Abstract
Non-native invasive plants are among the main threats to global biodiversity, including insects, and it is thus important to understand the mechanisms of how invasive plants impact native species. The community composition of nocturnal Lepidoptera was studied in the Czech Republic (Central Europe) in stands of native deciduous trees and in stands dominated by the invasive tree Robinia pseudoacacia, using automatic portable light traps together with an assessment of habitat characteristics. Native stands had more closed canopies and poorly developed understories. Conversely, R. pseudoacacia stands were more open and heterogeneous, with sparse canopies, well-developed shrub layers and a higher cover of taller herbs. Moth species richness, abundance and biomass were lower in R. pseudoacacia, likely due to the low richness of canopy herbivores not adapted to feed on the exotic host. However, feeding guilds associated with the understorey were more represented in stands of R. pseudoacacia, likely due to the more heterogeneous habitat structure. The Lepidopteran communities observed in stands of R. pseudoacacia resembled communities of open-forests or forest-steppe habitats. In contrast, native stands were dominated by Lepidoptera associated with trees, including forest specialists but also habitat generalists. From a conservation perspective, it appears that the invasive R. pseudoacacia created structurally more heterogeneous environment and more Lepidopteran open-forest guilds were associated with this habitat. However, further spread of R. pseudoacacia should be prevented because it reduces the species richness of Lepidoptera. Simultaneously, we recommend increasing the habitat heterogeneity of native forests to support functionally more diverse Lepidopteran communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plant species have broad ecological and economic impacts in both natural and human-altered environments (Higgins et al. 1997; Leung et al. 2002; Vilà et al. 2011), and are among the main global threats to biodiversity (Vitousek et al. 1996; Pauchard and Shea 2006; van Kleunen et al. 2015). In particular, they alter the structure and diversity of native plant communities (Vitousek et al. 1996; Vilà et al. 2011; Benesperi et al. 2012), affect the productivity of native plant species (Chambers et al. 2007) and significantly disrupt the trophic structure of ecosystems (Levin et al. 2006; Heleno et al. 2008; Tallamy et al. 2010; Schirmel et al. 2016), with prolonged impacts on diversity at higher trophic levels (Spafford et al. 2013; Bezemer et al. 2014; Litt et al. 2014; van Hengstum et al. 2014).
The impacts of plant invasions on arthropod assemblages strongly vary among different taxa (Spafford et al. 2013; Bezemer et al. 2014; Litt et al. 2014; van Hengstum et al. 2014; Buchholz et al. 2015). Specialized herbivores or pollinators, evolutionarily bound to a small number of plant species (Traveset and Richardson 2006; Aizen et al. 2008; Moroń et al. 2009; Burghardt et al. 2010) or parasitoids (Simao et al. 2010), usually respond to plant invasions negatively, by decreasing in diversity or abundance (Degomez and Wagner 2001; Spafford et al. 2013; Litt et al. 2014). On the other hand, non-specialized pollinators (Bezemer et al. 2014), predators (Pearson 2009; Hartley et al. 2010) and detritivores (Standish 2004; Litt et al. 2014) are often unaffected by invasions, or their diversity and abundance may even increase in novel habitats.
Among invasive plants, the ecological consequences of woody invaders are particularly profound, due to their strong effects on native habitats (Richardson 1998; Hierro and Callaway 2003). Alien woody plants, by eliminating native species as a consequence of interspecific competition (Vilà et al. 2011; Benesperi et al. 2012), can decrease the food supply in the forest canopy (Litt et al. 2014; Reif et al. 2016; Hejda et al. 2017), or change the habitat structure and therefore affect the composition of the whole community (Harris et al. 2004; Pawson et al. 2010; Litt et al. 2014; van Hengstum et al. 2014; Buchholz et al. 2015).
Arthropods are among the most diverse groups of animals (Ødegaard 2000) and significantly contribute to trophic interactions (Mooney et al. 2010). Therefore, a deeper understanding of the impact of woody invaders on arthropods is important, as the impacts of invasive plants on organisms at lower trophic levels may have consequences for the functioning of the whole ecosystem (Heleno et al. 2008; Tallamy et al. 2010; Bezemer et al. 2014; Litt et al. 2014; Reif et al. 2016; Schirmel et al. 2016; Hejda et al. 2017). Even though there are some studies that focus on the effects of woody invaders on arthropods (e.g. Bezemer et al. 2014; Litt et al. 2014; van Hengstum et al. 2014; Buchholz et al. 2015, Schirmel et al. 2016), our knowledge is still incomplete, given the enormous diversity of arthropods, and their ecological traits and life history strategies. For example, we can predict that herbivores bound to different layers of vegetation may vary in response to changes in light conditions or stand structure (Harris et al. 2004; Pawson et al. 2010), but the studies available have mostly used coarse groups of arthropod communities, and more detailed relationships remain unclear. In fact, woodland arthropod diversity may be more affected by habitat structure than by variability in plant diversity (Gardner et al. 1995; Highland et al. 2013).
The main objective of this study was to compare the assemblages of nocturnal Lepidoptera between stands invaded by a widespread invasive tree, the black locust (Robinia pseudoacacia) and forest stands formed by native tree species. Impacts of invasive Robinia pseudoacacia have been documented for various kinds of organisms (Degomez and Wagner 2001; Cierjacks et al. 2013; Buchholz et al. 2015; Rocca et al. 2016; Vítková et al. 2017). Nocturnal Lepidoptera (further called “moths”) are a well-studied group of arthropods in Central Europe, with detailed knowledge of their ecology (Summerville et al. 2004; Pavlikova and Konvicka 2012), known direct links to vegetation structure (Highland et al. 2013) as well as with a high diversity of larval feeding strategies, life-histories and other ecological traits (Strong et al. 1984; Pierce 1995). These moths therefore represent excellent study organisms for testing the effects of plant invasions on groups of species defined by their (ecological) traits. In particular, we asked: (i) Do stands of the invasive R. pseudoacacia differ in habitat structure compared to stands of native trees? (ii) Do these stand types differ in the species richness, abundance and biomass of moths? (iii) Do the moth assemblages associated with these stand types differ in their ecological traits and could the alteration of habitat structure explain these potential differences?
