Abstract
The simplification of native habitats leads to biodiversity decline in tropical terrestrial ecosystems. We evaluated how conversion of three types of native Cerrado vegetation (open grassland, typical savanna, and woodland savanna) to two human-managed land uses (Eucalyptus plantations and pastures) affects ant richness and composition in arboreal, epigaeic, and hypogaeic ant communities. We also sampled vegetation and soil characteristics to determine which specific features could be driving differences in ant communities with land use conversion. In general, biodiversity was negatively affected by conversion to Eucalyptus plantations and pastures regardless of vegetation type. But these impacts do not act in the same way in each ant strata or vegetation type. Grass and herbaceous cover was the most important environmental variable correlated with diversity in open grassland and plant richness and litter diversity were the most important environmental variables for ant species in typical and woodland savannas. Our results indicate that expanding Eucalyptus plantations may have stronger negative impacts from conversion of open vegetation types while pasture implementation may have stronger negative effects if implemented in closed vegetation types. Thus, we show the need of protection of the diversity of all native vegetation found in the Brazilian Cerrado (from open to forested habitats).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern landscapes are fragmented and include both patches of native vegetation and several other human-managed land use types (Kissling et al. 2012). These human-managed habitats are usually less diverse in species richness and differ in species composition and ecosystem functions (Barlow et al. 2007; Gardner et al. 2009). Thus, most native fauna and flora are retained in small patches of native vegetation (Turner and Corlett 1996). Replacement of native vegetation with pastures and plantations is one of the greatest threats to biodiversity (Newbold et al. 2015). Land use types like pastures and monocultures have the strongest impacts on biodiversity, dramatically changing diversity in comparison to primary forests (McGill 2015; Newbold et al. 2015). In general, anthropogenic impacts have reduced species richness by more than 10% and future species losses are already predicted (Newbold et al. 2015). However, despite strong negative effects of fragmentation and habitat transformation, some native environments are more or less resilient than others with different biodiversity impacts (Holling 1973; Marimon et al. 2014).
The Brazilian savanna, called Cerrado, is one of the world’s most diverse environments (Furley 1999; Klink and Machado 2005), and is the second largest biome in Brazil, covering 21% of the territory (Bridgewater et al. 2004), but the Cerrado is severely threatened by land use change (Furley 1999; Brannstrom et al. 2008; Espírito-Santo et al. 2016). The Cerrado is considered a Biodiversity Hotspot, supports high species richness and thousands of endemic species (Myers et al. 2000). Part of this huge biodiversity can be associated with the diversity of native vegetation types (e.g. grasslands, shrublands, typical savannas, and woodland savannas) that differ in grass cover, percentage of canopy cover, and dominant plant species as well as fire dynamics and water availability (Oliveira and Marquis 2002). Native vegetation types also differ in terms of soil characteristics; grasslands have shallower and rockier soils than do woodland savannas (Oliveira and Marquis 2002). Even though the Cerrado supports high levels of biodiversity, only 20% of native Cerrado vegetation is protected under Brazilian law. Thus, threats to this biome are growing, especially following changes to the Brazilian Forest Code enacted in 2012 that may allow increases in deforestation (Sparovek et al. 2012; Loyola 2014; Alarcon et al. 2015). The conversion of Cerrado into pastures and croplands is the most common human activity in the biome but some native habitats are also negatively impacted by mining and deforestation (Brannstrom et al. 2008).
Two of the major land use types that have replaced native vegetation in the Cerrado are pastures and Eucalyptus plantations. In the last 50 years, exotic grasses (e.g. African grasses) have been introduced in the Cerrado in attempts to intensify cattle production with intensive pasture management (Martha-Jr and Vilela 2002; Sano et al. 2010). In addition, conversion of Cerrado into Eucalyptus plantations to produce paper, charcoal, and wood for furniture has been intensified (Eldridge et al. 1993; Zinn et al. 2002). Typically, both pastures and Eucalyptus plantations support less biodiversity than native areas, however, the degree to which ecological interactions are affected may depend on the landscape in which they are implemented (Marinho et al. 2002; Braga et al. 2010; Neves et al. 2013; Schmidt et al. 2013; Beiroz et al. 2014). The conversion of Cerrado habitats to pastures and Eucalyptus plantations can be harmful in many ways. The introduction of African grasses into open grasslands may affect species communities due to management, such as plowing, fertilizers and addition of limestone (Martha-Jr and Vilela 2002) and to increasing soil compaction due to cattle trampling. On the other hand, typical and woodland savannas converted in pastures are environments that suffer more with simplification due to total or large vegetation suppression that hampers several animal-plant associations (Leite et al. 2016). Afforestation, such as that caused by Eucalyptus plantations in the Cerrado, can completely alter soil nutrient cycling in formerly grass-dominated environments. This phenomenon may also limit productivity of grass-dominated environments while reducing habitats for animals adapted to open environments (Veldman et al. 2015). So, conversion of native habitats in more dissimilar environments, in terms of vegetation and soil, can cause higher impacts in animal communities.
Some species or groups of organisms can be used to help us evaluate and compare these costs of habitat transformation (McGeoch 1998). Ants (Formicidae) are one of the most common insects in tropical systems (Hölldobler and Wilson 1990). They are associated with plants, mainly because their interactions with extrafloral nectaries (present in approximately 25% of Cerrado plant species) and plant-feeding trophobiont insects (Oliveira and Oliveira-Filho 1991). This close relationship with plants is one of the reasons that environmental complexity (e.g. richness and architecture of plant species) strongly correlates with ant abundance and richness at both local and landscape scales (Ribas et al. 2003; Schoereder et al. 2010; Costa et al. 2011; Pacheco and Vasconcelos 2012a). Ants have been used as indicators of environmental changes such as mining activities, deforestation or afforestation, urbanization, fire, and conversion of natural habitats to cropland and pastures (Andersen et al. 2002; Philpott et al. 2010; Ribas et al. 2012). Ant colonies are, in general, sessile and ants display certain characteristics that make them ideal indicators of habitat disturbance such as high diversity, known taxonomy, occupying different strata, easy and cheap to sample, and sensitivity to changes in habitat (Majer 1983; Philpott et al. 2010; Ribas et al. 2012).
In the Cerrado, ant studies have helped to evaluate the effects of fire (Maravalhas and Vasconcelos 2014; Vasconcelos et al. 2016), mining (Rabello et al. 2015), habitat edges (Brandão et al. 2011), agriculture (Frizzo and Vasconcelos 2013), and the importance of native vegetation remnants (Pacheco et al. 2013) on biodiversity and ecosystem functioning. However, there are two shortcomings in our knowledge. First, we do not know how transformation of native vegetation to Eucalyptus plantations and pastures affect ants or what are the specific vegetation or soil characteristics that are driving changes. Second, because research on ants has largely focused on arboreal and epigaeic strata (Schmidt and Solar 2010; Pacheco and Vasconcelos 2012b), we know little about how land use change affects ants from different foraging strata (mainly hypogaeic). Ants that forage in different strata may respond in different ways to changes in environmental complexity (e.g. vegetation structure) and soil compaction (Neves et al. 2013; Schmidt et al. 2013, 2016).
