Abstract
Eucalyptus benthamii is a threatened species with a restricted range to the southwest of Sydney, Australia. One extant subpopulation is relatively secure within a closed water catchment in the Kedumba Valley. However, the other main subpopulation, which once probably had a more-extensive distribution along the Nepean River, is now fragmented due to clearing for agriculture and urban development. This has impacted on reproductive success and recruitment. Planting of trees in urbanised areas and other locations along the Nepean River has been ongoing for over 20 years and is an important circa situm conservation measure. Government policy has been to plant trees that are of local provenance, however it is difficult to know whether this policy has been properly implemented: it is possible that planting stock may have been sourced from the Kedumba subpopulation. Microsatellite markers were used to examine the probable origins and kinship of 133 planted trees from 15 planted sites around Camden, NSW. Population assignment confirmed that the majority of planted trees are probably from the local Nepean River subpopulation, although some were identified that are very likely to have originated from the Kedumba Valley. While the overall diversity of the planted trees is high, kinship within individual planted stands is often elevated, increasing the potential for inbreeding and limiting their usefulness as seed sources. This probably reflects seed sourced from a narrow genetic base. We discuss the findings and their implications in the context of the species’ management and conservation strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus benthamii (common name Camden white gum or Nepean River gum; D’harawal language, Kai’yeroo) is a tall eucalypt, attaining heights of 25 to 35 m with a diameter at breast height of around 1.5 m when mature. It has a restricted distribution to the south west of Sydney where it grows on alluvial river flats of the Nepean River and its tributaries (Benson 1985) (see Fig. 1). It is of emerging industrial importance due to its capacity for rapid growth in subtropical climates combined with its tolerance of sudden, cold-weather events (Bush 2011). It is planted in the southern Unites States, Brazil and Uruguay for pulpwood and bioenergy production (Hart et al. 2016; Stanturf et al. 2018; Resquin et al. 2019). It has also been investigated for putative pharmaceutical properties (Döll-Boscardin et al. 2012; Akhtar et al. 2016). The species is of special cultural significance to the local D’harawal people, with links to Traditional Law and medicinal value (Bodkin & Bodkin-Andrews 2001; Akhtar et al. 2016).
Region to the west of Sydney and Wollongong, NSW. Features shown include the drainage system, the natural range of E. benthamii including extant (green) and extinct (red) subpopulations and locations where natural and/or planted populations referenced in this study occur. Two small, presumably-extant stands of the species on the Nattai River are indicated with an asterisk (*). The Nepean system is highly controlled, with dams creating Lakes Avon, Burragorang, Cataract, Cordeaux and Nepean. The dam on the Coxs River that created Lake Burragorang flooded the Coxs Valley, a region that is known to have been E. benthamii habitat. The largest extant population is found in the Kedumba River Valley towards the top of Lake Burragorang. Arrows marked on the rivers indicate their direction of flow
The species is susceptible to extinction in its natural range and is listed as Vulnerable by the Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth of Australia) and also by the Threatened Species Conservation Act 1995 (New South Wales). One relatively-large and well-protected population remains in the Kedumba Valley closed water catchment, while three remnant subpopulations exist at Camden, Bents Basin and Wallacia on the Nepean River (Butcher et al. 2005) (Fig. 1). A significant part of the species’ natural range has been lost due to clearance of native vegetation and flooding of the Coxs River valley during the construction of the Warragamba Dam (Butcher et al. 2005). It is not clear how extensive the population in the Coxs River valley would have been—the extent indicated in Fig. 1 simply includes those few trees for which herbarium records exist: the distribution was possibly wider. Benson (1985) also lists former subpopulations at The Oaks, on Werriberri Creek to the west of Camden, and to the west of Richmond, around the confluence of the Grose and Nepean Rivers, as extinct. According to records in the Atlas of Living Australia (www.ala.org.au) a small population has also been recorded on the Nattai River flats to the south of Lake Burragorang.
However, a recent survey carried out by KL and BP has revealed that there is a greater number of wild E. benthamii trees (~ 300) in the Camden area than previously thought, though most trees are in small, isolated patches as a result of habitat fragmentation. It is also clear from this survey and online records available at the Atlas of Living Australia that The Oaks subpopulation is still extant. Closer examination of other, relatively-inaccessible tributaries of the Nepean is ongoing.
The genetic relationship among the main subpopulations of E. benthamii (excluding those at The Oaks and from the Nattai River which were presumed-extinct and unknown, respectively, at the time) was investigated by Butcher et al. (2005). These authors estimated that there are around 6500 E. benthamii trees in the Kedumba Valley, around 300 in Bents Basin, 30 around Camden and nine at Wallacia. There is little discernible morphological difference among E. benthamii subpopulations from throughout its range, even when the trees are grown at a single “common garden” site, necessitating the use of molecular genetic markers for differentiation. They showed, using microsatellite molecular markers, that there is significant molecular genetic differentiation among the subpopulations situated at Camden, Wallacia and Bents Basin, despite their close proximity to each other on the Nepean River. These are also distinct from the larger Kedumba population that is situated approximately 40 km upstream, in a separate catchment of the river system.
While it is not always the case that eucalypts situated in fragmented stands will suffer negatively in terms of their mating system and offspring fitness (Bradbury and Krauss 2013), Butcher et al. (2005) indicated that trees at Camden and Wallacia, in particular, have higher-than-usual rates of inbred progeny, lower seed viability and high rates (> 20%) of interspecific hybrid offspring. This might be explained by poor pollen flow, resulting from isolation. Alternatively, light and/or sporadic flowering associated with the now highly-disturbed environment surrounding the stands, characterised by agricultural and urban development, and within the stands, where intense competition from woody weeds is common (Benson 1985), may also be a problem. Despite this issue, the genetic diversity of the remnant adult stands appears to remain high (Butcher et al. 2005). Maintenance of genetic diversity has similarly been observed in other eucalypts with formerly-widespread but presently-fragmented distributions (Byrne et al. 2008; Broadhurst 2013).
