Abstract
Urban expansion threatens global biodiversity through the destruction of natural and semi-natural habitats and increased levels of disturbance. Whilst woodlands in urban areas may reduce the impact of urbanisation on biodiversity, they are often subject to under or over-management and consist of small, fragmented patches which may be isolated. Effective management strategies for urban woodland require an understanding of the ecology and habitat requirements of all relevant taxa. Yet, little is known of how invertebrate, and in particular moth, assemblages utilise urban woodland despite being commonly found within the urban landscape. Here we show that the abundance, species richness, and species diversity of moth assemblages found within urban woodlands are determined by woodland vegetation character, patch configuration and the surrounding landscape. In general, mature broadleaved woodlands supported the highest abundance and diversity of moths. Large compact woodlands with proportionally less edge exposed to the surrounding matrix were associated with higher moth abundance than small complex woodlands. Woodland vegetation characteristics were more important than the surrounding landscape, suggesting that management at a local scale to ensure provision of good quality habitat may be relatively more important for moth populations than improving habitat connectivity across the urban matrix. Our results show that the planting of broadleaved woodlands, retaining mature trees and minimising woodland fragmentation will be beneficial for moth assemblages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban expansion threatens global biodiversity through the destruction of natural and semi-natural habitats and increased levels of disturbance (Grimm et al. 2008). Projections for 2030 estimate that urban land cover will have tripled compared to 2000 (Seto et al. 2012), so understanding how urbanisation affects different taxa, and what actions may reduce detrimental effects, is essential for biodiversity conservation.
Urban areas are one of the most dramatic forms of habitat conversions (McKinney 2006). However, green spaces within urban areas can be important for mitigating the impacts of urbanisation on biodiversity, and can hold relatively rich wildlife communities. Woodlands are the most important semi-natural habitat within European urban landscapes and have the capacity to accommodate a large number of species (Croci et al. 2008). For instance, bird species richness is often higher in urban compared to rural woodland due to greater food resources and more favourable microclimatic conditions in urban woodlands (Atchison & Rodewald 2006). Although site characteristics are important in determining species presence, the fragmented nature of urban woodland patches means that the surrounding landscape can be of similar importance. Woodland isolation, the proximity of buildings, and the extent of urbanisation in the surrounding landscape are known to influence the species richness of well-studied taxa such as birds and small mammals (Croci et al. 2008; Morimoto et al. 2006; Sadler et al. 2006). Management strategies for urban woodland are being developed in many countries as they are beneficial for human health (Matsuoka & Kaplan 2008; Takano et al. 2002) and biodiversity conservation (Cornelis & Hermy 2004; Croci et al. 2008). However, information is scarce or absent for many taxa and as a consequence there is a lack of consideration of these in management plans.
Moths (Lepidoptera) are an important component of terrestrial ecosystems due to their role as food resources for birds (Wilson et al. 1999) and small mammals (Vaughan 1997), as pollinators (Proctor et al. 1996; Devoto et al. 2011), and nutrient recyclers (Merckx et al. 2013). There have been substantial population declines in many moth species, including two-thirds of analysed common macromoth species in the UK (Conrad et al. 2006; Fox et al. 2013). The main drivers of decline are expected to include climate change (Fox et al. 2013), agricultural intensification (Merckx et al. 2012a) and afforestation with non-native trees and a decline in traditional management regimes for woodlands (Warren & Bourn 2011).
Although considerable efforts have been made to identify key drivers of moth abundance and diversity in agricultural landscapes (e.g. Fuentes-Montemayor et al. 2011; Merckx et al. 2012a; Jonason et al. 2013), the effects of urbanisation on moth populations remain poorly understood (Fox 2013). Despite the suggestion by Summerville & Crist (2008) that future research on forest Lepidoptera should include an understanding of the importance of urban woodland in retaining viable and diverse moth communities, research is lacking on this topic. In St Petersburg, Kozlov (1996) found that habitat fragmentation of urban woodland was the main driver of population declines in micromoths due to a reduction in colonization rates. Bates et al. (2014) found that species richness and abundance within urban gardens was negatively affected by urbanisation, although certain species did respond positively to the urban matrix. In contrast, in the San Francisco Bay Area, Rickman & Connor (2003) found that the extent of urbanization was not associated with species richness or total abundance of leaf-miner moth communities.
In agricultural woodlands, moth abundance and richness are positively related to a high diversity of tree species, a high proportion of native trees (Fuentes-Montemayor et al. 2012), and herbaceous plant species richness (Usher & Keiller 1998). However, the composition of urban woodlands is often quite different to that of rural woodlands. For example, understory vegetation in urban woodland tends to be dominated by short life-span (annual) species (Vallet et al. 2010), which may have negative consequences for moth species dependent on specific food plants. Additionally, stress factors including restrictive soil volume, high salinity, and trampling can restrict the tree species that are capable of successfully growing in urban environments (Alvey 2006). The response of moths to fragmented urban woodlands may therefore differ considerably from the response within agricultural landscapes. Woodland patch configuration (shape and size) is often a strong determinant of moth abundance in non-urban landscapes (Merckx et al. 2012b; Slade et al. 2013), and at the landscape scale, the presence of isolated trees, hedgerow trees and small woodland patches can function as ‘stepping stones’ for macromoths (Slade et al. 2013). In urban landscapes, reductions in garden size, switching from vegetated to hard surfaces, and the expansion of urban developments is expected to reduce resource availability for moths, but this has rarely been studied (Fox 2013; but see Bates et al. 2014).