Materials and methods
Focal invasive tree
Invasive black locust (Robinia pseudoacacia) occurs naturally in the southeast of the USA, where it represents an important part of early-successional forests, being eventually replaced by climax species (Boring and Swank 1984; Cierjacks et al. 2013). It was introduced to Europe at the beginning of the Seventeeth Century and has further spread worldwide (Cierjacks et al. 2013). At present, it is considered as one of the most widespread invasive species in Europe (Vítková et al. 2017). It was introduced to the Czech Republic at the beginning of Eighteenth Century (Slavík 1995), and was widely planted in warm areas, particularly on barren rocky slopes, for the stabilization of soil, and for wood and honey production (Vítková et al. 2017). Due to its nitrogen-fixing ability, it enriches habitats with nitrogen and supports the spread of nitrophilous herbs and shrubs (Benesperi et al. 2012; Vítková and Kolbek 2010; Vítková et al. 2017).
Study area and design
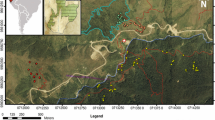
The fieldwork for this study was carried out in a forested lowland area of ca. 600 km2 (approximately between 49°56′N and 50°08′N, and 14°09′E and 14°26′E; 200–400 m a.s.l.) in central Bohemia, the Czech Republic, Europe (Fig. 1). This area is predominantly covered by stands of deciduous forests, human settlement, farmland and grassland. The forests are formed by native species of oak (Quercus spp.), hornbeam (Carpinus betulus), ash (Fraxinus excelsior), maples (Acer spp.) and limes (Tilia spp.). In the first half of the 20th century, large parts of this area were planted with R. pseudoacacia, which further spread spontaneously to the surroundings (Nožička 1957).
We established 20 study plots (100 m × 100 m) in stands of native deciduous trees (dominated by Quercus spp.; henceforth “native stands”) and 19 study plots in stands dominated by the invasive R. pseudoacacia (comprising more than 95% of tree cover; henceforth “R. pseudoacacia stands”) (Fig. 1, Online Resource 1). These study plots were established at least 100 m from the forest edges and the minimum distance between adjacent plots was 500 m (Beck and Linsenmair 2000; Hanzelka and Reif 2015). Mixed stands with both alien and native trees were avoided.
This study focused on moths of the superfamilies Hepialoidea, Cossoidea, Zygaenoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea and Noctuoidea. To sample moths in both stand types, we used 39 modified, automatic, portable light traps (Heaths 1965) with similar specifications as used by Brehm and Axmacher (2006). Moths were attracted to traps with two 8 W UV LED strip lights (total luminous flux of 400 lm in the wavelength range of 400–420 nm, powered by 7.2 Ah/12 V lead batteries) and then euthanized by evaporated chloroform. At each plot, a single portable trap was placed on the ground, approximately in the middle of the plot. All traps were exposed on the same night at the beginning of each month from April to November 2014, from dusk until dawn, when the weather was suitable (i.e. no strong wind and no strong rainfall) and the moon was quarter-sized at maximum. Such traps attract flying insects within a radius of a few tens of metres (Truxa and Fiedler 2012). Therefore, they should have attracted moths occurring almost exclusively within the area of the study plots, without sampling individuals from the surrounding habitats. Such a sampling design allowed us to cover all major phenological phases of moth species richness, throughout the part of the year when the moths were actively flying (e.g. Jonason et al. 2014; Tropek et al. 2014), and also to avoid short-term effects that may possibly affect the light trapping (Yela and Holyoak 1997).
Moth data and traits
The samples from the light traps were stored in paper bags and frozen at − 22 °C. In the laboratory, all moths from the target groups were identified to the species level. To measure their biomass (in terms of dry weight), the moths were dried in an oven at 80 °C for 12 h and then weighed on an analytical scale (van Langevelde et al. 2011). For each species, the evidence of its utilization of R. pseudoacacia as a host plant was determined from the literature (Kulfan 2012).
To analyse the possible effects of R. pseudoacacia resulting from differences in habitat characteristics, a species-traits approach was adopted (Pavlikova and Konvicka 2012). For each species, the following ecological characteristics were recorded (Table 1): (i) general traits, not specific to any particular stage of life cycle (voltinism, overwintering stage, preferred structure of habitat and vegetation layer), (ii) traits specific for the larval stage (length of larval development, diet specialisation and feeding guild) and (iii) traits specific for adults (wing span as a measure of body size and mobility, life span).
Habitat characteristics and environmental variables
To describe differences in the habitat structure of both R. pseudoacacia and native stands, 14 habitat characteristics expected to affect the diversity of arthropods (Strong et al. 1984; Gardner et al. 1995; Hartley 2002; Highland et al. 2013) were recorded in June 2014. At each plot, the age of the forest was recorded. Fallen (FALLEN TREES) and dead (DEAD TREES) trees were counted and other vegetation characteristics were estimated in three equal-sized subplots of 100 × 33 m, and mean values for each parameter were calculated (see Hanzelka and Reif 2016 for details). Specifically, by walking through the whole subplots, we visually estimated the percentage cover of herbs < 0.5 m height (HERB1), herbs > 0.5 m (HERB2), shrubs from 1 to 5 m in height (SHRUB), trees from 5 to 10 m (TREE1), trees > 10 m (TREE2), canopy cover (CANOPY) and clearings (CLEARINGS). Further we estimated the proportion of the number of trees with diameter at breast height (dbh) < 0.2 m (TREES); trees with dbh 0.2–0.5 m (TREEM) and trees with dbh > 0.5 m (TREET). We expressed the light conditions as the presence of a continuous canopy (CANYES/CANNO).