Conservation of the species by the CSIRO Australian Tree Seed Centre (ATSC) commenced in the mid 1990s, with establishment of an ex situ progeny trial comprising 86 families from the Kedumba Valley at Kowen Forest in the Australian Capital Territory. This stand is managed for seed production with many millions of viable seeds having been made available in Australia and overseas during the last 15 years. As trees along the Nepean River typically have very light or absent seed crops, and the aforementioned problems that are likely associated with isolation and inbreeding, a grafting programme commenced in 2014. This involves the capture of scions of isolated trees to create a grafted seed orchard or orchards to be located either ex situ or circa situm. Circa situm conservation involves planting trees sufficiently close to the wild population, such that pollen flow from the planted to the wild stand is possible, providing the opportunity for enhancement of genetic diversity and outcrossing (see for example Dawson et al. 2013). Circa situm plantings are typically not dedicated conservation plantings (Boshier et al. 2004); rather, they are stands planted to provide other functions such as on-farm wood production, or in this context, urban park and street amenity, as their primary purpose. As eucalypts are predominantly insect pollinated, it is important that circa situm plantings are situated quite close to the wild stands, as though eucalypt pollen can travel over distances of several kilometres (e.g. Sampson and Byrne 2008), more-reliable results in terms of outcrossing and seed set are achieved when the pollen and maternal parents are immediately adjacent.
Another earlier conservation initiative was a significant plantation of several hundred trees of E. benthamii at Harrington Park, a privately-owned urban residential development in Camden (Gardiner and Eldridge 1995). This planting, implemented by the property developers, was supposed to be based on genetic material sourced from the Nepean River subpopulation, though it has not been verified that this is what was actually used. It is possible that seedlings at this and numerous other plantings along the Nepean River and nearby towns may be based on seed from the Kedumba subpopulation, for which seed is more-readily available. To ensure that the remnant native population in Camden is properly conserved in line with local government guidelines and the wishes of the D’harawal indigenous people, the origin of this and other planted stands around Camden should be verified.
To ascertain the origin of planted trees, genetic features need to be measured and compared with those of potential ancestral wild populations. From the many modern molecular genetic markers, microsatellite DNA or SSR (Simple Sequence Repeats) are considered to be ideal for conservation genetic and genetic diversity studies as they are putatively neutral (i.e. not under selection) and codominant. This study was able to make use of the already-developed eucalypt markers proven effective for E. benthamii by Butcher et al. (2005).
The objectives of the study were to compare the genetic diversity and origins of planted trees in and around Camden, NSW, to those of the autochthonous Nepean subpopulation and also with the Kedumba subpopulation to determine the probable origins of the planted trees and their inter-relatedness. This information is critical for management of the threatened Nepean subpopulation, and the species as a whole, and for the potential use of the planted stands as seed sources for further revegetation works.
Methods
Population sampling
Leaf DNA samples were obtained from several sources representing wild and planted subpopulations. A total of 79 wild trees from the Nepean River system were drawn from a larger set of samples that were collected during a major new population survey conducted by KL and BP between 2013 and 2015. This survey revealed several new, small, wild stands and individual trees, often in less-accessible locations on the river reserve adjoining private property. This survey concentrated on trees that were thought highly likely to be wild, based on their position, and in many cases, age (large, old trees away from public spaces are unlikely to be planted given the local history of settlement and clearing in this district). In most cases where trees were growing in remnant stands along the Nepean River or creeks this was clear. In a few cases, where remnant patches abutted developed public spaces, large and apparently old trees were selected with lower certainty of their being wild, which was noted. For each tree, observations on morphological features including flower bud, flower and capsule presence (where these were assessable from the ground) were made and a leaf tissue sample was collected for DNA extraction (Table 1). Additional wild trees from Camden, Wallacia and The Oaks were accessed from a CSIRO grafted clone bank. The Wallacia subpopulation is very small: Butcher et al. (2005) estimated that there are only 9 wild trees remaining. A further 48 trees were sampled from the Kowen ex situ conservation stand in the Australian Capital Territory. Pedigree records indicated that this stand comprises the progeny of 57 wild, open-pollinated mothers collected from the Kedumba Valley. The trees in the ACT conservation stand were 15 years old at the time of sampling. A total of 133 samples from cultivated trees were collected from 15 planted stands around Camden, Picton and Tahmoor including a housing development at Harrington Park, public spaces such as sports fields and parks, and private property. The locations of wild and planted trees sampled for the study are shown in Fig. 2.