Effective management strategies for the conservation of biodiversity in urban woodlands require comprehensive data on the ecology and habitat requirements of all relevant taxa (Lindenmayer et al. 2006). Minimal management of urban woodland can lead to changes in vegetation structure (e.g. increased tree and shrub density, denser canopy cover) which may have contrasting species-specific effects (e.g. Smith & Gehrt 2010). Conversely, over-management, such as the removal of understory to enhance the recreational value of the woodland, can negatively affect a range of taxa (e.g. birds; Heyman 2010). It is not clear how moths may respond to urban woodland management. Also, the limited management advice available focuses on macromoths, with little attention given to the habitat requirements of the micromoths (Blakesley et al. 2010; Bland & Young 1996).
In this paper we investigate how woodland vegetation characteristics (e.g. tree species richness), patch configuration (e.g. woodland size), and the surrounding landscape (e.g. proportion of urban areas) influence moth assemblages. We aim to use this information to build an evidence base so that conserving moth diversity can be incorporated into management plans. Specifically, we address the following questions:
-
1.
How are moth assemblages determined by local woodland characteristics (vegetation structure and patch configuration) and the composition, spatial configuration and heterogeneity of the surrounding landscape?
-
2.
Are woodland site characteristics more important than the characteristics of the surrounding landscape in determining moth abundance, species richness and species diversity?
-
3.
What practical applications do our findings have for the management of urban woodland for moth assemblages?
Materials and methods
Site selection
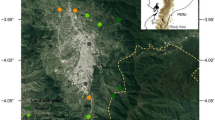
A total of 32 urban woodland study sites in central Scotland (Fig. 1) were identified using Ordnance Survey digital maps (EDINA Digimap Ordnance Survey Service 2013). Urban areas were designated as those where urban cover was the dominant land use within a 1 km grid square as categorised by the Centre for Ecology and Hydrology Land Cover Map 2000. Sites were selected by size, longitude, and degree of urbanisation in the surrounding 1 km using a stratified random sampling method. Selected woodlands were a minimum of 50 years old, were either broadleaved or consisted of a mixture of conifer and broadleaved trees, and were surveyed once between May 19th to September 1st 2011. Sites were surveyed in random order through the field season to avoid any spatial or temporal bias. We recognise that a single visit to each site provides only a coarse description of local moth assemblages, but we adopted this approach in order to maximise the number of sites and cover a wider range of characteristics when attempting to determine the factors influencing moths in urban woodlands.
Vegetation surveys
Daytime vegetation surveys were conducted within a week of each moth survey. Four circular plots with radii of 20 m were randomly located within each woodland patch (each within 50 m of a corresponding moth trap). At each of the four plots, all trees were counted, identified to at least genus level, and tree basal area measured (only trees ≥7 cm in diameter at breast height were measured). Dominant ground cover type (‘grass’, ‘ferns’, ‘moss’, ‘herbs’ or ‘bare ground’) and understory cover (%) using the Domin scale (Rodwell 2006) was visually estimated at 20 evenly spaced points within each plot. Results of the four vegetation plots were combined using the mean to provide a description of each woodland patch. Visual assessment of the remaining woodland showed that vegetation surveys were representative of the entire woodland patch.
Moth surveys
Moths were captured using portable 6 W heath light traps using E7586 9” actinic tube lights which were run on 12 V batteries. A total of four traps were placed within each woodland; two traps were placed along woodland edge and two within the woodland interior (≥20 m from the woodland edge). Traps were selectively positioned to ensure that similar light levels were emitted (i.e. ensuring that vegetation located around the trap did not obscure its range). When possible, traps were placed a minimum of 100 m apart to ensure independence (Dodd et al. 2008). The attraction radii of heath light traps are commonly between 10–30 m depending on moth family (Truxa & Fiedler 2012; Merckx & Slade 2014), so it is unlikely that even in smaller woodlands, where it was not possible to maintain a full 100 m separation, that distance between traps was an issue. Additionally, careful placement ensured that vegetation provided an additional physical and light-impermeable barrier between traps. Positioning traps in the vicinity of streetlights was avoided along the woodland edge. Lights were activated 30 min after sunset and remained on for the following four hours (the length of the shortest night in the study area). Captured insects were euthanized and stored for later identification. Surveys were only conducted in dry weather, when temperature was ≥10 °C and wind force ≤ Beaufort scale 4.