Finally, we considered six environmental variables known to affect arthropod communities (Novotny et al. 2015) expressed as proportions of the following land cover types in the surroundings of study plots: ARABLE—arable land; WATER—water bodies; ROCK; GRASS—grassland; URBAN—urban area; BROAD—broad-leaved forest; CONIF—coniferous forest. The proportions of these land cover types were estimated within a circular buffer of 500-m radius around each plot using ArcGIS version 10.2 (ESRI 2011).
Data analysis
To reduce the complexity of habitat and environmental data without substantial loss of information, the major dimensions of habitat structure and land cover characteristics of the R. pseudoacacia and native stands were determined by principal component analysis (PCA) in Canoco 5.0 (ter Braak and Šmilauer 2012). We ran two separate PCAs: one for vegetation structure and second for land cover characteristics. To determine the number of principal components, we used the screeplot method (Jackson 1993). Based on this criterion, we used the plot scores from the first two principal components of habitat structure (further called “VEG1” and “VEG2”) and land cover characteristics (further called “LAND1” and “LAND2”).
Since our data may have suffered from problems of spatial autocorrelation, we applied a method of generalized least squares (GLS) from the package “nlme” (Pinheiro et al. 2017) in all following univariate models. Geographic coordinates of plot centres were used to express the possible spatial effects, and different autocorrelation structures within the residuals (Gaussian, exponential, linear, rational quadratics and spherical) were compared. The parsimony of these models, as well as a model without residual autocorrelation, were assessed using the Akaike Information Criterion, AIC (Zuur et al. 2009). By comparing the AIC values, we selected the most appropriate autocorrelation structure. In all cases, the most parsimonious models (i.e. those with the lowest AIC value) turned out to be the models without spatial effects. Thus, we used linear models without accounting for spatial autocorrelation in further analyses.
To compare the habitat characteristics of native versus R. pseudoacacia stands, linear models were fitted with the principal components of habitat characteristics (VEG1 or VEG2) as respective response variables and the stand type (STAND: native trees or R. pseudoacacia) as the predictor.
To compare the species richness, abundance and biomass between the native and R. pseudoacacia stands, we used the number of moth species in each plot (SPECIES), number of all moth individuals per plot (INDIVIDUALS) and the total dry mass of moths per plot (BIOMASS; in grams) as the respective response variables, all transformed using the natural logarithm.
At first, a full linear model with all main predictors—stand type (STAND: native or R. pseudoacacia), LAND1 and LAND2 (principal components of land cover characteristics), VEG1 and VEG2 (principal components of habitat structure)—was constructed for each of the response variables (i.e. SPECIES, INDIVIDUALS, BIOMASS). Plots of the standardized residuals were checked against each continuous variable for possible polynomial trends. We thus added a quadratic term for VEG2 into the models. Interactions were not included, because there were no meaningful interpretations related to our hypotheses. In the next step, a multi-model inference framework was used (package “MuMIn”, Bartoń 2016) to obtain a minimum adequate set of predictors for each response variable. Due to the small sample size relative to the number of estimated parameters, the candidate models containing all possible predictor combinations were compared by AIC corrected for small sample sizes (AICc—Akaike 1974; Burnham and Anderson 2002). Models with ΔAICc (i.e. the difference between the AICc value of the focal model with the lowest AICc value) < 2 were selected as the best performing models. The predictors that appeared in these best performing models were considered as the minimum adequate set and were used for interpretations. All models were further validated for the assumption of normal distribution of errors, based on a visual inspection of the distribution of standardized residuals (Crawley 2013). All univariate models were fitted in the program R version 3.3.1 (R Core Team 2016).
To test if the stand types differed in the traits of the moths assemblages, redundancy analyses (RDA) were performed in Canoco 5.0 (ter Braak and Šmilauer 2012). However, this method does not take the geographic positions of study plots into account and thus its results may suffer from spatial autocorrelation in the data (Šmilauer and Lepš 2014). Therefore, we combined RDA with principal coordinates of neighbour matrices (PCNM) to account for spatial autocorrelation (Dray et al. 2006; Peres-Neto et al. 2006), following recommendations from the developers of this technique (Šmilauer and Lepš 2014).
In PCNM, the Euclidean distance matrix based on geographical distances of neighbouring sample plots was first calculated. This matrix was then processed by a principal coordinate analysis (PCoA) to obtain the spatial variables represented by respective PCoA axes (Šmilauer and Lepš 2014). Monte Carlo permutation tests (999 runs) were used to test the significance of each axis in the PCoA. From the PCoA output, we extracted the positions of each study plot along the significant PCoA axes (called “PCo scores”) and these scores were further used in all subsequent RDAs as covariate variables capturing the spatial information in the data.
In the next step, we fitted three RDA models, where each contained the functional traits as the response variables. The value of a particular functional trait for each plot was quantified as the number of all trapped individuals on a particular plot sharing an identical level of a given trait (e.g. SMALL body size; Table 1). These response variables were centred and standardized in all models. The first model (STAND model) included a single predictor, the stand type, and PCo scores as covariables. The second model (COVARIATE model) included land cover characteristics, LAND1 and LAND2, as predictors and PCo scores as covariables. The third model (STAND│COVARIATE model) included the stand type as a predictor and land cover characteristics and PCo scores as covariables.
