Abstract
Pseudomonas veronii strain UFZ B549, Acidovorax facilis strain UFZ B530, and a community of indigenous groundwater bacteria, adapted to oxygen limitation, were cultivated on chlorobenzene and its metabolites 2-chloro-cis,cis-muconate and acetate/succinate under hypoxic and denitrifying conditions. Highly sensitive approaches were used to maintain defined low oxygen partial pressures in an oxygen-re-supplying headspace. With low amounts of oxygen available all cultures converted chlorobenzene, though the pure strains accumulated 3-chlorocatechol and 2-chloro-cis,cis-muconate as intermediates. Under strictly anoxic conditions no chlorobenzene transformation was observed, while 2-chloro-cis,cis-muconate, the fission product of oxidative ring cleavage, was readily degraded by the investigated chlorobenzene-degrading cultures at the expense of nitrate as terminal electron acceptor. Hence, we conclude that oxygen is an obligatory reactant for initial activation of chlorobenzene and fission of the aromatic ring, but it can be partially replaced by nitrate in respiration. The tendency to denitrify in the presence of oxygen during growth on chlorobenzene appeared to depend on the oxygen availability and the efficiency to metabolize chlorobenzene under oxygen limitation, which is largely regulated by the activity of the intradiol ring fission dioxygenase. Permanent cultivation of a groundwater consortium under reduced oxygen levels resulted in enrichment of a community almost exclusively composed of members of the β-Proteobacteria and Bacteroidetes. Thus, it is deduced that these strains can still maintain high activities of oxygen-requiring enzymes that allow for efficient CB transformation under hypoxic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodegradation of hydrocarbons under oxygen-limited (hypoxic) conditions is a common situation in those environments where microbes are cut off from sufficient oxygen supply. For instance, in the polluted groundwater aquifer at Bitterfeld, Germany, poor oxygen availability limits the biotransformation of monochlorobenzene (CB) (Dermietzel and Vieth 2002; Vogt et al. 2004a).
CB degradation under aerobic conditions is initiated by a dioxygenase introducing an oxygen molecule into the aromatic structure (Fig. 1). Another molecule of oxygen is required for the subsequent ortho-cleavage of the central intermediate 3-chlorocatechol (CC) to 2-chloro-cis,cis-muconate (CM). Chloride elimination during the cycloisomerization of CM to a dienelactone and two further breakdown reactions yield 3-oxoadipate (Reineke and Knackmuss 1984), which after activation and cleavage to acetyl-CoA and succinate is funneled into the tricarboxylic acid cycle (TCC) (Reineke 2001). This pathway, most common in the degradation of many chlorinated aromatics via chlorocatechols, is usually designated the chlorocatechol or modified ortho-cleavage pathway (Reineke 2001; Schlömann 1994).
Chlorocatechol ortho-cleavage pathway for aerobic catabolism of CB in Proteobacteria (according to (Reineke and Knackmuss 1984; Reineke 2001). The following enzymes are involved: A, chlorobenzene 1,2-dioxygenase; B, chlorobenzene-cis-1,2-dihydrodiol dehydrogenase; C, chlorocatechol 1,2-dioxygenase; D, chloromuconate cycloisomerase; E, dienelactone hydrolase; F, maleylacetate reductase; G, terminal oxidoreductase, TCC = tricarboxylic acid cycle. Transfer of reducing equivalents [H] generated in the TCC to oxygen or nitrate takes place via the respiratory chain
Particular relevance to investigate the biodegradation of CB under reduced oxygen is given since sufficient activity of two dioxygenases is prerequisite prior to further catabolic reactions where oxygen can be potentially replaced.
Typically, microorganisms compensate for low oxygen availability by the expression of oxygen-requiring enzymes adapted to function in hypoxic environments as found for terminal oxidases (Otten et al. 2001; Rice and Hempfling 1978; Tseng et al. 1996) and for dioxygenases of different organisms (Balcke GU et al. 2007 (submitted); Krooneman et al. 1998; Kukor and Olsen 1996), or they compensate by elevating the synthesis of such enzymes in response to oxygen limitation (Dikshit et al. 1990).
Extended accumulation of CC in response to inadequate oxygen availability (Balcke GU et al. 2007 (submitted); Fritz et al. 1991; Krooneman et al. 1998; Vogt et al. 2004b) reflects insufficient activity of chlorocatechol 1,2-dioxygenase (CC12O). Also the excretion of CM has been observed under hypoxic conditions (Balcke et al. 2004 and 2007 (submitted)), but a simple biochemical explanation for its accumulation is elusive. Notably, no additional oxygen is required during further breakdown of CM. Thus, efficient anaerobic degradability is anticipated upon ring cleavage and formation of this metabolite when alternative electron acceptors can be utilized.
