Abstract
Multiple-use protected areas, in which sustainable levels of extractive livelihood activities are permitted, play an increasingly important role in the global protected area estate, and are expected to rise in prevalence. However, we know little about their effectiveness at conserving biodiversity. We surveyed bird and reptile communities in three areas across a forest disturbance gradient resulting from charcoal production and shifting cultivation within a multiple-use protected area in Madagascar’s sub-arid spiny forest. We scored individual species using a Conservation Value Index (CVI; a simple metric based on rarity, threat and distinctiveness), and estimated the total conservation value of each treatment by calculating the sum of frequency-weighted CVI scores across all present species. Bird and reptile community responses to forest disturbance were idiosyncratic. Bird richness was greatest in the moderate-disturbance treatment, but the low-disturbance treatment had the superior conservation value due to higher frequencies of locally-endemic species. Reptile richness was the same in low- and moderate-disturbance treatments, but the conservation value of the latter was greater. The high-disturbance areas had lowest richness and conservation value for both groups. For birds, increasing disturbance levels were accompanied by community turnover from high-value to low-value species, a pattern highlighted by CVI that is masked by assessing species richness alone. Although some endemic species appear to be resilient to degradation, multiple-use protected areas in Madagascar may lose biodiversity since most endemic species are forest-dependent. Stricter protected area models may be more appropriate in areas where much of the high-value biodiversity is sensitive to habitat degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impacts of human activity now threaten most of the Earth’s species and ecosystems (Ehrlich and Pringle 2008) and have precipitated the planet’s sixth mass extinction (Barnosky et al. 2011). Our primary strategy to stem this biodiversity loss is the creation and management of protected areas, which cover over 15 % of the world’s land area and constitute the largest planned land use in history (Juffe-Bignoli et al. 2014). All protected areas are spaces “recognised, dedicated and managed… to achieve the long-term conservation of nature with associated ecosystem services and cultural values” (Dudley 2008), but they vary greatly in management objective and approach. These differences form the basis for the World Conservation Union’s (IUCN) protected area categorisation system (Dudley 2008; Dudley et al. 2010). For simplicity’s sake the categories are often divided into ‘strict’ protected areas (generally categories I–IV), which seek to isolate nature from human processes that threaten it, and ‘multiple-use’ sites, which promote conservation through the sustainable extractive use of natural resources (category VI) or traditional land uses that sustain biodiversity (category V).
Recent decades have seen the number of multiple-use protected areas grow significantly in many parts of the world (Juffe-Bignoli et al. 2014). Although some strict sites have been downgraded (Mascia et al. 2014), this has been driven primarily by the predominance of multiple-use categories amongst new protected areas (Zimmerer et al. 2004). The trend can largely be attributed to: (i) the lack of remaining ‘wilderness’ areas, with a low human footprint, suitable for the creation of strict categories (Leroux et al. 2010); and, (ii) a paradigm shift in conservation, reflecting concern for the effects of exclusionary approaches on human wellbeing (Adams and Hutton 2007; Miller 2014), and the suggestion that sustainable use may be a more effective long-term conservation strategy than strict protection (Rosser and Leader-Williams 2010). As a result, only 45 % of the world’s protected areas are assigned to categories I–IV (Jenkins and Joppa 2009), and category VI sites expanded from 14 to 32 % of the world’s protected area estate (by area) between 1990 and 2010 (Bertzky et al. 2012). This trend is expected to become even more pronounced in the future (McDonald and Boucher 2011).
Signatories to the Convention of Biological Diversity are expected to increase the coverage of terrestrial protected areas to 17 % of their national territory by 2020 and ensure that they are “effectively managed” (CBD 2010, Aichi Target 11), a target that will require the most rapid expansion of protected areas in history (Venter et al. 2014). Thus, if new protected areas are expected to largely comprise multiple-use categories, it is important to know whether they are likely to be successful at achieving their objective—the long-term conservation of nature—in the face of authorised human impacts (Dudley et al. 2014; Watson et al. 2016). This is particularly apposite given longstanding debates over the contribution of multiple-use protected areas to conservation goals (Locke and Dearden 2005; Gaston et al. 2008; Shafer 2015).
The effectiveness of protected areas depends on both their coverage (i.e. ensuring that maximum biodiversity is represented within them) and their success in buffering the biodiversity from the processes that threaten its viability (Gaston et al. 2008; Watson et al. 2014). However, research tends to concentrate on the former (e.g. Montesino Pouzols et al. 2014; Venter et al. 2014; Butchart et al. 2015; Polak et al. 2015; Visconti et al. 2015), with the result that we know little about the success of protected areas in maintaining their condition over time (Cabeza 2013; Geldmann et al. 2013; Beaudrot et al. 2016; Watson et al. 2016). This knowledge gap is particularly acute with regards to multiple-use categories. Global studies comparing across categories have found stricter protected areas to be more effective at slowing deforestation in some regions (Joppa and Pfaff 2011; Scharlemann et al. 2010), whereas multiple-use sites demonstrate greater success in other countries (Ferraro et al. 2013; Nelson and Chomitz 2011). However, the use of remote sensed data within such analyses only allows us to quantify vegetation cover, therefore providing little insight into the ecological integrity of remaining natural vegetation and faunal communities beneath the canopy (Peres et al. 2006; Beaudrot et al. 2016). Less conspicuous changes to forest structure and composition (i.e. forest degradation) can stem from activities such as non-industrial selective logging, fuelwood collection, livestock grazing and the harvesting of non-timber forest products (NTFPs). Typically, these are precisely the types of activity that may be sanctioned within category V and VI protected areas (Dudley 2008). Indeed, conservationists still have a very limited understanding of species and community responses to habitat change, and our knowledge is largely derived from a small number of sites (Barlow et al. 2007; Gardner et al. 2009c, 2010). Furthermore, few researchers have investigated the impacts of subsistence activities on biodiversity (Borghesio 2008; Brown et al. 2013).
Madagascar is an example of a biodiversity-rich tropical developing country that is expanding its protected area system through the creation of new multiple-use sites. The island is a global conservation priority, boasting an unparalleled combination of species diversity and endemism (Brooks et al. 2006), with the majority of its endemic biota being forest dependent (Goodman and Benstead 2005). However, less than 16 % of the country retained forest cover by 2000 (Harper et al. 2007; McConnell and Kull 2014). Since 2003, Madagascar has been in the process of tripling the coverage of its protected area system, from 1.7 to over 6 million ha, in response to lobbying from international conservation organisations and funders (Corson 2014). Known as the ‘Durban Vision’ after the location of the fifth World Parks Congress at which it was launched, this ambitious programme has necessitated modifications to the country’s conception of protected areas and their governance. Previously, all protected areas were governed by the State, managed by the para-statal Madagascar National Parks, and comprised only strict categories (I, II and IV; Randrianandianina et al. 2003). Most of the new protected areas established as part of the Durban Vision are co-managed by non-governmental organisations (NGOs) and local communities, and are proposed or designated as categories V and VI (AGRECO 2012; Gardner 2011; Virah-Sawmy et al. 2014), with zoned areas where subsistence and low-level commercial natural resource use activities are permitted (e.g. Gardner et al. 2008; Virah-Sawmy et al. 2014; WWF 2010).