In addition to RDA models we used variation partitioning (Peres-Neto et al. 2006) to distinguish the marginal, conditional and shared effects of the three groups of predictors—stand type (native/R. pseudoacacia), habitat structure (VEG1 and VEG2) and environmental variables (significant PCo scores from PCNM and land cover characteristics, LAND1 and LAND2)—on the distribution of the ecological traits of moths. Marginal effects are the effects of a given predictor variable (or a group of variables) without taking the other predictors into account; conditional effects quantify the effects of a given predictor variable after controlling for the effects of other predictors; shared effects are the effects shared between a given predictor variable and the other predictors (Šmilauer and Lepš 2014).
Results
Habitat characteristics of native and invaded forest stands
Native and invaded forest stands differed in habitat characteristics (Fig. 2a). The first PC axis, VEG1 (explaining 55.42% of the variation in habitat characteristics), reflected a gradient from older stands with taller trees and a more developed and continuous canopy to younger, open stands with smaller trees, a more developed shrub layer and a higher number of fallen trees (Fig. 2a). Native stands had lower VEG1 scores than R. pseudoacacia stands (t = − 9.075, p < 0.001). The second axis, VEG2 (18.96%), reflected a gradient from plots with a more developed lower herb layer and small area of clearings to plots with a more developed taller herb layer and larger area of clearings (Fig. 2a), and was not significantly different between the native and the R. pseudoacacia stands (t = − 1.933, p = 0.061).
In case of the land cover characteristics, the first axis, LAND1 (63.45%), reflected mainly the gradient from a landscape with a large portion of broad-leaved forest to a landscape with a larger cover of urban area (Fig. 2b). The second axis, LAND2 (20.73%), reflected mainly the gradient from a landscape with a large share of coniferous forests to a landscape without coniferous forests.
Moth species richness, abundance and biomass
In total, 18,556 individuals of 384 moth species were captured (Online Resource 2), of which 346 species (mean ± s.e. [range] = 122 ± 27 [81–165]) were trapped in native stands and 304 species (90 ± 16 [61–120]) in R. pseudoacacia stands, with 266 species occurring in both stand types. Seventy eight species were more common in R. pseudoacacia and 164 species were more common in native stands. A total of 18 species that had been previously documented to feed on R. pseudoacacia were recorded, 15 of them in both stand types, two species only in native stands and one species only in R. pseudoacacia stands.
Relationships of moth species richness, abundance and biomass to the characteristics of the forest stands were estimated by linear models with performance assessed by AICc. Stand type was included in all except one of the best performing models (ΔAICc < 2) for all of the response variables (Table 2). Specifically, the species richness, the number of individuals, and the total biomass of captured moths were higher in native stands than in invaded stands (Table 3, Fig. 3). In addition, the best performing models for moth species richness, abundance and biomass also included VEG2 and the quadratic term of VEG2 (Table 2). The highest number of species and highest biomass were recorded in stands with intermediate values of VEG2 (Table 3a, c), i.e. with moderate proportions of clearings and both taller and shorter herbs in the understorey. Moreover, one model for moth abundance contained the effect of VEG1 (Table 3b), with increasing numbers of individuals towards stands with a closed canopy and less-developed shrub layer. Finally, the best performing models for moth species richness and abundance also included the effects of LAND2 (Table 2), with the number of species and individuals increasing towards stands surrounded by a higher coverage of coniferous trees (Table 3a, b). Some of the best performing models (m54 for moth abundance and m54 for moth biomass, see Table 2) contained “masquerading” variables and were thus not used for inference. Such variables are included among the terms of the best performing models, but do not improve the fit sufficiently to offset the penalty for their addition, compared to the more parsimonious models without this variable (Anderson 2008; Arnold 2010).
Composition of moth communities
The stand type explained 16.8% of the variation of moth traits (Table 4), and its effect remained significant even after controlling for environmental variables (STAND│COVARIATE models; Table 4). Moth communities in the native stands were characterised by a higher presence of univoltine moths, which are specialists of forest habitats and are associated with the canopy layer, and by habitat generalists (Fig. 4a). On the contrary, forest-steppe moths, which are associated with more open habitats or herb and shrub layers, with more generations per season and with chrysalis as an overwintering stage, were more numerous in the R. pseudoacacia stands. Adult moths in the native stands were larger, more mobile and longer-living, while the stands of R. pseudoacacia predominantly supported moths with a faster life cycle and a higher proportion of short-living adults of smaller body sizes (Fig. 4b). With respect to larval feeding guilds, larvae feeding on herb and shrub litter or on thallus were more common in R. pseudoacacia stands (Fig. 4c). Other feeding guilds showed weaker responses to stand type.
Redundancy analysis (RDA) relating the a general, b adult and c larval-stage ecological and biological traits of nocturnal Lepidoptera to the two types of stands studied: 20 plots of native stands and 19 plots dominated by the invasive Robinia pseudoacacia. Spatial autocorrelation and significant environmental variables were included as covariables in all these models. See the “Methods” section for plot characteristics and details on traits
Shared and marginal effects of all three groups of predictors (stand type, habitat structure, environmental variables) explained a substantial proportion of the variability in moths functional trait composition (Table 5). While the conditional effects of stand type and habitat characteristics were rather weak relative to the marginal effects (8.7 vs. 21.6% and 4.6 vs. 19.8%, respectively; Table 5), the effects of land cover characteristics, controlled for the spatial positions of plots, explained nearly half of the variability compared to the marginal effects (10.6 versus 22.7%; Table 5).
Discussion
Stands formed by native trees differed significantly in their habitat structure from stands of the invasive R. pseudoacacia. The native stands were mostly formed by taller trees with a closed canopy and with a higher cover of shorter herbs. On the other hand, R. pseudoacacia stands were characterized by a more open canopy, with a higher coverage of shrubs and taller herbs in the understorey. Similar to our study, Buchholz et al. (2015) reported a more developed understorey vegetation in R. pseudoacacia stands compared to stands of the native birch Betula pendula (with a significant effect on the cover of herbs and a marginally significant effect on the cover of shrubs). These effects are probably caused by the nitrogen-fixing ability of R. pseudoacacia enriching the soil in nitrogen (Boring and Swank 1984; Cierjacks et al. 2013; Vítková et al. 2017) and by the more open canopy of its stands, which allows for a better transmission of solar radiation into the understorey and consequently supports the growth of herbs and shrubs in the understorey layer.
