Abstract
Forest fragmentation creates forest edges, and the effect of those edges increases as the size of forest fragments decreases. Edge effects include changes to microclimatic conditions at the forest edge, which affect vegetation structure. No previous studies have directly tested the relationship between microclimate and vegetation structure (for instance, basal area, trees mean height, dead trees and damage trees) at the edge of forest fragments in the Atlantic Forest domain. We tested the following three hypotheses: (i) the microclimatic conditions differ between the edge and the interior of the forest, (ii) the forest structure differs between the edge and the interior of the forest and (iii) changes to microclimatic conditions at the forest edge negatively affect vegetation structure at the edges. Our results demonstrate that edge habitats are significantly more susceptible to strong winds, lower humidity and higher air temperatures than forest interiors. The microclimate may be considered the principal factor that explains the difference between the vegetation structure of the forest edge and the forest interior. Our results suggest that even large forest fragments in the Brazilian Atlantic Forest may be impacted by negative edge effects.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Fragmentation of tropical forests is one of the greatest threats to biodiversity and ecosystem function (Fahrig 2003; Pardini et al. 2010; Pütz et al. 2011; Magnago et al. 2014). Deforestation turns a continuous forest into a mosaic landscape with forest fragments of different sizes, which are usually surrounded by anthropogenic environments (Bennett and Saunders 2010). As a result of deforestation, species experience habitat loss, and there are changes in species composition (Laurance et al. 2006). The fragmentation process (see Fahrig 2003) creates many new edges, resulting in changes to the abiotic environment and additional biodiversity and biomass loss (Murcia 1995; Laurance et al. 1998; Harper et al. 2005; Pütz et al. 2011).
Many previous studies have reported that edges differ from the forest interior in microclimate (i.e., greater wind speed and air temperature), altered light conditions and decreased humidity (Kapos 1989; Chen et al. 1993; Camargo and Kapos 1995; Turton and Freiburger 1997; Williams-Linera 1990; Davies-Colley et al. 2000; Pinto et al. 2010). Some studies cite microclimatic changes as the primary factor that affects the forest structure in fragments; e.g., canopy damage, mortality rate and biomass loss (Ferreira and Laurance 1997; Laurance et al. 1998, 2000, 2002). To our knowledge, this is the first study that directly tests the relationship between microclimatic variables and vegetation structure at the forest edge and forest interior in the Brazilian Atlantic Forest domain.
Edges are responsible for a variety of disturbances in forest fragments, such as the loss of biodiversity and tree functionality (Laurance et al. 2002; Magnago et al. 2014). In tropical forests, a forest fragment may be impacted by edges effects, reaching more than 100 m from the edge (Laurance et al. 1998, 2011; Tabarelli et al. 1999, 2004; Pütz et al. 2011). The Brazilian Atlantic Forest harbors high biodiversity (Myers et al. 2000) and is almost entirely (83.4 %) composed of forest fragments that are smaller than 50 ha (Ribeiro et al. 2009); therefore, this domain is an excellent location to evaluate the effects of edges on forests and to improve our understanding of forest fragments. Understanding the relationship between microclimatic change and forest structure at the edges of large and primary forests is a conservation priority because (i) tropical forests are responsible for maintaining the carbon cycle at landscape and global scales (Laurance et al. 1997), and intact forests (i.e., large fragments or the interior of fragments) are able to store more carbon in biomass (Laurance et al. 1998, 2006), and (ii) large and primary tropical forest fragments are important to provide habitat that promotes biodiversity conservation and ecosystem function (Gibson et al. 2011; Magnago et al. 2014).
Our goal was to determine whether the microclimatic conditions at forest edges affected the vegetation structure of forest fragments in the Brazilian Atlantic Forest. This forest is a global hotspot of threatened biodiversity and endemism and has suffered extensive deforestation (Myers et al. 2000). Based on the knowledge of the impacts of edge effects, we tested the following three hypotheses: (i) microclimatic conditions differ between the forest edge and the forest interior, (ii) forest structure differs between the forest edge and the forest interior and (iii) microclimatic conditions at the forest edge have negative impacts on vegetation structure.