Locations and numbers of samples of 13 planted (red triangles) and nine groups (G1–G9) of wild (green circles) Eucalyptus benthamii sampled in urban and peri-urban areas near Camden, NSW. The inset map has an extent equivalent to Fig. 1, with the red bounding box delineating the extent of Fig. 2. The inset also shows two additional planted sampling locations at Picton and Tahmoor and additional wild samples were
Microsatellite analysis
DNA was extracted from each of 277 leaf samples that had been fully desiccated on silica gel. Genomic DNA was either extracted using the Invisorb® DNA Plant HTS 96 Kit (Stractec Biomedical AG, Germany) or as per the method of Broadhurst et al. (2015) with 5 mg of tissue per sample. A total of 16 pairs of microsatellite primers originally developed for E. globulus, E. grandis and E. sieberi were selected from those listed by Butcher et al. (2005) as being effective for E. benthamii and tested using a small subset of samples. The scored data from the Butcher et al. (2005) study was also re-examined to confirm PCR amplifications were undertaken as per the reagents and conditions in Broadhurst et al. (2015). The fluorescent dyes used for each marker are listed in Table 2. From these 16 SSRs, a subset of nine loci were selected, each producing clear amplification products at a single locus, except Eg91, which demonstrated peaks at two loci. The selected SSRs were amplified by PCR (Mastercycler, Eppendorf, Germany) and analysed using an Applied Biosystems 3130 XL Genetic Analyzer (Thermo Fisher, USA). Alleles were then identified as peaks using Genemapper 5 (Thermo Fisher, USA). To optimise the analytical process before the main genotyping runs, a subset of samples was re-run several times to confirm that the genotyping error rate was acceptably low and provided repeatable results. Several samples were re-run after the completion of the main genotyping runs where data were missing or difficult to score with certainty. The programme INest2.2 (Chybicki and Burczyk 2009) which implements a Bayesian method was used to simultaneously estimate the proportion of null alleles per locus and provide adjusted estimates of f (inbreeding coefficient). The software was run with 50,000 burn-in iterations followed by 500,000 iterations. Locus Eg86 was identified as potentially having a significant proportion of null alleles averaged over subpopulations, though the estimated proportion was markedly lower, 0.09 with a 95% confidence interval of 0–0.16 in the largest planted tree subpopulation and higher in the Kedumba subpopulation (0.26 with 95% CI 0.16–0.36). Other loci were identified as having a relatively small proportion of null alleles.
Data analysis
Data analysis was an iterative process. Based on previous analyses by Butcher et al. (2005), it was expected that there would be sufficient subpopulation differentiation, particularly between the Nepean (comprising Camden, Bents Basin and Wallacia provenances) and Kedumba subpopulations, to assess the origins of the planted stands. The Oaks provenance had not been previously assayed and is on a tributary of the Nepean (Fig. 1) separating it from Kedumba and other provenances on the Nepean River proper. Its affinity to the Kedumba and Nepean subpopulations was therefore unknown. A further complicating factor was uncertainty about the wild origins of a minority of the Nepean putatively-wild trees: we could not be absolutely certain that a small number of “wild” trees had not been planted, which left open the possibility that they may have had Kedumba origins. The population from the Kowen gene bank, on the other hand, was based entirely on seedlots collected from intact and relatively undisturbed natural forest, so there was a high level of certainty that this sample would be an accurate representative of the subpopulation. Our procedure was, therefore, to first screen the entire sample of trees to evaluate whether population differentiation was evident, and then to proceed to population assignment of the planted trees once putatively-wild individuals growing on the Nepean but suspected of having origins other than Nepean (i.e. Kedumba or indeterminate origin) had been removed from the dataset.
Analysis of population structure
Genetic differentiation among populations was assessed using methods including principal coordinates analysis (PCoA), analysis of molecular variance (AMOVA) and Bayesian clustering.
PCoA analysis of genetic distance matrices were carried out using GenAlEx 6.502 (Peakall and Smouse 2006). Population genetic structure was further examined in two ways. Firstly, FST statistics were calculated and analysis of molecular variance (AMOVA) was performed using GenAlex on the allele frequency data to partition the genetic variance among the wild and planted subpopulations and within subpopulations. Secondly, Bayesian clustering implemented in STRUCTURE (Pritchard et al. 2000) was carried out. We tested the assumption that the individuals belong to K populations with distinctive allele frequencies, from K = 1 (testing for panmixia) to K = 15, allowing for the wild subpopulations delineated by Butcher et al. (2005) as well as allowing for additional sub-structured populations. STRUCTURE assigns, without prior information that defines subpopulations, each individual to one or more groups with a relative frequency of predicted group membership summing to unity. We assumed no admixture among subpopulations. For each K we performed 30 analyses with 50,000 burn-in iterations and 500,000 iterations. We analysed the patterns of log-likelihood probability and the associated standard deviation (averaged across the 30 runs) to identify the most strongly-supported number of population clusters. We interpreted the results using the approaches of Pritchard et al. (2010) and Evanno et al. (2005).
Population genetic parameters
Observed heterozygosity (Ho), expected heterozygosity (He) and inbreeding coefficient (f) were calculated for each subpopulation. These parameters were all calculated using the GenAlEx software. Alleles per locus (A) and subpopulation private alleles, i.e. alleles unique to a single, wild subpopulation, were also determined. As population sizes were uneven, an adjusted estimate of allelic richness (Aadj) was made with a rarefaction correction (see Leberg 2002) for the major subpopulation groupings (Kedumba, Nepean and planted stand groups) using the hierfstat library for R statistical language (Goudet 2005).
Population assignment
Following analysis of population structure, population assignment was carried out for all individual wild trees. This was firstly done using the assignment in STRUCTURE at the optimal value of K. Secondly, the assignment method of Waser and Strobeck (1998) implemented in GenAlex was used. Unlike the method in STRUCTURE, that uses no prior information on the putative population of each tree, the Waser & Strobeck method is based on pre-assigned subpopulation data. Following this analysis, the small number of trees that appeared to be incorrectly assigned to their putative subpopulations, or that had ambiguous assignations, were removed from the dataset used in the following stages of analysis. These stages were (i) calculation of diversity statistics for the wild subpopulations and (ii) assignment of the likely origins of the planted trees.