In this study, we aimed to understand the structure (richness and composition) of ant communities in native vegetation types and human-managed land use types in the Cerrado. Our study specifically investigated the response of ant communities (arboreal, epigaeic, and hypogaeic) of native vegetation (open grassland, typical savanna, and woodland savanna) to conversion into two human-managed land use types (Eucalyptus plantation and exotic pasture). We asked the following research questions: i) Is ant species richness higher in native vegetation types than in human-managed land use types? ii) Does ant species composition differ between native vegetation types and human-managed land use types? and iii) Do changes in vegetation complexity and soil compaction correlate with any observed changes in ant species richness and composition? We want to know, in each case, the effects of conversion of the three vegetation types in Eucalyptus plantations and pastures on diversity. In general, we expected that the conversion of open grasslands into pastures as well as the conversion of woodland savannas into Eucalyptus plantations will be less damaging to ant communities because of similarities among native vegetation and human-managed land use types. Thus, typical savanna would present intermediate intensities of changes in ant communities. Specifically, we predicted that arboreal and epigaeic ants would suffer more from tree suppression in pasture converted sites in typical and woodland savannas due to the absence of or large decrease in vegetation complexity (e.g. lower plant richness and litter diversity). Further, we predicted that hypogaeic ant communities from open grasslands and typical savanna, on the other hand, would likely undergo greater changes in richness and composition patterns due to changes in compaction caused by conversion into Eucalyptus plantations that increase soil depth.
Materials and methods
Study region
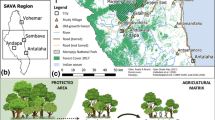
Our study was performed in Minas Gerais state, southeastern Brazil. Minas Gerais state is an important milk-producing region. However, in the last two decades, exotic Eucalyptus spp. plantations have expanded within the Cerrado biome, used mainly for the fence posts, paper, cellulose, wood, and charcoal production (AMS 2013), but tree monocultures (including Pinus spp. and other tree species) still cover a small part of the land in this state (Scolforo and Carvalho 2006; Rezende et al. 2013; Fernandes et al. 2016). Dairy farming is the main economic activity in many small cities, including Itutinga, Itumirim and Boa Esperança, where we conducted the fieldwork and now Eucalyptus plantations rises as alternative culture for many farmers. Our sampling sites are based on the Rio Grande basin which has a total area of 86,500 km2. This region is covered by Cerrado native vegetation (3.67% is open grasslands, 0.06% is typical savanna, and 1% is woodland savanna), more than 88% of total area is converted in pastures, monocultures, exposed soil, urban areas or other land use types and reforested areas, including Eucalyptus plantations that cover 0.30% of the total area, and the last part (~6–7%) is covered by other types of Cerrado and Atlantic Rainforest native vegetations (Scolforo and Carvalho 2006) (Fig. 1). Due to the characteristics of the distribution of the Cerrado vegetation (Oliveira and Marquis 2002) is not possible to find nearby areas that presented these three vegetation types to test the effects of conversion of native vegetation in land use systems simultaneously in Rio Grande basin.
a Map of the study landscapes in Itutinga, Itumirim, and Boa Esperança, southern Minas Gerais, Brasil and the experimental design used to sampling ants and environmental variables (above). b In each point in the map we installed a 200 m transect with ten sampling points each separated by 20 m. In each sampling point we installed pitfall traps (A Arboreal, E Epigaeic, and H Hypogaeic) to collect ants and measured environmental variables in a 6 × 6 m quadrant (below)
Study sites and experimental design
We conducted the fieldwork from January to March 2014. The study region is characterized by a dry winter (April to September) and a wet summer (October to March). The sampling sites were between 780 and 1045 m above sea level, and rainfall averages 1500 mm per year. In this region, we selected one area characterized by open grassland (near Itutinga), one area characterized by typical savanna (near Itumirim) and one area characterized by woodland savanna (near Boa Esperança) (Fig. 1a). All three areas have experienced land use conversion to both pasture and Eucalyptus plantations. In the Itutinga area, we sampled five open grassland sites, five Eucalyptus sites, and five pasture sites. In the Itumirim area, we sampled five typical savanna sites, three Eucalyptus sites, and five pasture sites. In the Boa Esperança area, we sampled five woodland savanna sites, four Eucalyptus sites, and five pasture sites. Thus, overall, we sampled a total of 42 sites, including five open grassland, five typical savanna, five woodland savanna, 12 Eucalyptus, and 15 pasture sites. All sampling sites were located in private farms containing pastures or Eucalyptus plantations as well as native vegetation remnants. Native remnants were not free of cattle grazing, fire (natural and managed), or vegetation extractivism (legal and illegal). Plantations varied from four to eight years in age, and rarely had understory vegetation (Appendix S1 in Supporting Information). Sites were separated by at least 200 m to ensure independence of the ant colonies sampled in each site. We established the minimum distance among sampling points and sites considering the foraging distance of ant species (Bernstein 1975; Kaspari 1996; Cuissi et al. 2015). In each site, we installed a 200 m transect with 10 sampling points each separated by 20 m (420 sampling points in total). In each sampling point we installed unbaited pitfall traps to collect ants and measured environmental variables in a 6 × 6 m quadrant (Fig. 1b).
Ant sampling
We sampled all ants with pitfall traps and groups of ants (e.g. arboreal, epigaeic, hypogaeic) were defined by the pitfall strata in which they were trapped. We considered as arboreal ants those captured in pitfall traps placed in plants, epigaeic ants as those collected in pitfalls placed on the ground and in leaf litter, and hypogaeic ants as those collected in subterranean pitfall traps. We used unbaited pitfall traps that consisted of plastic containers (diameter = 11 cm; height = 12 cm) with a liquid solution 200 ml of water, glycerol and salt (5 and 0.9% of total volume). We protected arboreal and epigaeic pitfall traps from rain and sun using a plastic roof. All pitfalls remained in the field for 48 h. We installed the arboreal pitfall traps at 1.3 m above ground level in trees. The pitfall traps were tied to the trees as close as possible to the trunk (Ribas et al. 2003) and in plants with >5 cm circumference at the base. We did not install arboreal pitfall traps in sites without trees. Epigaeic pitfalls were buried flush at ground level (Bestelmeyer et al. 2000). The hypogaeic pitfalls were buried 20 cm under the ground surface and had four lateral holes to allow access by hypogaeic ants (see Schmidt and Solar 2010).