Materials and methods
Study area
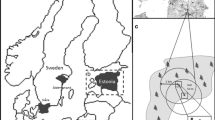
Our study was conducted in northern Espírito Santo State (19°06′08″S and 40°02′02″W) in southeastern Brazil. We surveyed two continuous forests with areas greater than 20,000 ha. One of these fragments, the Reserva Biológica de Sooretama (Biological Reserve of Sooretama), had an area of 23,442 ha and was owned by the federal government. The other fragment was owned by two private companies (Vale Natural Reserve and Fibria) and by the federal government. Together, these forest areas comprise more than 40,000 ha. This region has one of the largest remaining of tableland forest in the Atlantic Forest and is of substantial importance for conservation (Peixoto and Simonelli 2007; Peixoto et al. 2008). This region is considered one of the 14 centers of high plant diversity in Brazil (Peixoto and Silva 1997) and was recognized for its high species richness, trophic complexity, ecosystem function, and as a refuge for threatened species (Chiarello 1999; Srbek-Araujo and Chiarello 2006) and source of food resources for large frugivores (Magnago et al. 2014). The environmental matrix adjacent to this continuous forest is composed mainly of pastures and coffee, papaya and Eucalyptus spp. plantations (Rolim et al. 2005), in addition to other forest fragments of various sizes. All of the edges sampled in our study were adjacent to Eucalyptus spp. plantations.
The Brazilian Institute of Geography and Statistics (IBGE 1987) classifies the forests in our study as lowland ombrophilous dense forest. The region is also known as tertiary tableland because it occurs on Cenozoic sediments from the Barreiras group, and its altitude ranges from 28 to 65 m (Peixoto et al. 2008). Yellow Ultisols are the predominant soil type in this landscape (IBGE 1987). According to the Köppen classification system, the climate in this region is classified as tropical humid (Am), with an annual precipitation of 1403 mm and a dry season from May to September (Fig. 1).
Data collection
All of the forest edges at our field site were delineated by roads adjacent to the Eucalyptus spp. plantations. At forest edges, we sampled plots at a maximum distance of five meters from the edge. In the forest interior, we sampled the core area of each fragment (i.e., the most preserved area), a minimum distance of 300 m from any edge. All sampled plots were located on the same type of soil (Yellow Ultisols). We avoided sampling areas that were close to water and clearings or locations that had been logged in the past.
In each area (i.e., the forest edge and interior), we sampled thirty 10 × 10 m plots (100 m2 per plot), with each plot located at least 20 m from the nearest neighboring plot. In total, we sampled 60 plots in the continuous forest: 30 at the forest edge and 30 in the forest interior. In each plot, we collected data from all individual trees (including standing dead trees) with DBH ≥ 4.8 cm. We considered standing dead trees as an important type of tree mortality that can be caused by edge effects (see D’Angelo et al. 2004). Additionally, in the same plots, we sampled microclimatic variables, including maximum air temperature (°C), maximum wind speed (km/h) and relative humidity (%), using two Kestrel Weather and Environmental Meters, model 4500 (Kestrel Weather and Environmental Meters 4500, Nielsen-Kellerman Company, USA) as weather stations. To standardize microclimatic data collection among the sample plots, all measures were recorded during a 15-min period and at 1.5 m above the ground. Because microclimatic parameters show natural variation (e.g., warmer days have higher humidity and stronger winds than other days), we placed a Kestrel weather station in the surrounding matrix adjacent to each studied fragment, approximately 10 m from the edge. Thus, data collected at the edge and in the interior of forest fragments were expressed relative (i.e., percentage of change) to the matrix values to control for the effects of natural climatic variability.