Assignment of the planted trees to their most probable wild origins was done (i) using the assignment method of Waser and Strobeck (1998); (ii) using assignment methods implemented in the GENECLASS2 software (INRA, FRANCE) (Piry et al. 2004) including the methods of Baudouin and Lebrun (2001), Paetkau et al. (2004), and Rannala and Mountain (1997) and; (iii) by examination of assignment probability logs in STRUCTURE associated with the optimal K value. All of these methods, except that implemented in STRUCTURE which used no a priori assignments, used the wild tree dataset (with trees of apparently incorrect or ambiguous origin removed) as a basis for assignment. Information taken together from these analyses was used to identify the most-likely origin of each planted tree.
Relatedness
Relatedness of individual trees was estimated for the planted and wild trees around Camden. The coefficients of co-ancestry (or kinship) (θ) and relatedness (2θ) were calculated pairwise between trees using two maximum likelihood estimators (Milligan 2003; Wang 2007) that account for inbreeding due to the eucalypt mixed mating system, implemented with Coancestry software (Zoological Society of London, UK). The coefficient of co-ancestry is the probability that any two alleles sampled at random (one from each individual) are identical by descent, and it gives a measure of relatedness between two individuals (Wang 2007). For some typical cases, half-siblings have 2θ = 0.25 and full-siblings have 2θ = 0.5. Relatedness (2θ) between 0.5 and 1 is the result of inbreeding, for example self-full-siblings have 2θ = 1.
Results
Population structure and differentiation
Preliminary analyses indicated that differentiation of the wild subpopulations was likely to be sufficiently strong to accurately make population assignments of individual planted trees.
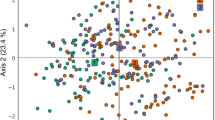
The PCoA plot for the wild subpopulations (wild trees from the Nepean system as well as trees from Kowen orchard which originate from Kedumba wild population) (Fig. 3) indicated clustering of samples from Nepean and Kedumba, though with an area of intergrade. The Oaks samples, from a provenance which had not been assayed by Butcher et al. (2005), appeared to cluster with the other provenances (i.e. geographic locations including Bents Basin, Camden, Wallacia) that together comprise the Nepean subpopulation. Strong differentiation among the provenances that constitute the Nepean subpopulation was not evident.
Principal coordinates analysis of a genetic distance matrix formed using 9 SSR loci for individual trees from Nepean and Kedumba wild subpopulations and trees from Kowen seed orchard grown from wild seed from the Kedumba subpopulation. Eight trees that were thought to be of wild Nepean origin but that were assigned to the Kedumba subpopulation using STRUCTURE and the Waser and Strobeck (1998) population assignment method are designated “suspect”
Private alleles (Table 2) were found in both Kedumba and Nepean subpopulations. Overall, 94% and 29% of Nepean and Kedumba samples, respectively, possessed a subpopulation-private allele at one or more loci. Alleles private to Bents Basin (3 alleles over 4 individuals), Wallacia (1 allele in 1 individual) and The Oaks (3 alleles over 5 individuals) provenances were also identified.
STRUCTURE
The usual techniques for interpreting the Bayesian STRUCTURE analysis were not completely effective for discriminating population structure for this dataset. The graphical method of Evanno et al. (2005) produced a major peak at K = 4 (detailed results provided in online supplementary material 1). It is difficult to provide a satisfactory biological explanation for K = 4 groups, with wild trees that are likely to be closely related, given their geographic proximity, assigned to different clusters in a number of cases. However, the K = 4 assignment did result in all trees from wild, Kedumba origins being assigned to the same group, while only a small number of trees from the putatively-wild Nepean subpopulation had affinity to this group (Fig. 4). All other wild Nepean trees were split between the three other groups in a way that was not simply interpretable, though most trees from the individual Groups 1 to 9 (see Fig. 2 for geographic locations of groups) were assigned to one main cluster. Trees from geographically-distant Wallacia and The Oaks made up the majority of members of a second cluster, though with a few trees in Groups 4 and 6 also having affinity to this cluster. A third cluster comprised trees from Groups 3 and 5 (but not Group 4 which is only 1 km from both Groups 3 and 5) at Camden airport, while the fourth cluster included members in all Groups except 9. Neither did runs for K = 2 or K = 3 give readily-interpretable results (results not given). Despite not giving a fully-interpretable result in terms of structural definition of the population, the method was highly useful for differentiating between trees of Kedumba and “non-Kedumba” origins at K = 4, i.e. it was an effective tool for population assignment of the planted trees.
Population assignments
The various population assignment methods (Waser and Strobeck (1998), Paetkau et al. (2004), Rannala and Mountain (1997) and Baudouin and Lebrun (2001)) produced results that were broadly in accord with those obtained from K = 4 STRUCTURE assignments. All methods assigned each Kedumba conservation stand tree to a single subpopulation (Kedumba). All but eight putatively-wild trees growing along the Nepean or Werriberri Creek (The Oaks provenance) were assigned to the Nepean subpopulation (online supplementary material 2). As a result, these eight trees were removed from the Nepean-wild dataset for the purposes of calculation of wild subpopulation genetic parameters and for the purposes of assignment of the cultivated trees to ancestral origins. These trees are also indicated in Fig. 3.
The assignment methods produced mostly-similar results when applied to the cultivated trees (online supplementary material 3). Most planted trees sampled from around the greater Camden area (Fig. 2) appear to be of Nepean origin. Some important exceptions exist. The large Harrington Park sample of 58 trees contained nine trees that are likely to be of Kedumba origin. Three of the five trees sampled from the Mt Annan Botanical Garden, a large, mature tree at Tahmoor railway station, all seven of the trees from the show ground and six trees from private property and public spaces are also likely to allochthonous. Overall, 19.5% of the planted population sample would appear likely to be of Kedumba origin. Table 3 summarises the assignments made to the Nepean and Kedumba subpopulations. All five methods of assignment returned the same result for all but 8 of the 133 planted trees.