Landscape analysis
Moth trap locations were plotted using ArcGIS 10 (ESRI Inc 2013) and the centre point of the four traps within each site determined. Buffers of 250, 500, 1,000, 1,500, 2,000, 2,500, and 3,000 m radius were created around this central point reflecting the range of spatial scales associated with non-migrating moth species (Merckx et al. 2009; Merckx et al. 2010a; Nieminen et al. 1999; Slade et al. 2013). Data from the OS MasterMap Topography Layer (EDINA Digimap Ordnance Survey Service 2013) was used to classify the landscape within each buffer into a set of discrete biotope types. These were (i) greyspace (buildings, structures, roads, and paths); (ii) green space (gardens, parkland, managed grassland, rough grassland, and scrubland); (iii) inland fresh water and (iv) woodland (coniferous, deciduous and mixed woodland). Woodland Euclidean nearest neighbour distance (ENN, the mean value of ENN distances between all woodland patches within the landscape) and the Shannon diversity index (SHDI, a measure of landscape heterogeneity incorporating the relative abundance of the four biotype types) were calculated as previous studies have found these variables to be important (Fuentes-Montemayor et al. 2012). The proportion of land covered by each biotope, woodland ENN, and SHDI were calculated for each buffer scale using Fragstats v4.0 (McGarigal et al. 2012).
Statistics
Statistical analyses were undertaken using R version 2.14 (R Core Team 2012). The lme4 package (Bates et al. 2013) was used for statistical analysis, whilst ggplot2 (Wickham 2009) and the effects package (Fox 2003) were used for graphics. The software package PAST (Hammer et al. 2001) was used to calculate diversity indices for macro- and micromoths. We selected Margalef diversity because it can deal with occasions where number of individuals in a trap is equal to number of species (a frequent occurrence), is commonly used as a measure of spatial species diversity, and because its biological interpretation is straightforward (Magurran 1988).
We performed a series of Generalised Linear Mixed-Effects Models (GLMMs; Zuur 2009) to incorporate both within and between-site variance. This allowed us to account for confounding factors which may cause within stand variance in sampling efficiency (i.e. background ambient light from the urban landscape). Using GLMMs we were able to quantify the influence of woodland characteristics and landscape metrics on moth abundance, richness, and diversity. We ran models using moths per trap (n = 128) as the response variable, with ‘site’ included in all models as a random (grouping) factor. Based upon the scientific literature on the ecology of woodland moths and typical management regimes undertaken in urban woodland the following predictor variables were included in the starting model: (i) woodland vegetation characteristics: tree species richness, percentage of native trees, average tree basal area and understory cover (covariates), and woodland type (fixed factor); (ii) patch configuration: woodland size and woodland shape (patch perimeter divided by the minimum perimeter possible for a maximally compact patch of the same areas; equals one when the patch is maximally compact and increases as shape becomes more irregular; McGarigal et al. 2012), both as covariates, trap location (woodland edge versus woodland interior; fixed factor); (iii) landscape metrics. Temperature (nightly average) and date were included in all models as covariates to account for any potential temporal bias in the results. Given the high collinearity between landscape metrics (i.e. between the proportions of different biotope types or the same biotope type at a variety of spatial scales) preliminary analyses were conducted to determine which landscape metrics should be included in each model. Individual GLMM models (one for each landscape parameter at each spatial scale) were constructed and marginal R2 values (determined following Nakagawa & Schielzeth (2012)) calculated to quantify the amount of variation in the data explained by each landscape parameter. For each model we used moth abundance, richness, or diversity (n = 128) as the response variable, a landscape parameter at a specific scale as a covariate, and ‘site’ as a random factor. We selected the landscape parameter which explained the most variation (i.e. highest marginal R2 value) and included it in the relevant model.
We also included interactions between woodland size and shape, woodland size and trap location, and woodland shape and trap location as these have previously been identified as important predictors of moth distributions (Fuentes-Montemayor et al. 2012). All predictor variables were tested for collinearity, however none were considered to be strongly correlated based upon a Pearson correlation coefficient of ≥0.6 and p ≤ 0.05. Continuous predictor variables were centred and standardized following Schielzeth (2010). Models containing response variables using count data (e.g. moth abundance or richness) were fitted with a Poisson distribution. Models containing continuous response variables (e.g. moth diversity) were fitted with a Gaussian distribution. All models were validated by visual examination of residuals (e.g. plotting residuals versus fitted values to check for constant variance; Crawley, 2012). Models were checked and found to be not spatially auto correlated using the Mantel test in the ade4 package within R (Dray and Dufour 2007).
We present the results of each full model including standardised parameters and confidence intervals for all explanatory variables. Inferences on the effect of each parameter were made by (i) comparing its standardized estimate with other predictor variables to determine relative importance, (ii) the upper and lower 95 % quantiles of each parameter distribution obtained from N = 2,000 simulated draws from the estimated distribution (Gellman & Hill 2007), and (iii) a comparison of models excluding each parameter in turn using likelihood ratio tests (LRTs) (Faraway 2005). LRTs of main effect parameters also involved in interactions were performed by comparing a model excluding the main effect term to a model including all main effects (but not interactions) only. Prediction plots were constructed by undertaking simulated draws (n = 2,000) from the estimated distribution of one explanatory variable whilst maintaining all other parameters in the model at their median observed values. This allowed the percentage increase (or decrease) and 95 % confidence intervals to be calculated for a predicted change in moth abundance, diversity, or species richness relative to changing one parameter.