Data analyses
We evaluate the forest structure by calculating basal area (m2), number of individuals and mean height (m) of individual trees in all plots. We also recorded individuals with canopy damage or standing dead trees to compare the tree damage or mortality between the edge and interior. Both of these metrics were measured as indicators of edge effects because (i) tree damage is used to show the effects of wind increase and other factors that are due to edge creation (Laurance et al. 1998) and (ii) standing dead trees are an important type of tree mortality that can be caused by edge effects (see D’Angelo et al. 2004). The numbers of individual trees in each diameter and height class were analyzed to compare the size structure of the forest edge and forest interior. To compare diameter classes, we applied pairwise comparisons using a Mann–Whitney U test (because these data were not normally distributed). The normally distributed height class data were compared using Student’s t test. We tested the normality of both sets of data using the Shapiro–Wilk test.
We used a partial t-test based on permutations (Peres-Neto et al. 2006) to test whether the vegetation structure (basal area, damaged trees, standing dead trees, mean height and individual density) and microclimatic variables (maximum air temperature, maximum wind speed and relative humidity) differed between forest edges and interiors. We used a partial t-test to separate the treatment effect (i.e., the variable tested) and the spatial autocorrelation (SAC) effect (Peres-Neto and Legendre 2010). The SAC was estimated using spatial filters (MEMs, Moran’s eigenvector maps; Dray et al. 2006) that were selected progressively (Blanchet et al. 2008). For this analysis, we followed the R code proposed by Eisenlohr (2014).
We used a generalized linear model (GLM) to analyze the relationship between microclimatic variables (maximum air temperature, maximum wind speed and relative humidity) and vegetation structure (basal area, damaged trees, standing dead trees and mean height of individual trees). A Gaussian distribution was used for the normally distributed response variables (mean tree height and log-transformed basal area), which were analyzed using Shapiro–Wilk tests. For the count data on proportions (damaged trees and standing dead trees), we applied a binomial family of errors. The binomial GLMs were constructed by weighting the response variable by the total of individual trees per plot, thus avoiding problems with differences in the total sampled individual trees per plot (Crawley 2007). We verified that there was no collinearity between the predictor variables (variance inflation factor <10; Quinn and Keough 2002).
We checked for spatial independence in the residuals of both the full and selected GLMs (Diniz-Filho et al. 2003, 2008) using correlograms, with Moran’s I coefficient as an indicator of SAC. The correlograms were constructed in SAM 4.0 (Rangel et al. 2010), and the number and size of distance classes followed the defaults for SAM. The significance of SAC was detected by sequential Bonferroni criteria (Fortin and Dale 2005). We found SAC in all of the full GLMs [i.e., prior to second-order Akaike information criterion (AICc) selection]. To address SAC, we obtained spatial filters (MEMs; Dray et al. 2006) using the ‘‘spacemakeR’’ package in R (The R Foundation for Statistical Computing 2014). We then progressively selected the spatial filters using forward selection (Blanchet et al. 2008) with the “packfor” package in R. The selected spatial filters for each response variable were used as fixed variables in all GLMs, whereas microclimatic variables were selected based on the lowest AICc values (Burnham and Anderson 2002) using the “model.sel” function of the “MuMIn” package in R. We tested the spatial independence of the residuals of the selected GLMs and found an absence of SAC. Finally, we assessed the model significance by comparing the best full model and the null model.
Results
Microclimatic conditions and forest structure differ between the edge and interior
We found that all of the microclimatic variables significantly differed between the forest edge and the forest interior. Air temperature and wind speed were greater at the edge than in the interior (F = 4.983; p = 0.033 for air temperature; F = 8.551; p = 0.010 for wind speed), and relative humidity was lower (F = 5.115; p = 0.022) (Table 1).
We sampled a total of 1,082 individual trees. There were fewer trees at the forest edge than in the forest interior (F = 5.071; p = 0.025). The vegetation structure also differed significantly between the forest edge and the interior. The total basal area of trees at edges (12.44 m2 ha−1) was significantly lower than the mean basal area in the forest interior (36.71 m2 ha−1) (F = 11.157; p = 0.005). The mean tree height at the edge (7.42 m) was significantly lower than the mean tree height in the interior (9.52 m) (F = 4.245; p = 0.047). There were no significant differences in damaged and standing dead trees between the forest edge and interior.