Although oxygen is the preferred electron acceptor, simultaneous denitrification has been observed under microaerobic and even aerobic conditions (Chen et al. 2003; Knowles 1982; Patureau et al. 2000). Denitrification is repressed in response to oxygen availability, depending on the strain under investigation (Knowles 1982; Wilson and Bouwer 1997; Zumft 1997). For aromatic substrates Wilson and Bouwer (1997) suggested a mechanism where two moles of oxygen were obligatory to cleave one mole of an aromatic toluene ring while nitrate could reduce the overall oxygen demand replacing oxygen as terminal electron acceptor of the respiratory chain. Albeit studies working with reduced oxygen levels showed enhanced BTEX degradation in the presence of nitrate (Leahy and Olsen 1997; Ma and Love 2001; Wilson Durant et al. 1999), such an effect has yet to be proven for chloroaromatic compounds such as CB (Vogt et al. 2004a, b). Notably, none of the studies cited could control the actual oxygen availability during the experimental course, which is a precondition to decide whether nitrate reduction occurs simultaneously or subsequently to the reduction of oxygen.
In this study we investigated the degradability of CB and its metabolites CM and acetate/succinate under hypoxic and denitrifying conditions. By means of a new, fluorescence-based oxygen detection method we conducted oxygen-fed batch cultivations where two pure bacterial strains and an enrichment culture adapted to hypoxic conditions were subjected to controlled oxygen limitation. Pseudomonas veronii strain UFZ B549 and Acidovorax facilis strain UFZ B530 were used since they are presumptive ‘key players’ in the CB-contaminated aquifer in Bitterfeld. Both strains are able to denitrify (Vogt et al. 2004b; unpublished results), but differ from each other in their ability to transform CC and CM under oxygen limitation (Balcke GU et al. 2007 (submitted); Vogt et al. 2004b). A groundwater consortium permanently cultivated on CB under hypoxic conditions was analyzed for key organisms in order to identify strains which coped best with oxygen limitation.
Materials and methods
Experimental setup
Duran glass bottles of 114 ml total volume filled with 65 ml, respectively, chloride-poor mineral salt medium were used as described previously (Balcke et al. 2004). The bottles were equipped with a teflon-coated magnetic stir bar and sealed by fresh Mininert lids (Supelco) with extra 2 mm-thick butyl septa. During the incubation, the bottles were stored in a plastic bag continuously flushed with argon to minimize undesired oxygen diffusion through the lids. Above and below the water surface, oxygen-sensitive optode spots (POF-PtSt3 and TOS7, Presens, Regensburg, Germany) were glued onto the inside of each glass bottle (Fig. 2). Setup and detection principle of fiber-optic oxygen measurements have been described elsewhere (Balcke GU et al. 2007 (submitted); Klimant et al. 1997; Tolosa et al. 2002).
Experimental setup for oxygen-fed batches. Nearly constant oxygen partial pressures were maintained by discontinuous oxygen gas spikes through a Mininert valve. Oxygen-sensitive optode spots attached above and below the water surface allowed for non-invasive analysis of oxygen in solution and headspace. For this, an oxygen-sensitive fluorophor embedded in a gas-permeable foil is excited through the glass wall by modulated light and the resulting fluorescence, which is quenched by traces of oxygen, can be measured simultaneously by means of a fiber-optic sensor. Tailored optode materials of different sensitivity allowed for obtaining signals specific to particular oxygen ranges (e.g., PtSt3 [A]: 0.6–700 μM dissolved oxygen; TOS7 [B]: 0.03–28 μM dissolved oxygen). Headspace oxygen partial pressures were converted into equilibrium dissolved oxygen saturations according to Henry’s Law (Dean 1992). Dissolved- and headspace oxygen concentrations are both expressed in micromole per liter aqueous solution
Each optode spot was calibrated separately prior to sterilization of the glassware using air-saturated mineral medium for PtSt3, or using medium saturated with a 1% (v/v) oxygen gas standard (Linde, Germany) for TOS7, respectively, and sodium dithionite (0% oxygen). Evaluating all associated headspace and dissolved oxygen data pairs, and applying the ideal gas law, as well as a Henry’s constant of 0.00135 mol kg−1 bar−1 (23°C), the absolute oxygen contents of microcosms were derived for each time step (Dean 1992). The cumulative oxygen demand was calculated from differences in the absolute oxygen contents of consecutive data pairs and was corrected for losses in total oxygen due to the withdrawal of samples for chemical analyses.
Cultivation and sampling
All experiments were conducted at 23°C and under constant stirring (450 rpm) in the dark. Prior to the inoculation each bottle was thoroughly flushed with a mixture of sterile N2/CO2 (approx. 95:5% v/v that balanced the pH of the medium to 6.7, spiked either to 500 μM CM, 500 μM each acetate/succinate, or with neat CB to 500 μM (assuming all CB to be dissolved in the water phase), and equilibrated for 2 h to dissolve CB blobs. CM was synthesized according to Kaschabek and Reineke (1994). Potassium nitrate was added to several microcosms from a sterile stock solution to give final concentrations of about 1800 μM. Sterile oxygen gas was added using gastight syringes. Samples for CB analyses and metabolites, acetate, succinate, nitrate, nitrite, and chloride were taken discontinuously using syringes with hot, flame-sterilized needles. Prior to sampling the Mininert lids were flamed using a micro-burner. For CB analysis 500 μl sample and 200 mg sodium dithionite were added to GC vials containing 4.5 ml water, shaken, and immediately frozen until analysis. Another 500 μl were filtered through a 0.2 μM PTFE filter prior to refrigeration and HPLC analysis.