Results
We recorded a total of 33 tree species/genera within our survey (Appendix 1), 23 of which are thought native to the British Isles. Tree species richness ranged from 2 to 13 species per site, whilst tree density varied from 207 to 1,766 trees per ha. Mixed woodland was composed of, on average, 24 % coniferous trees, 76 % broadleaved trees. Dominant ground cover was grouped into two categories (‘bare ground’ and ‘vegetated’) for analysis purposes; the dominant ground cover of 18 of the 34 sites was ‘bare ground’ whilst each descriptive subcategory of ‘vegetation’ (grass, ferns, moss, herbs) was dominant in fewer than five sites each .
We collected a total of 1,198 micromoths belonging to 72 species and 16 families and 1,656 macromoths from 103 species and eight families. A mean of 49 (±8) macromoths comprising 14 (±1) species were collected per woodland site. A mean of 34 (±14) micromoths of 6 (±0.5) species were collected per woodland site. We recorded three species of micromoth which are noted as nationally scarce (Davis 2012; Appendix 2) and seven species of macromoth which are classified as of conservation concern (Fox et al. 2006; Appendix 3).
The importance of landscape for macro and micromoths
There was considerable variety in the composition of the landscape surrounding each woodland; urban grey space ranged from 17 to 49 % coverage in the surrounding 1 km, whilst green space (including urban gardens) varied from 36 to 71 %. In general, the small effect size of the landscape parameters indicated that the composition of the surrounding urban matrix was a poor predictor of moth abundance, species richness, or diversity (Fig. 2). Macromoth populations showed the strongest relations to landscape parameters at a relatively small scale (250 m) whilst micromoths were influenced at larger scales (>1,000 m; Fig. 2). On average, the relative importance of the landscape for micromoths was double that of macromoths (Tables 1 & 2).
R2 values obtained from poisson GLMM models comparing the percentage of landscape covered by each biotope type at a variety of spatial scales and; a macromoth abundance, b micromoth abundance, c macromoth diversity, d micromoth diversity, e macromoth richness, f micromoth richness. We calculated marginal R2 values for mixed effect models a to f using moths per trap as the response variable
Macromoth abundance
The inclusion of temperature, average tree basal area, woodland type, trap location, and interactions between woodland size and shape, trap location and shape, and trap location and woodland size all significantly improved the fit of the macromoth abundance model (Table 1). Woodland type was the most important predictor within the model; moth abundance was 69 % (68–70 %) higher in broadleaved woodlands compared to mixed woodlands (Appendix 4.1). A change in average tree basal area from 20 to 40 cm2 within broadleaved woodlands is associated with an increase the abundance of macromoths from 14 to 24 individuals (65, 95 % CI 56–74 %; Fig. 3a). The interaction between woodland size and shape indicated that as woodland patch size increases, macromoth abundance increases in complex woodlands, but remains relatively constant in compact woodlands (Appendix 5.3). Additionally, the model was significantly improved by the interaction between trap location and woodland shape, indicating that macromoth abundance in the woodland interior increased with woodland shape but was lower at woodland edges. However, the effect size was marginal (Table 1; Appendix 5.1). In contrast, the interaction between trap location and woodland size was a relatively important predictor; macromoth abundance at the woodland edge marginally decreased as woodland size increased, whilst there was a considerable increase in abundance within the woodland interior with woodland size (Appendix 5.2).
Measurements of moth assemblages plotted against the strongest continuous predictor in each model. Dots are observed data whilst the lines are predictions of moth abundance, diversity and richness with varying levels of the continuous predictor. Dashed lines represent 95 % confidence intervals around the predictions. All prediction plots are calculated for broadleaved woodland and by setting all continuous parameters at their median observed values in the model. In models where there are no significant continuous predictor variables, the strongest categorical predictor is shown. Error bars represent 95 % confidence intervals around the predictions
Macromoth diversity
The inclusion of temperature, average tree basal area and woodland type all significantly improved the fit of the macromoth diversity model (Table 1). Macromoth diversity was 39 % (38–41 %) greater in broadleaved woodlands in comparison to mixed woodland (Appendix 4. 2), and an increase in tree basal area within broadleaved woodlands from 20 to 40 cm2 is associated with an increase the species diversity of macromoths by 23 % (15–29 %; Fig. 3b).
Macromoth richness
As with diversity, the model of macromoth species richness was significantly improved by the inclusion of temperature, average tree basal area, and woodland type (Table 1). The inclusion of the percentage of water in the surrounding 250 m of the woodland patch was also a significant predictor however had little predictive power due to a low effect size. Woodland type was the most important predictor within the model; moth species richness was 37 % (30–45 %) greater in broadleaved woodlands as compared to mixed woodland (Appendix 4. 3). Average tree basal area was also influential; an increase in average tree basal area within broadleaved woodlands from 20 to 40 cm2 is associated with an increase in macromoth richness from 7 to 10 species, an increase of 39 % (28–52 %; Fig. 3c).
