Abstract
Out of 10 thermophilic fungi isolated from wheat straw, farm yard manure, and soil, only three showed highest cellobiase, carboxymethyl cellulase, xylanase, and FPase activities. They were identified as Aspergillus nidulans (Th4), Scytalidium thermophilum (Th5), and Humicola sp. (Th10). A fungal consortium of these three fungi was used to compost a mixture (1:1) of silica rich paddy straw and lignin rich soybean trash. The composting of paddy straw for 3 months, during summer period in North India, resulted in a product with C:N ratio 9.5:1, available phosphorus 0.042% and fungal biomass 6.512 mg of N-acetyl glucosamine/100 mg of compost. However, a C:N ratio of 10.2:1 and highest humus content of 3.3% was achieved with 1:1 mixture of paddy straw and soybean trash. The fungal consortium was effective in converting high silica paddy straw into nutritionally rich compost thereby leading to economical and environment friendly disposal of this crop residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice–wheat cropping system is widely practiced in north-west India. As a consequence, crop residue yield of wheat and paddy range between 3.2–5.6 t ha−1 and 6.2–11.8 t ha−1 respectively. Paddy straw contains >9.0% silica, is rarely used as animal feed. A little portion of paddy straw is used for growing mushroom (Ross and Harris 1983; Stratsma et al. 1995) and in industries as packing material. The major portion of it is burnt in the field itself (Gupta et al. 2004). This results on one hand in a waste of renewable organic source in soil affecting C:N ratio and biota and on the other hand leads to emission of green house gases (Badrinath et al. 2006). Keeping in mind the harmful effects of burning paddy straw in the field, as well as the convenience of farmers, an economical, environment friendly and low labor intensive strategy should be adapted for effective utilization of this waste. The application of bio-degraded products of straw into soil has enormous potential to recycle nutrients and maintain soil fertility (Gaind et al. 2006). However, the presence of high lignin content makes the paddy straw less vulnerable to microbial attack. For effective utilization of lingo–cellulosic residues, several physical and chemical pre-treatments are required, that may not be convenient for farmers having small holdings. To make the process of lignin degradation economically viable, inoculation with lignocellulolytic microorganisms may prove beneficial. Since no single organism produces all the enzymes necessary for bioconversion of lignocellulose to optimum level, there is need to use a consortium of lignocellulolytic microorganism which can act synergistically for rapid bioconversion of agricultural residues without any chemical pretreatment. Mesophilic microbiota initiate decomposition by degrading readily available substrates present in composting mixture, but as the temperature during composting rises to 60–65°C, their population declines leading to ultimate effectiveness of the process. The decomposition rate is most rapid during the thermophilic stage, achieved with in first week of composting. Though, there may be some natural thermophilic microorganisms present in the substrate mixture, but they may not be able to execute the desired degradation of substrate due to lack of specific enzymes for cellulose and lignin degradation to optimum level. Therefore, there is need to inoculate thermophilic microorganisms capable of producing cellulolytic and lignolytic enzymes to degrade the crop waste like paddy straw. As fungi are saprophytic in nature and can easily grow on various crop residues (Ragunathan and Swami 2003), an idea of isolating thermophilic fungi having lignocellulolytic properties was subjected to exploration. This paper describes the isolation and selection of thermophilic fungi on the basis of hydrolytic enzymes activities, development of consortium of selected strains of fungi and testing of its efficacy to compost paddy straw as a primary substrate.
Material and methods
Substrates for isolation of fungal strains
Soil, wheat straw compost and farm yard manure were collected from Indian Agricultural Research Institute (IARI) Farm, experimental compost pits in the Division of Microbiology and Division of Agronomy, IARI, New Delhi respectively and used as supplement to isolate thermophilic fungi. Paddy straw was enriched with each of these supplements separately, moistened with Reese minimal medium (Reese and Mandel 1963) and incubated for 15 days at 50°C. Ten thermophilic fungal cultures were isolated from enriched materials, by dilution plating technique using Reese minimal medium with 1% swollen cellulose as the sole carbon source.
Screening of fungal strains
The fungal cultures were screened qualitatively for the production of cellulase, xylanase, laccase and lignin peroxidase at 50°C. The cellulase activity was tested by zone of clearing on Reese minimal medium with acid swollen cellulose as the sole carbon source (Rautela and Cowling 1966), while the presence of xylanase was tested by the method of Kluepfel (1988) using xylan as the carbon source. Laccase production was detected by modification of the method of Hankins and Anagnostakis (1975). Peroxidase production was confirmed by following the procedure of Egger (1986).
