Abstract
Increasing land conversion of agriculture and forest by urban growth in the southern United States has altered the landscape matrix surrounding pine forests, creating varying mixtures of urban and agricultural cover types. I investigated the avian diversity-environment relationship in the southern pine forests along an urban-agriculture-wildland gradient by quantifying multiple dimensions of biodiversity: taxonomic (Shannon-Wiener index), functional (RaoQ and its standardized effect size), and phylogenetic (mean pairwise distance, mean nearest taxon distance, and their standardized effect size) diversity. I also considered habitat guilds and single traits correlated with RaoQ. I used breeding bird data collected at 162 pine stands in Georgia. Taxonomic and functional diversity increased with agricultural cover within the landscape (1 km-radius area). In particular, shrub habitat guild and shrub nesters showed a strong positive response to the variable. Insectivores and tree nesters (two predominant traits in pine forests) responded negatively to urban and agricultural covers. Both taxonomic and functional diversity decreased and increased with increasing hardwood vegetation cover and herbaceous vegetation cover within a stand, respectively. Pine-grassland and shrub habitat guilds, omnivores, and shrub nesters also showed the similar responses to these variables. Phylogenetic diversity metrics were not associated with environmental variables. The findings of this study suggest that open habitat features within a stand are important to promote functional diversity as well as taxonomic diversity in pine forests. They also indicate that agricultural matrix does not act as an environmental filter and low to moderate levels of less intensive agricultural (hay/pasture) matrix may improve avian diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the southern United States, forests comprise 40% of land cover (Miller et al. 2009). Approximately 25% of the forestlands, about 22.3 million hectares are dominated by loblolly-shortleaf pine (Pinus taeda-P. echinata) forests, and 19% of the forestlands consist of planted pines (Huggett et al. 2013; Miller et al. 2009). Planted pine forest, or pine plantation, is also forecasted to increase to 24–35% by 2060 despite declines of total forest cover (Huggett et al. 2013). As natural pine forests have significantly decreased due to land conversion (agriculture and urbanization), fire suppression, and logging, numerous studies have assessed potential values of planted pine forests as well as remnant pine forests for biodiversity conservation, and have identified forestry practices or environmental features that are conservation-relevant at multiple spatial scales (Lee and Carroll 2014, 2018; Loehle et al. 2005, 2009; Miles et al. 2010; Veldman et al. 2014; Waldron et al. 2008).
At the local or stand scale, open condition within a pine patch (or stand as synonym) is one of key factors affecting plants and animals, especially birds of conservation concern, e.g., red-cockaded woodpecker (Leuconotopicus borealis), Bachman’s sparrow (Peucaea aestivalis), and northern bobwhite (Colinus virginianus) (Greene et al. 2019; McIntyre et al. 2019). Closed canopy and hardwood encroachment result in dense vegetation but very low herbaceous vegetation cover on the ground, simplifying vegetation structure within a patch and reducing diversity and abundance of birds (Allen et al. 1996; Melchiors 1991). At the landscape scale, heterogeneous stand age and amount of non-pine forests can have an impact on avian diversity in pine forests (Loehle et al. 2005; Mitchell et al. 2006). However, little attention has been given to pine forests in anthropogenic landscapes such as urbanized or agricultural landscapes (but see Lee and Carroll 2014, 2015). There is still a lack of research on how urbanization and agricultural land use affect avian diversity in pine forests.
In the United States, urban development is largely associated with conversion of agriculture and forest (McPhearson et al. 2013). The highest rate of urbanization in the United States has occurred in the southeastern regions (Smith et al. 2009). With rapid urban extension, urban land cover has become a significant part of the landscape matrices in which pine forests of these regions are embedded, creating a complex land mosaic composed of varying extents of urban and agricultural lands and fragmentated pine patches. Lee and Carroll (2014) reported positive effect of moderate urban development on occupancy of several common birds in pine forests. In another study, they found greatest species richness in pine forest embedded in a landscape with low agricultural cover (Lee and Carroll 2015). Although these studies bring new insights to understanding the relationships between human land uses and birds in pine forests, their focus has been on taxonomic diversity (species richness or Shannon-Wiener diversity).
Taxonomic diversity (TD) treats all species within a community as equally distinct: more species are often interpreted as representing more functional traits and more lineages. However, this interpretation is not always true as TD represents only one of multiple dimensions of biological diversity (Swenson 2014; Tilman 2001). Cumulative evidence indicates inconsistencies between TD and other biological diversity measures such as functional diversity and phylogenetic diversity, and exposes the limited information TD can convey (Cumming and Child 2009; Frishkoff et al. 2014; Monnet et al. 2014; Morelli et al. 2016; among others). Functional diversity (FD) quantifies the value and range of organismal traits that influence “ecosystem properties or species’ responses to environmental conditions” (Cadotte et al. 2011; Hooper et al. 2005; Tilman 2001). Phylogenetic diversity (PD) represents the evolutionary relatedness of all species within a community (Tucker et al. 2016; Webb et al. 2002). While interrelationships between TD, FD, and PD are not straightforward and still in debate (Mazel et al. 2018; Tucker and Cadotte 2013), there is a growing consensus that FD and PD may be better indicators for biodiversity assessment because they are associated with ecosystem functioning and stability (Cadotte et al. 2012; Dı́az and Cabido 2001; Flynn et al. 2011; Mouchet et al. 2010). Thus, FD and PD have been increasingly used to evaluate how land use intensification influences biodiversity, ecosystem service, and community assembly (Flynn et al. 2009; Grab et al. 2019; Pakeman 2011; among others).
Urbanization and agricultural land use are expected to act as an environmental filter, narrowing the range of ecological traits and lineages that can persist in these novel environments and consequently reducing FD and PD (Flynn et al. 2009; Frishkoff et al. 2014; Ibáñez- Álamo et al. 2017; Sol et al. 2017, 2020). But these patterns may differ at moderate or low level of the land uses (Endenburg et al. 2019; Sol et al. 2020). Urbanization and agricultural land use may also show variations in their effects. For example, Filippi-Codaccionia et al. (2009) found higher FD of farmland birds in urbanized areas than in agricultural landscapes. Weideman et al. (2020) observed greater species richness of African savanna birds in urban areas than in rural areas but no difference in FD and PD after accounting for variations in species richness.