We found that tree diameter distribution for the edge and the interior followed a negative logarithmic curve (Fig. 2a). There were no significant differences between the edge and the interior in the smallest three diameter classes. However, there were significant differences for three largest diameter classes, with the highest density of large trees found in the forest interior (see Fig. 2a). There were more trees at the forest edge in the first class of the height distribution (0–5 m) because shorter trees predominated in this system (Fig. 2b). The classes that comprised taller individuals (15–20, 30–35 and 35–40 m) were significantly more abundant in the forest interior (Fig. 2b).
Relationship between forest structure and microclimatic edge effects
Our best GLMs showed that the vegetation structure was strongly related to the microclimate of edge and interior habitats (Table 2; Fig. 3; see Appendix S1 in Supporting Information for the results of all tested models). Microclimatic conditions in the forest edges were related to the damage of individual trees. The decrease in relative humidity at the forest edge was positively associated with the increase in the proportion of dead trees (Table 2a; Fig. 3a). However, other plausible models (ΔAICc < 2) showed significant effects of the maximum air temperature and wind speed on the proportion of dead trees (Appendix S1). In addition, our models showed that the proportion of tree canopy damage significantly increased with increasing wind speed (Table 2b; Fig. 3b). Finally, the models indicated that mean tree height and basal area significantly decreased when the air temperature and wind speed increased and the relative air humidity decreased (Table 2c, d; Fig. 3c–g).
The best models of forest structure variables in relation to microclimatic changes within large fragments of tableland Atlantic forest. a The effect of proportional relative air humidity on damaged individual trees. b The effect of proportional wind speed on the proportion of standing dead trees. c, d The effect of proportional maximum air temperature, relative air humidity and wind speed on forest basal area. e–h The effect of proportional maximum air temperature, relative air humidity and wind speed on mean tree height. The response variables are represented by the fitted values from the GLMs, considering all selected MEMs. The numerical results graphically shown here are also presented in Table 2
Discussion
We report that forest structure, tree damage and standing dead trees are strongly related to the microclimate of edge habitats. We showed that the mean height and mean basal area of tree species are the best structural metrics with which to examine edge effects. For both metrics, we found that all of the microclimatic variables that we examined were selected in their respective best-fit GLMs, with significant effects and similar patterns. We also demonstrated that standing dead trees are strongly related to microclimatic variables, which can increase tree mortality by changes to physiological (humidity and temperature) and physical (wind speed) variables. We found a positive relationship between wind speed and canopy damage.
Similar differences in the microclimatic variables between the forest edge and interior have also been reported for other forest types, such as tropical forests in the Amazon, Panama and Australia (Kapos 1989; Chen et al. 1993; Camargo and Kapos 1995; Turton and Freiburger 1997; Williams-Linera 1990; Davies-Colley et al. 2000), Atlantic Forest (Ramos and Santos 2006; Pinto et al. 2010; Ewers and Banks-Leite 2013) and temperate forests (Chen et al. 1993). However, the relationships between microclimatic changes and forest structure are poorly investigated overall, particularly in fragments of the Atlantic Forest. These microclimatic distinctions may be the most important cause of the forest structural differences between the edge and the interior (Laurance et al. 1998, 2002; Harper et al. 2005).
The mean basal area and mean height were lower at the forest edge than in the forest interior, illustrating how edge effects may disrupt the forest structure of fragmented landscapes (also see Laurance et al. 1998, 2002; Harper et al. 2005; Carvalho et al. 2007). Our results on the tree density of the forest edge and interior are not consistent with some previous findings for tropical forests; others have reported the tree density at the edge generally exceeds that in the interior (Williams-Linera 1990; Murcia 1995). However, other studies (see Oosterhoorn and Kappelle 2000) have found the same pattern of tree density as we documented in this study. To note, trees density can vary, increasing or decreasing, over time with fragmentation dynamics (Williams-Linera 1990; Laurance et al. 2002; Harper et al. 2005).