Population differentiation (AMOVA and FST)
Following the removal of the eight putatively-wild Nepean trees that did not have clear Nepean subpopulation origins from the dataset, AMOVA of wild populations indicated that 5% of observed variance is among populations, 26% is among individuals and 69% is within individuals. Estimated FST among populations (Table 4) shows that the planted trees assigned to the Nepean subpopulation by the earlier techniques have most affinity to the Camden provenance. Trees from Bents Basin and the small sample from Wallacia appear to be quite distinct by this measure, while The Oaks are closest to Camden (and the planted Nepean trees). Despite the similarity between The Oaks and Wallacia implied by the STRUCTURE analysis (Fig. 4), FST between these subpopulations is not particularly low.
Population genetic parameters
Referring to Table 5, the Nepean wild subpopulation was the most diverse, with high expected heterozygosity and alleles per locus. However, the diversity of planted stands around Camden is similar to that of the wild Nepean subpopulation, regardless of whether trees of Kedumba origin are included or not. Observed heterozygosity (Ho) figures are all below expected heterozygosity (He), which may be due either to inbreeding, population sub-structure not accounted for in this analysis, or both. Null alleles are another possible cause of low heterozygosity estimates. Correcting for these using iNest resulted in overall estimates of 0.75 and 0.80 for Ho and He respectively. Adjusting the inbreeding coefficient (f) for a proportion of null alleles at each locus reduced the estimates for all subpopulations, especially for Kedumba. Locus Eg86 made the largest contribution to the elevated estimates in the uncorrected data, and there was a particularly high excess of homozygous alleles in the Kedumba subpopulation accounting for the very marked correction therein.
Kinship
Both the Wang (2007) and Milligan (2003) co-ancestry estimators gave similar results, with a Pearson correlation between estimated dyads from the two methods of 0.98. Due to the comparatively low number of markers used, individual dyad estimates were of low precision (Table 6), though for estimates above approximately 0.3, co-ancestry among individuals (i.e. individuals that share alleles that are identical by descent) is highly likely.
Wild trees around Camden, at Bents Basin and The Oaks (Fig. 2) show some close relatedness within groups, particularly G2 and G5, but are generally not highly-related between groups (Fig. 5). An exception is among G3, G4 and G5, which though now isolated, are less than 1.5 km apart and may have been a more-continuous stand in the past.
Pairwise estimated relatedness (2θ matrix for Camden wild trees with heat map spectrum from red (low relatedness) to green (high relatedness). Samples are grouped into nine subpopulations (G1–G9). The locations are shown in Fig. 2
Average pairwise relatedness (2θ) between groups of planted trees growing in various locations around Camden and further afield is shown in Fig. 6. In nearly all cases, trees from within individual planted stands (shown on the diagonal of Fig. 6) show elevated co-ancestry. There is also some evidence that trees cultivated at different locations share common ancestry, for example those growing at Ron Dine oval, the netball courts, Kings Bush Reserve, private property C and Engesta Reserve appear to be inter-related. This may be evidence that seed had been collected from wild stands that lacked diversity, and/or from only one or a few trees. Referring to Fig. 7, it seems that the most-likely origin of these aforementioned plantings is Bents Basin. The numerous trees at Harrington Park are, on average, not closely related. However, within this planting, some small clusters of close relatives were also identified (data not shown).
Pairwise estimated relatedness (2θ) matrix for planted trees with heat map spectrum from red (low relatedness) to green (high relatedness). A value of 0.25 indicates that the trees are half-sibs on average, whereas a value of 0.5 indicates that they are full-sib on average. Relatedness values of one reflect single-tree samples at a location, effectively the relatedness of the tree to itself
Wild trees at Cut Hill (Group 6), The Oaks (Group 7), and Wallacia (Group 9) do not appear to be well represented among the planted trees in our dataset, which we consider likely to be a representative sample of planted trees in the area.
Discussion
Genetic differentiation between planted stands and wild populations
The results of the genetic distance-based cluster analysis and population assignment techniques taken together indicate that planted stands around Camden are likely, in most cases, to be derived from the Nepean River subpopulation. Butcher et al. (2005) indicated that the Kedumba subpopulation is genetically distinct from those of the Nepean including Camden, Bents Basin and Wallacia. We also found that the Kedumba subpopulation is distinct, but there was less evidence for strong differentiation among the provenances from the Nepean (Camden, Bents Basin, Wallacia) and the previously-unstudied stand at The Oaks on Werriberri Creek, in a catchment separate from both the Kedumba and Nepean subpopulations (Fig. 1). Possible reasons for the less-marked differentiation relative to Butcher et al. (2005) include the fact that we used a subset of the markers employed by them (9 of 21) and that we had a significantly larger sample of trees (67 vs 16) from locations near the Camden township. We took the decision to treat the Nepean (including The Oaks) provenances together as a subpopulation, distinct from the Kedumba subpopulation, with the primary objective of population assignment of the planted trees. Notwithstanding this decision, we certainly found some evidence that particular Nepean provenances are genetically distinct to some degree. Private alleles present in The Oaks and Wallacia populations are evidence that geneflow along the Nepean and its tributaries has been restricted for a long period, as pointed out by Butcher et al. (2005). An additional study has commenced that involves all (approximately 400) of the wild trees sampled by KL and BP that uses several thousand single nucleotide polymorphism (SNP) markers that will further elucidate population structure.