We sorted and identified ants to genus level according to Baccaro et al. (2015). Ant identification to the species level was carried out by T. S. R. da Silva and G. Camacho from Laboratório de Sistemática e Biologia de Formigas, Universidade Federal do Paraná (UFPR) where all voucher specimens were deposited. They used the following keys for identification: (DeAndrade and Baroni-Urbani 1999; Mayhé-Nunes and Brandão 2002, 2005; Wilson 2003; Lattke et al. 2007).
Environmental variable sampling
We also sampled vegetation and soil variables that represent environmental complexity in each sampling point (Ribas et al. 2003; Neves et al. 2013; Queiroz et al. 2013; Schmidt et al. 2016). At each sampling point, we measured canopy cover, percentage grass and herbaceous cover, litter weight and diversity, tree number, density, height and diameter (circumference at basis height at 30 cm above ground level >5 cm), and soil compaction. We measured canopy cover with digital images using a fish-eye lens attached to a camera positioned at 1.5 m above ground level, and analyzed images with Gap Light Analyser 2.0 software (Frazer et al. 1999). We estimated the percentage of grass and herbaceous cover within a 1 × 1 m quadrat placed on the ground. Within the same quadrat, we collected litter from the ground. We dried litter samples for 96 h at 60 °C and then weighed them with a precision balance. We assessed litter diversity (invD—Inverse Simpson) by counting the number of different leaves, branches and sticks (modified from Queiroz et al. 2013). We counted the density, and richness of woody plants (e.g. trees and shrubs), estimated plant height and measured the circumference at basis height (CBH) of those plants for diameter data. Finally, we measured soil compaction by dropping a pointed sharp metal knife from 1.5 m above ground and then measured the depth (cm) mark.
Data analyses
We generated extrapolation curves to compare ant species richness from each stratum (arboreal, epigaeic, hypogaeic) among different habitats (native vegetation, Eucalyptus, pasture) in each vegetation type (open grassland, typical savanna, woodland savanna) using ‘iNEXT’ package (Hsieh et al. 2016). We used extrapolation curves with confidence intervals of 95%, generated by permutations, to allow us to statistically compare richness where sampling intensity and capture rates differed.
We examined if ant species composition from three strata in three habitats change with the conversion of native vegetation to pastures and Eucalyptus plantations with multivariate analysis. We performed a non-metric multidimensional scaling (NMDS) to assess the species composition of ants among habitats, strata, and vegetation type, and then performed analysis of similarities (ANOSIM) to detect the differences of composition among areas. We used ant frequency data from each site and Bray-Curtis index. We did not test differences in arboreal ant composition between native vegetation types and pastures due to low captures of arboreal ant species within pastures.
To test for correlations between ant species richness (response variable) and environmental variables (explanatory variable) we used generalized linear models (GLMs). We used generalized linear models with a Poisson distribution, adjusted to Quasipoisson when necessary (Crawley 2013). We selected the variables according to strata, as described above, and simplified the complete model removing the non-significant explanatory variables. For each ant group, we selected four environmental variables most relevant to that group. For arboreal ants we included plant richness, plant density, circumference at base height, and canopy cover. For epigaeic ants we used litter diversity, litter dry weight, grass and herbaceous cover, and canopy cover. For hypogaeic ants we used soil compaction, litter dry weight, grass and herbaceous cover and canopy cover. Finally, we submitted our models to residual analysis to evaluate the adequacy of the error distribution (Crawley 2013).
To examine which environmental variables influenced ant species composition we performed a multivariate regression procedure with the same variables mentioned above. The models showed which environmental variables were correlated with changes in species composition. The models were fit against a redundancy analysis (RDA) (Legendre and Legendre 2012), a constrained ordination, and we used ant frequency data from each transect and Bray-Curtis index with the ‘vegan’ package (Oksanen et al. 2015).
All data analyses were carried out with R software 3.2.2 (R Development Core Team 2015). For all data analyses, we constrained comparisons among the native vegetation type and the two human-managed land uses (open grassland, typical savanna, woodland savanna, and respectives Eucalyptus plantations and pastures) to sites located in the same study area as depicted in Fig. 1a. We made analyses for each stratum (9 comparisons for analysis). We did not use the above-mentioned analyzes to presume cause-effect relationships.
Results
We collected a total of 217 species (82 identified at species level and 135 morpho-species), distributed in 46 genera, and seven subfamilies (Appendix S2 in Supporting Information). The richest subfamily was Myrmicinae, with 122 species and 24 genera. The richest genera were Pheidole (38), Camponotus (27) and Solenopsis (14). Pheidole oxyops was the most common species in our study. We collected 76 species in open grassland, 105 species in typical savanna, and 102 species in woodland savanna native sites. We collected 48, 46, and 58 species in their respective converted Eucalyptus plantations, and 54, 56, and 62 in their converted pastures. Considering each vegetation type and strata, all native vegetation areas presented higher species richness and higher mean number of species (Table 1).
Species richness patterns
In most of our species extrapolation curves, with confidence intervals of 95%, we observed native vegetation richer than Eucalyptus plantations and pastures (Fig. 2). In the open grassland area, arboreal ant species richness was similar between open grassland and pastures, but was lower in Eucalyptus plantations (Fig. 2a). For epigaeic and hypogaeic ants, open grassland showed higher species richness than Eucalyptus plantations but did not differ between open grassland and pasture (Fig. 2b, c). In the typical savanna area, arboreal ant species richness was higher in typical savanna than in Eucalyptus plantation (Fig. 2d), but pastures were intermediate between these areas. Epigaeic ant species richness was higher in typical savanna than in Eucalyptus plantations or pastures, but we did not find differences between the two human-managed land uses (Fig. 2e). Hypogaeic ant species richness was similar across all habitats (Fig. 2f). Finally, in the woodland savanna area, arboreal and epigaeic ant species richness was higher in woodland savanna than in Eucalyptus plantations and pastures (Fig. 2g, h), but hypogaeic ant richness did not differ between woodland savanna and the two human-managed land uses (Fig. 2i).