Large tree abundance was greater in the forest interior than at the edge, where the mean basal area was also higher (~2.5 times) than in the edge habitat. Similarly, the mean height of the trees in the interior was 2.1 m higher than the mean height of the trees at the edge. Therefore, forest interiors contain significantly more large trees and, thus, greater basal areas, which are indicators of lower vegetation disturbance (Ferreira and Laurance 1997; Laurance et al. 1998, 2000, 2007; Carvalho et al. 2007). The high basal area in the forest interior, which are indicators of the carbon-storage potential in the tree biomass (Phillips et al. 1998; Laurance et al. 2004, 2009), suggest that the above ground biomass in the forest interior can contain more carbon than that at the forest edge.
At forest edges, wind is one of the principal sources of trees mortality (Laurance et al. 1998, 2000; Ferreira and Laurance 1997; Laurance and Curran 2008). Although wind speed significantly differed between the edges and interior in our study, this variable was not the best predictor for the standing dead tree density gradient; instead, the standing dead tree density was better predicted by humidity. Thus, we can infer that the greater numbers of standing dead trees at the forest edge could be more likely due to factors that affect trees ecophysiology, such as variations in relative humidity and air temperature (Table S1 in Supporting Information). Extreme events that involve low relative humidity and high temperature have been strongly suggested to be the principal causes of tree mortality. According to Kapos (1989) and Laurance et al. (2001), the lack of water and increased air temperature at the forest edge may cause physiological changes that play an important role in tree mortality. In contrast, wind is considered to be a cause of physical disturbance (loss of vegetation biomass, sensu Grime 2001) in forests, an idea supported by the relationship between wind speed and the increased damage to tree canopies.
Our models show that the decrease in mean basal area and mean tree height at edges was related to an increase in wind speed. This relationship may be explained by the occurrence of increased tree canopy damage (also see Laurance et al. 1998, 2000). Higher wind speeds have been found to cause greater crown damage and a decrease in mean forest height. However, as proposed by O’Brien et al. (1995) and Chave et al. (2005), tree height is also associated with basal area. In this case, greater basal area is associated with greater tree height. Thus, wind causes larger trees to fall and decreases their resilience (Laurance et al. 2000), which in turn decreases the mean basal area and height of trees at the forest edge over the long term (in support of this idea, a linear Pearson’s correlation between the basal area and the mean height of trees in our study area resulted in F = 11.421; p = 0.001).
Other microclimatic conditions recorded at the forest edge, i.e., low humidity and high temperature, are responsible for biomass loss in tropical forests (Kapos 1989; Laurance et al. 2001; Rolim et al. 2005) and result in negative effects on forest height and basal area (see Williams-Linera 1990; Laurance et al. 2000; Harper et al. 2005). In our study, the results of the GLMs between forest structure and microclimatic variables could therefore be influenced by the following two factors: (i) the rapid mortality of pioneer tree species (which increases the number of standing dead trees) (see Laurance et al. 2002; Santos et al. 2012) and the regeneration of species that naturally only reach small sizes (which decreases the forest structure development) at edges (Van Den Berg and Oliveira-Filho 1999) and (ii) the microclimatic conditions at forest edges can induce physiological and physical changes in trees and cause them to become unable to reach large sizes (basal area and height) (see Murcia 1995; Laurance et al. 2002; Harper et al. 2005).
However, the impact of microclimatic conditions at edges on the vegetation structure within forest fragments is dependent on the landscape matrix and can be minimized to the point that microclimatic differences between the edge and interior are not noticeable (Pinto et al. 2010). At our field site, all the forest edges were delineated by roads adjacent to Eucalyptus sp. plantations. Although these areas are considered forest plantations, they did not minimize the microclimatic differences found between the forest edge and interior (Table 1). Alternatively, the mitigation was not sufficient to amend the impact of edge effects on forest structure.