The goals of the expanded Madagascar Protected Area System (SAPM) are to conserve the country’s unique biodiversity and its cultural heritage, as well as promoting the sustainable use of natural resources for poverty alleviation and development (Commission SAPM 2006). The simultaneous achievement of these goals is particularly complex because most forms of traditional land and resource use in Madagascar have negative impacts on biodiversity (Gardner 2009, 2011; Irwin et al. 2010). Planning the management of new multiple-use protected areas requires an understanding of species and community responses to habitat degradation arising from permitted resource use, yet our knowledge of the influence this has on biodiversity is patchy for the country as a whole, and particularly for the globally-important spiny forest ecoregion (Irwin et al. 2010). Moreover, existing studies in Madagascar tend to mirror patterns in global research (Burivalova et al. 2014) by summarising assemblage-level change via species richness (e.g. Randriamiharisoa et al. 2015; Scott et al. 2006). In other words, while studies may investigate the ecological or other attributes of species remaining in degraded habitats, their results are usually reported in terms of species richness, but this measure has been criticised because it can mask community turnover from specialists to generalists (Barlow et al. 2007; Gardner et al. 2010). Here we investigate bird and reptile community responses to habitat change in a new protected area in the spiny forest ecoregion to ascertain the impacts of permitted and illegal livelihood activities (charcoal production and shifting cultivation respectively) on the conservation value of the vertebrate fauna. To overcome the issues associated with species richness as a metric, we use a novel Conservation Value Index (CVI) to examine the influence of habitat degradation on the two taxonomic assemblages.
Methods
Study site
Madagascar’s spiny desert (or spiny forest), is a global priority ecoregion (Olson and Dinerstein 1998) and Endemic Bird Area (Stattersfield et al. 1998) with extremely high rates of local floral endemism (Phillipson 1996). Between 1990 and 2010 it suffered the fastest rates of deforestation of any ecoregion in the country (Harper et al. 2007; ONE et al. 2013) and, prior to 2003, it was the least represented ecoregion within the country’s protected area network (Fenn 2003).
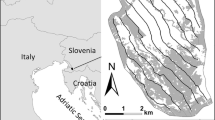
Ranobe PK32 is a new protected area that received temporary protected status within the Durban Vision framework in 2008, and is co-managed by local community associations, the regional Forest Service and the international NGO WWF (Virah-Sawmy et al. 2014). Lying north of the regional capital Toliara between the Fiherenana and Manombo rivers (Fig. 1), it is the richest landscape in the ecoregion in terms of its bird, reptile and lemur fauna (Gardner et al. 2009a, b, 2015a). However, the area is inhabited by approximately 90,000 people (WWF 2010), many of whom depend on natural resources from within and around the protected area for their subsistence and household income (Gardner and Davies 2014; Gardner et al. 2015b). Ranobe PK32 is thus proposed as a category VI protected area in which subsistence and low-level commercial livelihood activities (such as timber cutting, fuelwood collection and charcoal production, grazing and the harvesting of NTFPs) are permitted in sustainable use zones which cover 86.5 % of the protected area’s 148,554 ha (Virah-Sawmy et al. 2014; WWF 2010).
Map of: a Ranobe PK32 protected area (dotted line) showing location of five strict conservation zones (grey shading), wetlands and rivers (black shading/lines) and Ranobe village; and b location of three vegetation treatments used to survey bird and reptile communities across a gradient of degradation (forest cover, grey shading; wetlands, double line). Inset shows location of Ranobe PK32 within Madagascar (black square) and limits of spiny forest ecoregion following Goodman and Raherilalao (2013) (grey shading)
Charcoal is primarily produced in the western part of the protected area, due to the presence of the Route Nationale 9 (RN9) road that facilitates transportation. The industry is driven by the close proximity of Toliara, a city of approximately 20,0000 people in which 98 % of households use wood or charcoal for cooking; demand from the city tripled between 2000 and 2007, and is largely met by anarchic charcoal production along the RN9 (Gardner et al. 2015b; Partage 2008). Since the region lacks fuelwood plantations, charcoal is produced entirely from natural forests (Bertrand et al. 2010). Charcoal producers select only hardwood trees (Randriamalala et al. 2016), thus causing forest degradation rather than outright deforestation (Casse et al. 2004).
We conducted our study in the vicinity of Ranobe, a complex of three villages with a total population of approximately 2000 people (Gardner and Davies 2014), where the surrounding forests had been subjected to both charcoal production and shifting cultivation within recent years. We selected three areas within 3 km of the main village which, until recently, were part of a contiguous and relatively homogeneous forest block. Subsequently, the three areas have suffered varying levels of disturbance that are indicative of the habitat degradation gradient found across the whole landscape: (i) a forest area showing minimal impacts of human activity (low-disturbance, hereafter Low); (ii) a forest area subject to intensive charcoal production (moderate-disturbance, Mod); and, (iii) an area regenerating following shifting cultivation (high-disturbance, High). While Low and Mod retained a complex three-dimensional structure and can be termed forest, High was an open area dominated by shrubs, with only scattered trees (Fig. 1; Table 1). As there were no areas of forest near Ranobe that had not been subject to any human disturbance, it was not possible to include a control site representing intact habitat. Birds and reptiles were surveyed between January and March 2010 in the rainy season, when both groups are most active (Glaw and Vences 2007; Safford and Hawkins 2013).
Bird survey protocol
We established 48 census stations within each area and used the point count method (Bibby et al. 1998) to estimate bird relative abundance. Access to the forest interior was hindered by the impenetrable nature of the vegetation at Low and Mod, so census stations were placed on a stratified random grid along existing ox-cart tracks. We positioned all stations at a perpendicular distance of 75 m from a track (following Jones et al. 1995) to minimise the influence of edge effects, and at least 150 m apart to minimise the risk of double counting.
We surveyed each census station for 15 min (following a settling period of 4 min after arrival), during which we recorded all visual and auditory contacts within 50 m of the census station. To reduce time-of-day and weather-related effects, surveys were limited to between 06.00 and 08.00 and were not conducted on rainy or windy days. The majority of bird contacts in spiny forest (>85 % at Low and Mod) were auditory due to the dense vegetation, thus making it difficult to generate reliable distance estimates for bird contacts and, as such, we did not employ distance sampling methods. However, the non-visual nature of most contacts reduces the likelihood of a detectability bias arising from surveying in forests of varying degradation levels (Bibby and Buckland 1987). The auditory nature of most contacts also meant that we could not accurately count the number of individuals for social species, and thus we recorded the presence of groups not individuals. We did not include contacts with juvenile birds in our data analysis to reduce seasonality effects. Point count observations yielded both relative frequency (defined as the proportion of counts in which a given species was recorded) and relative abundance (mean number of contacts of a given species per count) data.