Population assignment of individual trees using STRUCTURE and the Waser and Strobeck (1998) method allowed us to identify a small number of putatively-wild trees along the Nepean River that may have been planted using seed sourced from the Kedumba Valley. Survey notes show that three of these trees (all adjacent and within Group 1) were questionable at the time of sampling, as though they are on the edge of a wild stand, they also border a public area containing other planted trees. The remaining five, due to their age and locations, seem less likely to have been planted and may possess low-frequency alleles that are common to the Kedumba population, leading to incorrect or ambiguous assignment. Overall, the population assignment methods appear to have yielded robust results with only a low proportion of wild trees that are of doubtful origin.
It is also worth noting that if the abovementioned, putatively-wild trees are in fact planted, and from Kedumba, they would appear to be well adapted to their current environment based on their large size and age. ATSC experience in plantation forestry with Kedumba-based seedstock indicates that trees of this size are very likely to be reproductively fit in this environment. Their apparent vigour lessens concerns that their intraspecific hybrid progeny might be maladapted due to introgression of exotic alleles, a commonly-cited reason for avoiding plantings using allochthonous subpopulations near to autochthonous ones (e.g. Byrne et al. 2011; Santini et al. 2018). This is more likely to be a serious issue for species that have wide geographic or clinal variation and also when large plantations, with the capacity for large-scale geneflow, are being established near autochthonous stands. In the present case, the Kedumba subpopulation inhabits a quite-similar environment to that of the Nepean, and both subpopulations appear to be quite plastic in terms of adaptation to a broad range of climates (e.g. Gardner 2007). Due to this, and the comparatively small scale of planting of Kedumba-origin trees that could potentially be a source of geneflow into the native stands, we do not believe that the native stands around Camden are under threat from intraspecific genetic pollution. Rather, the ongoing isolation of the remaining small stands and the lack of recruitment due to inbreeding and weed infestation in the understorey are the main causes for concern.
We were also able to identify a number of planted trees in the Harrington Park planting and at other locations around Camden that are probably of Kedumba origin. However, these trees were in the minority, less than 20% of our sample of planted trees. This is a broadly-positive finding, because it suggests that the remnant wild patches around Camden are not likely to suffer from large amounts of “genetic pollution”, i.e. introgression of non-local genes (Potts et al. 2003; Barbour et al. 2005), due to large numbers of planted individuals from Kedumba. One strategy to minimise the chance of introgression of exogenous Kedumba genes into wild Nepean stands would be to cull the planted trees of suspected Kedumba origin. This strategy has been recommended by Santini et al. (2018) in the context of extensive allochthonous forestry plantations of Abies alba that might provide substantial geneflow into native stands. We do not recommend removal of the planted Kedumba trees, as the plantings are not extensive and are probably “better than nothing” given the levels of inbreeding and interspecific hybridisation detected by Butcher et al. (2005) in the wild stands. Rather, a policy of more-extensive plantings of Nepean origin and gradual replacement of the Kedumba trees with those of the local subpopulation would be an effective, practical measure.
It should also be noted that introduction of a broader genetic base from exogenous subpopulations can sometimes be a highly beneficial conservation technique, particularly when fragmentation has resulted in loss of diversity in the subpopulation of interest. However, the Nepean subpopulation, taken as a whole, is not yet at this stage. Genetic diversity in the wild stands was relatively high (HE estimates between 0.7 and 0.8), at the upper end of observations for eucalypts with a localised or regional distribution (Moran and Hopper 1983, 1987; Sampson et al. 1988; Bush and Thumma 2013), and similar to the estimates previously made by Butcher et al. (2005). Overall diversity in the planted stands was similarly high, indicating that collectively, the planted resource around Camden is a valuable gene repository.
Given the comparatively high estimated genetic diversity in the Nepean wild and planted stands, a strategy of circa situm planting with material from the local, Nepean provenance should lead to positive outcomes. This would need to be coupled with measures to reduce weed competition effects on flowering and recruitment in the natural stands. In contrast, the Wallacia stand, which only consists of nine trees, is unlikely to constitute a viable self-sustaining subpopulation given the expected strongly-negative consequences of eucalypt inbreeding depression (e.g. Griffin and Cotterill 1988; Hardner and Potts 1997; Hardner and Tibbits 1998; Costa e Silva et al. 2010). Butcher et al. (2005) showed that while the stand is genetically distinct, it produces low quality offspring comprising a high proportion of selfs and interspecific hybrids. In this case, providing circa situm plantings based on other Nepean subpopulations and clonally capturing the remaining individuals for inclusion in a broadly-based Nepean-subpopulation seed orchard is a practical and appropriate conservation strategy.
While planted trees around Camden are probably mostly of local origin, and collectively genetically diverse, trees within planted patches are often closely related to each other (i.e. θ > 0). This may reflect inbreeding in the remnant patches around Camden and/or lack of seed trees to collect from—collections from a single or only a few trees resulting in plantings comprising close relatives. The planted stands may therefore be susceptible to inbreeding, i.e. they may produce inbred offspring because the trees in each location are themselves related. Some of the planted stands are therefore not ideal seed sources for carrying out further revegetation work. To a lesser extent, they are not ideal circa situm conservation plantings, as they lack the genetic diversity that they could potentially supply if they were based on a broader selection of mothers from the Nepean River subpopulation.