We report that the microclimatic conditions found at forest edges have significant impacts on vegetation structure and caused the forest edges to be more susceptible to structural disturbance and tree mortality (Williams-Linera 1990; Murcia 1995; Kapos 1989; Laurance et al. 1998, 2002; Harper et al. 2005). These results confirm our initial three hypotheses. Our results also suggest that the Brazilian Atlantic Forest is suffering from serious impacts of edge effects. Because edge effects may penetrate up to 100 m into the interiors of a tropical forest fragment (Laurance et al. 1998, 2011; Tabarelli et al. 1999, 2004; Pütz et al. 2011), substantial areas of large forest fragments may be experiencing negative effects from the creation of forest edges.
References
Bennett AF, Saunders DA (2010) Habitat fragmentation and landscape change. In: Sodhi N, Ehrlich P (eds) Conservation biology for all. Oxford University Press, Oxford, pp 88–106
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Camargo JLC, Kapos V (1995) Complex edge effects on soil moisture and microclimate in central Amazonian forest. J Trop Ecol 11:205–211
Carvalho WAC, Oliveira-Filho AT, Fontes MAL, Curi N (2007) Variação espacial da estrutura da comunidade arbórea de um fragmento de floresta semidecídua em Piedade do Rio Grande, MG, Brasil. Rev Bras Bot 30:315–335
Chave J, Andalo C, Brown S, Cairns MA et al (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oceologia 145:87–99
Chen J, Franklin JF, Spies TA (1993) Contrasting microclimates among clearcut, edge, and interior of old growth Douglas-fir forest. Agric For Meteorol 63:219–237
Chiarello AG (1999) Effects of fragmentation of the Atlantic forest on mammal communities in southeastern Brazil. Biol Conserv 89:71–82
Crawley MJ (2007) The R Book. John Wiley & Sons, West Sussex
D’Angelo SA, Andrade ACS, Laurance SG, Laurance WF, Mesquita RCG (2004) Inferred causes of tree mortality in fragmented and intact Amazonian forests. J Trop Ecol 20:243–246
Davies-Colley RJ, Payne GW, Van Elswijk M (2000) Microclimate gradients across a forest edge. N Z J Ecol 24:111–121
Diniz-Filho JAF, Bini LM, Hawkins BA (2003) Spatial autocorrelation and red herrings in geographical ecology. Glob Ecol Biogeogr 12:53–64
Diniz-Filho JAF, Rangel TFLVB, Bini LM (2008) Model selection and information theory in geographical ecology. Glob Ecol Biogeogr 17:479–488
Dray S, Legendre P, Peres-Neto P (2006) Spatial modeling: a comprehensive framework for principal coordinate analysis of neighbor matrices (PCNM). Ecol Model 196:483–493
Eisenlohr PV (2014) Persisting challenges in multiple models: a note on commonly unnoticed issues regarding collinearity and spatial structure of ecological data. Braz J Bot. doi:10.1007/s40415-014-0064-3
Ewers RM, Banks-Leite C (2013) Fragmentation impairs the microclimate buffering effect of tropical forests. PLoS One 8(3):e58093
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Ann Rev Ecol Evol Syst 34:487–515
Ferreira LV, Laurance WF (1997) Effects of forest fragmentation on mortality and damage of selected tree in central Amazonia. Conserv Biol 20:243–246
Fortin MJ, Dale MRT (2005) Spatial analysis: a guide for ecologists. Cambridge University Press, Cambridge
Gibson L, Lee TM, Koh LP et al (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–381
Grime JP (2001) Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Wiley, Chichester
Harper KA, Macdonald SE, Burton PJ et al (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
IBGE (Fundação Instituto Brasileiro de Geografia e Estatística) (1987) Folha SF.34 Rio Doce: geologia, geomorfologia, pedologia, vegetação e uso potencial da terra. Projeto Radambrasil, Rio de Janeiro