Reptile survey protocol
We calculated the relative abundance of reptiles based on capture in pitfall traps and area constrained refuge searches (transects), because observation and capture-based methods permit the sampling of different components of the reptile fauna (Raselimanana 2008). For pitfall trapping we followed a standard protocol widely used in Madagascar (D’Cruze et al. 2007; Raselimanana 2008). The traps consisted of plastic buckets (270 mm deep, 290 mm internal diameter at top, 220 mm internal diameter at base) placed 10 m apart and buried in the ground with the rim level with the surface. Drainage holes were drilled in the bottom of each bucket and the handles were removed. Buckets were connected by a drift fence 500 mm high, passing directly over the centre of each bucket, constructed from a sheet of plastic supported by wooden stakes. The lower 50 mm of the fence was buried in the soil and covered with leaf litter to prevent animals passing underneath. Within each treatment we established three trap lines (each of 10 or 11 buckets), placed randomly, but at least 150 m apart. Traps were constructed in the morning and left open for 13 nights, equating to 403 trap nights in total per area, and were checked at 07.00 and 16.00 each day. All captured animals were marked on the hind leg or ventral surface with nail polish, and released at the site of capture. Recaptured individuals were excluded from the data analysis.
We also established 38 transects along which we conducted active refuge searches. Each transect consisted of a 50 m rope erected adjacent to forest tracks based on a stratified random grid. Each transect was at least 150 m apart, ran perpendicular to a track and started 10 m into the forest to reduce the influence of edge effects. We established each transect 24 h prior to surveying to minimise disturbance effects. During surveys, two observers moved slowly along each transect and searched for reptiles within 2 m of the central line, scanning the trunks and branches of trees, searching within tree holes, under bark, in the leaf litter and under/within dead branches. All reptiles initially observed within 2 m of the central line were recorded. Transects were walked from 08.00 to 10.00 (n = 22/site) and 15.00–17.00 (n = 16/site); we did not survey during periods of rain or thick cloud cover to minimise variation in weather-related detectability, which reduced the number of appropriate afternoon survey periods. Juveniles were omitted from the dataset to minimise any bias that might be associated with the effects of the breeding season. Transects and pitfalls generated density and capture rate data, respectively: we pooled the data and used total contacts for further analyses (not including rarefaction).
Data analysis
In order to compare species richness between treatments and estimate the completeness of our sampling, we generated individual-based observed species richness rarefaction curves and associated 95 % confidence intervals using EstimateS v.9.0 (Colwell 2013). For reptiles, we combined the two datasets by assigning species to one or other method on the basis of substrate use, following a protocol adapted from Bicknell et al. (2015), whenever a species was recorded by both methods. Thus all arboreal species were assigned to transects and all terrestrial and fossorial lizards were assigned to pitfall traps. Remaining terrestrial species (snakes and a tortoise) were assigned to the method by which they were most frequently recorded. We used Chi squared contingency tables to test for homogeneity of observed species relative frequency (birds) or total contacts (reptiles) across treatments.
Conservation value index
All species are not equal, and may differ in their value to conservationists on the basis of endemism, extinction risk (Mace et al. 2008), evolutionary distinctiveness (Tucker et al. 2012; Hidasi-Neto et al. 2015), public appeal (Smith et al. 2012) or other attributes (Humphries et al. 1995; Joseph et al. 2009). This variation forms the basis of a range of protocols designed to elucidate the conservation value of species and, in turn, support the prioritisation of conservation actions or funding allocations (e.g. Huang et al. 2016; Isaac et al. 2007; Joseph et al. 2009). However, these protocols tend to be methodologically complex and require the collection of large datasets, diminishing their applicability in situations where non-academic conservation practitioners lack the training to apply them or where the necessary data are unavailable (Gardner et al. 2015a). In this study, we therefore used the CVI (adapted from Gardner et al. 2015a) to quantify the conservation value of individual species because it uses only readily available data and does not require the use of specialist software. As such, it can be easily applied in day-to-day decision-making by conservation practitioners. We assigned CVI scores to individual species of bird and reptile before combining them to assess the impacts of natural resource use, and subsequent habitat degradation, on the conservation value of spiny forest habitats.
For the CVI we assigned scores to each individual species based on four attributes that reflect rarity, distinctiveness and threat. We use different combinations of attributes for the two taxonomic groups because the variation in conservation value within each group is driven by different factors. We scored rarity using geographical scale of endemism (G) and representation within SAPM (R), distinctiveness by taxonomic level of endemism (E), and threat on the basis of hunting and collection pressure (C) and degradation tolerance (T). We did not use E for reptiles because all species are endemic and there are no endemic families, so variation in the attribute is limited. Similarly, we did not use C for birds because most species in the Ranobe area are subject to comparable hunting pressure (Gardner and Davies 2014).
Introduced species were removed from the dataset and scores assigned to indigenous taxa on a scale of 1–5 for each attribute (Table 2). For G we used different scoring systems for reptiles and birds because species distributions of the two taxonomic groups are best explained by different biogeographical models (Pearson and Raxworthy 2009). For birds we used distribution maps from Safford and Hawkins (2013) and followed Stattersfield et al. (1998) to classify microendemic species, whereas for reptiles we visually estimated range criteria using maps in Glaw and Vences (2007) and adopted 10,000 km2 as the threshold for microendemic species (following Gardner et al. 2015a). E was assigned on the basis of taxonomy in Safford and Hawkins (2013), R scores were assigned on the basis of occurrence in 14 (birds) or 15 (reptiles) protected areas in the dry regions of Madagascar derived from the literature (Online resource 1), and values for C were based on occurrence in CITES (Convention on International Trade in Endangered Species) appendices and the literature on reptile declines in Madagascar. T was attributed following the methods outlined in Gardner et al. (2015a) for reptiles, and were based on the literature (Safford and Hawkins 2013; Wilmé 1996) for birds. Species for which no degradation tolerance data were available were scored as intolerant on the basis of the precautionary principle.
The individual species CVI scores were calculated, producing a value in the range of 4–100, using the following formulae for reptiles and birds:
The conservation value of a site can be considered a function of: (i) the value of the species occurring there; and, (ii) their abundance, because an area with a large population of a valuable species is more important than one with a small population. To understand the relative conservation value of each habitat treatment, we therefore wanted a metric that combined the CVI of each species with their relative abundance. However, simply weighting the CVI score by the relative frequency would heavily bias common species at the expense of rarer ones which are recorded only infrequently. We thus gave each species weightings standardised to the treatment where it was most frequent (e.g., a species with relative frequency of 0.36, 0.18 and 0.12 across each of the three treatments would be given weightings of 1, 0.5 and 0.33 respectively). In each treatment the CVI was then multiplied by the weighting to produce a frequency-weighted CVI score for each species, before these were summed to produce a conservation value score for each treatment.