Given increasing land cover change and landscape complexity surrounding pine forests in the southern United States, it is imperative to build comprehensive knowledge on how human land uses affect avian diversity in pine forests. This also provides an opportunity to evaluate the value of pine forests for conservation in anthropogenic landscapes as well as natural landscapes (“wildland” in this study). It is important to go beyond simple counts of species as human land use may lead to a functionally redundant or phylogenetically close bird community despite more species, which could jeopardize ecosystem stability (Cadotte et al. 2011, 2012). Here, I quantified changes in multiple dimensions of avian diversity in pine forests including plantations and remnant pine patches along an urban-agriculture-wildland gradient. This study aimed to understand (1) how urban and agricultural land uses affect patterns of avian taxonomic, functional, and phylogenetic diversity in pine forests, and (2) what other environmental factors at local and landscape scales are associated with these patterns.
Methods
Study sites
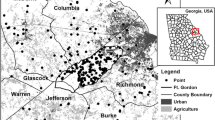
I performed this study in 162 mid-aged (20–75 years old) pine patches located in Sand Hills, Coastal Plain Red Uplands, and Southern Outer Piedmont ecoregions of Georgia, USA (Fig. 1). Most stands were dominated by loblolly pines and approximately 15% of stands by longleaf pine (P. palustris). In some areas, loblolly pines were slightly mixed with slash (P. elliottii) or shortleaf (P. echinate) pines.
Pine patches used for this study were embedded in a matrix of varying degrees of urban development and agricultural land use (mostly pasture and hay fields). I pre-selected sample patches considering separation distance between patches to minimize spatial dependency and percentage of urban and agricultural lands surrounding pine patches based on 2006 National Land Cover Dataset (NLCD) and 1998 Digital Orthophoto Quarter Quads (DOQQs). More patches were selected within Fort Gordon because it maintained pine stands that their local conditions resembled natural southern pine forests (pine-grassland ecosystems) as well as commercial pine forests with moderate and high basal area (Lee and Carroll 2018). I finalized the selection of sample patches following ground-truth surveys. However, the location and the number of sample patches outside Fort Gordon were significantly limited by landowner permission and within Fort Gordon partly by accessibility.
Bird and local environmental data
Within each patch, I established one sample point randomly at 50–70 m away from an edge of the patch. All points were 1.9 ± 1.3 km (mean and standard deviation of nearest distance, ranging from 0.54 to 7.83 km) apart. Bird surveys were performed three times during breeding season (May-June) in 2011, using fixed-radius point counts (Ralph et al. 1993). At a sample point, an observer recorded species detected visually or aurally within a 50 m radius of a sample point for 10 min between dawn and 11:00 EDT. Birds flying over the 50 m radius area were recorded separately. I alternated survey order to minimize the effect of time-of-day and rotated two observers between points to reduce observer effects. Surveys were not conducted during periods of high wind or rain.
Vegetation sampling was performed between mid-June and July. At a sample patch, I established four 5 m radius circular plots and four 10 m radius plots in each cardinal direction at a fixed distance of 30 m from a sample point. Within a 5 m radius circular plot, we visually estimated the percent cover of vegetation in the herb (< 0.5 m in height), shrub (0.5–5 m in height), and tree (>5 m in height) layers following Point Reyes Bird Observatory Point Count Veggie (Relevé) Protocol (www.prbo.org/cadc/songbird/pc/relevepr.html). Tree layer vegetation was also divided into two categories: softwood (pine tree) and hardwood (mainly sweetgum [Liquidambar styraciflua], oak [Quercus spp.], sassafras [Sassafras albidum], black cherry [Prunus serotina], and flowering dogwood [Cornus florida]). For each of four vegetation covers, I averaged the values estimated from the four circular plots. Within each 10 m circular plot, the diameter at breast height (DBH) of hardwoods and softwoods (≥ 8 cm in diameter) was measured. I calculated hardwood basal area and softwood basal area separately within a plot using the DBH data and for each basal area, averaged the value across all four plots. Most of these vegetation features are often considered important to wildlife in pine forests (Dickson et al. 1993; Lee and Carroll 2014, 2018; McIntyre et al. 2019; Melchiors 1991).
Land cover data
To create a land cover map precise enough for analysis, I on-screen digitized 2010 digital orthophoto images (National Agriculture Imagery Program) and 1998 DOQQs in ArcGIS version 9.2 and conducted ground-truth surveys. By modifying the classification scheme of 2006 NLCD, I delineated 9 land cover types within a 1 km radius area surrounding a sample point and calculated percent cover of each land cover type (Table S1 for summary; Lee and Carroll 2014). The landscape size, i.e., a 1 km radius area, is similar to ‘‘the size of a typical residential development’’ (Marzluff 2001), large enough to encompass home range size of songbirds (Lee and Carroll 2015). All sample points were embedded in a landscape where agricultural cover (except one point) or urban cover (except five points) was < 50%. I calculated the Shannon diversity index for 5 natural/semi-natural land covers that could be used by birds: pine forest, hardwood forest, mixed forest, shrubland, and open water. The Shannon diversity index measures landscape heterogeneity (LandH) that represents habitat heterogeneity at the landscape scale. I also calculated an interspersion and juxtaposition index of pine patches (IJI-Pine) within a 1 km radius area to describe the distribution of adjacencies among pine patches (McGarigal and Marks 1995). It represents the spatial arrangement of pine patches and increases as pine patches are equally adjacent to each other. All calculations were performed using FRAGSTATS version 4 (McGarigal et al. 2012).
Taxonomic and functional diversity of birds
To calculate diversity metrics, I included in analysis bird species observed at least once but excluded flyovers, resulting in a total of 59 species (Table S2 for species list). For abundance of each species, I used the maximum count recorded over all visits.
Taxonomic diversity was measured with Shannon-Wiener diversity index (SW). Earlier studies have shown that species’ habitat type or assemblage influences the bird species-environment relationship in southern pine forests (Wilson et al. 1995; Canterbury et al. 2000; Lane et al. 2011; Lee and Carroll 2014). I grouped birds into three general habitat guilds (forest, open habitat [open-woodland and grassland], and shrub) and one habitat guild unique to southern pine forest (pine-grassland). Although open habitat contained all pine-grassland species, I included pine-grassland guild because it is closely related with pine ecosystems and among 17 species of regional concern, 9 are pine-grassland species (Table S2). I calculated SW of each of 4 guilds, as well as of whole community (total species).