The quantitative estimation of enzymes produced by fungal isolates involved the release of reducing sugars from filter paper (FPase), carboxy methyl cellulose (CMCase), cellobiose (cellobiase) and xylan (xylanase). The cultures were grown in Reese minimal broth supplemented with 1% cellulose, xylan and paddy straw separately as the sole carbon source. Sterilized Reese minimal broth was inoculated with 6 mm disc cut from a 7 day old fungal culture of each strain separately and incubated at 50°C. After 15 days of incubation, the broth was filtered through Whatman 44. The filtrate was stored at 4°C and assayed for FPase, CMCase, cellobiase (Ghose 1987), and xylanase (Bailey et al. 1992).
Selection of fungi for consortium
Out of 10 thermophilic fungal isolates, the three most efficient cultures (Th4, Th5, and Th10) were used for solid state fermentation (SSF) of paddy straw under laboratory conditions. In 500 ml Schott Duran bottles, 10 g shredded paddy (2.5 cm pieces) and 30 ml of Reese minimal broth was taken, mixed thoroughly and sterilized at 1.1 kg cm2 pressure for 15 min. Each of the three fungal cultures was grown separately in Petri dishes for 15 days at 50°C on Potato Dextrose Agar (PDA). A 6 mm disc of each culture was punched out and transferred aseptically to each bottle as per the schedule given in Table 2. All the bottles along with control were incubated at 50°C for 15 days. The degraded product was analyzed for dry matter loss, organic matter, organic carbon and total nitrogen by following the standard methods (Jackson 1973). Humus was estimated by the method of Kononova (1966).
Compatibility test
All the three selected cultures were tested for their compatibility with each other. They were co-inoculated on PDA plate and incubated at 50°C for 1 week. None of the culture inhibited the growth of other culture, but grew well.
Inoculum preparation
Inoculum of the selected fungi was raised on boiled sorghum seeds mixed with 2% CaSO4 and 4% CaCO3. Flasks containing sorghum grains were inoculated with 6 mm disc of each culture, grown on PDA, and incubated at 50°C. After 2 weeks, all the three cultures were mixed in equal proportion and used as inoculum for compost preparation.
Preparation of substrate mixture for compost production
Paddy straw and soybean trash, the primary substrates used for composting were collected from IARI farms and chopped to a size of 10–15 cm. The initial wide C:N ratio of paddy straw (88:1) was narrowed down to about 60:1 by adding 0.45% urea in one set of the treatment and by adding soybean trash in the ratio of 1:1 to the other set of treatment. About 20 kg of each substrate mixture was filled in the plastic containers having lids and inoculated with fungal consortium @ 1% (w/w) as per treatment schedule (Table 4). The inoculum was mixed thoroughly. The substrate was allowed to decompose for 3 months during summer season under natural conditions. The contents in each container were turned upside down at monthly intervals and the moisture was maintained at 60% (w/v). The composted sample were drawn at maturity and divided into two parts. One part was dried in oven at 60°C and ground to pass through 2 mm sieve. The powdered samples were analyzed for organic matter, organic carbon and total nitrogen by following the standard method (Jackson 1973). Crude protein was calculated by multiplying the nitrogen percent with 6.25. Available phosphorus was estimated by the method of Olsen et al. (1954). Total humus content (humic acid, fulvic acid and humin) in matured compost was estimated by the method of Kononova (1966) using 0.1 M sodium pyrophosphate as an extractant followed by dialysis of filtrate under running water for 24 h.
The fungal biomass was estimated by the method of Aidoo et al. (1981). 100 mg oven dried sample was mixed with 5 ml of 2 N HCl in a screw capped tube and incubated in boiling water bath for 2 h. The contents were filtered through Whatman 42 filter paper. 1 ml filtrate was mixed with 1 ml sodium nitrite (5% w/v) and 1 ml potassium hydrogen sulphate (5%). The tubes were incubated at room temperature for 15 min. To this 1 ml of ammonium sulphamate (12.5% w/v) was added. After shaking for 5 min 1.0 ml 3-methyl-2-benzothiazole hydrazone hydrochloride (MBTH 0.5% w/w) was added and the tubes were kept in boiling water bath for 5 min. After cooling, 1.0 ml ferric chloride (0.83% w/v) was added. The contents after thorough mixing were left for 30 min for color development and OD was measured at 650 nm against reagent blank.
Another set of samples were air dried and ground to pass through 2 mm sieve. The levels of different enzymatic activities viz dehydrogenase, alkaline phopshatase, cellulase, cellobiase and xylanase were assayed in matured compost samples following the standard procedures.