Based on the light-trapping data, we found significantly lower total species richness, abundance and biomass of nocturnal Lepidoptera in stands dominated by the invasive R. pseudoacacia. This is in accordance with the general pattern of decreasing diversity, abundances or biomass of herbivores caused by invasive plants (Liu and Stiling 2006; Gerber et al. 2008; Spafford et al. 2013; Litt et al. 2014; van Hengstum et al. 2014; Schirmel et al. 2016). Similarly, Degomez and Wagner (2001) found in northern Arizona a nearly 30% loss of species diversity in stands of non-native R. pseudoacacia in contrast to stands of native Robinia species. However, some of the studied groups, e.g., the hyperdiverse Hymenoptera and Diptera, did not exhibit losses in diversity (Degomez and Wagner 2001). Also, other studies comparing the diversity of native and R. pseudoacacia stands did not find differences in species diversity of predators (Buchholz et al. 2015) or saproxylic beetles (Rocca et al. 2016). Based on these findings and on the results of our study, it seems that the response of arthropods to the invasion of R. pseudoacacia depends on their feeding strategy, with prevailing negative effects on herbivores.
The lower species richness and lower number of individuals observed in R. pseudoacacia stands could be explained by the paucity of canopy moths, which are also mostly leaf-chewing herbivores (Degomez and Wagner 2001). Despite three centuries of occurrence in Central Europe (Slavík 1995), the spectrum of species able to feed on R. pseudoacacia remains limited (Kulfan 2012). Higher abundances of canopy dwellers in the native stands could also explain the higher proportion of moths overwintering in the egg stage found in the native stands, including polyphagous forest pests with generally higher population densities (Alford 2000). In contrast to understorey species, canopy species are often strongly associated with spring leaf germination (Van Asch and Visser 2007; Hikisz and Soszyńska-Maj 2015), when leaves are more palatable. Since they are probably not able to feed on the alien R. pseudoacacia, they lack a suitable feeding niche in canopies dominated by this tree. Differences in the number of leaf-chewing moths between R. pseudoacacia stands and native stands could also affect the frequency distribution of adult body sizes, because species restricted to the canopy of native trees tend to be larger in body size (Heleno et al. 2008). Thus the lack of canopy species in R. pseudoacacia stands can also explain lower total biomass of moths in these stands.
The species diversity and the number of individuals were also weakly positively affected by the proportion of coniferous stands in the surroundings of the study plots (represented by LAND2). This is in accordance with the known effects of woody plant diversity in the surroundings of traps on the diversity and abundance of moths (Novotny et al. 2015). Even among forest species specialized on coniferous trees, larger and mobile moths can be found (e.g. larger geometrids, hawkmoths, lappets), dispersing occasionally to deciduous stands and thus increasing the total species diversity and abundance.
Our results also showed that aspects of habitat structure, not accounted for by the distinction between native and invaded stands, had important effects on species diversity, abundance and total biomass. Specifically, sites with an intermediate proportion of clearings and an intermediate proportion of lower and taller herbs in the herb layer (the quadratic term of VEG2) had the highest number of species and individuals and the highest biomass of moths. We also detected the direct effects of vegetation structure on the functional composition of moths. Therefore, vegetation structure plays an important role in moth community assembly.
The moth assemblages of native forests were only partly formed by forest canopy specialists. Another guild occurring more frequently in the native stands were generalists, without distinctive habitat specialization. This is not consistent with some studies on ubiquitous species (Yoshioka et al. 2010, 2014), showing a higher abundance of generalists in invaded habitats. However this discrepancy may be due to the fact that those studies were conducted in non-forest habitats while our research was performed in forest stands. A majority of generalists in our study were migrants or pests with good dispersal ability (Slade et al. 2013). Such species probably disperse more easily through the more permeable native stands, formed by tall trees and without a well-developed shrub layer, than through stands of R. pseudoacacia, with a dense understorey. The more complex structure of invaded forests may therefore represent a dispersal barrier for insect habitat generalists (Barbaro et al. 2005). Similarly, in contrast to predictions and results showing a higher occurrence of diet specialists in native stands (Liu and Stilling 2006; Burghardt et al. 2010; Litt et al. 2014), we found no difference in preferences for stand type in the herbivore monophages and oligophages. This is probably because the loss of canopy diet specialists in R. pseudoacacia stands is compensated by dietary specialists gained in the better developed understorey.
Many studies on detritivores in invaded habitats have shown that the diversity or abundances of detritivores is higher in invaded stands than in native stands due to the higher amount of ground litter and decaying vegetation in non-native vegetation (Standish 2004; Levin et al. 2006; Litt et al. 2014). Interestingly, we found that moths with larvae feeding on litter leaves of herbs or shrubs were more common in stands of R. pseudoacacia. This may be related to the higher cover of shrubs and taller herbs, dominated by native plant species (Hejda et al. 2017), in the R. pseudoacacia stands.
It is interesting that moths with faster life-cycles (i.e. those having shorter larval development, shorter adult lifespans and more generations per season) occurred more frequently in R. pseudoacacia stands. This may be caused by a warmer and drier microclimate in these stands because leaves of R. pseudoacacia, unlike the leaves of native trees, rotate during strong summer heat to be less exposed to solar radiation (Xu et al. 2009), making them less effective in buffering heat stress in the understorey than native trees.