Micromoth abundance
The inclusion of temperature and woodland type significantly improved the fit of the micromoth abundance model (Table 2). The standardized effect size of woodland type was almost double that of any other predictor variable; micromoth abundance was 795 % (550–1,040 %) greater in broadleaved woodlands compared to mixed woodland (Appendix 4. 4). Average tree basal area was a marginally significant predictor of micromoth abundance (Table 2); an increase in average tree basal area within broadleaved woodlands from 20 to 40 cm2 appears to increases the abundance of micromoths from 6 to 12 individuals (102, 95 % CI 91–114 %; Fig. 3d).
Micromoth diversity
The inclusion of temperature and woodland type were the only significant variables within the micromoth diversity model (Table 2), with diversity found to be 57 % (45–64 %) greater in broadleaved woodlands than mixed woodland (Fig. 3e).
Micromoth richness
The model of micromoth richness was significantly improved by the inclusion of temperature and woodland type, and marginally improved by the addition of the percentage of woodland in the surrounding 2 km (Table 2). Micromoth richness was 104 % (88–120 %) greater in broadleaved woodlands compared to mixed woodland (Fig. 3f). The inclusion of percentage woodland in the surrounding 2 km was marginally significant in improving model fit; an increase in woodland cover from 10 to 20 % in the surrounding 2 km is associated with a decrease in micromoth richness by 41 % (26–53 %).
Discussion
Understanding the impact of urbanisation on global biodiversity is vital as the rate of urban expansion continues to accelerate (Aronson et al. 2014). Urban spread is often cited as a contributory factor in the population decline and range contraction of many moth species, despite relatively little research having being conducted within the urban matrix (Fox 2013). Here, we show how moths in fragmented urban woodland respond to vegetation characteristics, patch configuration, and the surrounding landscape. Our results contribute to a greater understanding of how management of urban woodland can incorporate the needs of often neglected taxa such as moths.
The number of macromoths captured per woodland in this study was approximately half of that captured in a study conducted in the same region, in similar environmental conditions, and using the same methods, but within agricultural woodlands (Fuentes-Montemayor et al. 2012). Urban woodlands contained, on average, a third fewer macromoths species than agricultural woodlands. Similarly, abundance of micromoths was approximately a third higher in agricultural woodlands, but for this group, a similar number of species were recorded. This suggests that urban woodlands are of poorer quality than those on farmland, although the underlying cause(s) are unclear. A caveat to this is that the two studies were conducted in different years, which is likely to introduce temporal differences, although the environmental conditions (e.g. temperature) were similar.
The strongest predictor of moth assemblages for all models was woodland type; woodland comprising only of broadleaved trees contained a higher abundance, species richness, and diversity of moths than mixed woodland. A preference for broadleaved woodlands, in comparison to mixed woodlands, has also been found to be important for moth communities in agricultural landscapes (Fuentes-Montemayor et al. 2012). We might have expected species richness to be higher in mixed woodland given that species adapted to benefit from human activity, such as those feeding on exotic conifer species introduced into gardens, are showing population increases (Conrad et al. 2004; Fox et al. 2013). It may be that the relative scarcity of conifer species within urban woodlands (24 % of all trees identified in this survey) means that fragmented mixed woodlands are unable to support viable populations of conifer moth specialists. In addition, the presence of conifer species will reduce the availability and quality of broadleaved habitat available.
The abundance, species richness and diversity of macromoth assemblages and the abundance of micromoths were higher in woodlands with large average tree basal areas, a trait associated with mature woodlands (Fuentes-Montemayor et al. 2012). Younger woodlands may contain smaller and more species-poor moth assemblages because colonisation rates may be slower within a fragmented landscape. Although the permeability of the surrounding matrix and dispersal ability will determine colonisation rates of individual moth species, it is likely that mature woodlands will have remained relatively undisturbed during the process of urbanisation. Similar trends between patch age and colonisation rates explain plant species distribution in urban vegetation fragments (Bastin & Thomas 1999). Additionally, woodlands containing larger trees may provide more niches and therefore more foraging opportunities (Summerville & Crist 2008).
The differences between moth assemblages at the woodland edge and woodland interior were accentuated by woodland size and shape. Macromoth abundance was greater within the woodland interior of larger woodlands indicating an edge effect within smaller woodlands that reduces abundance. Edge effects often strongly influence insect communities in fragmented landscapes (McGeoch & Gaston 2000). Slade et al. (2013) found that forest fragments need to be larger than five hectares and require interior woodland habitat a minimum of 100 m from the woodland edge to sustain populations of forest specialist macromoth species, whilst Usher & Keiller (1998) suggest that woodlands of less than 1 ha fail to support characteristic woodland moth communities. Similarly, the shape of the woodland patch in our study appears to determine the extent of impact that the edge effect with moth assemblages in the woodland interior negatively affected as woodland shape complexity increased. These findings support Usher & Keiller (1998); Fuentes-Montemayor et al. (2012) and Merckx et al. (2012b) who suggest that compact woodland patches which expose proportionally less edge to the surrounding matrix can support larger and more diverse moth communities. Woodland edges in the urban matrix are often adjacent to habitats under high anthropogenic pressure which may determine the tree species and invertebrate populations that are able to tolerate such conditions (Lehvävirta et al. 2006). Edge effects may also explain the interaction between shape and size; macromoth abundance is highest in large complex woodlands demonstrating that woodlands of sufficient size can maintain core woodland habitat despite being irregular or elongated. Micromoth species richness was the only descriptor of moth assemblages which was influenced by its surrounding landscape. Micromoth species richness was negatively influenced by woodland cover at a variety of spatial scales, with the strongest effect at 2,000 m. These findings contrast with those of Ricketts et al. (2001); Summerville & Crist (2004); and Fuentes-Montemayor et al. (2012) who found a strong positive influence between woodland cover in the surrounding landscape and moth presence.