I used 5 types of traits that are functionally important or strongly related with resource-use to characterize functional diversity (Flynn et al. 2009; Lee and Carroll 2018; Luck et al. 2012; among others): one continuous trait-body mass; and four categorical traits-diet (insectivorous, granivorous, and omnivorous), foraging behavior and location (aerial foraging, foliage gleaning, bark gleaning, and ground foraging), migratory status (migrant and resident), and nest placement (tree, shrub, ground, and others [e.g., cliff, building/house]). Body mass data were compiled from Dunning (2008) and the other trait data from The Birds of North America online database (http://bna.birds.cornell.edu/BNA/). Habitat guild and trait of each of 59 species are described in Table S2.
As an index of functional diversity, I used abundance-weighted Rao’s quadratic entropy Q (RaoQ), which represents functional divergence and partly functional richness (Mouchet et al. 2010). RaoQ is high when there is less empty functional space and/or when trait dissimilarity is high between the most abundant species. RaoQ can be sensitive to community assembly rules as well as species richness. A recent simulation study shows that the standardized effect size of RaoQ (SES.RaoQ) is unaffected by differences in species richness among communities while retaining good power to detect assembly processes along an environmental gradient (Mason et al. 2013). SES.RaoQ measures the deviation of an observed RaoQ from the expected RaoQ while removing bias associated with variations in species richness (Swenson 2014). Thus, I considered both RaoQ and SES.RaoQ.
SES. RaoQ was calculated following a null model approach (Gotelli and Rohde 2002): SES.RaoQ = (observed RaoQ—mean expected RaoQ)/standard deviation of expected RaoQ, where expected RaoQ values are calculated from random communities (Gotelli and Rohde 2002). I simulated 999 communities by randomly choosing species from the species pool and randomly selecting abundance of each species but maintaining species richness and the pattern of abundance as constant at each point. Mean and standard deviation of expected RaoQ were calculated from the RaoQ values of the 999 random communities. RaoQ was calculated using “dbFD” function in FD package (Laliberté et al. 2014) in R. The Randomization was carried out using picante package (Kembel et al. 2010). I did not standardize body mass as dbFD standardizes traits automatically when not all traits are numeric (Laliberté et al. 2014). In bird data, species richness was correlated with RaoQ (Pearson’s correlation r = 0.40, P < 0.001) but not with SES.RaoQ (r = 0.14, P = 0.24), confirming the independent relationship between SES.RaoQ and species richness.
In addition to RaoQ (multi-trait index), I computed the community-weighted mean (CWM) for each trait as a single-trait index (Garnier et al. 2004) using “dbFD” function. For continuous traits, CWM represents the mean value of trait weighted by relative abundance of species in a given community. For categorical or binary traits, it is relative abundance of a given individual category in a community. Although CMW may not represent functional diversity because it measures “a central tendency” rather than variability, CWM defines dominant traits and describes functional composition of community (Ricotta and Moretti 2011). I used CWM to identify traits associated with the observed pattern of RaoQ and to evaluate how environmental features influenced these individual traits. Body mass was log-transformed for normalization prior to the calculation of its CWM. I examined correlations between RaoQ and CWM of each trait. For further analysis, I focused on CWMs of traits moderately and highly correlated with RaoQ and SES.RaoQ (r ≥ |0.5|).
Phylogenetic diversity
I obtained 1,000 trees of 59 species from each of two backbones containing the complete phylogeny of birds available at BirdTree (http://birdtree.org): the Ericson backbone phylogeny and the Hackett backbone phylogeny (Jetz et al. 2012). For each backbone phylogeny, I constructed one consensus tree (maximum clade credibility tree) that was used to calculate phylogenetic diversity indices from the 1000 trees in TreeAnnotator v 2.6.2 of the BEAST 2 package (Drummond and Rambaut 2007).
As a measure of phylogenetic diversity, I choose distance-based metrics that are widely adopted to quantify the phylogenetic similarity of species within a community (Swenson 2014): the mean pairwise distance, MPD, and the mean nearest taxon distance, MNTD. MPD is the overall average of phylogenetic distance between all species within a community, whereas MNTD is the average of shortest distance for each species to its closest relative. MNTD can deliver more detail information than MPD as closely related species are likely to have stronger interaction than distantly related species. These metrics were also abundance-weighted.
There were little differences between values calculated from the Hackett backbone phylogeny and those from the Ericson backbone phylogeny (r = ~0.99, P < 0.0001 for both MPD and MNTD). Thus, I used the values from the Hackett backbone for subsequent analysis. MPD and MNTD were correlated with species richness: MPD, r = − 0.34, P < 0.001; MNTD, r = − 0.45, P < 0.001. The standardized effect size of MPD (SES.MPD) and MNTD (SES.MNTD) were calculated following the same method (i.e., a null model approach) used for SES.RaoQ.
Statistical analysis
Final analysis included 8 explanatory variables: 5 landscape variables (landscape heterogeneity, IJI-Pine, percent cover of agricultural land, urban development, and hardwood vegetation), and 3 local variables (softwood basal area, percent cover of hardwood vegetation in the tree layer and grasses and forbs in the herb layer). The relationship between avian diversity and environmental characteristics was quantified using generalized linear models with beta distribution for RaoQ, Gaussian distribution for guild-level SW and three SES variables, gamma distribution for MPD and MNTD, and beta or Gaussian distribution for CWMs depending on traits. These distributions were determined based on not only data type of response variable but also whether the model satisfied homoscedasticity assumption. Spatial error model was used for SW of all species due to significant spatial autocorrelation (see below). I standardized all environmental variables to normalize model residuals.