Species richness curves comparing ant richness across all land use systems and strata. Letters represent each comparison: arboreal (a), epigaeic (b), and hypogaeic (c) in open grasslands (OGR), (d, e, f) in typical savanna (TSA), and (g, h, i) in woodland savanna (WSA). Different symbols illustrate native vegetation (circles), Eucalyptus plantation (squares), pasture (diamonds). Shaded colors represent the confidence interval (95%). (Color figure online)
Species composition patterns
We found several differences in species composition between habitat types for all ant groups. In the open grassland area, arboreal ant species composition did not differ by habitat type (p = 0.105, R = 0.105), but epigaeic ant composition was different in all three categories compared (p < 0.001, R = 0.796). Further, hypogaeic ant composition was similar only between open grassland and Eucalyptus plantations (p = 0.002, R = 0.435). In the typical savanna area, arboreal, epigaeic, and hypogaeic ant species composition all differed among typical savanna, Eucalyptus plantations, and pastures (arboreal: p = 0.001, R = 0.522; epigaeic: p = 0.001, R = 0.486; hypogaeic: p = 0.001, R = 0.531). Finally, in the woodland savanna area, arboreal ant species composition differed between woodland savanna and Eucalyptus plantations (p = 0.007, R = 0.528), epigaeic ant species composition differed among woodland savanna, Eucalyptus plantations, and pastures (p < 0.001, R = 0.876), and hypogaeic ant composition differed between woodland savanna and human-managed land uses (p = 0.008, R = 0.575). More details and results of pairwise comparisons in Appendix S3 and S4 in Supporting Information.
Ant richness and environmental variables
We found different patterns in the study region characterized by open grasslands compared with those characterized by typical or woodland savanna (Table 2, Appendix S5 in Supporting Information). In the study area characterized by open grassland, differences in arboreal ant species richness did not correlate with differences in plant richness, plant density, circumference at base height, and canopy cover, but epigaeic ant richness was positively correlated with grass and herbaceous cover. In the study area characterized by typical savanna and the study area characterized by woodland savanna, arboreal and epigaeic species richness were positively correlated with plant richness and litter diversity, respectively. We did not find any correlation between hypogaeic ant species richness and the environmental variables in our models for any of the three study areas.
Ant composition and environmental variables
We also found differences in species composition as environmental variables changed (Table 2; Fig. 3). In the study area characterized by open grassland, arboreal ant composition did not correlates with environmental variables (Fig. 3a), but changes in epigaeic ant composition were correlated with all environmental variables (litter diversity and weight, grass and herbaceous cover and canopy cover) (Fig. 3b), and changes in hypogaeic ant composition were correlated with canopy cover (Fig. 3c). In the study region characterized by typical savanna changes in arboreal ant composition were correlated with plant richness and basal plant circumference (Fig. 3d); changes in arboreal ant composition were also correlated with plant richness in woodland savanna areas (Fig. 3g). In both typical and woodland savanna areas, changes in epigaeic ant composition were correlated with litter diversity and litter dry weight (Fig. 3e, h) and changes in hypogaic ant composition were correlated with litter dry weight and grass and herbaceous cover (Fig. 3f, i).
Redundancy analysis of ant composition at three land use systems [native vegetation (NAT), Eucalyptus plantation (EUC), and pasture (PAS)] and eight environmental variables [plant richness (PRI), plant diversity (PDI), circumference at basis height (CBH), canopy cover (CCO), litter diversity (LDI), liter dry weight (LDW), grass and herbaceous cover (GCO), and soil compaction (SCO)]. Letters represent each comparison: arboreal (a), epigaeic (b), and hypogaeic (c) in open grasslands (OGR), (d, e, f) in typical savanna (TSA), and (g, h, i) in woodland savanna (WSA). Different symbols illustrate native vegetation (circles), Eucalyptus plantation (squares), pasture (diamonds). (Color figure online)
Discussion
We found similar patterns of species loss between typical and woodland savannas that differed from patterns in open grasslands. The conversion of Cerrado to Eucalyptus plantations and pastures leads to communities with fewer species and distinct ant species composition. But these impacts do not act in the same way in each ant strata or vegetation type. Grass and herbaceous cover was the most important environmental variable correlated with diversity in open grassland and plant richness and litter diversity were the most important environmental variables for ant species in typical and woodland savannas. Thus, both ant strata and the original vegetation type may have important implications for understanding how land conversion will affect ant communities. This is the first study that evaluates and shows differences in the responses of ant biodiversity in the Cerrado after conversion into Eucalyptus plantations and pastures in distinct strata and vegetation types. With these results, we can explore the links between land-use and biodiversity, and provide useful information for supporting biological conservation in the Cerrado (Mattison and Norris 2005).
In this study, we collected dozens of common ants in Cerrado biome. Pheidole oxyops dominates in the samples and is widespread through Cerrado landscapes (Wilson 2003). We also found the presence of species belonging to common genera in arboreal and epigaeic strata (e.g. Cephalotes, Pseudomyrmex, and Ectatomma) (Brandão et al. 2011; Schoereder et al. 2010). In the study region characterized by open grasslands, genera such as Tapinoma and Dorymyrmex were often sampled and these are ants typically collected in open Cerrado areas (Pacheco and Vasconcelos 2012a). Azteca, Crematogaster, Nesomyrmex and Hypoponera were more common when tree cover in savannas increased (Pacheco and Vasconcelos 2012a). Cephalotes atratus was found only in the typical savanna study area while Cephalotes pusillus was collected in the both the typical and woodland savanna areas which was expected given that these ants associate with native vegetation and related to Cerrado flora (Ribas et al. 2003; Pacheco and Vasconcelos 2012a). Ectatomma, especially E. edentatum, is an indicator of Cerrado habitats (Pacheco et al. 2013) and was common in all native habitats sampled in our study. Interestingly, we found Ochetomyrmex, maily, in native areas, Nomamyrmex exclusively in the native open grassland area, and many Trachymyrmex exclusively in the typical savanna area - both Ochetomyrmex and Nomamyrmex are uncommon genera in Minas Gerais (Janicki et al. 2016). The occurrence of Cyatta abscondita (Sosa-Calvo et al. 2013), an endemic species from Brazil, in woodland savanna areas reinforces that there are many rare species in these increasingly fragmented habitats.
Structure of ant communities
Conversion to human-managed habitats affected ant richness and composition in most of our comparisons. Conversion of native vegetation into simple human-managed habitats can affect species communities (Philpott et al. 2008) and Cerrado native vegetation supports a higher number of ant species (both above and below ground) than human-managed areas (Pacheco et al. 2013). In this study, we confirm the findings of previous studies showing losses of ant richness and differences in species composition after Cerrado simplification to pastures, Eucalyptus plantations, and other disturbed habitats (Almeida et al. 2011; Gries et al. 2012; Frizzo and Vasconcelos 2013; Pacheco et al. 2013; Rabello et al. 2015). In addition, we show that even though Eucalyptus plantations increase tree abundance, these plantations did not support arboreal ant diversity likely because Eucalyptus trees do not provide necessary ant resources that are provided by native Cerrado plants (Oliveira and Oliveira-Filho 1991).