Results
Degradation impacts on birds
We recorded 2385 bird contacts, comprising 53 species, in point counts across all treatments. Rarefaction curves approach an asymptote in all treatments, indicating that bird communities were sufficiently sampled (Fig. S1). Although observed richness was highest in the moderate-degradation treatment (Low—36 spp.; Mod—43 spp.; High—37 spp.), rarefaction curves show no significant differences in richness since the 95 % confidence intervals overlap (Online resource 2). Total richness is estimated at 42.0 (Low), 46.8 (Mod) and 39.7 (High) species in the three treatments. Twenty-four species (45.3 %) were recorded in all treatments, one species (1.9 %) was restricted to Low, five species (9.4 %) were restricted to Mod, and seven (13.2 %) species were restricted to High: 17 species (32.1 %) were recorded only in forest habitats (Low and Mod).
Observed patterns of species relative frequency differed significantly for 22 species (41.5 %) across the three treatments. Three of these species (Cuculus rochii, Hypsipetes madagascariensis and Dicrurus forficatus) were observed more frequently in the low-degradation treatment, one species (Ploceus sakalava) in the moderate-degradation treatment, and six species (Turnix nigricollis, Oena capensis, Agapornis canus, Cisticola cherina, Acridotheres tristis and Foudia madagascariensis) in the high-degradation treatment. A further 12 species were recorded less frequently in the high-degradation treatment than in forest habitat (Low or Mod) (Online resource 3).
Patterns of species endemism varied across the degradation gradient (Fig. 2). While the proportion of endemic species was approximately equal in all treatments, the high-degradation treatment contained a lower proportion of regionally-endemic birds (defined as restricted to Madagascar and the islands of the western Indian Ocean) and a higher proportion of non-endemic species. The vast majority (97.9 %) of contacts with introduced species (A. tristis) occurred in the high-degradation treatment.
Endemism status of birds at Ranobe expressed as a percentage of contacts from 48 point counts at three sites across a gradient of degradation. Black, Madagascar endemic; dark grey, regional endemic; light grey, indigenous non-endemic; white, introduced. Regional endemic species are defined as restricted to Madagascar and the western Indian Ocean islands (Comoros, Mascarene and Seychelles archipelagos)
Degradation impacts on reptiles
We recorded 661 reptile contacts comprising 32 species, 27 of which were recorded at Low and Mod, and 15 species at High. Twenty-two species were observed during transects, and 27 were captured in pitfall traps (Online resource 4). Twelve species (37.5 %) were recorded in all treatments, 17 species (53.1 %) were only recorded in forest habitats, and one species (Lygodactylus tuberosus) was recorded only in the high-disturbance site. Rarefaction curves indicate that Low and Mod had significantly higher species richness than High, as there is no overlap between confidence intervals (Online resource 5). Total richness is estimated at 30.5 (Low), 34.2 (Mod) and 19.1 (High) species in the three treatments.
Observed patterns of reptile abundance, based on total contacts, were significantly heterogeneous for 11 species (34.4 %). Three species were recorded more frequently in the low-degradation treatment (Chalarodon madagascariensis, Lygodactylus verticillatus and Oplurus cyclurus), two species in the moderate-degradation treatment (Madascincus cf. igneocaudatus and Tracheloptychus petersi), and three species in the high-degradation treatment (L. tuberosus, Paroedura picta and Typhlops arenarius). A further three species (Geckolepis c.f. polylepis, Phelsuma mutabilis and Trachylepis elegans) were recorded more frequently in the two forest areas than in the high-degradation treatment.
Forest disturbance affected distinct components of the reptile community differently, depending on their foraging substrate (Online resource 6). Terrestrial species decreased in frequency (capture rate and/or density) with increasing disturbance, while arboreal species demonstrated reduced frequency at Mod and reduced richness at High compared to the less degraded site. Fossorial and litter dwelling species reached peak frequency under conditions of moderate-intensity disturbance.
Conservation value of species and sites
The CVI allowed us to weight species on the basis of their conservation value. The six highest scoring bird species were locally-endemic forest specialists (Table 3), while the highest scoring reptile was the heavily harvested (and thus Critically Endangered) tortoise Pyxis arachnoides (Table 4). The relative conservation value of each treatment varied for the two taxonomic groups. Total bird conservation value was highest in Low, while total reptile conservation value was highest in Mod, although in both cases the differences between the two forest areas were small (Table 5). The high-degradation treatment had the lowest conservation value for both taxa.
Discussion
We have generated some of the first data on the impacts of permitted livelihood activities within Madagascar’s new generation of multiple-use protected areas. Our results show that charcoal production, an authorised activity within much of the Ranobe PK32 protected area, resulted in an overall reduction in the conservation value of habitats, although the responses of reptile and bird communities varied. However the impacts of charcoal production were less severe than the impacts of illegal shifting cultivation for both groups.
Although the impacts of habitat degradation on Madagascar’s biodiversity have been well studied (reviewed in Gardner 2009; Irwin et al. 2010), the vast majority of research has been conducted in the country’s humid and dry forests, ecosystems which greatly differ from the spiny forest in terms of biotic communities and abiotic conditions (Moat and Smith 2007; Goodman and Raherilalao 2013). Within the spiny forest, degradation has been found to reduce species richness in both birds (Randriamiharisoa et al. 2015) and reptiles (Theisinger and Ratianarivo 2015). However, in our study, richness was maintained for reptiles and increased for birds at moderate degradation levels. Indeed the conservation value of reptiles was greatest at the moderate-disturbance site, perhaps reflecting an increase in microhabitat heterogeneity or structural complexity (MacArthur and MacArthur 1961; Tews et al. 2004). Bird communities were more responsive than reptiles to habitat degradation, undergoing extensive community turnover. This was reflected in the greater prevalence of birds adapted to open areas, and a decrease in the frequency of certain high-value, locally-endemic species such as Monias benschi, Coua cursor and Newtonia archboldi, with increasing degradation intensity.
Wilmé (1996) suggests that “the tolerance of [Madagascar’s] endemic forest avifauna to forest degradation is proportional to its degree of taxonomic endemism”. However, we recorded seven members of endemic genera (C. cursor, M. benschi, Neomixis striatigula, Newtonia brunneicauda, N. archboldi, Vanga curvirostris and Xenopirostris xenopirostris) previously thought to occur only in undisturbed or slightly disturbed habitats, within a largely deforested habitat in our high-disturbance treatment. These findings lend some support to the hypothesis that faunal species of Madagascar’s dry and spiny forests may be more tolerant of degradation than those same or congeneric species in the country’s humid east and north (Gardner 2009). This may arise due to the more ‘gentle’ habitat modifications occurring in dry forests compared to rainforests (Irwin et al. 2010): for example, the increased light penetration in forest gaps is thought to make little difference to the understory in the spiny forest, because the sparse, deciduous nature of the canopy already allows illumination at ground level (Seddon and Tobias 2007). However, while tropical dry forests are thought to be more resilient than humid forests in terms of regeneration capacity (Lebrija-Trejos et al. 2008), little is known about the relative disturbance sensitivity of their respective faunas. Such research should be considered a priority since it has important repercussions for the implementation of multiple-use protected areas in different bioclimatic contexts.