In this study, the standardized effect size of functional and phylogenetic diversity metrics was used to remove their dependency on species richness. However, SES values can also be used to explore community assembly processes, especially environmental filtering vs. limiting similarity (Mouchet et al. 2010; Webb et al. 2002). Negative and positive SES values are often considered to indicate a stronger role of environmental filtering and limiting similarity in structuring community assembly, respectively, although the relative effects of these two drivers on community phylogenetic structure are more complicated (Mayfield and Levine 2010). Environmental filtering tends to result in assemblages of species with more similar traits and possibly closer relatedness. Limiting similarity can drive assemblages of species with more distinct traits and likely less related species. I took significantly low (<− 1.96) and high (> 1.96) SES values, especially SES.RaoQ as an evidence for environmental filtering and limiting similarity, respectively.
I performed Moran’s I test to examine spatial autocorrelation using the packages spdep in R (Bivand et al. 2013). There were no significant cases of Moran’s I test (P > 0.1) except SW of total species (P < 0.001). To deal with spatial autocorrelation, I adopted spatial autoregressive modeling and built a spatial error model for SW of total species (Kissling and Carl 2008). Moran’s I test of the spatial error model did not show a sign of spatial dependence (P = 0.45). I also computed variation inflation factor values. All values were between 1.1 and 2.0, indicating that multicollinearity would have a negligible effect on results. The plots of residuals vs. fitted values of final models did not show any patterns, suggesting no violation of homoscedasticity assumption. Each model was also compared with its own null model (intercept-only model) using likelihood ratio test to examine a model fit.
Results
General patterns
There were over 2,800 detections across 59 species. Of 59 species, Carolina chickadee (Poecile carolinensis), pine warbler (Dendroica pinus), northern cardinal (Cardinalis cardinalis), and summer tanager (Piranga rubra) were the most common, occurring at > 75% of sample points (Table S2). Carolina chickadee also showed the greatest mean abundance per point, followed by northern cardinal, brown-headed nuthatch (Sitta pusilla), and pine warbler. While most species were observed at wildland as well as in urbanized or agricultural landscapes, Bachman’s sparrow and red-cockaded woodpecker were detected only at wildland and eastern meadowlark (Sturnella magna) in an agricultural landscape.
A bird community was dominated by two traits, insectivorous diet and tree nest (Fig. S1). The sum of mean abundance of insectivores and tree nesters were 7 times and 4 to 14 times higher than other diet traits and nest placement traits, respectively (Table S2). Although resident species were twice as abundant as migrant species, species richness of migrant birds was slightly greater (Fig. S1). Forest guild showed greater species richness (29 species) compared to open habitat (20), shrub (7), and pine-grassland (14) guilds.
Avian diversity-environment relationships at community level
Both taxonomic and functional diversity responded similarly to local variables: SW of total species, RaoQ, and SES.RaoQ decreased with increasing hardwood vegetation cover in the tree layer but increased with percent cover of herbaceous vegetation (grasses and forbs) in the herb layer within a pine patch (Table 1). The significant responses of SES.RaoQ indicated that the loss or gain of functional diversity could be greater than expected by chance given species richness.
Agricultural land use showed a positive impact on SW of total species, RaoQ, and SES.RaoQ (Table 1). Although urban land cover was positively associated with SES.RaoQ, P value was marginal (0.045), indicating their weak association. Landscape heterogeneity also influenced taxonomic diversity positively.
Among polygenetic diversity metrics, MNTD responded positively or negatively to several variables, but these responses were dependent on changes in species richness given insignificant responses of SES.MNTD (Table 1). SES.MPD was positively associated with local hardwood vegetation cover. MPD was not related to any environmental variables. However, both models of SES.MPD and MPD did not differ from their null models based on insignificant result of likelihood ratio test (P = 0.12 and P = 0.48, respectively) and lower Akaike Information Criterion (AIC) values of their null models than full models, suggesting little association between these explanatory and response variables. All other response variables showed significant result of likelihood ratio test (P < 0.01) and lower AIC value of full model than null model (AIC difference > 4).
SES.RaoQ values were highly variable at wildland (Fig. 2, A). However, as agricultural cover increased from low to moderate, some values were significantly positive (> 1.96) and SES.RaoQ tended to be positive. Significantly negative values (<− 1.96) were not found with increasing agricultural cover. The relationship between SES.RaoQ and hardwood vegetation cover in the tree layer showed similar patterns except direction in the response: a few significantly negative cases and no significantly positive value with increasing the vegetation cover (Fig. 2, B). For other explanatory variables, SES.RaoQ values did not vary significantly, except at low level which had both negative and positive values (Fig. S2).
Plot of SES.RaoQ (the standardized effect size of RaoQ) along a gradient of agricultural land cover at the landscape scale (a) and hardwood vegetation cover in the tree layer at the local scale (b). Both environmental variables were standardized. Note that there are no significantly negative (<− 1.96) and positive (> 1.96) values along the gradient of agricultural land cover and hardwood vegetation cover, respectively
Responses of habitat guilds and single traits
Of 4 habitat guilds, SW of shrub was positively affected by both urban and agricultural land covers and SW of open habitat negatively by urban land cover (Fig. 3). SW of Pine-grassland species was more positively related to agricultural land cover than urban land cover although it was insignificant. All guilds except forest species, their SW decreased with increasing local hardwood vegetation cover. Local herbaceous vegetation cover and softwood basal area showed positive and negative effect on SW of pine-grassland species, respectively (Fig. 3, Table S3). SW of forest species increased with landscape heterogeneity and open habitat species showed a similar trend (Table S3).
Relationships between Shannon-Wiener diversity of each habitat guild and environmental variables. Estimate and error bar represent parameter estimate (coefficient) from linear models and 95% confidence interval, respectively. See Table S3 for all estimates. Abbreviation: Agriculture, agricultural land cover; Urban, urban land cover; HW_local, local hardwood vegetation cover; GrFo, local herbaceous vegetation (grasses and forbs) cover
CWMs of five traits were moderate or highly correlated with RaoQ (and SES.RaoQ) (Table S4): 2 diet (insectivorous and omnivorous), 2 nest placement (tree and shrub), and 1 foraging (ground) traits, suggesting that the degree of trait dissimilarity between species in a community may be linked to an increase or decrease of birds with these traits. While CWM of insectivorous diet decreased with agricultural and urban land covers, CWMs of omnivorous diet, ground foraging, and shrub nest increased (Fig. 4). At the local scale, CWMs of shrub nest and omnivorous diet were high at open pine patches with low hardwood vegetation and high herbaceous vegetation covers; however, CWM of tree nest was low at those patches and high at densely vegetated pine patches (Fig. 4, Table S5).