In the study region characterized by open grassland, the impacts of pasture conversion on ant richness were smaller than those caused by Eucalyptus plantations. It is possible that the structural similarity in pastures and native vegetation can present similarities in ant species requirements (Audino et al. 2014; Solar et al. 2015; Queiroz and Ribas 2016). However, land use change affects ant species composition in epigaeic and hypogaeic strata (Pacheco and Vasconcelos 2012b; Schmidt et al. 2013). We observe that hypogaeic ant species composition was similar in Eucalyptus plantations and native open grasslands, therefore we suppose that soil structure found in pastures can be more affected by the soil management (e.g. soil tillage) (DeBruyn 1999).
The study regions characterized by typical and woodland savannas shared many similarities in terms of shifts in richness and composition resulting from land use change. Shifts to both pastures and Eucalyptus plantations had extreme impact on typical and woodland savanna communities of all strata as has been demonstrated previously (Pacheco et al. 2013). In this sense, we highlight two inferences: first, the exchange of native trees for Eucalyptus does not seem to effective in maintaining the native biodiversity of ants. Second, and perhaps most importantly, the conversion of typical and woodland savannas into pastures leads to a true mass extinction of arboreal ant fauna—one of the richest in tropical biomes (Oliveira and Freitas 2004; Schoereder et al. 2010). In addition, we saw a large decrease in hypogaeic ant richness in pastures likely due to environmental shifts resulting from beef production—a practice that is most common in the typical savanna area (pers. obs.). Cattle grazing reduces ant species richness (Boulton et al. 2005), and we supose that the degree to which species are lost may depend on the cattle handling and the management intensity of the cattle farming (Rogers et al. 1972; Crist and Wiens 1996; Jerrentrup et al. 2014). Even though these observed shifts in ants may be due to current land practices, we cannot discard historic land use, as not all native vegetation was directly converted to Eucalyptus or pastures. In addition, factors such as distance to native habitats and soil texture may also be important factors driving ant diversity and community composition in these areas.
Environmental variables and ant communities
There are large differences in ant richness and composition between native and human made habitats and most of the environmental factors are also likely to differ massively between them. Open grassland, for example, a native habitat with naturally rare shrubs and trees, did not show a distinct arboreal ant community and patterns for arboreal ants. In the other hand, the decrease in epigaeic ant richness is in the open grassland area was correlated with decreases in grass and herbaceous cover. Thus, conversion of open grasslands into pastures may be slightly less harmful to ants than the conversion into Eucalyptus plantations due to the similarity in environmental structure (Frizzo and Vasconcelos 2013). Surprisingly, we found similarity between species from native open grasslands and Eucalyptus plantations in hypogaeic stratum, correlated with the canopy cover. We consider the soil exposure as a possible explanation. Soil exposure, which is higher in pastures (less covered by plants, litter or thick tufts of grass), receive more solar radiation. Once soil temperature and moisture affect ants (Rivas-Arancibia et al. 2014) hypogaeic ants can indirectly suffer without plant cover and try to find wet soils to establish, and thereby likely avoid pastures.
Higher plant richness is an important factor to arboreal ant diversity in the study regions characterized by typical and woodland savannas and even ecological interactions of ants (Ribas et al. 2003; Pacheco et al. 2009; Lange and Del-Claro 2014). In the woodland savanna region, we found higher similarity between the arboreal ant community of native vegetation and Eucalyptus plantations. Epigaeic communities were highly dissimilar between native vegetation and pastures that were correlated with differences in litter diversity. We presume that plant richness and litter diversity regulate ant species richness and composition in typical and woodland savannas. In other studies, litter presence and diversity is also an important food and nesting resource for ants (Campos et al. 2007; Paolucci et al. 2010; Queiroz et al. 2013). Moreover, in our study, the difference in hypogaeic ant species composition in typical savanna area was related to litter weight. The presence of higher amounts of litter in Eucalyptus plantations can increase moisture, which would hinder ants to colonize these soils (Kaspari and Weiser 2000). Grass and herbaceous cover was the most important agent affecting hypogaeic ant species composition in the study region characterized by woodland savanna. In this case, only native vegetation areas have native herbaceous cover, and many ant species do associate with particular species of native herbaceous plants (Christianini et al. 2012).
Conclusions
We found different intensity of impacts associated with the conversion of Cerrado to Eucalyptus plantations and pastures in the different vegetation types and the strata where ants were sampled. In general, biodiversity was negatively affected by conversion to Eucalyptus plantations and pastures regardless of vegetation type. Thus, we show the need of protection of the diversity of all native vegetation found in the Brazilian Cerrado (from open to woodland savanna habitats). Yet we did find some differences in the magnitude of effects on ants in different human-managed land uses. Our results indicate that expanding Eucalyptus plantations may have stronger negative impacts from conversion of open vegetation types while pasture implementation may have stronger negative effects if implemented in closed vegetation types.
Pastures are common in the Cerrado, mainly in typical savanna areas, but Eucalyptus plantations are currently spreading into all Cerrado vegetation types. We conclude that impacts of Eucalyptus plantations and pastures are not uniform when implemented in areas with different natural histories. We recommend that legislators take certain steps to promote biodiversity conservation such as: (a) propose increases in the amount of protected areas within farms where the major crops affect biodiversity more intensely; (b) increase the amount of protected areas for rare vegetation types in the Cerrado such as woodland savanna; (c) imposing penalties (e.g. fines) for farmers and companies that do not comply with the new law or proposals or reward those farmers that follow the recommendations of good management. Without implementing some of these changes, the recent changes confirmed after the revision of the Brazilian Forest Code may result in rapid biodiversity loss with worrying consequences (Soares-Filho et al. 2014).
