazard Mater 84:72–83
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25:113–152
Mohan D, Singh KP (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Res 36:2304–2318
Noghabi KA, Zahiri HS, Yoon SC (2007) The production of a cold-induced extracellular biopolymer by Pseudomonas fluorescens BM07 under various growth conditions and its role in heavy metals absorption. Process Biochem 42:847–855
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol 97:2321–2329
Rakhshaee R, Khosravi M, Ganji MT (2006) Kinetic modeling and thermodynamic study to remove Pb (II), Cd (II), Ni(II) and Zn(II) from aqueous solution using dead and living Azolla filiculoides. J Hazard Mater B134:120–129
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1026
Rees DA (1981) Polysaccharide shape and their interactions—some recent advances. Pure Appl Chem 53:1–14
Sag Y, Kutsal T (1996) Fully competitive biosorption of chromium (VI) and iron(III) ions from binary metal mixtures by R. arrhizus: use of the competitive Langmuir model. Process Biochem 31:561–579
Sankar M, Sekaran G, Sadulla S, Ramasami T (1999) Removal of diazo and triphenylmethane dyes from aqueous solutions through an adsorption process. J Chem Technol Biotechnol 74:337–344
Sarı A, Tuzen M (2008) Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J Hazard Mater 152:302–308
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141
Sheng PX, Ting YP, Chen JP (2007) Biosorption of heavy metal ions (Pb, Cu, and Cd) from aqueous solutions by the marine alga Sargassum sp. in single- and multiple-metal systems. Ind Eng Chem Res 46:2438–2444
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133:304–308
Wang XS, Qin Y, Li ZF (2006) Biosorption of zinc from aqueous solutions by rice bran: kinetics and equilibrium studies. Sep Sci Technol 41:747–756
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89:31–60
Yabe MJS, de Oliveira E (2003) Heavy metals removal in industrial effluents by sequential adsorbent treatment. Adv Environ Res 7:263–272
Yu Q, Matheickal JT, Yin P, Kaewsarn P (1999) Heavy metal uptake capacities of common marine macro algal biomass. Water Res 33:1534–1537
Yun YS (2004) Characterization of functional groups of protonated Sargassum polycystum biomass capable of binding protons and metal ions. J Microbiol Biotechnol 14:29–34
Acknowledgments
The authors acknowledge the kind support of Dr. P. K. Ghosh, Dirctor, CSMCRI during the course of the study. The financial support received from GSBTM, Department of Science and Technology, Govt. of Gujarat and Ministry of Earth Sciences, Govt. of India for carrying out this project is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jha, B., Basha, S., Jaiswar, S. et al. Biosorption of Cd(II) and Pb(II) onto brown seaweed, Lobophora variegata (Lamouroux): kinetic and equilibrium studies. Biodegradation 20, 1–13 (2009). https://doi.org/10.1007/s10532-008-9194-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-008-9194-2