Relationships between environmental variables and community-weighted mean trait values (CWMs) of five traits that were associated with RaoQ. Estimate represents parameter estimate (coefficient) from linear models. Error bars are 95% confidence intervals. See Table S5 for all estimates and Fig. 3 for abbreviations
Discussion
This study reveals that local vegetation features can have a strong impact on trait differences as well as species diversity in bird communities of pine forests. Taxonomic and functional diversity of whole community and most habitat guilds and single traits showed consistent responses to local variables: hardwood vegetation cover had a negative effect on these diversity metrics, whereas herbaceous vegetation cover showed a positive effect. In particular, taxonomic diversity of pine-grassland species was strongly affected by the two variables and basal area (negative response). Pine-grassland habitat guild includes species of conservation concern such as Bachman’s sparrow, northern bobwhite, and red-cockaded woodpecker (endangered species) in southern pine forests. In pine forests, heterogeneous habitat structure (more vegetation layers and their even distribution) decreases when canopy closes (Allen et al. 1996; Melchiors 1991). Pine forests with closed canopy become biologically barren without proper management. Low species richness or occurrence of birds, especially shrub species and pine-grassland species has been reported at pine forests of which basal area or canopy cover is high (Canterbury et al. 2000; Lee and Carroll 2018). Increasing basal area and hardwood vegetation is likely to make vegetation dense, interrupt the establishment of herbaceous plants on the ground, dimmish grassland-like understory cover, and subsequently degrade open conditions within a pine forest. These environmental changes could be strong enough to filter out traits. More cases of significantly negative SES.RaoQ values with increasing hardwood vegetation cover is partly congruent with this potential mechanism, which further indicates that environmental filtering may drive bird community structure at local scale in pine forests. Hardwood reduction while increasing ground cover of grasses and forbs is frequently recommended for pine forest management because it can benefit endangered species or species of conservation concern as well as other species (Dunning and Watts 1990; Provencher et al. 2000). The findings of this study are consistent with the recommendation. They further suggest that managing hardwood vegetation cover and understory vegetation can improve functional diversity as well.
This study also found that agricultural lands may serve as a good quality matrix that could improve avian diversity in pine stands. Agricultural cover had a significantly positive impact on both taxonomic and functional diversity at community level, whilst urban cover did not influence any dimensions of diversity strongly. In general, human land uses reduce taxonomic diversity of birds with some variations between ecological guilds (Chace and Walsh 2006; Marzluff 2001). However, most significant effects are often found in high levels of urban or agricultural cover, for example, occupying over 50% of a landscape (Endenburg et al. 2019). Species richness or abundance can be great in moderately urbanized landscapes (Chace and Walsh 2006; Marzluff 2001), landscapes with low agricultural cover or less intensive agricultural land use, and heterogeneous agricultural systems (Endenburg et al. 2019; Frishkoff et al. 2014; Lee and Carroll 2015). Results from recent large-scale studies also show varying trends in how urbanization and agricultural land use affect avian functional diversity. For example, Matuoka et al. (2020) reported a negative effect of urbanization on functional diversity but an insignificant effect of agricultural land use. The more significant cases of agricultural land cover than urban land cover at community level could be associated with the type of agriculture or agricultural intensity. Open pine forest that resembles both pine woodland and savanna ecosystem is prioritized for conservation management of plants and animals in the southern United States (McIntyre et al. 2019). Hay/pasture-dominant agricultural lands are common throughout the study area. These lands may provide open semi-natural habitat complementing the open condition of pine forest. Hay/pasture is considered less intensive agriculture that could maintain slightly higher functional diversity of birds than intensive arable agriculture (Bregman et al. 2016). While green spaces are one of key factors affecting urban biodiversity (Aronson et al. 2014; Beninde et al. 2015), open green space in urban areas consists mostly of turfgrass lawn that is intensively managed, e.g., frequent mowing and herbicide/pesticide and fertilizer usage, which is common in US cities (Robbins and Birkenholtz 2003). Previous study also found a tendency of lower herbaceous vegetation cover and higher woody vegetation cover in pine patches in urban areas than in rural areas (Lee and Carroll 2015). Thus, the current agricultural type (hay/pasture) could form a more amenable matrix for birds in pine forests compared to urban environment.
Agricultural land surrounding pine stands may also facilitate functional differentiation among coexisting species or increase trait divergence within a bird community in pine forests. As agricultural land cover increased, relative abundance of species with less dominant traits (omnivores, ground foragers, and shrub nesters) increased in pine patches and relative abundance of species with predominant traits (insectivores and tree nesters) decreased. These changes may prevent certain traits from being too dominant and at the same time increase trait differences between abundant species in a community. This is supported by positive SES.RaoQ values with increasing agricultural cover, which indicates a relatively strong role of limiting similarity on determining bird community structure in pine forests in agricultural landscapes. It should also be pointed out that the negative response of predominant traits, especially tree nesters could be driven by species preferring dense forests (non-open pine forests), given the positive response of tree nesters to local hardwood vegetation cover and softwood basal area but the negative response to local herbaceous vegetation cover.
While the similar patterns were found between urban land cover and most single traits, estimates (coefficients) of urban cover were higher compared to agricultural cover. That it, changes in urban land cover could shift dominance of these traits more significantly. Bird community gains and loses birds of certain traits (for example, omnivores and insectivores, respectively) rapidly with increasing urban land cover. New individuals added to the community could be more functionally similar in urbanized landscapes than in agricultural landscapes. They are likely species preferring dense forests considering the negative response of open habitat guild to urban land cover. Dominance of omnivores and scarcity of insectivores in bird community have been reported in urban areas (Chace and Walsh 2006; Kark et al. 2007), which is partly congruent with the pattern observed in this study.