Woodland site characteristics were consistently more important than the surrounding landscape in determining the abundance, species richness and diversity of moths. Our findings are in accordance with those of Wood & Pullin (2002) who found that some butterfly species within the urban landscape were limited more by the availability of suitable habitat than their ability to move among habitat patches. Similarly, Angold et al. (2006) demonstrated the importance of local habitat variables over landscape variables within the urban matrix in determining carabid species distribution. Additionally, Bates et al. (2014) found that distance to woodland did not significantly influence moth species richness or abundance within urban gardens. However, our findings contrast with moth communities within fragmented woodland in an agricultural setting which are strongly influenced by the landscape (Fuentes-Montemayor et al. 2012). It may be that fragmented agricultural woodlands are pockets of suitable habitat within an ecologically poor landscape, whilst the widespread presence of urban gardens and green space in urban landscapes may facilitate dispersal. Urban gardens can maintain relatively high levels of invertebrate species richness (Smith et al. 2006) and therefore may minimise patch isolation by providing additional habitat for moth species outside of the fragmented woodland (Bates et al. 2014). The marked difference in the abundance and diversity of moths in agricultural woodlands may reflect that although the urban landscape is more permeable for moths, urban woodland is of poorer quality which restricts the abundance and diversity of moths.
Practical implications for the management of urban woodland for moth assemblages
Although rarer species (i.e. nationally scarce or of conservation concern) may require specific management plans to enhance their populations (but see Merckx et al. 2010b), the management of fragmented urban woodland patches to increase moth abundance, species richness, and species diversity should take into consideration the following general points:
-
1.
Maintaining broadleaved woodlands will support high moth abundance, species richness, and species diversity. Habitat action plans exist for the management of some urban woodland (e.g. London Borough of Richmond upon Thames 2013) which emphasise the role of planting and supporting the natural regeneration of native broadleaved species. We found no significant relationship between moth assemblages and native species which may reflect that naturalised species such as Acer pseudoplatanus (the most frequently recorded tree species during this study) are providing a suitable habitat. Although A. pseudoplatanus is negatively perceived from a management perspective as it is a non-native, our findings support those of Peterken (2001) who found little evidence that it reduces native biodiversity.
-
2.
Management strategies to retain the presence of mature trees and stands are important not only for macromoth assemblages, but also provide preferred habitats for many other taxa (e.g. bats; Perry et al. 2007), in addition to meeting the needs of the public’s perception of aesthetically pleasing woodland (Ode & Fry 2002). Old trees in urban woodlands are often perceived as a public danger due to the risk of falling branches, however minimising the removal of deadwood or retaining it within the woodland once it has fallen may prove beneficial for both macromoth assemblages and other invertebrate species (e.g. saproxylic beetles; Carpaneto et al. 2010).
-
3.
Differences in the abundance of macromoths between the woodland edge and woodland interior may result from high anthropogenic pressure in the surrounding urban matrix. Light intensity was higher at the woodland edge than within the woodland interior (Lintott et al. unpublished), which can have a detrimental impact on moth populations (Conrad et al. 2006). Integrating public safety concerns such as the preference for lit pathways through urban green space into biodiversity management plans (Luymes & Tamminga 1995) requires care as ‘edge effects’ can unintentionally be created within the woodland interior. Although not addressed in this paper, future work investigating the direct impact of anthropogenic disturbance (i.e. extent of recreational use within woodland, noise/light intensity) on urban moth assemblages would be of value.
Limitations
-
(i)
Temporal variations: Each site was surveyed only once, which provides only a coarse description of local moth assemblages within urban areas. However, we ensured that weather conditions were relatively similar during surveys and that surveying order was randomised to ensure that woodland patches with different characteristics (i.e. size or shape) were evenly surveyed throughout the season. In addition, previous studies have shown that patterns of moth community composition in relation to, for example, patch area effects remain consistent despite seasonal species turnover (Summerville and Crist 2003). Therefore, temporal variations are unlikely to have influenced the patterns detected during our study and the conclusions drawn from them. Similar techniques have provided insights into how moths respond to agricultural woodland (Fuentes-Montemayor et al. 2012); this paper uses the same methodology to give, for the first time, an indication of the species richness, abundance, and diversity of moth populations within fragmented urban woodland.