References
Alarcon GG, Ayanu Y, Fantini AC, Farley J, Schmitt AL, Koellner T (2015) Weakening the Brazilian legislation for forest conservation has severe impacts for ecosystem services in the Atlantic Southern Forest. Land Use Policy 47:1–11. doi:10.1016/j.landusepol.2015.03.011
Almeida S, Louzada J, Sperber C, Barlow J (2011) Subtle land-use change and tropical biodiversity: dung beetle communities in Cerrado grasslands and exotic pastures. Biotropica 43:704–710. doi:10.1111/j.1744-7429.2011.00751.x
AMS—Associação Mineira de Silvicultura (2013) Florestas Plantadas, p. 63. http://silviminas.com.br/
Andersen AN, Hoffmann BD, Müller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17. doi:10.1046/j.1365-2664.2002.00704.x
Audino LD, Louzada J, Comita L (2014) Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol Conserv 169:248–257. doi:10.1016/j.biocon.2013.11.023
Baccaro FB, Feitosa RM, Fernández F, Fernandes IO, Izzo TJ, Souza JLP, Solar R (2015) Guia para os gêneros de formigas do Brasil. Editora INPA
Barlow J, Mestre LAM, Gardner TA, Peres CA (2007) The value of primary, secondary and plantation forests for Amazonian birds. Biol Conserv 136:212–231. doi:10.1016/j.biocon.2006.11.021
Beiroz W, Audino L, Queiroz ACM, Rabello AM, Boratto I, Silva Z, Ribas CR (2014) Structure and composition of edaphic arthropod community and its use as bioindicators of environmental disturbance. AEER 12:481–491
Bernstein RA (1975) Foraging strategies of ants in response to variable food density. Ecology 56:213–219. doi:10.2307/1935314
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WL Jr, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) Ants. Standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp 122–144
Boulton AM, Davies KF, Ward PS (2005) Species richness, abundance, and composition of ground-dwelling ants in northern California grasslands: role of plants, soil, and grazing. Environ Entomol 34:96–104. doi:10.1603/0046-225X-34.1.96
Braga DL, Louzada JN, Zanetti R, Delabie JHC (2010) Rapid evaluation of ant diversity in land use systems in southern Bahia, Brazil. Neotrop Entomol 39:464–469. doi:10.1590/S1519-566X2010000400002
Brandão CRF, Silva RR, Feitosa RM (2011) Cerrado ground-dwelling ants (Hymenoptera: Formicidae) as indicators of edge effects. Zoologia (Curitiba) 28:379–387. doi:10.1590/S1984-46702011000300012
Brannstrom C, Jepson W, Filippi AM, Redo D, Xu Z, Ganesh S (2008) Land change in the Brazilian Savanna (Cerrado), 1986–2002: comparative analysis and implications for land-use policy. Land Use Policy 25:579–595. doi:10.1016/j.landusepol.2007.11.008
Bridgewater S, Ratter JA, Ribeiro JF (2004) Biogeographic patterns, β-diversity and dominance in the cerrado biome of Brazil. Biodivers Conserv 13:2295–2317. doi:10.1023/B:BIOC.0000047903.37608.4c
Campos RB, Schoereder JH, Sperber CF (2007) Small-scale patch dynamics after disturbance in litter ant communities. Basic Appl Ecol 8:36–43. doi:10.1016/j.baae.2006.03.010
Christianini AV, Mayhé-Nunes AJ, Oliveira PS (2012) Exploitation of fallen diaspores by ants: are there ant-plant partner choices? Biotropica 44:360–367. doi:10.1111/j.1744-7429.2011.00822.x
Costa FV, Neves FS, Silva JO, Fagundes M (2011) Relationship between plant development, tannin concentration and insects associated with Copaifera langsdorffii (Fabaceae). Arthropod Plant Interact 5:9–18. doi:10.1007/s11829-010-9111-6
Crawley MJ (2013) The R book. Wiley, Chichester
Crist TO, Wiens JA (1996) The distribution of ant colonies in a semiarid landscape: implications for community and ecosystem processes. Oikos 76:301–311. doi:10.2307/3546202
Cuissi RG, Lasmar CJ, Moretti TS, Schmidt FA, Fernandes WD, Falleiros AB, Schoereder JH, Ribas CR (2015) Ant community in natural fragments of the Brazilian wetland: species–area relation and isolation. J Insect Conserv 19:531–537. doi:10.1007/s10841-015-9774-5
DeAndrade ML, Baroni-Urbani C (1999) Diversity and adaptation in the ant genus Cephalotes, past and present. Stuttg Beitr Naturkd Serie B 271:1–889
DeBruyn LL (1999) Ants as bioindicators of soil function in rural environments. Agric Ecosyst Environ 74:425–441. doi:10.1016/S0167-8809(99)00047-X
Eldridge KG, Davidson J, Harwood CE, Wyk GV (1993) Eucalypt domestication and breeding. Clarendon Press, Oxford
Espírito-Santo MM, Leite ME, Silva JO, Barbosa RS, Rocha AM, Anaya FC, Dupin MGV (2016) Understanding patterns of land-cover change in the Brazilian Cerrado from 2000 to 2015. Philos Trans R Soc B 371:20150435. doi:10.1098/rstb.2015.0435
Fernandes GW, Coelho MS, Machado RB, Ferreira ME, de Souza Aguiar LM, Dirzo R, Scariot A, Lopes CR (2016) Afforestation of savannas: an impending ecological disaster. Nat Conserv. doi:10.1016/j.ncon.2016.08.002
Frazer GW, Canham CD, Lertzman KP (1999) Gap light analyzer (GLA): Imaging software to extract canopy structure and gap light transmission indices from truecolour fisheye photographs, user manual and program documentation. Simon Fraser University, Burnaby, British Colombia and The Institute of Ecosystem Studies, Millbrook. New York
Frizzo TLM, Vasconcelos HL (2013) The potential role of scattered trees for ant conservation in an agriculturally dominated Neotropical landscape. Biotropica 45:644–651. doi:10.1111/btp.12045
Furley PA (1999) The nature and diversity of neotropical savanna vegetation with particular reference to the Brazilian cerrados. Glob Ecol Biogeogr 8:223–241. doi:10.1046/j.1466-822X.1999.00142.x
Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA, Sodhi NS (2009) Prospects for tropical forest biodiversity in a human-modified world. Ecol Lett 12:561–582. doi:10.1111/j.1461-0248.2009.01294.x
Gries R, Louzada J, Almeida S, Macedo R, Barlow J (2012) Evaluating the impacts and conservation value of exotic and native tree afforestation in Cerrado grasslands using dung beetles. Insect Conserv Divers 5:175–185. doi:10.1111/j.1752-4598.2011.00145.x
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Holling CS (1973) Resilience and stability of ecological systems. Ann Rev Ecol Syst 4:1–23
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. doi:10.1111/2041-210X.12613