To prevent the entry of oxygen during sampling of anoxic and hypoxic microcosms, the bottles were transferred into a plastic bag permanently flushed with argon gas. Sampling was achieved through a small hole in the bag. Before and after sampling, the oxygen concentration in the batches was measured via the TOS7 optode spot.
Chemical analyses
Metabolite quantification was carried out at 280 nm on a Dionex Summit HPLC which was equipped with a diode array detector PDA100 and an Ultrasep ES Phenol 7MY reversed-phase column (Sepserv, Berlin, Germany). Separations were achieved using a linear gradient of 10–90% (v/v) acetonitrile, containing 1% (w/v) acetic acid. 3-Chlorocatechol and 2-chloro-cis,cis-muconic acid were available as authentic standards (Kaschabek and Reineke 1994; Mason 1947). CB was extracted by solid phase micro-extraction (85 μm polyacrylate fiber, Supelco, 25 min) and determined by GC-FID analyses (see Balcke et al. (2004) for other details). Chloride, nitrite, and nitrate were measured using an ion suppression system (Dionex ICS2000) equipped with an AS14-anion exchange column (Dionex). Acetate and succinate were separated on a Polyspher OAHY column (30 cm by 6.5 mm; Merck, Darmstadt, Germany) in 5 mM H2SO4 at a flow rate of 1.4 ml min−1 using an HPLC-system (Jasco) and detected at 210 nm. Nitrous oxide was sampled from the headspace using a gas-tight syringe and detected by a GC-ECD system (Shimadzu, 60°C isotherm, packed column Haye Q 80/100 mesh, Vici International) with valve injection using a 1 ml injection coil. Ammonia was analyzed as described by Martienssen et al. (2006).
Preparation of the inocula
An indigenous bacterial community was obtained from the CB-polluted, virtually oxygen-free quaternary aquifer at Bitterfeld, Germany. Under protective gas atmosphere (95% nitrogen, 5% carbon dioxide), 12 l groundwater were filtered through sterile 0.2 μm Isopore cellulose acetate filters (Millipore). About 50 ml of the re-suspended filter pellet (in 100 ml medium) were used to inoculate 400 ml mineral medium spiked to 500 μM CB. During 3 months the cells were cultivated at reduced oxygen levels with a maximum available oxygen concentration of 0.12% (1.6 μM equilibrium concentration) in the vessel headspace. After three passages into fresh medium and repeated additions of CB 0.5 ml of this cell suspension were used as inoculum (approximately 1.0*104 cells/ml final concentration).
A. facilis B530 and P. veronii B549 were isolated from sediment of the same site during a hydrogen peroxide treatment. Both strains use the chlorocatechol ortho-cleavage pathway for degradation of CB as indicated by positive enzyme assays for chlorocatechol 1,2-dioxygenase (CC12O), and negative enzyme assays for chlorocatechol 2,3-dioxygenase using CB-grown cells (Vogt et al. 2004a; unpublished results). For tests on anaerobic CM degradation the two pure strains were pre-cultured on 1 mM CB in mineral medium under aerobic conditions until a cell density of 2.0*107 cells/ml was achieved. Residual oxygen and CB were purged from actively-growing cultures by N2/CO2 before 500 μM CM were added. For hypoxic oxygen-fed batches pre-cultivation occurred on 1 mM CB in presence of 1800 μM nitrate with a molar excess of CB in relation to oxygen. The oxygen was allowed to become depleted. Cells were harvested at a density of 1.2*107 cells/ml, concentrated 40-fold by centrifugation (2,822 × g, 5 min, 4°C) and washed twice in substrate/chloride-free mineral medium. About 1 ml of the re-suspended cell pellets was used to inoculate each main batch to start with a cell density of 6*104 cells/ml.
Community analysis
DNA of centrifuged cells (2,822 × g, 5 min, 4°C) of 50 ml aliquots taken from the culture at the end of the hypoxic cultivation was extracted using the Fast DNA SPIN Kit for Soil (Q•BIOgene, Germany) with Lysing Matrix E tubes supplied with the kit. For efficient lysis per tube 10 μl β-mercaptoethanol were added to the lysis buffer. The extracts were checked for DNA concentration by calculation with NanoDrop ND-100 (NanoDrop, Wilmington, USA). DNA extracts were stored at −20°C until further use.