Overall, moth assemblages in R. pseudoacacia stands were similar to those of open-forests or forest-steppe habitats with better light conditions, but lacked canopy species, while forest and canopy dwellers dominated in native stands.
Conservation implications
The lower moth species richness in stands dominated by the invasive R. pseudoacacia indicates that this habitat does not favour Lepidopteran species richness in central European forests. Moreover, we did not record any moth species of conservation concern (sensu Farkač et al. 2005) in the invaded stands, while several such species were recorded in the native stands. Therefore, we suggest that the further spread of this invasive tree should be prevented and its eradication from sites of conservation concern should be prioritized.
At the same time, we found remarkable differences in the proportions of various ecological groups of moths between the native and invasive stands, which were likely caused by differences in habitat structure. Specifically, the native forests had more closed canopies and a less developed understorey than the studied invasive stands. Due to the higher light availability and well-developed understorey vegetation (Buchholz et al. 2015), stands of R. pseudoacacia resembled open forests, which are among the most threatened and vanishing habitats in Europe (Miklín and Čížek 2014). Therefore, from the perspective of moths restricted to the forest understorey, forest-steppe and open habitats, the conservation potential of the studied native stands with the currently prevailing vegetation structure is limited. The second message from our study for the conservation of moths in central European lowland forests is therefore the need to increase the heterogeneity of the habitat structure and canopy openness of native forests (see also Sebek et al. 2015). Even though the composition of native stands supports the diversity of some functional groups of moth fauna due to long-term adaptations (e.g. canopy feeders), the diversity of moth fauna and its functional guilds may be limited by the large-scale homogeneity of native stands, with closed canopies and relatively homogenous age structure. In this respect, the management of native lowland forests may consider active measures (planned clearings, coppicing, disturbances, grazing, creating small-scale gaps or selective cutting) to promote the patch dynamics of new versus old stands, as well as closed canopies versus more open areas (Merckx et al. 2012; Pavlikova and Konvicka 2012; Sebek et al. 2015).
References
Aizen MA, Morales CL, Morales JM (2008) Invasive mutualists erode native pollination webs. PLoS Biol 6:396–403
Alford DV (2000) Pest and disease management handbook. Blackwell Science, Oxford
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York
Arnold T (2010) Uninformative parameters and model selection using akaike’s information criterion. J Wildl Manage 74:1175–1178
Barbaro L, Pontcharraud L, Vetillard F, Guyon D, Jactel H (2005) Comparative responses of bird, carabid, and spider assemblages to stand and landscape diversity in maritime pine plantation forests. Ecoscience 12:110–121
Bartoń K (2016) Package ‘MuMIn’. Model selection and model averaging based on information criteria (AICc and alike). R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn. Accessed 10 April 2017
Beck J, Linsenmair E (2000) Feasibility of light-trapping in community research on moths: attraction radius of light, completeness of samples, nightly flight times and seasonality of Southeast-Asian hawkmoths (Lepidoptera: Sphingidae). J Res Lepidoptera 39:18–37
Benesperi R, Giuliani C, Zanetti S, Gennai M, Lippi MM et al (2012) Forest plant diversity is threatened by Robinia pseudoacacia (black-locust) invasion. Biodivers Conserv 21:3555–3568
Berwaerts K, Van Dyck H, Aerts P (2002) Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct Ecol 16:484–491
Bezemer TM, Harvey JA, Cronin JT (2014) Response of native insect communities to invasive plants. Annu Rev Entomol 59:119–141
Boring LR, Swank WT (1984) The role of black-locust (Robinia pseudoacacia) in forest succession. J Ecol 72:749–766
Brehm G, Axmacher JC (2006) A comparison of manual and automatic moth sampling methods (Lepidoptera: Arctidae, Geometridae) in a rain forest in Costa Rica. Environ Entomol 35:757–764
Buchholz S, Tietze H, Kowarik I, Schirmel J (2015) Effects of a major tree invader on urban woodland arthropods. PLoS ONE 10:e0137723
Burghardt KT, Tallamy DW, Philips CH, Shropshire KJ (2010) Non-native plants reduce abundance, richness, and host specialization in lepidopteran communities. Ecosphere 1:1–22
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information—theoretic approach. Springer-Verlag, New York
Chambers JC, Roundy BA, Blank RR, Meyer SE, Whittaker A (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol Monogr 77:117–145
Cierjacks A, Kowarik I, Joshi J, Hempel S, Ristow M et al (2013) Biological flora of the British Isles: Robinia pseudoacacia. J Ecol 101:1623–1640
Crawley MJ (2013) The R book, 2nd edn. Wiley Publishing, Chichester
Degomez T, Wagner RM (2001) Arthropod diversity of exotic vs. native Robinia species in northern Arizona. Agric For Entomol 3:19–27
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Model 196:193–483
ESRI (2011) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Farkač J, Král D, Škorpík M (eds) (2005) List of threatened species in the Czech Republic. Invertebrates. Agentura ochrany přírody a krajiny ČR, Prague
Gardner SM, Cabido MR, Valladares GR, Diaz S (1995) The influence of habitat structure on arthropod diversity in Argenthine semi-arid Chaco forest. J Veg Sci 6:349–356
Gerber E, Krebs C, Murrell C, Moretti M, Rocklin R, Schaffner U (2008) Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol Conserv 141:646–654