Conservation implication
Though the planted stands around Camden are not ideal circa situm conservation plantings, as they lack diversity (individually but not taken collectively) and often comprise closely-related individuals within individual plantings, they are likely to be of overall benefit, given the highly-fragmented and vulnerable wild populations. Circa situm conservation planting should be continued and expanded, but it would be beneficial to facilitate wider crossing in the relict patches of wild trees along the Nepean River. This could be achieved by ensuring new plantings are more genetically diverse. Trees growing at The Oaks, Camden—Cut Hill area and Wallacia should be targeted in particular, as the analysis of co-ancestry indicated that these stands may not have been previously used as seed sources for urban planting. The planned ex situ grafted seed orchard for the Nepean subpopulation is likely to be useful for producing seed that is outcrossed and that represent a large amount of the subpopulation’s genetic diversity. This is in contrast to the strategy recommended by Li et al. (2005) who assess a similar situation with highly-fragmented subpopulations of Metasequoia glyptostroboides. They argue for single-subpopulation conservation stands, to avoid the risks of outbreeding depression and maladaptation of outcrossed offspring, even in their situation, where subpopulations consist of only a few trees or tens of trees. Outbreeding depression does not appear to be common in forest trees, in fact there is some evidence to the contrary in Eucalyptus that wide intraspecific crosses can result in heterosis (see discussion in Costa e Silva et al. 2014). Moreover, the certainty of inbreeding and likelihood of inbreeding depression that would occur in trying to manage subpopulations of only a few trees make this strategy impractical.
One of the major objectives of the study was to assess the ancestry of the large Harrington Park planting. Our results show that this stand is quite genetically diverse, though it does contain a proportion of trees that are likely to be from Kedumba. As it is located at quite a long distance from the natural stands, and eucalypts are predominantly insect pollinated, this is not likely to be a major source of pollen that might be introgressed into wild populations. Due to its genetic diversity, the stand is likely to be one of the best sources of outcrossed seed for planting in the region. If the entire stand could be screened and the Kedumba-origin trees removed or temporarily coppiced, it would be a highly valuable seed production area. However, because of its private ownership and challenges of managing such a stand for seed production in an urban area, this may not be practical.
Conclusion
The origins of planted stands that form a circa situm conservation measure for threatened E. benthamii along the Nepean River were successfully determined using SSR markers and wild reference populations. It is probable that the majority of trees planted in and around the town of Camden, where many of the surviving Nepean subpopulation trees are situated, are from the local subpopulation rather than from the geographically-distant and genetically-distinct Kedumba subpopulation. While the planted subpopulation, taken as a whole, has genetic diversity that appears to be similar to the wild subpopulation, individual stands often comprise close relatives representing a narrow genetic base. They are therefore not highly suitable for seed collection, as they are likely to produce inbred progeny, and are suboptimal pollen sources, as they lack diversity. Despite these shortcomings the plantings are undoubtedly of value, and this could be increased by augmentation with further plantings and/or gradual replacement with trees of carefully-selected genetic origin. Establishment of pedigreed seed orchards with genetically-diverse Nepean selections and further circa situm plantings using material that is of known, genetically-diverse origins is a high priority. The approach of using molecular markers to assay fragmented wild and planted subpopulations of trees was highly successful and would be broadly applicable to conservation projects involving other tree species. Having access to information about background levels of diversity, population structure and origins of both wild and planted subpopulations is a key tool for management of fragmented stands of threatened species in environments where these are likely to interbreed.
References
Akhtar MA, Raju R, Beattie KD, Bodkin F, Münch G (2016) Medicinal plants of the Australian aboriginal Dharawal people exhibiting anti-inflammatory activity. Evid Based Complement Altern Med 2016:8
Barbour R, Potts B, Vaillancourt R (2005) Pollen dispersal from exotic eucalypt plantations. Conserv Genet 6:253–257
Baudouin L, Lebrun P (2001) An operational Bayesian approach for the identification of sexually reproduced cross fertilized populations using molecular markers. Acta Hortic 546:81–93
Benson DH (1985) Aspects of the ecology of a rare tree species, Eucalyptus benthamii, at Bents Basin, Wallacia. Cunninghamia 1:371–383
Bodkin F, Bodkin-Andrews G (2001) D'harawal dreaming stories: the story of The Seven Peacekeepers and The Forgotten Ones. Dharawalstories.com, Sydney, Australia
Boshier DH, Gordon JE, Barrance AJ (2004) Prospects for circa situm tree conservation in Mesoamerican dry-forest agro-ecosystems. In: Frankie GW, Mata A, Vinson SB (eds) Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest. University of California Press, Berkeley, p 352
Bradbury D, Krauss SL (2013) Limited impact of fragmentation and disturbance on the mating system of tuart (Eucalyptus gomphocephala, Myrtaceae): implications for seed-source quality in ecological restoration. Aust J Bot 61:148–160
Broadhurst LM (2013) A genetic analysis of scattered yellow box trees (Eucalyptus melliodora A. Cunn. ex Schauer, Myrtaceae) and their restored cohorts. Biol Conserv 161:48–57
Broadhurst L, Fifield G, Vanzella B, Pickup M (2015) Evaluating the evolutionary potential of two Yellow Box (Eucalyptus melliodora A. Cunn. ex Schauer, Myrtaceae) seed production areas. Aust J Bot 63:455–466
Bush D (2011) Plantation eucalypt species: recent trends in testing and planting. IUFRO Working Group 20803: improvement and culture of eucalypts. IUFRO, Porto Seguro, Bahia, Brazil, pp 23–25