For PCR the Taq polymerase Master Mix (Promega, Mannheim, Germany) was used together with 1.5 mM MgCl2, 0.3 μM of each primer, 5% DMSO and corresponding DNA aliquots (1 μl), respectively. The PCR conditions for total 16S rDNA amplification with the primer pair UniBac27F (Lane, 1991) and Univ1492R (Lane, 1991) (MWG-Biotech AG, Ebersberg, Germany) were as reported by Alfreider et al. (2002). PCR products were detected by electrophoresis on 1.5% (w/v) agarose in 0.5xTBE and purified with the E.Z.N.A. Cycle Pure Kit (PeqLab, Erlangen, Germany).
16S rDNA amplificates were ligated into the pGEM vector using the Promega pGEM-T vector system (Promega, Madison, WI, USA). Transformation and screening were performed according to the manufacturer’s protocol. For differentiation of clones ARDRA (amplified rDNA restriction analysis) fingerprints were prepared. Cloned DNA fragments were amplified according to the recommendations of Promega with the primers pUC/M13 Forward (24mer) and pUC/M13 Reverse (22mer) of the kit (Promega, Madison, WI, USA), digested with restriction endonuclease HaeIII (BioLabs, New England) (37°C, 2 h), and separated on a 2% (w/v) metaphor agarose gel (BMA, Rockland, ME, USA). Staining with 0.003% SYBR green solution (MoBiTec, Göttingen, Germany) for 30 min followed prior to analysis with a fluorescent image analyzer FLA-3000 (Fujifilm, Berthold, Australia) and the evaluation software „Image Reader FLA-3000 Series” Version 1.11 and „AIDA” (Advanced Image Data Analyzer) Version 2.31. ARDRA patterns were discriminated by calculation with Phoretix 1D software, Version 5.20 (Nonlinear Dynamics Ltd., UK). At least one representative of each pattern was identified by partial sequence analysis using an Applied Biosystems BigDye RR Terminator AmpliTaqTM FS Kit version 3.1 and the primers UniBac27F and UniBac519R (Lane, 1991). Electrophoresis and data collection were carried out on an Applied Biosystems ABI PRISM 3100 Genetic Analyzer. Data were analyzed by the ABI PRISM DNA sequencing analysis software, and sequences of both complementary strands were assembled by the ABI PRISM autoassembler software. The BLASTN program (Altschul et al. 1990) was used to search for similar sequences in public nucleotide sequence databases.
Sequences were aligned to a database of small subunit rRNA and rDNA sequences based on the secondary structure of the SSU rRNA with the help of the ARB database system (Ludwig, 2004) and alignments were manually corrected for best secondary structure. All dendrograms in this communication were calculated using DNA parsimony. The overview dendrogram was calculated considering only positions of the alignment, where the position was conserved in at least 50% of the sequences within the Bacteria. The dendrogram of Acidovorax-related sequences was calculated including sequence positions with 50% homology within the Comamonadaceae. Full-length reference sequences were first included into the calculation in all cases. The shorter sequences obtained in this study were then included into the dendrogram without changing the topology of the dendrogram using the parsimony tool of the ARB programme and applying the same filter as for the full-length sequences. The DNA sequences obtained in this study were submitted to GenBank (www.ncbi.nlm.nih.gov) and can be retrieved under the accession numbers DQ905965-DQ905992.
Results and discussion
CB degradation under hypoxic conditions
As inferred by the film theory, limited oxygen re-supply via a gas–liquid interface results from decreasing oxygen mass transfer rates with decreasing oxygen partial pressure (Holocher et al. 2003). We made use of this phenomenon to maintain low oxygen re-supply to hypoxic cultures. By periodical oxygen dosages to the cultures the headspace oxygen partial pressure was periodically adjusted to a low target value, which theoretically would be in equilibrium with 5–8 μM dissolved oxygen (oxygen-fed batch). However, irrespective of the oxygen permanently present in the batch headspace, steady state oxygen concentrations in parts even well below 1 μM dissolved oxygen emerged in solution during the course of the CB conversion (Fig. 3). Irrespective of the lowest initial cell densities, the bacterial consortium previously adapted to hypoxic conditions brought the steady state dissolved oxygen concentrations down to the lowest values (0.3–0.8 μM). In contrast, transformation of CB and CC in batches of A. facilis and P. veronii was accompanied with higher steady state concentrations of 1–3 and 4–7 μM dissolved oxygen, respectively. Interestingly, these characteristic oxygen levels appeared to be buffered to distinct values irrespective of increasing oxygen demand (Fig. 3A), e.g., caused by growth of biomass (not shown).