Enzyme assay
Estimation of dehydrogenase
Dried and sieved compost sample (1 g) was treated with 3% triphenyl tetrazolium chloride (TTC) in distilled water for 24 h at 28°C in darkness. The triphenylformazone (TPF) formed was extracted with 20 ml methanol by shaking vigorously for 1 min and filtered through Whatman 42 filter paper. TPF was measured spectrophotometrically at 485 nm following the method of Casida et al. (1964).
Alkaline phosphatase
About 4 ml of modified universal buffer (pH 11.0) and 1 ml p-nitrophenyl phosphate disodium (0.025 M) was added to 1 g compost sample and incubated at 37°C for 60 min. It was followed by the addition of 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M NaOH. Mixture was centrifuged at 4000g for 5 min. p-nitrophenol was determined spectrophotometrically at 400 nm and phosphatase activity expressed as μmoles of PNP released g−1 h−1 with reference to standard curve of p-nitrophenol (Tabatabai and Bremner 1969).
Estimation of cellulase, cellobiase, xylanase, phenol and soluble protein
Enzyme was extracted from compost with citrate buffer of pH 7.0. A known sample (0.5 ml) of filtrate was incubated with respective substrates (filter paper strips/cellobiose/xylan) and volume was made up to 1 ml with 0.05 M citrate buffer of pH 4.8. All the tubes were incubated at 50°C for 1 h for cellulase and 30 min for CMCase cellobiase and xylanase. Reducing sugars liberated by action of enzyme was estimated by adding 3 ml dinitrosalicylic acid and keeping in boiling water bath for 16 min (Wood and Bhat 1988; Bailey et al. 1992). Enzyme concentration was represented as international unit (IU g−1). 1 IU is defined as μ mol of product produced ml−1 min−1 for filtrate. Cellulose, hemicellulose and lignin content in the primary substrates were estimated by the method of Goering and Van Soest (1970). Phenol and soluble protein were estimated in buffer extracts of compost samples by the method Bray and Thorpe (1954) and Lowry et al. (1951) respectively.
Statistical analysis
Through out the study, three replications of each treatment were kept. The data were subjected to analysis of variance (ANOVA) using least significance difference test and comparing the difference between the specific treatments. Test of significance was determined at P < 0.05 (Panse and Sukhatme 1978).
Results and discussion
Qualitative screening of the fungal cultures revealed that all the 10 strains expressed cellulase, cellobiase, CMCase, xylanase, and peroxidase. However, laccase was expressed by only one strain Th4.
The quantitative estimation of enzyme production in Reese’s minimal medium with paddy straw (1% w/v) as the sole carbon source showed significant variation in the abilities of the strains (Table 1). Maximum activity of FPase (11.43 IU ml−1) by Th10, cellobiase (134.21 IU ml−1) by Th4, CMCase (30.36 IU ml−1), and xylanase (236.43 IU ml−1) by Th5 were recorded. The most promising cultures were identified as Humicola sp.Th10, Aspergillus nidulans Th4, and Scytalidium thermophilum Th5 at Indian Type Culture Collection Centre, Division of Mycology and Plant Pathology, IARI, New Delhi.
Preliminary analysis of samples taken after 15 days of paddy straw decomposition by solid state fermentation under laboratory conditions revealed that maximum loss of dry matter, least C:N ratio and maximum humus was achieved when inoculation was made with mixed cultures of fungi (Table 2).Therefore, the consortium with all the three fungal cultures was used for composting of paddy straw.
The physico-chemical analysis of different substrates used in the study is given in Table 3. There was not much variation in the cellulose content of paddy straw and soybean trash, but lignin content of latter was almost three times to that of former. Hemicellulose content of paddy straw was almost double to that of soybean. Soybean due to high content of nitrogen had the most desired C:N ratio to initiate the decomposition. Therefore, it was used as an organic source of nitrogen supplement substituting urea in some treatments.
The chemical analysis of mature compost prepared from paddy straw and mixture of paddy straw + Soybean trash (1:1) is given in Table 4. The pH being an important parameter controlling the availability of nutrients like P, Fe, and Zn should get due attention while recommending the compost to be mature and safe for soil application. It was found to be in the near neutral range of 6.3–7.5, the acceptable values for the final product. Fungal inoculation resulted in lowering of pH of all the treatments compared to control, barring soybean amended fungal inoculated treatment that had slightly higher pH (7.5), compared to its uninoculated counterpart. This may be attributed to greater production of ammonia during the process of decomposition. Electrical conductivity, a measure of dissolved salts ranged between 1.3 mS and 2.4 mS, and was far below the safe limit of 3 mS. The effect of inoculation was well pronounced in reducing the electrical conductivity.