Janicki J, Narula N, Ziegler M, Guénard B, Economo EP (2016) Visualizing and interacting with large-volume biodiversity data using client–server web-mapping applications: the design and implementation of antmaps. org. Ecol Inform 32:185–193. doi:10.1016/j.ecoinf.2016.02.006
Jerrentrup JS, Wrage-Mönnig N, Röver KU, Isselstein J (2014) Grazing intensity affects insect diversity via sward structure and heterogeneity in a long-term experiment. J Appl Ecol 51:968–977. doi:10.1111/1365-2664.12244
Kaspari M (1996) Testing resource-based models of patchiness in four Neotropical litter ant assemblages. Oikos 76:443–454. doi:10.2307/3546338
Kaspari M, Weiser MD (2000) Ant activity along moisture gradients in a Neotropical forest. Biotropica 32:703–711. doi:10.1646/0006-3606(2000)032[0703:AAAMGI]2.0.CO;2
Kissling WD, Dormann CF, Groeneveld J, Hickler T, Kühn I, McInerny GJ, Montoya JM, Römermann R, Schiffers K, Schurr FM, Singer A, Svenning JC, Zimmermann NE, O’Hara RB (2012) Towards novel approaches to modelling biotic interactions in multispecies assemblages at large spatial extents. J Biogeogr 39:2163–2178. doi:10.1111/j.1365-2699.2011.02663.x
Klink CA, Machado RB (2005) Conservation of the Brazilian Cerrado. Conserv Biol 19:707–713. doi:10.1111/j.1523-1739.2005.00702.x
Lange D, Del-Claro K (2014) Ant-plant interaction in a tropical savanna: may the network structure vary over time and influence on the outcomes of associations? PLoS ONE 9:e105574. doi:10.1371/journal.pone.0105574
Lattke JE, Fernández F, Palacio EE (2007) Identification of the species of Gnamptogenys Roger in the Americas. Mem Am Entomol Inst 80:254–270
Legendre P, Legendre LF (2012) Numerical ecology. Elsevier, Amsterdam
Leite GL, Veloso RV, Zanuncio JC, Alonso J, Ferreira PS, Almeida CI, Fernandes GW, Serrão JE (2016) Diversity of Hemiptera (Arthropoda: Insecta) and their natural enemies on Caryocar brasiliense (Malpighiales: Caryocaraceae) trees in the Brazilian Cerrado. Fla Entomol 99:239–247. doi:10.1653/024.099.0213
Loyola R (2014) Brazil cannot risk its environmental leadership. Divers Distrib 20:1365–1367. doi:10.1111/ddi.12252
Majer JD (1983) Ants: bio-indicators of minesite rehabilitation, land-use, and land conservation. Environ Manage 7:375–383. doi:10.1007/BF01866920
Maravalhas J, Vasconcelos HL (2014) Revisiting the pyrodiversity–biodiversity hypothesis: long-term fire regimes and the structure of ant communities in a Neotropical savanna hotspot. J Appl Ecol 51:1661–1668. doi:10.1111/1365-2664.12338
Marimon BS, Marimon-Junior BH, Feldpausch TR, Oliveira-Santos C, Mews HA, Lopez-Gonzalez G, Lloydbh J, Franczakf DD, Oliveira EA, Maracahipes L, Miguel A, Lenza E, Phillips OL (2014) Disequilibrium and hyperdynamic tree turnover at the forest–cerrado transition zone in southern Amazonia. Plant Ecol Divers 7:281–292. doi:10.1080/17550874.2013.818072
Marinho CGS, Zanetti R, Delabie JHC, Schlindwein MN, Ramos LS (2002) Diversidade de formigas (Hymenoptera: Formicidae) da serapilheira em eucaliptais (Myrtaceae) e área de cerrado de Minas Gerais. Neotrop Entomol 31:187–195. doi:10.1590/S1519-566X2002000200004
Martha-Jr G, Vilela L (2002) Pastagens no Cerrado: baixa produtividade pelo uso limitado de fertilizantes. Embrapa Cerrados
Mattison EH, Norris K (2005) Bridging the gaps between agricultural policy, land-use and biodiversity. Trends Ecol Evol 20:610–616. doi:10.1016/j.tree.2005.08.011
Mayhé-Nunes AJ, Brandão CRF (2002) Revisionary studies on the attine ant genus Trachymyrmex Forel. Part 1: definition of the genus and the opulentus group (Hymenoptera: Formicidae). Sociobiology 40:667–698
Mayhé-Nunes AJ, Brandão CRF (2005) Revisionary studies on the attine ant genus Trachymyrmex Forel. Part 2: the Iheringi group (Hymenoptera: Formicidae). Sociobiology 45:271–305
McGeoch MA (1998) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev Camb Philos 73:181–201
McGill B (2015) Biodiversity: land use matters. Nature 520:38–39. doi:10.1038/520038a
Myers N, Mittermeier RA, Mittermeier CG, DaFonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. doi:10.1038/35002501
Neves FS, Queiroz-Dantas KS, DaRocha WD, Delabie JHC (2013) Ants of three adjacent habitats of a transition region between the Cerrado and Caatinga biomes: the effects of heterogeneity and variation in canopy cover. Neotrop Entomol 42:258–268. doi:10.1007/s13744-013-0123-7
Newbold T, Hudson LN, Hill SLL et al (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. doi:10.1038/nature14324
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) The vegan package. Community ecology package, version 2-3-1. http://vegan.r-forge.r-project.org
Oliveira PS, Freitas AV (2004) Ant–plant–herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 91:557–570. doi:10.1007/s00114-004-0585-x
Oliveira PS, Marquis RJ (2002) The cerrados of Brazil: Ecology and natural history of a neotropical savanna. Columbia University Press, New York
Oliveira PS, Oliveira-Filho AT (1991) Distribution of extrafloral nectaries in the woody flora of tropical communities in Western Brazil. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant–animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 163–175
Pacheco R, Vasconcelos HL (2012a) Habitat diversity enhances ant diversity in a naturally heterogeneous Brazilian landscape. Biodivers Conserv 21:797–809. doi:10.1007/s10531-011-0221-y
Pacheco R, Vasconcelos HL (2012b) Subterranean pitfall traps: is it worth including them in your ant sampling protocol? Psyche. doi:10.1155/2012/870794
Pacheco R, Silva RR, Morini MS, Brandão CR (2009) A comparison of the leaf-litter ant fauna in a secondary atlantic forest with an adjacent pine plantation in southeastern Brazil. Neotrop Entomol 38(1):55–65. doi:10.1590/S1519-566X2009000100005
Pacheco R, Vasconcelos HL, Groc S, Camacho GP, Frizzo TLM (2013) The importance of remnants of natural vegetation for maintaining ant diversity in Brazilian agricultural landscapes. Biodivers Conserv 22:983–999. doi:10.1007/s10531-013-0463-y
Paolucci LN, Solar RRC, Schoereder JH (2010) Litter and associated ant fauna recovery dynamics after a complete clearance. Sociobiology 55:133–144
Philpott SM, Arendt WJ, Armbrecht I, Bichier P, Diestch TV, Gordon C, Greenberg R, Perfecto I, Reynoso-Santos RO, Soto-Pinto LO, Tejeda-Cruz CE (2008) Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv Biol 22:1093–1105. doi:10.1111/j.1523-1739.2008.01029.x