Time course of concentrations during hypoxic CB degradation by a bacterial consortium adapted to oxygen limitations (representative examples of 2 replicates); (A) without nitrate, (B) with nitrate present in the culture medium. CB (●); released Cl- (□); dissolved O2 concentration in medium (▲); nitrate (▼). A low oxygen concentration in the headspace (Δ) of the microcosms was reestablished by numerous oxygen gas spikes (dotted lines). The target range of headspace oxygen concentrations (5–6 μM) is represented by dashed lines. For comparison dissolved and headspace oxygen concentrations are both expressed in micromole per liter aqueous solution
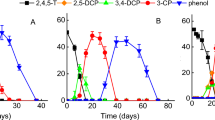
Dissolved oxygen levels below the equilibrium concentration reflect steady state conditions simultaneously influenced by oxygen phase transfer kinetics and oxygen demand kinetics due to activities of three oxygen utilizing enzymes (Fig. 1). Different levels as observed during CB conversion are assumed to be stabilized by different CC12Os activities specific to each investigated culture and were shown to correspond to the oxygen affinity of the respective CC12O (Balcke GU et al. 2007 (submitted)). This is of major importance because the accumulation of catechols is critical due to inhibitory effects on the degradation of aromatic compounds (Fritz et al. 1991; Pérez-Pantoja et al. 2003; Schweigert et al. 2001), and only few bacterial strains were reported to achieve productive turnover of catechols at severe oxygen limitation (Krooneman et al. 1998; Kukor and Olsen 1996). Extremely low steady state dissolved oxygen concentrations as measured for the consortium (Fig. 3) are indicative for remarkably high activities of the initial and the ring fission dioxygenase. This is corroborated since CB was efficiently transformed by the consortium within 15 ± 1.5 days under simultaneous dechlorination, since no metabolite accumulation could be observed, and since the oxygen demand throughout the experimental course implied oxygen demand rates >2 moles oxygen per mol CB transformed (not shown). In contrast to the consortium, both pure strains excreted CC (thereby producing a pink color) and CM to the culture media. Consequently, CB depletion and dechlorination did not occur simultaneously (Fig. 4). Whereas in hypoxic batches with A. facilis accumulating CC was further transformed with concomitant oxygen demand, and extensive dechlorination occurred within 8 ± 1 days, CC conversion by P. veronii was strongly inhibited, and hence, during the course of the experiment P. veronii showed only <50% of the dechlorination theoretically possible (Fig. 4B).
Time course of concentrations during hypoxic CB degradation by (A) Acidovorax facilis B530 and (B) Pseudomonas veronii B549 (representative examples of 2 replicates). Symbols: CB (●); released Cl- (□); dissolved O2 concentration in medium (▲); 3-chlorocatechol (♦), 2-chloro-cis,cis-muconate (◊). A low oxygen concentration in the headspace of the microcosms was maintained by numerous oxygen gas spikes. Dashed lines represent the target range of headspace oxygen concentration (6–8 μM)
When 500 μM acetate/succinate were provided as sole carbon sources, under similar conditions, for both strains complete turnover of this mixture also occurred within 8 ± 1.5 days but much lower steady state oxygen levels between 0.2 and 0.8 μM were detected in the media of all three cultures (data not shown). Since acetate and succinate can be directly funneled into the TCC, these decreased oxygen levels can be associated with an oxygen demand solely based on aerobic respiration and affirm indirectly the diverse activity the three cultures were capable of maintaining under oxygen limitation.
Influence of nitrate
In hypoxic batches containing the bacterial consortium nitrate and oxygen were utilized simultaneously during the transformation of CB (Fig. 3B). By contrast, in batches with nitrate present but omitting the presence of traces of oxygen no CB transformation was observed within 120 days (data not shown). As for cultivations omitting nitrate additions, the dissolved oxygen level decreased to values <1 μM. Under these strongly oxygen-limited conditions nitrate reduction was initiated, and once induced, it continued also at concentrations up to 5 μM dissolved oxygen subsequent to complete CB transformation. Similar CB transformation and dechlorination rates (14 ± 0.5 days) compared to experiments omitting nitrate (15 ± 1.5 days) do not come as a surprise, since oxygen is an obligatory prerequisite for initial ring attack and ring cleavage whereas nitrate is not involved in these reactions. However, with nitrate present a by 16 ± 3% lower cumulative oxygen demand was estimated, which is in good accordance with the amount of nitrate disappeared a complete reduction to dinitrogen given.
Finally, although our data did not prove that nitrate reduction was coupled to any electron transport chain, several observations point at a respiration of nitrate simultaneous to the oxygen reduction. Concomitant to cell growth in the consortium large amounts of nitrate vanished (440 μM), whereas intermediate nitrite did not accumulate and only trace amounts of nitrous oxide were detected. Sufficient ammonia levels of 2500–2800 μM were measured throughout the cultivation, thus making a nitrate assimilation unlikely. Since nitrate reduction accelerated upon increasing metabolization of CB, nitrate respiration coupled to the degradation of intermediates and cell residuals is anticipated. It can not be excluded that this was performed by members of the complex mixed culture, which were not involved in the oxidation of CB and CC but capable of denitrifying under the conditions applied.