Hanzelka J, Reif J (2015) Responses to the black locust (Robinia pseudoacacia) invasion differ between habitat specialists and generalists in central European forest birds. J Ornithol 156:1015–1024
Hanzelka J, Reif J (2016) Effects of vegetation structure on the diversity of breeding bird communities in forest stands of non-native black pine (Pinus nigra A.) and black locust (Robinia pseudoacacia L.) in the Czech Republic. Forest Ecol Manag 379:102–113
Harris RJ, Toft RJ, Dugdale JS, Williams PA, Rees JS (2004) Insect assemblages in a native (kanuka—Kunzea ericoides) and an invasive (gorse—Ulex europaeus) shrubland. N Z J Ecol 28:35–47
Hartley MK (2002) Rationale and methods for conserving biodiversity in plantation forests. For Ecol Manag 155:81–95
Hartley MK, Rogers WE, Siemann E (2010) Comparisons of arthropod assemblages on an invasive and native trees: abundance, diversity and damage. Arth-Plant Int 4:237–245
Heath J (1965) A genuienly portable UV light trap. Entomol Rec J Var 77:236–238
Hejda M, Hanzelka J, Kadlec T, Štrobl M, Pyšek P, Reif J (2017) Impacts of an invasive tree across trophic level: species richness, community composition and resident species’ traits. Divers Distrib 23:997–1007
Heleno RH, Ceia RS, Ramos JA, Memmott J (2008) Effects of alien plants on insect abundance and biomass: a food-web approach. Conserv Biol 23:410–419
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256:29–39
Higgins SI, Azorin EJ, Cowling RM, Morris MJ (1997) A dynamic ecological-economic model as a tool for conflict resolution in an invasive-alien-plant, biological control and native-plant scenario. Ecol Econ 22:141–154
Highland SA, Miller JC, Jones JA (2013) Determinants of moth diversity and community in a temperate mountain landscape: vegetation, topography, and seasonality. Ecosphere 4:1–22
Hikisz J, Soszyńska-Maj A (2015) What moths fly in winter? The assemblage of moth active in a temperate deciduous forest during the cold season in central Poland. J Entomol Res Soc 17:59–71
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Jonason D, Franzén M, Ranius T (2014) Surveying moths using light traps: effects of weather and time of year. PLoS ONE 9:e92453
Kulfan M (2012) Lepidoptera on the introduced Robinia pseudoacacia in Slovakia, Central Europe. Check List 8:709–711
Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G (2002) An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc R Soc Lond B 269:2407–2413
Levin LA, Neira C, Grosholz ED (2006) Invasive cordgrass modified wetland trophic function. Ecology 87:419–432
Litt AR, Cord EE, Fulbright TE, Schuster GL (2014) Effects of invasive plants to Arthropods. Conserv Biol 28:1532–1549
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545
Macek J, Dvořák J, Traxler L, Červenka V (2007) Lepidoptera and caterpillars of the central Europe. Nocturnal Lepidoptera I. Academia, Prague
Macek J, Dvořák J, Traxler L, Červenka V (2008) Lepidoptera and caterpillars of the central Europe. Nocturnal Lepidoptera II. Academia, Prague
Macek J, Procházka P, Traxler L (2012) Lepidoptera and caterpillars of the central Europe. Nocturnal Lepidoptera III. Academia, Prague
Merckx T, Feber RE, Hoare DJ, Parsons MS, Kelly CJ et al (2012) Conserving threatened Lepidoptera: towards an effective woodland management policy in landscapes under intense human land-use. Biol Conserv 149:32–39
Miklín J, Čížek L (2014) Erasing a European biodiversity hot-spot: Open woodlands, veteran trees and mature forests succumb to forestry intensification, succession, and logging in a UNESCO Biosphere Reserve. J Insect Conserv 22:35–41
Mooney KA, Gruner DS, Barber NA, Van Bael SA, Philpott SM, Greenberg R (2010) Interactions among predators and the cascading effects of vertebrate insectivores on arthropod communities and plants. PNAS 107:7335–7340
Moroń D, Lenda M, Skórka P, Szentgyörgyi H, Settele J, Woyciechowski M (2009) Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscapes. Biol Conserv 142:1322–1332
Novotny D, Zapletal M, Kepka P, Benes J, Konvicka M (2015) Large moths captures by a pest monitoring system depend on farmland heterogenity. J Appl Entomol 139:390–400
Nožička J (1957) Overview of the development of our forests. SZN, Prague
Öckinger E, Schweiger O, Crist TO, Debinski DM, Krauss J et al (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979
Ødegaard F (2000) How many species of arthropods? Erwin’s estimate revised. Biol J Linn Soc 71:583–597
Pauchard A, Shea K (2006) Integrating the study of non-native plant invasions across spatial scales. Biol Invasions 8:399–413
Pavlikova A, Konvicka M (2012) An ecological classification of Central European macromoths: habitat associations and conservation status returned from life history attributes. J Insect Conserv 16:187–206
Pawson SM, McCarthy JK, Ledgard NJ, Didham RK (2010) Density-dependent impacts of exotic conifer invasion on grassland invertebrate assemblages. J Appl Ecol 47:1053–1062
Pearson DE (2009) Invasive plant architecture alters trophic interactions by changing predator abundance and behavior. Oecologia 159:549–558
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pierce NE (1995) Predatory and parasitic Lepidoptera: carnivores living on plants. J Lepid Soc 49:412–453
Pinheiro J, Bates D, DebRoy S, Sarkar D and R Core Team (2017) nlme: linear and nonlinear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reif J, Hanzelka J, Kadlec T, Štrobl M, Hejda M (2016) Conservation implications of cascading effects among groups of organisms: the alien tree Robinia pseudacacia in the Czech Republic as a case study. Biol Conserv 198:50–59
Richardson DM (1998) Forestry trees as invasive aliens. Conserv Biol 12:18–26
Rocca FD, Stefanelli S, Bogliani G (2016) Robinia pseudoacacia as a surrogate for native tree species for saproxylic beetles inhabiting the riparian mixed forests of northern Italy. Agric For Entomol 18:250–259