Philpott SM, Perfecto I, Armbrecht I, Parr CL (2010) Ant diversity and function in disturbed and changing habitats. In: Lach L, Parr CL, Abott KL (eds) Ant ecology. Oxford University Press, New York, pp 137–157
Queiroz ACM, Ribas CR (2016) Canopy cover negatively affects arboreal ant species richness in a tropical open habitat. Braz J Biol. doi:10.1590/1519-6984.02015
Queiroz ACM, Ribas CR, França FM (2013) Microhabitat characteristics that regulate ant richness patterns: the importance of leaf litter for epigaeic ants. Sociobiology 60:367–373. doi:10.13102/sociobiology.v60i4.367-373
R Development Core Team (2015) R: a language and environment for statistica computing. R Foundation for Statistical Computing, Vienna http://www.rproject.org
Rabello AM, Queiroz ACM, Lasmar CJ, Cuissi RG, Canedo-Júnior EO, Schmidt FA, Ribas CR (2015) When is the best period to sample ants in tropical areas impacted by mining and in rehabilitation process? Insectes Soc 62:227–236. doi:10.1007/s00040-015-0398-2
Rezende JB, Pereira JR, Botelho DO (2013) Expansão da cultura do eucalipto nos municípios mineiros e gestão territorial. Cerne 19:1–7. doi:10.1590/S0104-77602013000100001
Ribas CR, Schoereder JH, Pic M, Soares SM (2003) Tree heterogeneity, resource availability, and larger scale processes regulating arboreal ant species richness. Austral Ecol 28:305–314. doi:10.1046/j.1442-9993.2003.01290.x
Ribas CR, Campos RB, Schmidt FA, Solar RR (2012) Ants as indicators in Brazil: a review with suggestions to improve the use of ants in environmental monitoring programs. Psyche. doi:10.1155/2012/636749
Rivas-Arancibia SP, Carrillo-Ruiz H, Bonilla-Arce A, Figueroa-Castro DM, Andrés-Hernández AR (2014) Effect of disturbance on the ant community in a semiarid region of central México. Appl Ecol Environ Res 12:703–716. doi:10.15666/aeer/1203_703716
Rogers LE, Lavigne RJ, Miller JL (1972) Bioenergetics of the western harvest ant in the shortgrass plains ecosystems. Environ Entomol 3:994–997. doi:10.1093/ee/1.6.763
Sano EE, Rosa R, Brito JLS, Ferreira LG (2010) Mapeamento do uso do solo e cobertura vegetal-bioma cerrado: ano base 2002. MMA, Ministério do Meio Ambiente, Brasília
Schmidt FA, Solar RRC (2010) Hypogaeic pitfall traps: methodological advances and remarks to improve the sampling of a hidden ant fauna. Insectes Soc 57:261–266. doi:10.1007/s00040-010-0078-1
Schmidt FA, Ribas CR, Schoereder JH (2013) How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol Indic 24:158–166. doi:10.1016/j.ecolind.2012.05.031
Schmidt FA, Schoereder JH, Caetano MD (2016) Ant assemblage and morphological traits differ in response to soil compaction. Insectes Soc. doi:10.1007/s00040-016-0532-9
Schoereder JH, Sobrinho TG, Madureira MS, Ribas CR, Oliveira PS (2010) The arboreal ant community visiting extrafloral nectaries in the Neotropical cerrado savanna. Terr Arthropod Rev 3:3–27. doi:10.1163/187498310X487785
Scolforo JR, Carvalho LMT (2006) Mapeamento e inventário da flora nativa e dos reflorestamentos de Minas Gerais. Universidade Federal de Lavras, Lavras
Soares-Filho B, RajãoR MacedoM, Carneiro A, Costa W, Coe M, Rodrigues H, Alencar A (2014) Cracking Brazil’s forest code. Science 344:363–364. doi:10.1126/science.1246663
Solar RRC, Barlow J, Ferreira J, Berenguer E, Lees AC, Thomson JR, Louzada J, Maués M, Moura NG, Oliveira VH, Chaul J (2015) How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecol Lett 18:1108–1118. doi:10.1111/ele.12494
Sosa-Calvo J, Schultz TR, Brandão CRF, Klingenberg C, Feitosa RM, Rabeling C, Bacci-Jr M, Lopes CT, Vasconcelos HL (2013) Cyatta abscondita: taxonomy, evolution, and natural history of a new fungus-farming ant genus from Brazil. PLoS ONE 8:e80498. doi:10.1371/journal.pone.0080498
Sparovek G, Berndes G, Barretto AG, Klug IL (2012) The revision of the Brazilian Forest Act: increased deforestation or a historic step towards balancing agricultural development and nature conservation? Environ Sci Policy 16:65–72. doi:10.1016/j.envsci.2011.10.008
Turner IM, Corlett TR (1996) The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol Evol 11:330–333. doi:10.1016/0169-5347(96)10046-X
Vasconcelos HL, Maravalhas JB, Cornelissen T (2016) Effects of fire disturbance on ant abundance and diversity: a global meta-analysis. Biodivers Conserv. doi:10.1007/s10531-016-1234-3
Veldman JW, Overbeck GE, Negreiros D, Mahy G, Le Stradic S, Fernandes GW, Durigan G, Buisson E, Putz FE, Bond WJ (2015) Where tree planting and forest expansion are bad for biodiversity and ecosystem services. BioScience. doi:10.1093/biosci/biv118
Wilson EO (2003) Pheidole in the new world. A dominant, hyperdiverse ant genus. Harvard University Press, Cambridge
Zinn YL, Resck DV, Silva JE (2002) Soil organic carbon as affected by afforestation with Eucalyptus and Pinus in the Cerrado region of Brazil. Forest Ecol Manag 166:285–294. doi:10.1016/S0378-1127(01)00682-X
Acknowledgements
Our thanks to D.J. Botelho, M.J. Costa, P.J. Costa, T. Costa, P.S. Paula, S. Pereira, F. Reis, L.F.B. Simões, and other farmers from Itutinga, Itumirim and Boa Esperança cities. We thank G. Alves, P. Borges, R. Carvalho, R.G. Cuissi, C.J. Lasmar, and M. Imata for helping us with fieldwork. We are thankful to T.S.R. Silva and G. Camacho for their help to confirm the ant identification, to H.L. Vasconcelos for his comments in the previous versions of this project, and L. Zanella and R. Solar for their help with figures and statistical analysis. During the study, A.C.M. Queiroz and A.M. Rabello received scholarships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Capes (PDSE processes #8794/2014-06 and #4934/2014-08, respectively), and C.R. Ribas received scholarship from Fundação de Amparo a Pesquisa de Minas Gerais—FAPEMIG (CRA PPM-00243/14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jens Wolfgang Dauber.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Queiroz, A.C.M., Rabello, A.M., Braga, D.L. et al. Cerrado vegetation types determine how land use impacts ant biodiversity. Biodivers Conserv 29, 2017–2034 (2020). https://doi.org/10.1007/s10531-017-1379-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1379-8