Cultures of A. facilis and P. veronii did not significantly reduce nitrate during the transformation of CB under hypoxic conditions. However, as in batches omitting the addition of nitrate, higher dissolved oxygen levels between 1–2 and 3–8 μM, respectively, prevailed during CB transformation (not shown). By contrast, the degradation of acetate/succinate resulted in much lower steady state dissolved oxygen levels of 0.2–0.7 μM for both strains, and denitrification concomitant to oxygen reduction occurred simultaneously (not shown). About 1,800 μM nitrate were reduced but 43–49% of the vanished nitrate accumulated as nitrite. Simultaneous utilization of nitrate had no effect on the steady state oxygen level that emerged in media of cultures actively degrading acetate/succinate. Both compounds were degraded within 8 days as observed for tests in the absence of nitrate. Furthermore, when acetate/succinate were supplied as carbon source to P. veronii and A. facilis, respectively, with nitrate present 21 ± 5% and 23 ± 5% less oxygen were consumed as compared to cases were only oxygen was present. Note that these and the above results were obtained from cumulative oxygen measurements treating all oxygen data pairs according to the law of error propagation.
Compared to the consortium, slow turnover of CC and CM by the pure cultures is expected to produce less electron pressure by the TCC, which could have assisted in the observed exclusive respiration of oxygen. Due to lower activities of chlorocatechol 1,2-dioxygenase the dissolved oxygen level was largely kept to concentrations >1 μM over the most part of the CB transformation, and the formation of enzymes required for denitrification in A. facilis and P. veronii might have been suppressed (Knowles 1982; Zumft 1997). On the other hand, the supply of acetate/succinate, as metabolites that can be directly funnelled into the TCC (Fig. 1), easily initiated a significant denitrification concomitant to oxygen reduction. Dissolved oxygen levels <<1 μM during the conversion of acetate/succinate might have allowed concomitant nitrate reduction besides the activity of oxidoreductases. The oxygen level upon which bacteria begin to denitrify simultaneously to oxygen reduction has been described to vary widely (Zumft 1997).
Degradation of CM
CM was degraded by the groundwater consortium with oxygen as electron acceptor (≥180 μM, within 14 ± 2 days) as well as under strictly anoxic and denitrifying conditions (within 42 ± 4 days) (Fig. 5). In anoxic cultures without nitrate, almost no CM was converted over a 42-day period. Although high amounts of sulfate (8.3 mM) were present in the medium, sulfate reduction was not observed in any of the cultures.
Degradation of CM by a bacterial consortium under aerobic and anoxic conditions providing or omitting nitrate as alternative electron acceptor. Data illustrate one of two replicates representative for the culture studied. Symbols: aerobic (●); anoxic with nitrate present (■); anoxic omitting nitrate (▲); solid symbols represent CM, open symbols represent chloride released to the medium
In the absence of oxygen cultures of A. facilis and P. veronii were also capable of converting CM with nitrate as an electron acceptor (Fig. 6A, B) as illustrated by an equimolar chloride formation and concomitant decrease in nitrate, though both strains showed a lower tendency to reduce intermediary accumulating nitrite as compared to the community. A. facilis converted the major fraction of CM already within the first 14 days, reduced the whole amount of the 1,850 μM nitrate present, and slowly transformed accumulating nitrite, while P. veronii converted less than half of the CM within 28 days slowly reducing nitrate to nitrite, which was not further reduced during the experiment.
This demonstrates that denitrifying bacteria isolated from an anoxic CB-polluted aquifer were capable of degrading the ring fission product 2-chloromuconate without the need of oxygen.
Composition of hypoxic enrichment culture
The anoxic original Bitterfeld groundwater community was found to be very diverse (Alfreider et al. 2002). About 87 different bacterial 16S rDNA genes of a clone bank were dominated by a bacterial consortium affiliated with different subdivisions of Proteobacteria. The most frequently occuring clone type was related to the β-Proteobacteria most represented by Alcaligenes faecalis, Rhodoferax fermentans, and Acidovorax species, but also matches with α- and δ-Proteobacteria as well as with Firmicutes were found. Notably, within the γ-Proteobacteria members of the genus Pseudomonas were not identified. Compared to anaerobic conditions preferentially high G + C gram-positive (Rhodococcus spp.), γ-Proteobacteria (Pseudomonas spp.), and α-Proteobacteria (Xanthobacter spp.) enriched upon treatment of CB contaminated groundwater with high concentrations of H2O2, while β-Proteobacteria such as Acidovorax spp. contributed only minimally to the aerated groundwater community (Vogt et al. 2004a). Consecutive aerobic-anaerobic cultivation conditions, however, led to a dominance of the genera Acidovorax and Pseudomonas (Balcke et al. 2004).
Upon permanent cultivation under strictly hypoxic conditions we found a dramatic reduction of diversity in comparison to the indigenous groundwater community. Evaluation of 79 ARDRA patterns, sequence analysis of 28 clones (at least one representative for each ARDRA pattern), and search for closest relatives in public data bases (Fig. 7) reflected a bacterial community >97% composed of β-Proteobacteria and Bacteriodetes. The most frequently occurring patterns could be associated with the genera Simplicispira/Acidovorax/Variovorax (53 clones) in the Comamonadaceae family, followed by Wautersia (12 clones) and Thauera (1 clone). Within the phylum Bacteriodetes, the Cytophaga-Flavobacterium group contributed to the community with two strains (11 clones). Only one clone each related to the genera α-Proteobacteria (Mesorhizobium) and γ-Proteobacteria (Pseudomonas). Notably, no gram-positive bacteria persisted under permanent hypoxic conditions.