A reduction in total organic carbon (TOC) content was observed in all the composts. This points the mineralization of organic material present in each of the substrates. Both the substrates must be having some easily mineralizable fraction of organic matter. Reduction in total carbon content was 36% in soybean supplemented paddy straw compared to 25% in urea amended and inoculated paddy straw. Though the lignin content of soybean trash was high, but fungal consortium was effective in degradation of organic matter and brought significant reduction in total organic carbon. The N content in most of the treatments improved due to a concentration effect caused by degradation of labile organic carbon compounds which reduced the weight of the composting mass. It is believed that when organic matter loss is greater than loss of NH3, nitrogen concentration usually increases (Bernal et al. 1998).
The improvement in nitrogen and lowering of carbon percent resulted in lowering of C:N ratio, an important criterion for compost to be fully mature, as well as an adequate predictor of amendments impact on N cycling on its incorporation in soil. Though, most of the tested composts had the C:N < 20, in this study, the lowest C:N (9.54:1) was observed in compost prepared by using paddy straw amended with 0.45% urea and inoculated with fungal consortium. Unamended, but inoculated paddy straw after decomposition had comparatively higher C:N ratio (14.6:1). On the contrary, soybean supplemented paddy straw had C:N 12.4:1. This showed the importance of initial C:N in the substrate mixture used for composting. Low initial C:N ratio caused fast degradation of cellulose and hemicellulose, while high initial C:N resulted in low degradation (20%) of both cellulose and hemicellulose. These results were in agreement with the findings of Eiland et al. (2004). However, the most acceptable C:N ratio of 10.3:1 and highest humus content was achieved when paddy straw was amended with soybean trash. This showed the effectiveness of fungal consortium and supplementation with soybean trash on the degradation of paddy straw. It was also seen that by replacing urea with soybean, most desired C:N was achieved and the compost so obtained was a dark brown colored mass with no odour but with high organic value.
Highest fungal biomass (6.512 mg of N-acetyl glucosamine/100 mg substrate) was recorded in urea amended paddy straw followed by soybean amended treatment. High level of N-acetyl glucosamine was considered to be due to the higher growth of fungi in easily available nitrogen supplemented treatment (Aidoo et al. 1981).
Inoculation improved the dehydrogenase activity to a considerable extent as compared to control (Table 5). As this activity is linked to microbial respiratory process, the maximum value of dehydrogenase activity in soybean amended treatment (1928.58 μg TPF g−1h−1) showed the higher proliferation of both native and inoculated flora. This may be due to high N and P % in this treatment.
Not much variation in values of alkaline phosphatase was observed among all the treatments. An inverse relation in available P content and alkaline phosphatase was observed. This was in agreement with the findings (Nannipieri 1990; Gaind and Lata 2004), due to inhibition of phosphatase activity in presence of soluble phosphorous.
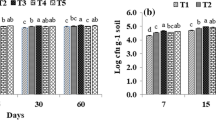
Xylanase activity and soluble protein content were maximum in paddy straw inoculated treatment whereas cellulase activity was maximum in soybean supplemented and fungal inoculated paddy straw (2664.92 IU g−1) (Table 5). Expression of high xylanase activity must have resulted in the maximum saccharification of lignocellulosic material in paddy straw (Bernier et al. 1983).
Highest production of phenol (1422.12 μg g−1) was recorded in unamended, fungal inoculated paddy straw compost. This showed that lignin was degraded into monomeric phenolic compounds due to action of phenol oxidase and lignin peroxidase. However, low values of phenol in soybean amended, fungal inoculated treatment in spite of high content of humus may be attributed to the fact that fungal consortium was highly effective in enzymatic oxidation of the toxic compounds. Inoculation with specific fungi with the capability of degrading phenolic compounds also explains the phenolic reduction.
Conclusions
The enhancement of nutritive values and reduction in volume of agricultural wastes using thermophilic fungi has also been reported by Abraham et al. (1992). The fungal consortium proved effective in converting high silica paddy straw into valuable product as compost. The supplementation of paddy straw with soybean trash and inoculation with fungal consortium yielded compost that was nutritionally comparable to compost prepared with paddy straw supplemented with urea and fungal inoculation. The compost prepared without the use of urea may be of great use in organic farming as it will be free from chemicals.