-
(ii)
Trapping effects: Even though light trapping is the most effective technique for general moth recording (in terms of the wide spectrum of species it attracts relative to the sampling effort; Waring & Townsend 2003), many moth species are not attracted to light. However, our trapping method allowed us to detect general patterns in moth abundance/richness associated with urban woodland, even if this does not reflect the habitat preferences of all moth species. Higher background ambient light along the woodland edges may have a direct impact on moth populations, however it may also impair sampling efficiency (i.e. Yela & Holyoak 1997). As trap location (edge versus interior) was not influential on all measures of moth assemblages, it is likely that where an edge effect was observed (i.e. macromoth abundance) this was a true trend rather than simply existing as an artefact of the sampling method. Determining the extent to which the surrounding ambient urban light may be impacting moth populations within urban woodland is, however, beyond the scope of this paper”.
Conclusions
In summary, woodland vegetation character, woodland patch configuration and the surrounding landscape all influence moth populations in urban woodland to different extents. The creation and maintenance of large, compact, mature, broadleaved woodland patches will enhance moth populations within the urban landscape. The importance of the surrounding landscape in determining moth distributions is less pronounced in urban landscapes than in alternative landscapes under intense human land-use including agricultural areas. The urban matrix may not be limiting the dispersal of moths, with the exception of micromoths in scarcely wooded landscapes. The detrimental impacts of edge effects make moth populations in small fragmented urban woodland vulnerable.
References
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Green 5:195–201
Angold P, Sadler JP, Hill MO, Pullin A, Rushton S, Austin K et al (2006) Biodiversity in urban habitat patches. Sci Total Environ 360:196–204
Aronson M F, La Sorte F A, Nilon C H Katti M, Goddard M A, Lepczyk C A, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proceedings of the Royal Society B: Biological Sciences 281(1780), 20133330
Atchison KA, Rodewald AD (2006) The value of urban forests to wintering birds. Nat Areas J 26:280–288
Bastin L, Thomas CD (1999) The distribution of plant species in urban vegetation fragments. Landsc Ecol 14:493–507
Bates D, Maechler M, Bolker B (2013) lme4: linear mixed-effects models using S4 classes. R package version 0.999999-2
Bates AJ, Sadler JP, Grundy D, Lowe N, Davis G et al (2014) Garden and landscape-scale correlates of moths of differing conservation status: significant effects of urbanization and habitat diversity. PLoS One 9(1):e86925. doi:10.1371/journal.pone.0086925
Blakesley D, Buckley GP, Blakesley T (2010) Managing your woodland for wildlife. Pisces Publications, Newbury
Bland K, Young M R (1996) Priorities for conserving scottish moths. Conserving Scottish Insects (eds G.E. Rotheray & I. MacGowan), pp. 27–36. Edinburgh Entomological Club, Edinburgh
Carpaneto GM, Mazziotta A, Coletti G, Luiselli L, Audisio P (2010) Conflict between insect conservation and public safety: the case study of a saproxylic beetle (Osmoderma eremita) in urban parks. J Insect Conserv 14:555–565
Conrad KF, Woiwod IP, Parsons M, Fox R, Warren MS (2004) Long-term population trends in widespread British moths. J Insect Conserv 8:119–136
Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP (2006) Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv 132:279–291
Cornelis J, Hermy M (2004) Biodiversity relationships in urban and suburban parks in Flanders. Landsc Urban Plan 69:385–401
Crawley, M J (2012) The R book. John Wiley & Sons
Croci S, Butet A, Georges A, Aguejdad R, Clergeau P (2008) Small urban woodlands as biodiversity conservation hot-spot: a multi-taxon approach. Landsc Ecol 23:1171–1186
Davis AM (2012) A review of the status of microlepidoptera in Britain. Butterfly Conservation, Wareham
Devoto M, Bailey S, Memmott J (2011) The ‘night shift’: nocturnal pollen-transport networks in a boreal pine forest. Ecol Entomol 36:25–35
Dodd LE, Lacki MJ, Rieske LK (2008) Variation in moth occurrence and implications for foraging habitat of Ozark big-eared bats. For Ecol Manage 255:3866–3872
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22(4):1–20
EDINA Digimap Ordnance Survey Service (2013). OS MasterMap Topography Layer. http://edina.ac.uk/digimap
Emmet AM, Heath J (1991) The moths and butterflies of Great Britain and Ireland, vol 7, part 2. Harley Books, Essex
ESRI Inc (2013) ArcGIS 10, http://www.esri.com. (Accessed September 2013)
Faraway JJ (2005) Extending the linear model with R: generalized linear, Mixed Effects and Nonparametric Regression Models. CRC press
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8:1–27
Fox R (2013) The decline of moths in Great Britain: a review of possible causes. Insect Conserv Divers 6:5–19