Kapos V (1989) Effects of isolation on the water status of forest patches in the Brazilian Amazon. J Trop Ecol 5:173–185
Laurance WF, Curran TJ (2008) Impacts of wind disturbance on fragmented tropical forests: a review and synthesis. Austral Ecol 33:399–408
Laurance WF, Laurance SG, Ferreira LV, RankindeMerona JM, Gascon C, Lovejoy TE (1997) Biomass collapse in Amazonian forest fragments. Science 278:1117–1118
Laurance WF, Ferreira LV, Merona JMR, Laurance SG (1998) Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79:2032–2040
Laurance WF, Delamonica P, Laurance SG, Vasconcelos HL, Lovejoy TE (2000) Rainforest fragmentation kills big trees. Nature 404(6780):836
Laurance WF, Williamson GB, Delamonica P, Oliveira A, Lovejoy TE, Gascon C, Pohl L (2001) Effects of a strong drought on Amazonian forest fragments and edges. J Trop Ecol 17:771–785
Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, Laurance SG, Sampaio E (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Laurance WL et al (2004) Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428:171–175
Laurance WF, Nascimento H, Laurance SG, Andrade A, Ribeiro J, Giraldo J, Lovejoy TE, Condit R, Chave J, D’Angelo S (2006) Rapid decay of tree community composition in Amazonian forest fragments. Proc Natl Sci USA 103:19010–19014
Laurance WF, Nascimento HEM, Laurance SG, Andrade A, Ewers RM (2007) Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS One 2(10):e1017
Laurance SGW et al (2009) Long-term variation in Amazon forest dynamics. J Veg Sci 20:323–333
Laurance WF, Camargo JLC, Luizão RCC, Laurance SG et al (2011) The fate of Amazonian forest fragments: a 32-year investigation. Biol Conserv 144:56–67
Magnago LFS, Edwards DP, Edwards FA, Magrach A, Martins SV, Laurance WF (2014) Functional attributes change but functional richness is unchanged after fragmentation of Brazilian Atlantic forests. J Ecol 102(2):475–485
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Tree 10:58–62
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
O’Brien ST, Hubbell SP, Condit PSR, Foster RB (1995) Diameter, height, crown, and age relationship in eight neotropical tree species. Ecology 76:1926–1939
Oosterhoorn M, Kappelle M (2000) Vegetation structure and composition along an interior-edge-exterior gradient in a Costa Rican montane cloud forest. For Ecol Manag 126:291–307
Pardini R, Bueno ADA, Gardner TA, Prado PI, Metzger JP (2010) Beyond the fragmentation threshold hypothesis: regime shifts in biodiversity across fragmented landscapes. PLoS One 5(10):e13666
Peixoto AL, Silva IM (1997) Tabuleiro forests of northern Espirito Santo, Southeastern Brasil. In: Davis SD, Heywood VH (eds) Centres of plant diversity—a guide and strategy for their conservation, 1st edn. WWF and IUCN Publisher, Cambridge, pp 369–372
Peixoto AL, Simonelli M (2007) Florestas de Tabuleiro. In: Simonelli M, Fraga CN (eds) Espécies da flora ameaçadas de extinção no estado do Espírito Santo, 1st edn. IPEMA, Vitória, pp 33–44
Peixoto AL, Silva I, Pereira OJ, Simonelli M, Jesus RM, Rolim SG (2008) Tabuleiro Forests North of the Rio Doce: Their Representation in the Vale do Rio Doce Natural Reserve, Espírito Santo, Brazil. Memoirs of the New York Botanical Garden, New York, pp 319–350
Peres-Neto PR, Legendre P (2010) Estimating and controlling for spatial structure in the study of ecological communities. Glob Ecol Biogeogr 19:174–184
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Phillips OL, Malhi Y, Higuchi N et al (1998) Changes in the carbon balance of tropical forests: evidence from long-term plots. Science 282:439–442
Pinto SRR, Mendes G, Santos AMM, Dantas M, Tabarelli M, Melo FPL (2010) Landscape attributes drive complex spatial microclimate configuration of Brazilian Atlantic forest fragments. Trop Conserv Sci 3:389–402