Although phylogenetic profiling based on 16S rDNA sequences is not suited to make statements about the oxygen affinity of functional genes, it appears that only two groups of the Eubacteria dominated the mixed culture upon CB degradation under hypoxic conditions. By comparison of sequences encoding the oxygen requiring enzyme CC12O, Alfreider et al. (2003) could assign clone library data of strains isolated from Bitterfeld groundwater to two distinct clusters, each one of high similarity. Previously, we found that the CC12O of Pseudomonas veronii B549 (a strain with low apparent oxygen affinity) shows high identity with one cluster, whereas a representative of the family Comamonadaceae (Acidovorax facilis B530, a strain with high apparent oxygen affinity) expressed a CC12O with high sequence convergence towards the other CC12O encoding cluster (Balcke GU et al. 2007 (submitted)). These results suggest the existence of high and low-affinity type CC12Os. Although the affiliation to the high-affinity type is anticipated, the assignment of CC12Os of members of the hypoxic community to one of these clusters has yet to be proven. Furthermore, syntrophic growth of strains, which do not cleave the aromatic ring themselves but utilize metabolites is assumed to account for abundance in the clone library.
Conclusions
It could be demonstrated that hypoxic bacterial consortia can efficiently degrade CB under largely reduced oxygen levels (e.g., 0.3 μM) also under concomitant denitrification, while pure strains, isolated from Bitterfeld groundwater, accumulated metabolites and did not demonstrate simultaneous denitrification upon growth on CB.
Keys to the activation of enzymes involved in nitrate respiration are the availabilities of oxygen and of ring fission metabolites produced if there are sufficient enzyme activities of the CB catabolism present (e.g., Fig. 1). Since during aerobic degradation of CB the availability of oxygen for terminal oxidases is affected by the activity of the dioxygenases involved in the preceding CB catabolism, strains which possess initial and ring fission dioxygenases, which are not suited to maintain high activities at reduced oxygen concentrations will produce reduction equivalents only slowly. As a result residual dissolved oxygen concentrations equilibrate to levels high enough to suppress denitrification. However, pure strains or consortia that can maintain high activities of both dioxygenases are able to suppress the dissolved oxygen concentration to levels (e.g., 1 μM) that allow for hypoxic denitrification. For complex mixtures the question if denitrifying bacteria are likewise CB degraders remains unsolved. However, under anoxic conditions, CM, the fission product of oxidative ring cleavage, is readily degraded by all studied CB-degrading cultures at the expense of nitrate as the sole terminal electron acceptor. Hence, it is concluded, if oxygen becomes depleted, denitrification is expected to become the dominating respiratory process as long as non-aromatic metabolites are available. Oxygen is an obligatory requirement for activation and fission of the aromatic CB ring but can be replaced by nitrate in respiration when oxygen is limiting.
Under hypoxic conditions a microbial community mainly composed of β-Proteobacteria developed which is different from aerobic isolates of the same groundwater. Isolation and characterization of microaerophilic CB degrading specialists has yet to be done. Yet, compared to aerobic enrichments, hypoxic cultivation is expected to give a more representative picture of the key microorganisms that will develop upon aeration of polluted groundwater where oxygen re-supply was limiting.