The finding that moderate levels of degradation provoked an increase in richness of birds, and maintained richness in reptiles, is consistent with Connell’s (1978) ‘intermediate disturbance hypothesis’, and reflects a pattern widely reported from other tropical environments, at least for some guilds (Burivalova et al. 2014; Child et al. 2009; Gray et al. 2007; Martin and Blackburn 2010; Pons and Wendenburg 2005). However, all species are not equal, and the greater richness may often mask a turnover from range-restricted specialists to widespread generalists (Canaday 1997; Christian et al. 2009; Holbech 2005; Petit and Petit 2003; Scott et al. 2006). The latter are of less importance to conservationists precisely because they adapt well to anthropogenic disturbance and thus do not require conservation actions, such as protected areas, to maintain them (Harris and Pimm 2004; Gardner et al. 2009c). The use of species richness as a measure of conservation value has been widely criticised for this reason (Barlow et al. 2007; DeClerck et al. 2010; Fermon et al. 2005; Norris et al. 2010), but remains persistent (e.g., studies reviewed by Burivalova et al. 2014). Our use of the CVI provides further evidence of the inadequacies of richness in prioritising between sites or habitats, as the use of richness would indicate that forests degraded by charcoal production are more valuable for bird conservation in the spiny forest than less degraded habitats. Of course, the CVI does not represent a definitive quantification of conservation value, but is a useful heuristic tool to help conservationists prioritise action to where it is most needed (i.e. high-value species), and can be used without training, complex software or collecting new data.
Although the use of CVI provides novel insights into the impacts of habitat change on the conservation value of spiny forest bird and reptile assemblages, our results must be interpreted with caution. We carried out surveying during the rainy season when both groups are most active, and surveyed each site sequentially for logistical reasons. However, biases may have arisen due to changes in species detectability related to the advancing breeding season. In addition, the entry of new cohorts may have increased population size as surveying progressed. We minimised the latter problem by excluding all records of juveniles from the analysis, although it would have been preferable to repeat data collection over multiple years, or to survey each site simultaneously using multiple teams. Nonetheless, the latter approach has a number of drawbacks, including the extensive training needed to minimise the biases associated with potential differences in the bird detection abilities and/or identification skills of research assistants.
Although our observations appear to suggest that the majority of bird and reptile species in Ranobe are somewhat resilient to moderate or high levels of degradation, the presence of a species does not necessarily equate to its viability. It should not be assumed that local populations in disturbed areas will persist in the long-term because there are likely to be time lags associated with the impacts arising from perturbation, meaning that the degraded habitats at Ranobe may be carrying an ‘extinction debt’ (Kuussaari et al. 2009; Tilman et al. 1994). This is particularly true given that the habitat modifications that are the focus of this study are relatively recent (range: 3–15 years across the treatments). In addition, the persistence of some species within degraded habitats may be the result of source-sink dynamics, with populations maintained only by immigration from nearby areas of higher quality habitat (Hylander and Ehrlén 2013; Pulliam 1988; Tilman et al. 1994). The degraded habitats at Ranobe may therefore experience future local extinctions, even without further modification, and we may have over-estimated the value of these areas for bird and reptile diversity (Barlow et al. 2007; Sekercioglu et al. 2007). The scale of extinction debt can be influenced by habitat quantity, quality, or connectivity (Hylander and Ehrlén 2013). As such, when destructive activities such as charcoal production cannot be prevented within the ‘sustainable use zones’ of multiple-use protected areas, both the structural and functional connectivity between high-quality habitat patches should be maximised in order to maintain biodiversity and mitigate the negative impacts associated with resource exploitation.
The suggestion that Madagascar’s new generation of multiple-use protected areas may suffer the continued erosion of biodiversity as a result of the impacts of authorised livelihood activities has important ramifications for the objectives and management of multiple-use sites worldwide. In a multi-taxon assessment across a continuum of protection levels in East Africa, Gardner et al. (2007) found that multiple-use protected areas provide significant and complementary conservation services to strictly-protected sites, maintaining species richness but conserving significantly different faunal communities to those occurring in national parks. Thus a spectrum of protected area categories may be appropriate to conserve the full complement of biodiversity in continental regions, if these possess a range of faunal assemblages adapted to a continuum of habitat types from dense forests to wooded savannahs and grasslands (Borghesio 2008; Gardner et al. 2007; Pons et al. 2003).
Madagascar, however, differs from continents in that the vast majority of the endemic biota is forest-dependent (Goodman and Benstead 2005), while non-forest areas typically contain floristically- and faunistically-impoverished assemblages characterised by non-endemic species of low conservation value (Irwin et al. 2010; Koechlin et al. 1974; Lowry et al. 1997). In this context, multiple-use sites essentially conserve the same communities as strict protected areas, but may do so less successfully than the latter. Thus, while multiple-use categories may be the only politically, ethically and logistically feasible option for many of Madagascar’s new generation of protected areas, given the socioeconomic importance to rural communities of remaining forest resources (Gardner et al. 2013), it should not be assumed that they will be successful in maintaining the biodiversity they were established to conserve. Given that range-restricted habitat specialists are disproportionately likely to go extinct in modified habitats (Posa and Sodhi 2006; Scales and Marsden 2008), and are of greatest conservation interest worldwide, careful attention must be paid to the choice of protected area models in different contexts; in regions where the majority of priority species are disturbance-sensitive, strict protected areas may be a more appropriate model if they can be managed effectively.