Pütz S, Groeneveld J, Alves LF, Metzger JP, Huth A (2011) Fragmentation drives tropical forest fragments to early successional states: a modelling study for Brazilian Atlantic forests. Ecol Model 222(24):1986–1997
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Ramos FN, Santos FAM (2006) Microclimate of Atlantic forest fragments: regional and local scale heterogeneity. Braz Arch Biol Technol 49:935–944
Rangel TFLVB, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Ribeiro MC, Metzer JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Rolim SG, Jesus RM, Nascimento HEM, Couto HTZ, Chambers JQ (2005) Biomass change in an Atlantic tropical moist forest: the ENSO effect in permanent sample plots over a 22 year period. Oecologia 142:387–410
Santos GGA, Santos BA, Nascimento HEM, Tabarelli M (2012) Contrasting demographic structure of short- and long-lived pioneer tree species on Amazonian forest edges. Biotropica 44:771–778
Srbek-Araujo AC, Chiarello AG (2006) Registro recente de harpia, Harpia harpyja (Linnaeus) (Aves, Accipitridae), na Mata Atlântica da Reserva Natural Vale do Rio Doce, Linhares, Espírito Santo e implicações para a conservação regional da espécie. Rev Bras Zool 23:1264–1267
Tabarelli M, Mantovani W, Peres CA (1999) Effects of habitat fragmentation on plant guild structure in the montane Atlantic forest of southeastern Brazil. Biol Conserv 91:119–127
Tabarelli M, Silva MJC, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of neotropical forests. Biodivers Conserv 13:1419–1425
The R Foundation for Statistical Computing (2014) R: a language and environment for statistical computing. Vienna (Austria). http://www.R-project.org/. Accessed 15 Apr 2014
Turton SM, Freiburger HJ (1997) Edge and aspect effects on the microclimate of a small tropical forest remnant on the Atherton Tableland, Northeastern Australia. In: Laurance WF, Bierregaard RO Jr (eds) Tropical forest remnants. Ecology, management and conservation of fragmented communities. University of Chicago Press, Chicago, pp 45–54
Van Den Berg E, Oliveira-Filho AT (1999) Spatial partitioning among tree species within an area of tropical montane gallery forest in south-eastern Brazil. Flora 194(2/3):249–266
Williams-Linera G (1990) Vegetation structure and environmental conditions of forest edges in Panama. J Ecol 78:356–373
Acknowledgments
We are grateful to CAPES for a doctoral scholarship in Brazil and for an overseas doctoral scholarship (the Sandwich Program) provided to the first and second authors. We thank the Projeto Floresta-Escola, FAPEMIG and CNPQ (Grant No. 477780/2009-1) for financial support. We thank Vale Natural Reserve, especially Gilberto Terra, for logistical support. We thank Sooretama Biological Reserve for allowing us to conduct this study, and Fibria Celulose S. A. for logistical support and for access to the study areas. We also thank Fabio A. Matos, Renata Pagotto, Vinicius Guss, Stephano, Glaúcia Tolentino, Túlio, Átila, Talissa Harb and Domingos Folli, among others, for their help with field activities. JAAMN was awarded a CNPq scholarship for scientific productivity. LFSM is supported by a PNPD program of CAPES. MFR was supported by Floresta Ecolola project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jefferson Prado, Pedro V. Eisenlohr and Ary T. de Oliveira-Filho.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Magnago, L.F.S., Rocha, M.F., Meyer, L. et al. Microclimatic conditions at forest edges have significant impacts on vegetation structure in large Atlantic forest fragments. Biodivers Conserv 24, 2305–2318 (2015). https://doi.org/10.1007/s10531-015-0961-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-015-0961-1