References
Alfreider A, Vogt C, Babel W (2002) Microbial diversity in an in situ reactor system treating monochlorobenzene contaminated groundwater as revealed by 16S ribosomal DNA analysis. Syst Appl Microbiol 25:232–240
Alfreider A, Vogt C, Babel W (2003) Expression of cholorocatechol 1, 2-dioxygenase and cholorocatechol 2,3-dioxygenase genes in chlorobenzene-contaminated subsurface samples. Appl Environ Microbiol 69: 1372–1376
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Balcke GU, Turunen LP, Geyer R, Wenderoth DF, Schlosser D (2004) Chlorobenzene biodegradation under consecutive aerobic-anaerobic conditions. FEMS Microbiol Ecol 49:109–120
Chen F, Xia Q, Ju L-K (2003) Aerobic denitrification of Pseudomonas aeruginosa monitored by online NAD(P)H fluorescence. Appl Environ Microbiol 69:6715–6722
Dean JA (1992) Lange’s handbook of chemistry. 14 edn. McGraw-Hill, New York
Dermietzel J, Vieth A (2002) Chloroaromatics in groundwater: chances of bioremediation. Environ Geol 41:683–689
Dikshit KL, Dikshit RP, Webster DA (1990) Study of Vitreoscilla globin (vgb) gene-expression and promoter activity in E. coli through transcriptional fusion. Nucleic Acids Res 18:4149–4155
Fritz H, Reineke W, Schmidt E (1991) Toxicity of chlorobenzene on Pseudomonas sp. strain RHO1, a chlorobenzene-degrading strain. Biodegradation 2:165–170
Holocher J, Peeters F, Aeschbach-Hertig W, Kinzelbach W, Kipfer R (2003) Kinetic model of gas bubble dissolution in groundwater and its implications for the dissolved gas composition. Environ Sci Technol 37:1337–1343
Kaschabek SR, Reineke W (1994) Synthesis of bacterial metabolites from haloaromatic degradation. 1. Fe(III)-catalyzed peracetic acid oxidation of halocatechols, a facile entry to cis,cis-2-halo-2,4-hexadienedioic acids and 3-halo-5-oxo-2(5H)-furanylideneacetic acids. J Org Chem 59:4001–4003
Klimant I, Kuhl M, Glud RN, Holst G (1997) Optical measurement of oxygen and temperature in microscale: strategies and biological applications. Sensor Actuat B-Chem 38:29–37
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Krooneman J, Moore ERB, van Velzen JCL, Prins RA, Forney LJ, Gottschal JC (1998) Competition for oxygen and 3-chlorobenzoate between two aerobic bacteria using different degradation pathways. FEMS Microbiol Ecol 26:171–179
Kukor JJ, Olsen RH (1996) Catechol 2,3-dioxygenases functional in oxygen-limited (hypoxic) environments. Appl Environ Microbiol 62:1728–1740
Leahy JG, Olsen RH (1997) Kinetics of toluene degradation by toluene-oxidizing bacteria as a function of oxygen concentration, and the effect of nitrate. FEMS Microbiol Ecol 23:23–30
Ma GH, Love NG (2001) BTX biodegradation in activated sludge under multiple redox conditions. J Environ Eng 127:509–516
Martienssen M, Fabritius H, Kukla S, Balcke GU, Hasselwander E, Schirmer M (2006) Determination of naturally occurring MTBE biodegradation by analysing metabolites and biodegradation by-products. J Contam Hydrol 87:37–53
Mason HS (1947) The allergenic principles of poison ivy . VI. Note on the synthesis of 3-substituted catechols. J Am Chem Soc 69:2241–2242
Otten MF, Stork DM, Reijnders WNM, Westerhoff HV, Van Spanning RJM (2001) Regulation of expression of terminal oxidases in Paracoccus denitrificans. Eur J Biochem 268:2486–2497
Patureau D, Bernet N, Delgenès JP, Moletta R (2000) Effect of dissolved oxygen and carbon-nitrogen loads on denitrification by an aerobic consortium. Appl Microbiol Biotechnol 54:535–542
Pérez-Pantoja D, Ledger T, Pieper DH, González B (2003) Efficient turnover of chlorocatechols is essential for growth of Ralstonia eutropha JMP134(pJP4) in 3-chlorobenzoic acid. J Bacteriol 185:1534–1542
Reineke W, Knackmuss H-J (1984) Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol 47:395–402
Reineke W (2001) Aerobic and anaerobic biodegradation potentials of microorganisms. In: Beek B (ed) The handbook of environmental chemistry, biodegradation and persistence 2K vol. Springer, Heidelberg, Berlin, pp 1–161
Rice CW, Hempfling WP (1978) Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol 134:115–124
Schlömann M (1994) Evolution of chlorocatechol catabolic pathways. Conclusions to be drawn from comparisons of lactone hydrolases. Biodegradation 5:301–321
Schweigert N, Zehnder AJB, Eggen RIL (2001) Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ Microbiol 3:81–91
Tolosa L, Kostov Y, Harms P, Rao G (2002) Noninvasive measurement of dissolved oxygen in shake flasks. Biotechnol Bioeng 80:594–597
Tseng CP, Albrecht J, Gunsalus RP (1996) Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol 178:1094–1098
Vogt C, Alfreider A, Lorbeer H, Hoffmann D, Wünsche L, Babel W (2004a) Bioremediation of chlorobenzene-contaminated ground water in an in situ reactor mediated by hydrogen peroxide. J Contam Hydrol 68:121–141
Vogt C, Simon D, Alfreider A, Babel W (2004b) Microbial degradation of chlorobenzene under oxygen-limited conditions leads to accumulation of 3-chlorocatechol. Environ Toxicol Chem 23:265–270
Wilson Durant LP, D’Adamo PC, Bouwer EJ (1999) Aromatic hydrocarbon biodegradation with mixtures of O2 an NO −3 as electron acceptors. Environ Eng Sci 16:487–499
Wilson LP, Bouwer EJ (1997) Biodegradation of aromatic compounds under mixed oxygen/denitrifying conditions: a review. J Ind Microbiol Biotechnol 18:116–130
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nestler, H., Kiesel, B., Kaschabek, S.R. et al. Biodegradation of chlorobenzene under hypoxic and mixed hypoxic-denitrifying conditions. Biodegradation 18, 755–767 (2007). https://doi.org/10.1007/s10532-007-9104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-007-9104-z