References
Abraham TK, Sen S, Chakrabarty SL (1992) Biodegradation and utilization of agricultural wastes by thermophilic fungi. In: Subbarao NS, Balagopalan C, Ramakrishna SV (eds) New Trends in Biotechnology. Oxford and IBH, New Delhi, India, pp 399–406
Aidoo KE, Hendry R,Wood BJB (1981) Estimation of fungal growth in a solid state fermentation system. European J Appl Microbiol Biotechnol 12:6–9
Badrinath KVS, Kiranchand TR, Krishna Prasad V (2006) Agriculture crop burning in the Indo-Gangetic plains—a study using IRS-P6AWiFS satellite data. Curr Sci 91(8):1085–1089
Bailey MJ, Biley P, Poutanen K (1992) Inter laboratory testing of methods for assay of Xylanase activity. J Biotechnol 23:257–270
Bernal MP, Pardes C, Sanchez-Monedero MA, Cegarra J (1998) Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour Technol 63:91–99
Bernier RJR, Desrochers M, Jurasek L, Paice MG (1983) Isolation and characterization of Xylanase from Bacillus subtilis. Appl Environ Microbiol 46:511–514
Bray HG, Thorpe WV (1954) Analysis of phenolic compounds of interests in metabolism. Methods Biochem Anal 1:27–52
Casida LE, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Egger KN (1986) Substrate hydrolysis pattern of post fire ascomycetes (Pezizales). Mycologia 78:771–780
Eiland FM, Klamer A, Lind N, Leth M, Baath E (2004) Influence of initial C:N ratio on chemical and microbial composition during long term composting of straw. Microbial Ecol 41(3):272–280
Gaind S, Lata (2004) Quantitative evaluation of exoenzymes during composting. Indian J Microbiol 44(3):175–179
Gaind S, Pandey AK, Lata (2006) Microbial biomass, P nutrition and enzymatic activities of wheat soil in response to phosphorus enriched organic and inorganic manures. J Environ Sci Health part B Pest food contam agric wastes 41:177–187
Ghose TK (1987) Measurements of cellulase activities. Pure Appl Chem 59:257–268
Goering HK, Van Soest PJ (1970) Forage fiber analysis. Agriculture Hand book No. 379, U.S.D.A., Washington DC, pp 1–12
Gupta PK, Sahai S, Singh N, Dixit CK, Singh DP, Sharma C, Tiwari MK, Gupta RK, Garg SC (2004) Residue burning in rice wheat cropping system: causes and implications. Curr Sci 87:1713–1715
Hankin L, Anagnostakis SL (1975) The use of solid media for detection of enzyme production by fungi. Mycologia 67:597–607
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India, New Delhi, p 663
Kluepfel D (1988) Screening of prokaryotes for cellulose and hemicellulose degrading enzymes. Methods Enzymol 160:180–185
Kononova MM (1966) Soil organic matter, its nature, its role in humus formation and soil fertility. Pergamon, Oxford, p 325
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin as phenol reagent. J Biol Chem 193:265–277
Nannipieri P (1990) The potential use of soil enzymes as indicator of productivity, sustainability and pollution. In: Panhkurst CE, Doube, DM, Gupta, WSR, Grace PR (eds) Soil biota management in sustainable farming system, CSIRO, East Melbourne, Victoria, Australia, pp 238–244
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. United States Department of Agriculture, Circular No 939, Govt Printing Office, Washington, pp 1–9
Panse VG, Sukhatme PV (1978) Statistical methods for agricultural workers. I.C.A.R., New Delhi
Ragunathan R, Swaminathan K (2003) Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chem 80:371–375
Rautela GS, Cowling EB (1966) Simple cultural test for relative cellulolytic activity of fungi. Appl Microbiol 4:39–45
Reese ET, Mandels M (1963) Enzymatic hydrolysis of cellulose and its derivatives. Methods carbohydrate chem 3:139–142
Ross RC, Harris PJ (1983) The significance of thermophilic fungi in mushroom compost preparation. Scientia horticulturae 20:61–70
Stratsma G, Samson RA, Olignsma TW, Gerritis JPG, Op-De-Camp HJM, Cariensven LJLD (1995) Bioconversion of cereal straw into mushroom compost. Can J Bot 73(SUPI, Sections, E. H.):S1019–S1024
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Wood TM, Bhat KM (1988) Methods for measuring cellulose activities. Methods Enzymol 160:87–112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Gaind, S. & Nain, L. Evaluation of thermophilic fungal consortium for paddy straw composting. Biodegradation 19, 395–402 (2008). https://doi.org/10.1007/s10532-007-9145-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-007-9145-3