References
Adams WM, Hutton J (2007) People, parks and poverty: political ecology and biodiversity conservation. Conserv Soc 5:147–183
AGRECO (2012) Analyse des coûts et sources de financement du système des aires protégées de Madagascar (Octobre 2010—Janvier 2012). AGRECO, Antananarivo
Barlow J, Gardner TA, Araujo IS, Avila-Pires TC, Bonaldo AB, Costa JE, Esposito MC, Ferreira LV, Hawes J, Hernandez MIM et al (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. PNAS 104:18555–18560
Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC et al (2011) Has the earth’s sixth mass extinction already arrived? Nature 471:51–57
Beaudrot L, Ahumada JA, O’Brien T, Alvarez-Loayza P, Boekee K, Campos-Arceiz A, Eichberg D, Espinosa S, Fegraus E, Fletcher C et al (2016) Standardized assessment of biodiversity trends in tropical forest protected areas: the end is not in sight. PLoS Biol 14:e1002357
Bertrand A, Ramamonjisoa B, Montagne P (2010) Les filières péri-urbaines d’approvisionnement en bois énergie des grandes villes de Madagascar. In: Montagne P, Razafimahatratra S, Rasamindisa A, Crehay R (eds) ARINA, le charbon de bois à Madagascar: entre demande urbaine et gestion durable. CITE, Antananarivo, pp 23–36
Bertzky B, Corrigan C, Kemsey J, Kenney S, Ravilious C, Besançon C, Burgess N (2012) Protected planet report 2012: tracking progress towards global targets for protected areas. IUCN, Gland and UNEP-WCMC
Bibby CJ, Buckland ST (1987) Bias of bird census results due to detectability varying with habitat. Acta Oecol 8:103–112
Bibby C, Jones M, Marsden S (1998) Expedition field techniques: bird surveys. Royal Geogr Soc, London
Bicknell JE, Struebig MJ, Davies ZG (2015) Reconciling timber extraction with biodiversity conservation in tropical forests using reduced-impact logging. J Appl Ecol 52:379–388
Borghesio L (2008) Effects of human subsistence activities on forest birds in northern Kenya. Conserv Biol 22:384–394
Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL (2006) Global biodiversity conservation priorities. Science 313:58–61
Brown KA, Johnson SE, Parks KE, Holmes SM, Ivoandry T, Abram NK, Delmore KE, Ludovic R, Andriamaharoa HE, Wyman TM et al (2013) Use of provisioning ecosystem services drives loss of functional traits across land use intensification gradients in tropical forests in Madagascar. Biol Conserv 161:118–127
Burivalova Z, Şekercioğlu CH, Koh LP (2014) Thresholds of logging intensity to maintain tropical forest biodiversity. Curr Biol 24:1893–1898
Butchart SHM, Clarke M, Smith RJ, Sykes RE, Scharlemann JPW, Harfoot M, Buchanan GM, Angulo A, Balmford A, Bertzky B et al (2015) Shortfalls and solutions for meeting national and global conservation area targets. Conserv Lett 8:329–337
Cabeza M (2013) Knowledge gaps in protected area effectiveness. Anim Conserv 16:381–382
Canaday C (1997) Loss of insectivorous birds along a gradient of human impact in Amazonia. Biol Conserv 77:63–77
Casse T, Milhøj A, Ranaivoson S, Randriamanarivo JR (2004) Causes of deforestation in southwestern Madagascar: what do we know? For Policy Econ 6:33–48
CBD (Convention on Biological Diversity) (2010) Decision adopted by the Conference of the Parties to the Convention on Biological Diversity at its tenth meeting [Decision X/2] Nagoya, Aichi Prefecture, Japan, 18–29 October 2010. Secretariat to the Convention on Biological Diversity, Montreal
Child MF, Cumming GS, Amano T (2009) Assessing the broad-scale impact of agriculturally transformed and protected area landscapes on avian taxonomic and functional richness. Biol Conserv 142:2593–2601
Christian K, Isabelle LV, Frédéric J, Vincent D (2009) More species, fewer specialists: 100 years of changes in community composition in an island biogeographical study. Divers Distrib 15:641–648
Commission SAPM (2006) Système d’aires protégées Malagasy: orientations générales sur les catégories et les types de gouvernance. SAPM Commission, Antananarivo
Connell JH (1978) Diversity in tropical rainforests and coral reefs. Science 199:1302–1310
Cornet A (1974) Essai de Cartographie Bioclimatique à Madagascar. Orstom, Paris
Corson C (2014) Conservation politics in Madagascar: the expansion of protected areas. In: Scales I (ed) Conservation and environmental management in Madagascar. Routledge, London, pp 193–215
D’Cruze N, Sable J, Green K, Dawson J, Gardner C, Robinson J, Starkie G, Vences M, Glaw F (2007) The first comprehensive survey of amphibians and reptiles at Montagne des Français, Madagascar. Herpetol Conserv Biol 2:87–99
DeClerck FAJ, Chazdon R, Holl KD, Milder JC, Finegan B, Martinez-Salinas A, Imbach P, Canet L, Ramos Z (2010) Biodiversity conservation in human-modified landscapes of Mesoamerica: past, present and future. Biol Conserv 143:2301–2313
Dudley N (ed) (2008) Guidelines for applying protected area management categories. IUCN, Gland
Dudley N, Parrish JD, Redford KH, Stolton S (2010) The revised IUCN protected area management categories: the debate and ways forward. Oryx 44:485–490
Dudley N, Groves C, Redford KH, Stolton S (2014) Where now for protected areas? setting the stage for the 2014 World Parks Congress. Oryx 48:496–503
Ehrlich PR, Pringle RM (2008) Where does biodiversity go from here? A grim business-as-usual forecast and a hopeful portfolio of partial solutions. PNAS 105:11579–11586
Fenn MD (2003) The spiny forest ecoregion. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago, pp 1525–1529
Fermon H, Waltert M, Vane-Wright RI, Muhlenberg M (2005) Forest use and vertical stratification in fruit-feeding butterflies of Sulawesi, Indonesia: impacts for conservation. Biodivers Conserv 14:333–350
Ferraro PJ, Hanauer MH, Miteva DA, Canavire-Bacarreza GJ, Pattanayak SK, Sims KR (2013) More strictly protected areas are not necessarily more protective: evidence from Bolivia, Costa Rica, Indonesia and Thailand. Environ Res Lett 8:025011
Gardner CJ (2009) A review of the impacts of anthropogenic habitat change on terrestrial biodiversity in Madagascar: implications for the design and management of new protected areas. Malagasy Nat 2:2–29
Gardner CJ (2011) IUCN management categories fail to represent new, multiple-use protected areas in Madagascar. Oryx 45:336–346
Gardner CJ, Davies ZG (2014) Rural bushmeat consumption within multiple-use protected areas: qualitative evidence from southwest Madagascar. Hum Ecol 42:21–34
Gardner TA, Caro T, Fitzherbert EB, Banda T, Lalbhai P (2007) Conservation value of multiple-use areas in East Africa. Conserv Biol 21:1516–1525
Gardner CJ, Ferguson B, Rebara F, Ratsifandrihamanana AN (2008) Integrating traditional values and management regimes into Madagascar’s expanded protected area system: the case of Ankodida. In: Mallarach JM (ed) Protected landscapes and cultural and spiritual values. Kasparek Verlag, Heidelberg, pp 92–103
Gardner CJ, Kidney D, Thomas H (2009a) First comprehensive avifaunal survey of PK32-Ranobe, a new protected area in south-western Madagascar. Phelsuma 17:20–39
Gardner CJ, Fanning E, Thomas H, Kidney D (2009b) The lemur diversity of the Fiherenana-Manombo Complex, southwest Madagascar. Madag Conserv Dev 4:38–43
Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA, Sodhi NS (2009c) Prospects for tropical forest biodiversity in a human-modified world. Ecol Lett 12:561–582
Gardner TA, Barlow J, Sodhi NS, Peres CA (2010) A multi-region assessment of tropical forest biodiversity in a human-modified world. Biol Conserv 143:2293–2300
Gardner CJ, Nicoll ME, Mbohoahy T, Oleson KLL, Ratsifandrihamanana AN, Ratsirarson J, René de Roland LA, Virah-Sawmy M, Zafindrasilivonona B, Davies ZG (2013) Protected areas for conservation and poverty alleviation: experiences from Madagascar. J Appl Ecol 50:1289–1294
Gardner CJ, Raxworthy CJ, Metcalfe K, Raselimanana AP, Smith RJ, Davies ZG (2015a) Comparing methods for prioritising between existing protected areas: a case study using Madagascar’s dry forest reptiles. PLoS ONE 10:e0132803
Gardner CJ, Gabriel FUL, St John FAV, Davies ZG (2015b) Changing livelihoods and protected area management: a case study of charcoal production in south-west Madagascar. Oryx. doi:10.1017/S0030605315000071
Gaston KJ, Jackson SF, Cantu-Salazar L, Cruz-Pinon G (2008) The ecological performance of protected areas. Annu Rev Ecol Evol Syst 39:93–113
Geldmann J, Barnes M, Coad L, Craigie ID, Hockings M, Burgess N (2013) Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol Conserv 161:230–238
Glaw F, Vences M (2007) A field guide to the amphibians and reptiles of madagascar, 3rd edn. Vences and Glaw Verlags GbR, Köln
Goodman SM, Benstead JP (2005) Updated estimates of biotic diversity and endemism for Madagascar. Oryx 39:73–77
Goodman SM, Raherilalao MJ (2013) Atlas of selected land vertebrates of Madagascar. Association Vahatra, Antananarivo
Gray MA, Baldauf SL, Mayhew PJ, Hill JK (2007) The response of avian feeding guilds to tropical forest disturbance. Conserv Biol 21:133–141
Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F (2007) 50 years of deforestation and forest fragmentation in Madagascar. Environ Conserv 34:325–333
Harris GM, Pimm SL (2004) Bird species’ tolerance of secondary forest habitats and its effects on extinction. Conserv Biol 18:1607–1616
Hidasi-Neto J, Loyola R, Cianciaruso MV (2015) Global and local evolutionary and ecological distinctiveness of terrestrial mammals: identifying priorities across scales. Diversity Distrib 21:548–559
Holbech LH (2005) The implications of selective logging and forest fragmentation for the conservation of avian diversity in evergreen forests of south-west Ghana. Bird Conserv Int 15:27–52
Huang J, Lu X, Huang J, Ma K (2016) Conservation priority of endemic Chinese flora at family and genus levels. Biodivers Conserv 25:23–35
Humphries CJ, Williams PH, Vane-Wright RI (1995) Measuring biodiversity value for conservation. Annu Rev Ecol Evol Syst 26:93–111
Hylander K, Ehrlén J (2013) The mechanisms causing extinction debts. Trends Ecol Evol 28:341–346
Irwin MT, Wright PC, Birkinshaw C, Fisher B, Gardner CJ, Glos J, Goodman SM, Loiselle P, Rabeson P, Raharison JL et al (2010) Patterns of species change in anthropogenically disturbed habitats of Madagascar. Biol Conserv 142:2351–2362
Isaac NJB, Turvey ST, Collen B, Waterman C, Baillie JEM (2007) Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 2:e296
Jenkins CN, Joppa L (2009) Expansion of the global terrestrial protected area system. Biol Conserv 142:2166–2174
Jones MJ, Linsley MD, Marsden SJ (1995) Population sizes, status and habitat associations of the restricted-range bird species of Sumba, Indonesia. Bird Conserv Int 5:21–52
Joppa LN, Pfaff A (2011) Global protected area impacts. Proc R Soc B 278:1633–1638
Joseph LN, Maloney RF, Possingham HP (2009) Optimal allocation of resources among threatened species: a project prioritisation protocol. Conserve Biol 23:328–338
Juffe-Bignoli D, Burgess ND, Bingham H, Belle EMS, de Lima MG, Deguidnet M, Bertzky B, Milam AN, Martinez-Lopez J, Lewis E et al (2014) Protected planet report 2014. UNEP-WCMC, Cambridge
Koechlin J, Guillaumet JL, Morat P (1974) Flore et végétation de Madagascar. J. Cramer Verlag, Vaduz
Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Öckinger E, Pärtel M, Pino J, Rodà F et al (2009) Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol 24:564–571
Lebrija-Trejos E, Bongers F, Pérez-Garcia EA, Meave JA (2008) Successional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40:422–431
Leroux SJ, Krawchuk MA, Schmiegelow F, Cumming SG, Lisgo K, Anderson LG, Petkova M (2010) Global protected areas and IUCN designations: do the categories match the conditions? Biol Conserv 143:609–616
Locke H, Dearden P (2005) Rethinking protected area categories and the new paradigm. Environ Conserv 32:1–10
Lowry PP, Schatz GE, Phillipson PB (1997) The classification of natural and anthropogenic vegetation in Madagascar. In: Goodman SM, Patterson BD (eds) Natural change and human impact in Madagascar. Smithsonian Institution Press, Washington, pp 93–123
MacArthur RH, MacArthur JW (1961) On bird diversity. Ecology 42:594–598
Mace GM, Collar NJ, Gaston KJ, Hilton-Taylor C, Akçakaya HR, Leader-Williams N, Milne-Gulland EJ, Stuart SN (2008) Quantification of extinction risk: iUCN’s system for classifying threatened species. Conserv Biol 22:1424–1442
Martin TE, Blackburn GA (2010) Impacts of tropical forest disturbance upon avifauna on a small island with high endemism: implications for conservation. Conserv Soc 8:127–139
Mascia MB, Pailler S, Krithivasan R, Roshchanka V, Burns D, Mlotha MJ, Murray DR, Peng N (2014) Protected area downgrading, downsizing and degazettement (PADDD) in Africa, Asia and Latin America and the Caribbean, 1900–2010. Biol Conserv 169:355–361
McConnell WJ, Kull CA (2014) Deforestation in Madagascar: debates over the island’s forest cover and challenges of measuring forest change. In: Scales IR (ed) Conservation and environmental management in Madagascar. Routledge, Abingdon, pp 65–104
McDonald RI, Boucher TM (2011) Global development and the future of the protected area strategy. Biol Conserv 144:383–392
Miller DC (2014) Explaining global patterns of international aid for linked biodiversity conservation and development. World Dev 59:341–359
Moat J, Smith P (2007) Atlas of the vegetation of Madagascar. Royal Botan Gard, Kew
Montesino Pouzols F, Toivonen T, Di Minin E, Kukkala A, Kullberg P, Kuusterä J, Lehtomäki J, Tenkanen H, Verburg PH, Moilanen A (2014) Global protected area expansion is compromised by projected land-use and parochialism. Nature 516:383–386
Nelson A, Chomitz KM (2011) Effectiveness of strict vs. multiple use protected areas in reducing tropical forest fires: a global analysis using matching methods. PLoS ONE 6:e22722
Norris K, Asase A, Collen B, Gockowski J, Mason J, Phalan B, Wade A (2010) Biodiversity in a forest-agriculture mosaic—the changing face of West African rainforests. Biol Conserv 143:2341–2350
Olson DM, Dinerstein E (1998) The Global 200: a representation approach to conserving the earth’s most biologically valuable ecoregions. Conserv Biol 12:502–515