Bush D, Thumma B (2013) Characterising a Eucalyptus cladocalyx breeding population using SNP markers. Tree Genet Genomes 9:741–752
Butcher PA, Skinner AK, Gardiner CA (2005) Increased inbreeding and inter-species gene flow in remnant populations of the rare Eucalyptus benthamii. Conserv Genet 6:213–226
Byrne M, Elliott CP, Yates CJ, Coates DJ (2008) Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv Genet 9:97–105
Byrne M, Stone L, Millar MA (2011) Assessing genetic risk in revegetation. J Appl Ecol 48:1365–1373
Chybicki I, Burczyk J (2009) Simultaneous estimation of null alleles and inbreeding coefficients. J Hered 100:106–113
Costa e Silva J, Hardner C, Tilyard P, Pires AM, Potts BM (2010) Effects of inbreeding on population mean performance and observational variances in Eucalyptus globulus. Ann For Sci 67:605
Costa-e-Silva J, Potts BM, Lopez GA (2014) Heterosis may result in selection favouring the products of long-distance pollen dispersal in Eucalyptus. PLoS ONE 9:e93811
Dawson IK, Guariguata MR, Loo J, Weber JCR, Lengkeek A, Bush D, Cornelius J, Guarino L, Kindt R, Russell J, Jamnadass R (2013) What is the relevance of smallholders’ agroforestry systems for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? A review. Biodivers Conserv 22:301–324
Döll-Boscardin PM, Sartoratto A, Sales Maia BHLdN, Padilha de Paula J, Nakashima T, Farago PV, Kanunfre CC (2012) In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid Based Complem Altern Med 2012:8
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Gardiner C, Eldridge K (1995) Eucalyptus benthamii for Harrington Park. CSIRO Australian Tree Seed Centre, Canberra
Gardner R (2007) Investigating the environmental adaptability of promising subtropical and cold-tolerant eucalypt species in the warm temperate climate zone of KwaZulu-Natal, South Africa. South Hemisphere For J 69:27–38
Goudet J (2005) hierfstat, a package for r to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186
Griffin AR, Cotterill PP (1988) Genetic variation in growth of outcrossed, selfed and open-pollinated progenies of Eucalyptus regnans and some implications for breeding strategy. Silvae Genet 37:124–131
Hardner CM, Potts BM (1997) Postdispersal selection following mixed mating in Eucalyptus regnans. Evolution 51:103–111
Hardner C, Tibbits W (1998) Inbreeding depresion for growth, wood and fecundity traits in Eucalyptus nitens. For Genet 5:11–20
Hart PW, Johnson J, Paim R (2016) Status update on development of a eucalyptus plantation program in the southeastern United States and higher elevations of southern Brazil. TAPPI J 15:148–155
Leberg PL (2002) Estimating allelic richness: effects of sample size and bottlenecks. Mol Ecol 11:2445–2449
Li Y-Y, Chen X-Y, Zhang XIN, Wu T-Y, Lu H-P, Cai Y-W (2005) Genetic differences between wild and artificial populations of Metasequoia glyptostroboides: implications for species recovery. Conserv Biol 19:224–231
Milligan BG (2003) Maximum-likelihood estimation of relatedness. Genetics 163:1153–1167
Moran GF, Hopper SD (1983) Genetic diversity and the insular population structure of the rare granite rock species, Eucalyptus caesia Benth. Aust J Bot 31:161–172
Moran GF, Hopper SD (1987) Conservation of the genetic resources of rare and widespread eucalypts in remnant vegetation. In: Saunders DA, Arnold GW, Burbidge AA, Hopkins AJM (eds) Nature conservation: the role of remnants of native vegetation. Surrey Beatty and Sons, Sydney, pp 151–162
Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13:55–65
Peakall R, Smouse PE (2006) Genalex 6: genetic analysis in Excel Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Potts BM, Barbour RC, Hingston AB, Vaillancourt RE (2003) Genetic pollution of native eucalypt gene pools: identifying the risks. Aust J Bot 51:1–25
Pritchard J, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pritchard JK, Wen X, Falush D (2010) Documentation for structure software: Version 23. University of Chicago, Chicago
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci 94:9197–9201
Resquin F, Navarro-Cerrillo RM, Carrasco-Letelier L, Casnati CR (2019) Influence of contrasting stocking densities on the dynamics of above-ground biomass and wood density of Eucalyptus benthamii, Eucalyptus dunnii, and Eucalyptus grandis for bioenergy in Uruguay. For Ecol Manag 438:63–74
Sampson JF, Byrne M (2008) Outcrossing between an agroforestry plantation and remnant native populations of Eucalyptus loxophleba. Mol Ecol 17:2769–2781
Sampson J, Hopper S, James S (1988) Genetic diversity and the conservation of Eucalyptus crucis Maiden. Aust J Bot 36:447–460
Santini F, Andrisano T, Leonardi S, Ciaschetti G, Labriola M, Vendramin GG, Piotti A (2018) Tracking the origin of silver fir plantations along the boundary between different genetic clusters in central Apennines: implications for their management. For Ecol Manag 408:220–227
Stanturf JA, Young TM, Perdue JH, Dougherty D, Pigott M, Guo Z, Huang X (2018) Productivity and profitability potential for non-native Eucalyptus plantings in the southern USA. For Policy Econ 97:210–222
Wang J (2007) Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet Res Camb 89:135–153
Waser PM, Strobeck C (1998) Genetic signatures of interpopulation dispersal. Trends Ecol Evol 13:43–44
Acknowledgements
The survey and sample collection work was partially supported by a Eucalypt Australia (Bjarne K Dahl Trust) Small Grants Program Grant to KL. The molecular marker work was supported by a CSIRO NRCA vacation scholarship program grant to LH. We acknowledge the cooperation of numerous private landowners, local government and Peter Cuneo and Richard Johnstone at the Australian Botanic Garden, Mt Annan, for allowing access to the trees sampled in this study. We thank the D’harawal Elders for sharing the cultural significance of the species with us, and respectfully acknowledge that the study was conducted on D’harawal Country.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, L., Love, K., Peace, B. et al. Origin of planted Eucalyptus benthamii trees in Camden NSW: checking the effectiveness of circa situm conservation measures using molecular markers. Biodivers Conserv 29, 1301–1322 (2020). https://doi.org/10.1007/s10531-020-01936-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-01936-4