Among other landscape variables, landscape heterogeneity had a positive effect on taxonomic diversity of total species and forest species. Open habitat guild also tended to respond positively. Heterogeneous environments are often assumed to increase animal diversity because these environments can provide spatially and temporarily diverse resources to animals (Fahrig et al. 2011; Tews et al. 2004). While the positive response of taxonomic diversity supports the well-known assumption, insignificant response of functional and phylogenetic diversity indicates little differences in traits and phylogenetic relatedness between bird assemblage of pine forest in homogeneous matrix and that in heterogeneous matrix. These inconsistent patterns show that the positive biodiversity-landscape heterogeneity relationship can vary depending on the aspect of biodiversity measure, indicating the importance of considering multiple aspects of biodiversity to comprehensively understand the relationship.
Compared to taxonomic and functional diversity, relationships between phylogenetic diversity and environmental features were weak. Loss of phylogenetic diversity has been reported in disturbed systems including urban or agricultural environments, although part of the loss may be mitigated by “the entrance of opportunistic species” (Ibáñez-Álamo et al. 2017; Sol et al. 2017). Evolutionary unique species or some branches of the tree of life that have fewer relatives can be more vulnerable to novel environmental changes. In that case, a bird community in these systems contains more closely related species (phylogenetic clustering; Frishkoff et al. 2014; Sol et al. 2017). Unclear responses of phylogenetic diversity metrics may be explained by a long history of disturbance across the study region. Pine-grassland ecosystems, especially longleaf pine ecosystem, which is the most biodiversity rich ecosystem outside tropics, had dominated the southern forests until the late 1800’s (Brockway et al. 2005). However, it has drastically declined during the 1900 s, losing 97% of its historic range due to timber production, land conversion, and changes in forest practices (Van Lear et al. 2005; Jose et al. 2006). Evolutionary distinctive bird species associated with the old-growth pine forest could already be lost or their populations could have significantly declined in the process. As a result, mean phylogenetic distance of all species pairs (MPD) may not be substantially different in pine forests across the study region. Although phylogenetic diversity at the terminal of the tree of life (i.e., mean phylogenetic distance between closest relatives, MNTD) varied along some environmental gradients, the pattern was driven by changes in species richness.
Limitation and future study
This study considered multiple dimensions of avian diversity; however, each dimension of diversity was measured within a community, i.e., α diversity, because this study was interested in the specific focal habitat and its matrix. It was also not feasible to survey multiple land covers due to logistic issues. However, if higher diversity is largely fueled by immigrants from adjacent natural or semi-natural habitats including agriculture lands (i.e., spillover effect), if diversity loss is compensated by those immigrants, or if biotic homogenization occurs at much larger scale (Gossner et al. 2016; Sax et al. 2002), β diversity (turnover or dissimilarity among bird communities) can be low. α and β diversity can also respond differently to environmental features (Flohre et al. 2011; Meynard et al. 2011). Although partitioning diversity requires different study design and intensive sampling, comparisons between α and β diversity will enable us to assess habitat value of pine forest at regional scale and to test potential biotic homogenization driven by human land use. They may also help clarify the ambiguous responses of phylogenetic diversity to environmental variables. Future research also needs to account for forest management history. Forestry practices (e.g., thinning method, even-aged vs. uneven-aged management, prescribed fire usage and frequency) affect vegetation structure at local and landscape scales and subsequently species richness and abundance of birds (Dickson et al. 1993; Melchiors 1991). I could not incorporate any information about forest management practices because such information was not available at most stands. Although local vegetation features could partly capture variations in management practices among stands, I acknowledge that some of unclear results may be influenced by how the stands have been managed.
Conclusions
This study demonstrates the importance of managing vegetation features within pine forests to conserve not only taxonomic diversity of birds but also their functional diversity. The findings of this study provide valuable insights on the human land use-avian diversity relationship. When urban or agricultural lands within a landscape are not too extensive (e.g., < 50% cover within a 1 km circular area), they may neither form a low quality matrix for embedded southern pine forests, nor serve as an environmental filter at the spatial scale of this study. In particular, less intensive agricultural matrix (hay/pasture land) may complement open condition of pine forests while providing resources for species uncommon in pine forests. This has important implications for conservation management. Species inhabiting a habitat patch within a high quality matrix may require a lesser amount of optimal habitat for species to persist (Fahrig 2001). Remnant pine patches in anthropogenic landscapes, which is relatively small, may still be suitable habitat for birds. Pine patches in a landscape with low to moderate level of hay/pasture agricultural cover can serves as a good quality matrix or secondary habitat (Lee and Carroll 2015). However, it should not be interpreted that these pine patches can replace ones in natural landscapes. Some species of conservation concern (Bachman’s sparrow and red-cockaded woodpecker) could be susceptible to human land uses. Overall, while conservation priority should be given to pine patches in natural landscapes, the findings of this study suggest that in pine plantations, pine stands adjacent to agricultural lands can contribute to promoting avian diversity especially if they maintain open habitat conditions through forest management such as reducing basal area and hardwood vegetation and increasing herbaceous vegetation cover.