ONE (Office National pour l’Environnement), DGF/MEF, Conservation International, FTM, Madagascar National Parks (2013) Evolution de la couverture de forêts naturelles à Madagascar 200–52010. Office National pour l’Environnement, Antananarivo
Partage (2008) Analyse de l’offre et de la demande en énergie domestique au niveau de la commune de Toliara. Partage, Antananarivo
Pearson RG, Raxworthy CJ (2009) The evolution of local endemism in Madagascar: watershed versus climatic gradient hypotheses evaluated by null biogeographic models. Evolution 63:959–967
Peres CA, Barlow J, Laurance WF (2006) Detecting anthropogenic disturbance in tropical forests. Trends Ecol Evol 21:227–229
Petit LJ, Petit DR (2003) Evaluating the importance of human-modified lands for neotropical bird conservation. Conserv Biol 17:687–694
Phillipson PB (1996) Endemism and non-endemism in the flora of south-west Madagascar. In: Lourenço WR (ed) Biogéographie de Madagascar. Editions ORSTOM, Paris, pp 125–136
Polak T, Watson JEM, Fuller RA, Joseph LN, Martin TG, Possingham HP, Venter O, Cawardine J (2015) Efficient expansion of global protected areas requires simultaneous planning for species and ecosystems. R Soc Open Sci 2:150107
Pons P, Wendenburg C (2005) The impact of fire and forest conversion into savanna on the bird communities of west Madagascan dry forests. Anim Conserv 8:183–193
Pons P, Lambert B, Rigolot E, Prodon R (2003) The effects of grassland management using fire on habitat occupancy and conservation of birds in a mosaic landscape. Biodivers Conserv 12:1843–1860
Posa MRC, Sodhi NS (2006) Effects of anthropogenic land use on forest birds and butterflies in Subic Bay, Philippines. Biol Conserv 129:256–270
Pulliam HR (1988) Sources, sinks, and population regulation. Amer Nat 132:652–661
Randriamalala JR, Radosy HO, Razanaka S, Randriambanona H, Hervé D (2016) Effects of goat grazing and woody charcoal production on xerophytic thickets of southwestern Madagascar. J Arid Environ 128:65–72
Randriamiharisoa LO, Rakotondravony D, Raherilalao MJ, Ranirison A, Wilmé L, Ganzhorn JU (2015) Effects of transhumance route on the richness and composition of bird communities in Tsimanampesotse National Park. Madag Conserv Dev 10:110–115
Randrianandianina BN, Andriamahaly LR, Harisoa FM, Nicoll ME (2003) The role of protected areas in the management of the island’s biodiversity. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. The University of Chicago Press, Chicago, pp 1423–1432
Raselimanana AP (2008) Herpétofaune des forêts sèches malgaches. In: Goodman SM, Wilmé L (eds). Les forêts sèches de Madagascar. Malagasy Nature 1: 46–75
RKColwell2013EstimateS: Statistical estimation of species richness and shared species from samples Consultado en: http://viceroy. eeb. uconn. edu/estimates
Rosser AM, Leader-Williams N (2010) Protection or use: a case of nuanced trade-offs? In: Leader-Williams N, Adams WM, Smith RJ (eds) Trade-offs in conservation: deciding what to save. Wiley-Blackwell, Oxford, pp 135–156
Safford R, Hawkins F (eds) (2013) Birds of Africa, vol. VIII: the Malagasy region. Christopher Helm, London
Scales BR, Marsden SJ (2008) Biodiversity in small-scale tropical agroforests: a review of species richness and abundance shifts and the factors influencing them. Environ Conserv 35:160–172
Scharlemann JPW, Kapos V, Campbell A, Lysenko I, Burgess ND, Hansen MC, Gibbs HK, Dickson B, Miles L (2010) Securing tropical forest carbon: the contribution of protected areas to REDD. Oryx 44:352–357
Scott DM, Brown D, Mahood S, Denton B, Silburn A, Rakotondraparany F (2006) The impacts of forest clearance on lizard, small mammal and bird communities in the arid spiny forest, Southern Madagascar. Biol Conserv 127:72–87
Seddon N, Tobias JA (2007) Population size and habitat associations of the long-tailed ground-roller Uratelornis chimaera. Bird Conserv Int 17:1–12 (287–304)
Sekercioglu CH, Loarie SR, Oviedo Brenes F, Ehrlich PR, Daily GC (2007) Persistence of forest birds in the Costa Rican agricultural countryside. Conserv Biol 21:482–494
Shafer CL (2015) Cautionary thoughts on IUCN protected area management categories V–VI. Global Ecol Conserv 3:331–348
Smith RJ, Veríssimo D, Isaac NJB, Jones KE (2012) Identifying cinderella species: uncovering mammals with conservation flagship appeal. Conserv Lett 5:205–212
Stattersfield AJ, Crosby MJ, Long AJ, Wege DC (1998) Endemic bird areas of the world: priorities for biodiversity conservation. BirdLife International, Cambridge
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Theisinger O, Ratianarivo MC (2015) Patterns of reptile diversity loss in response to degradation in the spiny forest of Southern Madagascar. Herpetol Conserv Biol 10:273–283
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Tucker CM, Cadotte MW, Davies TJ, Rebelo TG (2012) Incorporating geographical and evolutionary rarity into conservation prioritisation. Conserv Biol 26:593–601
Venter O, Fuller RA, Segan DB, Cawardine J, Brooks T, Butchart SHM, Di Marco M, Iwamura T, Joseph L, O’Grady D et al (2014) Targeting global protected area expansion for imperilled biodiversity. PLoS Biol 12:e1001891
Virah-Sawmy M, Gardner CJ, Ratsifandrihamanana AN (2014) The Durban vision in practice: experiences in participatory governance of Madagascar’s new protected areas. In: Scales IR (ed) Conservation and environmental management in Madagascar. Routledge, London, pp 216–252
Visconti P, Bakkenes M, Smith Joppa L, Sykes RE (2015) Socio-economic and ecological impacts of global protected area expansion plans. Phil Trans R Soc B 370:20140284
Watson JEM, Dudley N, Segan DB, Hockings M (2014) The performance and potential of protected areas. Nature 515:67–73
Watson JEM, Darling ES, Venter O, Maron M, Walston Possingham HP, Dudley S, Hockings Barnes M, Brooks TM (2016) Bolder science needed now for protected areas. Conserv Biol 30:243–248
Wilmé L (1996) Composition and characteristics of bird communities in Madagascar. In: Lourenço WR (ed) Biogéographie de Madagascar. Editions ORSTOM, Paris, pp 349–362
WWF (2010) Plan d’aménagement et de gestion de PK32-Ranobe. WWF Madagascar and Western Indian Ocean Programme Office, Antananarivo
Zimmerer KS, Galt RE, Buck MV (2004) Globalization and multi-spatial trends in the coverage of protected-area conservation (1980–2000). Ambio 33:520–529
Acknowledgments
This research was funded by an African Bird Club conservation grant to CJG. We would like to thank the community of Ranobe for hosting us during our research. In addition, we are grateful to E. Rasolondranaly, B. Rasolonandrasana and M. Virah-Sawmy for logistical support, and B. Morgan and J.E. Bicknell for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephen Garnett.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gardner, C.J., Jasper, L.D., Eonintsoa, C. et al. The impact of natural resource use on bird and reptile communities within multiple-use protected areas: evidence from sub-arid Southern Madagascar. Biodivers Conserv 25, 1773–1793 (2016). https://doi.org/10.1007/s10531-016-1160-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-016-1160-4