Schirmel J, Bundschuh M, Entling MH, Kowarik I, Buchholz S (2016) Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: a global assessment. Glob Change Biol 22:594–603
Slade EM, Merckx T, Riutta T, Bebber DP, Redhead D et al (2013) Life-history traits and landscape functional traits predict macro-moth responses to forest fragmentation. Ecology 94:1519–1530
Simao MCM, Flory SL, Rudgers JA (2010) Experimental plant invasion reduces arthropod abundance and richness across multiple trophic levels. Oikos 119:1553–1562
Slavík B (1995) Flora of the Czech Republic 4. Academia, Prague
Spafford RD, Lortie CJ, Butterfield BJ (2013) A systematic review of arthropod community diversity in association with invasive plants. Neobiota 16:81–102
Standish RJ (2004) Impact of an invasive clonal herb on epigaeic invertebrates in forest remnants in New Zealand. Biol Conserv 116:49–58
Strong DR, Lawton JH, Southwood R (1984) Insects on plants: community patterns and mechanisms. Blackwell Scientific, Oxford
Summerville KS, Ritter LM, Crist TO (2004) Forest moth taxa as indicators of lepidopteran richness and habitat disturbance: a preliminary assessment. Biol Conserv 116:9–18
Sebek P, Bace R, Bartos M, Benes J, Chlumska Z et al (2015) Does a minimal intervention approach threaten the biodiversity of protected areas? A multi-taxa short-term response to intervention in temperate oak-dominated forests. For Ecol Manag 358:80–89
Šmilauer P, Lepš J (2014) Multivariate analysis of ecological data using Canoco 5, 2nd edn. Cambridge University Press, Cambridge
Tallamy DW, Ballard M, Amico VD (2010) Can alien plants support generalist insect herbivores? Biol Invasions 12:2285–2292
ter Braak CJF, Šmilauer P (2012) CANOCO reference manual and user’s guide: software for ordination, version 5.0. Microcomputer Power, Ithaca
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Tropek R, Cerna I, Straka J, Kadlec T, Pech P et al (2014) Restoration management of fly ash deposits crucially influence their conservation potential for terrestrial arthropods. Ecol Eng 73:45–52
Truxa C, Fiedler K (2012) Attraction to light—from how far do moths (Lepidoptera) return to weak artificial sources of light? Eur J Entomol 109:77–84
van Asch M, Visser ME (2007) Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol 52:37–55
van Hengstum T, Hooftman DAP, Oostermeijer JGB, van Tienderen PH (2014) Impact of plant invasion on local arthropod communities: a meta-analysis. J Ecol 102:4–11
van Kleunen M, Dawson W, Essl F, Pergl J, Winter M et al (2015) Global exchange and accumulation of non-native plants. Nature 525:100–103
van Langevelde F, Ettema JA, Donners M, WallisDeVries MF, Groenendijk D (2011) Effect of spectral composition of artificial light on the attraction of moths. Biol Conserv 144:2274–2281
Vilà M, Espinar LJ, Hejda M, Hulme EP, Jarošík V et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Vítková M, Kolbek J (2010) Vegetation classification and synecology of Bohemian Robinia pseudoacacia stands in a Central European context. Phytocoenologia 40:205–241
Vítková M, Müllerová J, Sádlo J, Pergl J, Pyšek P (2017) Black locust (Robinia pseudoacacia) beloved and despised: a story of an invasive tree. For Ecol Manag 384:287–302
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:468–478
Xu F, Guo W, Wang R, Xu W, Du N et al (2009) Leaf movement and photosynthetic plasticity of black locust (Robinia pseudoacacia) alleviate stress under different light and water conditions. Acta Physiol Plant 31:553–563
Yela JL, Holyoak M (1997) Effects of moonlight and meteorological factors on light and bait trap catches of Noctuid moths (Lepidoptera: Noctuidae). Environ Entomol 26:1283–1290
Yoshioka A, Kadoya T, Suda S, Washitani I (2010) Impacts of weeping lovegrass (Eragrostis curvula) invasion on native grasshopers: responses of habitat generalist and specialist species. Biol Invasions 12:531–539
Yoshioka A, Takada MB, Washitani I (2014) Landscape effects of a non-native grass facilitate source populations of a native generalist bug, Stenotus rubrovittatus, in a heterogeneous agricultural landscape. J Insect Sci 14:110
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank J. Skala for his help during light trapping in the field, J. Rom, Prague City Hall, and P. Heřman, Bohemian Karst PLA, for their help with getting permits for the fieldwork and P. Chajma for fruitful comments.
Funding
This study was funded by the Czech Science Foundation (Grant No. 18-26542S), the Internal Grant Agency of the Faculty of Environmental Sciences, Czech University of Life Sciences Prague (Grant No. 20164222), and the Charles University Research Centre (Grant No. 204069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by David Hawksworth.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10531_2018_1560_MOESM1_ESM.pdf
Online Resource 1 Figures showing the different habitat structure of the selected stands of (a) Robinia pseudoacacia and (b) native stands in this study. R. pseudoacacia stands were more open, with a discontinuous canopy, well-developed shrub layer and higher coverage of taller herbs. Native stands had a more closed canopy, poorly developed shrub layer and higher coverage of shorter herbs. Supplementary material 1 (PDF 11327 kb)
10531_2018_1560_MOESM2_ESM.xlsx
Online Resource 2 List of all moth species captured in 2014 in 20 plots in native forest stands and in 19 plots in stands dominated by the invasive Robinia pseudoacacia. Supplementary material 2 (XLSX 27 kb)
Rights and permissions
About this article
Cite this article
Kadlec, T., Štrobl, M., Hanzelka, J. et al. Differences in the community composition of nocturnal Lepidoptera between native and invaded forests are linked to the habitat structure. Biodivers Conserv 27, 2661–2680 (2018). https://doi.org/10.1007/s10531-018-1560-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1560-8