References
Allen AW, Bernal YK, Moulton RJ (1996) Pine plantations and wildlife in the southeastern United States: an assessment of impacts and opportunities. US Department of the Interior National Biological Service Information and Technology. Report 3
Aronson MFJ, La Sorte FA, Nilon CH et al (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B 281:20133330
Beninde J, Veith M, Hochkirch A (2015) Biodiversity in cities needs space: a metaanalysis of factors determining intra-urban biodiversity variation. Ecol Lett 18:581–592
Bivand RS, Pebesma E, Gomez-Rubio V (2013) Applied spatial data analysis with R, 2nd edn. Springer, New York
Bregman TP, Lees AC, MacGregor HEA, Daski B, de Moura NG, Aleixo A, Barlow J, Tobias JA (2016) Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc R Soc B 283:20161289
Brockway DG, Outcalt KW, Tomczak DJ, Johnson EE (2005) Restoration of Longleaf Pine Ecosystems. Gen Tech Rep GTR-SRS-83 USDA For. Serv Southern Res Sta Asheville, NC
Cadotte MW, Cascadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087
Cadotte MW, Dinnage R, Tilman D (2012) Phylogenetic diversity promotes ecosystem stability. Ecology 93:S223–S233
Canterbury GE, Martin TE, Petit DR, Petit LJ, Bradford DF (2000) Bird communities and habitat as ecological indicators of forest conditions in regional monitoring. Conserv Biol 14:544–558
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landscape Urban Plan 74:46–69
Cumming GS, Child MF (2009) Contrasting spatial patterns of taxonomic and functional richness offer insights into potential loss of ecosystem services. Philos Trans R Soc B 364:1683–1692
Díaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Dickson JG, Thompson FR III, Conner RN, Franzreb KE (1993) Effects of silviculture on neotropical migratory birds in central and southeastern oak pine forests. In: Finch DM, Stangel PM (eds) Status and management of neotropical migratory birds, Gen Tech Rep RM-229 USDA For Serv Colorado, pp 374–385
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Dunning JB Jr (2008) CRC handbook of avian body masses, 2nd edn. CRC Press, Boca Raton, Florida
Dunning JB Jr, Watts BD (1990) Regional differences in habitat occupancy by Bachman’s sparrow. Auk 107:463–472
Endenburg S, Mitchell GW, Kirby P, Fahrig L (2019) The homogenizing influence of agriculture on forest bird communities at landscape scales. Landscape Ecol 34:2385–2399
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin J-L (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Filippi-Codaccionia O, Clobert J, Julliard R (2009) Urbanisation effects on the functional diversity of avian agricultural communities. Acta Oecol 35:705–710
Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33
Flynn DFB, Mirotchnick N, Jain MI, Palmer S, Naeem (2011) Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 92:1573–1158
Flohre A, Fischer C, Aavik T et al (2011) Agricultural intensification and biodiversity partitioning in European landscapes comparing plants carabids and birds. Ecol Appl 21:1772–1781
Frishkoff LO, Karp DS, M’Gonigle LK, M’Gonigle LK, Mendenhall CD, Zook J, Kremen C, Hardly EA, Daily GC (2014) Loss of avian phylogenetic diversity in neotropical agricultural systems. Science 345:1343–1346
Garnier E, Cortez J, Billes G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Gossner MM, Lewinsohn TM, Kahl T et al (2016) Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540:266–269
Gotelli NJ, Rohde K (2002) Co-occurrence of ectoparasites of marine fishes: A null model analysis. Ecol Lett 5:86–94
Grab H, Branstetter MG, Amon N, Urban-Mead KR, Park MG, Gibbs J, Blitzer EJ, Poveda K, Loeb G, Danforth BN (2019) Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363:282–284
Greene RE, Iglay RB, Evans KO (2019) Providing open forest structural characteristics for high conservation priority wildlife species in southeastern US pine plantations. Forest Ecol Manag 453:117594
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Huggett R, Wear DN, Li R, Coulston J, Liu S (2013) Forecasts of forest conditions. In: Wear DN, Greis JG (eds) The southern forest futures project. Gen Tech Rep SRS-178 USDA For Serv Southern Res Sta, pp 73–101
Ibáñez-Álamo JD, Rubio E, Benedetti Y, Morelli F (2017) Global loss of avian evolutionary uniqueness in urban areas. Global Change Biol 23:2990–2998
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Jose S, Jokela EJ, Miller DL (2006) The longleaf pine ecosystem: an overview. In: In: Jose S, Jokela EJ, Miller DL (eds) The longleaf pine ecosystem: ecology silviculture and restoration. Springer, New York, pp 3–8
Kark S, Iwaniuk A, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an ‘urban exploiter’? J Biogeor 34:638–651
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kissling WD, Carl G (2008) Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob Ecol Biogeogr 17:59–71
Laliberté E, Legendre P, Shipley B (2014) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12
Lane VR, Miller KV, Castleberry SB, Cooper RJ, Miller DA, Wigley TB, Marsh GM, Mihalco RL (2011) Bird community responses to a gradient of site preparation intensities in pine plantations in the Coastal Plain of North Carolina. For Ecol Manage 262:1668–1678
Lee M-B (2013) Avian biodiversity along an urban–rural/agriculture–wildlife gradient. Dissertation, University of Georgia
Lee M-B, Carroll JP (2014) Relative importance of local and landscape variables on site occupancy by avian species in a pine forest urban and agriculture matrix. Forest Ecol Manag 320:161–170
Lee M-B, Carroll JP (2015) Avian diversity in pine forests along an urban-rural/agriculture—wildland gradient. Urban Ecosyst 18:685–700
Lee M-B, Carroll JP (2018) Effects of patch size and basal area on avian taxonomic and functional diversity in pine forests: implication for the influence of habitat quality on the species-area relationship. Ecol Evol 8:6909–6920
Loehle C, Wigley TB, Rutzmoser S et al (2005) Managed forest landscape structure and avian species richness in the southeastern US. Forest Ecol Manag 214:279–293
Loehle C, Wigley TB, Schilling E, Tatum V, Beebe J, Vance E, Van Deusen P, Weatherford P (2009) Achieving conservation goals in managed forests of the Southeastern Coastal Plain. Environ Manage 44:1136–1148
Luck GW, Lavorel S, McIntyre S, Lumb K (2012) Improving the application of vertebrate trait-based frameworks to the study of ecosystem services. J Anim Ecol 81:1065–1076
Marzluff JM (2001) Worldwide urbanization and its effects on birds. In: Marzluff JM, Bowman R, Donnelly RE (eds) Avian conservation and ecology in an urbanizing world. Norwell Kluwer, pp 19–47
Mason NWH, de Bello F, Mouillot D, Pavoine S, Dray S (2013) A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J Veg Sci 24:794–806
Matuoka MA, Benchimol M, de Almeida-Rocha JM, Morante-Filho JC (2020) Effects of anthropogenic disturbances on bird functional diversity: a global meta-analysis. Ecol Indic 116:106471
Mayfield MM, Levine JM (2010) Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol Lett 13:1085–1093
Mazel F, Pennell M, Cadotte M, Diaz S, Riva GD, Grenyer R, Leprieur F, Mooers A, Mouillot D, Tucker C, Pearse W (2018) Prioritizing phylogenetic diversity captures functional diversity unreliably. Nat Commun 9:288
McGarigal K, Marks BJ (1995) FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. Gen Tech Rep PNW-GTR-351 USDA For. Serv Pacific Northwest Res Sta Portland
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: Spatial pattern analysis program for categorical and continuous maps. Available at http://www.umassedu/landeco/research/fragstats/fragstats.html
McIntyre RK, Conner LM, Jack SB, Schlimm EM, Smith LL (2019) Wildlife habitat condition in open pine woodlands: field data to refine management targets. Forest Ecol Manag 437:282–294
McPhearson T, Auch R, Alberti M (2013) Regional Assessment of North America: urbanization trends biodiversity patterns and ecosystem services. In: In: Elmqvist T, Fragkias M, Goodness J et al (eds) Urbanization biodiversity and ecosystem services: challenges and opportunities. Springer, New York, pp 279–286
Melchiors MA (1991) Wildlife management in southern pine regeneration systems. In: In: Duryea ML, Dougherty PM (eds) Forest Regeneration Manual. Kulwer Academic Publishers, The Netherlands, pp 391–420
Meynard CN, Devictor V, Mouillot D, Thuiller W, Jiguet F, Mouquet N (2011) Beyond taxonomic diversity patterns How do a b and g components of bird functional and phylogenetic diversity respond to environmental gradients across France? Global Ecol Biogeogr 20:893–903
Miller DA, Wigley TB, Miller KV (2009) Managed forests and conservation of terrestrial biodiversity in the southern United States. J Forest 107:197–203
Miles AC, Castleberry ST, Miller DA, Conner LM (2010) Multi-scale roost‐site selection by Evening bats on pine‐dominated landscapes in Southwest Georgia. J Wild Manag 70:1191–1199
Mitchell MS, Rutzmser SH, Wigley TB, Loehle C, Gerwin JA, Keyser PD, Lancia RA, Perry RW, Reynolds CJ, Thill RE, Weih R, White D, Wood PB (2006) Relationship between avian richness and landscape structure at multiple scales using multiple landscapes. Forest Ecol Manag 221:155–169
Monnet AC, Jiguet F, Meynard CN, Mouillot D, Mouquet N, Thuiller W, Devictor V (2014) Asynchrony of taxonomic functional and phylogenetic diversity in birds. Global Ecol Biogeogr 23:780–788
Morelli F, Benedetti Y, Ibáñez-Álamo JD, Jokimäki J, Mäand R, Tryjanowski P, Møller AP (2016) Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Global Ecol Biogeogr 25:1284–1293
Mouchet MA, Villeger S, Mason NWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876
Pakeman RJ (2011) Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J Ecol 99:1143–1151
Provencher L, Gobris NM, Brennan LA, Gordon DR, Hardesty JL (2000) Breeding bird response to midstory hardwood reduction in Florida Sandhill longleaf pine forests. J Wild Manag 66:641–661
Ralph CJ, Geupel GR, Pyle P, Martin TE, DeSante DF (1993) Handbook of field methods for monitoring landbirds. Gen Tech Rep PSW-GRT-144 USDA For Serv Pacific Southwest Res Sta
Ricotta C, Moretti (2011) CWM and Rao’s quadratic diversity: a unified framework for functional ecology. Oecologia 167:181–188
Robbings P, Birkenholtz T (2003) Turfgrass revolution measuring the expansion of the American lawn. Land Use Policy 20:181–194
Sax DF, Gaines SD, Brown JH (2002) Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am Nat 160:766–783
Smith WB, Miles PD, Perry CH, Pugh SA (2009) Forest Resources of the United States 2007. Gen Tech Rep WO-78 USDA For Serv Washington DC
Sol D, Bartomeus I, Gonzalez-Lagos C, Pavoine S (2017) Urbanisation and the loss of phylogenetic diversity in birds. Ecol Lett 20:721–729
Sol D, Trisos C, Múrria C, Jeliazkov A, González-Lagos C, Pigot AL, Ricotta C, Swan CM, Tobias JA, Pavoine S (2020) The worldwide impact of urbanisation on avian functional diversity. Ecol Lett 23:962–972
Swenson NG (2014) Functional and phylogenetic ecology in R. Springer, New York
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of key structures. J Biogeogr 31:79–92
Tilman D (2001) Functional diversity. In: Levin SA (ed) Encyclopedia of biodiversity. Academic Press, pp 109–120
Tucker CM, Cadotte MW (2013) Unifying measures of biodiversity: understanding when richness and phylogenetic diversity should be congruent. Divers Distrib 19:845–854
Tucker CM, Cadotte MW, Carvalho SB et al (2016) A guide to phylogenetic metrics for conservation community ecology and macroecology. Biol Rev 92:698–715
Van Lear DH, Carroll WD, Kapeluck PR, Johnson R (2005) History and restoration of the longleaf pine-grassland ecosystem: implications for species at risk. Forest Ecol Manag 211:150–165
Veldman JW, Brudvig LA, Damschen EI, Orrock JL, Mattingly WB, Walker JL (2014) Fire frequency agricultural history and the multivariate control of pine savanna understorey plant diversity. J Veg Sci 25:1438–1449
Waldron JL, Welch SM, Bennett SH (2008) Vegetation structure and the habitat specificity of a declining North American reptile: a remnant of former landscapes. Biol Conserv 141:2477–2482
Weideman EA, Slingsby JA, Thomson RL, Coetzee BTW (2020) Land cover change homogenizes functional and phylogenetic diversity within and among African savanna bird assemblages. Landscape Ecol 35:145–157
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505
Wilson CW, Masters RE, Bukenhofer GA (1995) Breeding bird response to pine grassland community restoration for red-cockaded woodpeckers. J Wildlife Manage 59:56–67
Acknowledgements
This work was supported by McIntire-Stennis Project GEOZ 136 and GDAS Special Project of Science and Technology Development 2020GDASYL-20200103089. I would like to thank Georgia Ornithological Society for additional research grant. I also thank all staff at the Fort Gordon Natural Resources Branch for their help, field technicians for their hard work, Dr. J. Carroll for advice on this project, and Dr. J. Rotenberry for comments and edits on the draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Not applicable: Research grant is indicated above (Acknowledgements).
Conflicts of interest
There are no conflicts of interest.
Additional information
Communicated by Grzegorz Mikusinski.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, MB. Multiple facets of avian diversity in pine forests along an urban-agricultural gradient. Biodivers Conserv 31, 497–516 (2022). https://doi.org/10.1007/s10531-021-02345-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02345-x