Fox R, Conrad KF, Parsons MS, Warren MS, Woiwod IP (2006) The state of Britain’s larger moths. Butterfly Conserv Rothamsted Res, Dorset
Fox R, Parsons MS, Chapman JW, Woiwod IP, Warren MS, Brooks DR (2013) The state of Britain’s larger moths 2013. Butterfly Conserv Rothamsted Res, Wareham
Fuentes-Montemayor E, Goulson D, Park KJ (2011) The effectiveness of agri-environment schemes for the conservation of farmland moths: assessing the importance of a landscape-scale management approach. J Appl Ecol 48:532–542
Fuentes-Montemayor E, Goulson D, Cavin L, Wallace JM, Park KJ (2012) Factors influencing moth assemblages in woodland fragments on farmland: implications for woodland management and creation schemes. Biol Conserv 153:265–275
Gellman A, Hill, J (2007) Data analysis using regression and multilevel/hierarchical models
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760
Hammer Ø, Harper D, Ryan P (2001) PAST-Palaeontological statistics. http://www.uv.es/~pardomv/pe/2001_1/past/pastprog/past.pdf, acessado em, 25, 2009
Heyman E (2010) Clearance of understory in urban woodlands: assessing impact on bird abundance and diversity. For Ecol Manag 260:125–131
Jonason D, Franzén M, Pettersson LB (2013) Transient peak in moth diversity as a response to organic farming. Basic Appl Ecol 14(6):515–522
Kozlov M (1996) Patterns of forest insect distribution within a large city: microlepidoptera in St Peterburg, Russia. J Biogeogr 23:95–103
Lehvävirta S, Kotze DJ, Niemelä J, Mäntysaari M, O’Hara B (2006) Effects of fragmentation and trampling on carabid beetle assemblages in urban woodlands in Helsinki, Finland. Urban Ecosyst 9:13–26
Lindenmayer D, Franklin J, Fischer J (2006) General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol Conserv 131:433–445
London Borough of Richmond upon Thames (2013) Richmond upon Thames Habitat Action Plan Broad-leaved Woodland. Available at: http://www.richmond.gov.uk/broad-leaved_woodland_hap1.pdf Accessed January 2014
Luymes DT, Tamminga K (1995) Integrating public safety and use into planning urban greenways. Landsc Urban Plan 33:391–400
Magurran A E (1988) Ecological diversity and its measurement. Princeton University Press,Princeton
Matsuoka RH, Kaplan R (2008) People needs in the urban landscape: analysis of < i > Landscape And Urban Planning contributions. Landsc Urban Plan 84:7–19
McGarigal K, Cushman S A, Ene E (2012) FRAGSTATS v4: Spatial pattern analysis program for categorical and continuous maps. Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst. http://www.umass.edu/landeco/research/fragstats/fragstats.html
McGeoch M, Gaston K (2000) Edge effects on the prevalence and mortality factors of Phytomyza ilicis (Diptera, Agromyzidae) in a suburban woodland. Ecol Lett 3:23–29
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Merckx T, Slade EM (2014) Macro-moth families differ in their attraction to light: implications for light-trap monitoring programmes. Insect Conserv Divers. doi:10.1111/icad.12068
Merckx T, Feber RE, Dulieu RL, Townsend MC, Parsons MS, Bourn NA et al (2009) Effect of field margins on moths depends on species mobility: field-based evidence for landscape-scale conservation. Agric Ecosyst Environ 129:302–309
Merckx T, Feber RE, Mclaughlan C, Bourn NA, Parsons MS, Townsend MC, Riordan P, Macdonald DW (2010a) Shelter benefits less mobile moth species: the field-scale effect of hedgerow trees. Agric Ecosyst Environ 138:147–151
Merckx T, Feber RE, Parsons MS, Bourn NA, Townsend MC, Riordan P, Macdonald DW (2010b) Habitat preference and mobility of Polia bombycina: are non-tailored agri-environment schemes any good for a rare and localised species? J Insect Conserv 14:499–510
Merckx T, Marini L, Feber RE, Macdonald DW (2012a) Hedgerow trees and extended-width field margins enhance macro-moth diversity: implications for management. J Appl Ecol 49:1396–1404
Merckx T, Feber RE, Hoare DJ, Parsons MS, Kelly CJ, Bourn NA, Macdonald DW (2012b) Conserving threatened Lepidoptera: towards an effective woodland management policy in landscapes under intense human land-use. Biol Conserv 149:32–39
Merckx T, Huertas B, Basset Y, Thomas J (2013) A Global Perspective on Conserving Butterflies and Moths and their Habitats In: Key Topics in Conservation Biology 2 (Edited by: DW Macdonald & KJ Willis) pp 237-257. Oxford: Wiley
Morimoto T, Katoh K, Yamaura Y, Watanabe S (2006) Can surrounding land cover influence the avifauna in urban/suburban woodlands in Japan? Landsc Urban Plan 75:143–154
Nakagawa S, Schielzeth H (2012) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nieminen M, Rita H, Uuvana P (1999) Body size and migration rate in moths. Ecography 22:697–707
Ode ÅK, Fry GL (2002) Visual aspects in urban woodland management. Urban For Urban Green 1:15–24
Perry RW, Thill RE, Leslie DM Jr (2007) Selection of roosting habitat by forest bats in a diverse forested landscape. For Ecol Manage 238:156–166
Peterken GF (2001) Ecological effects of introduced tree species in Britain. For Ecol Manage 141: 31–42
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins, London
Ricketts TH, Daily GC, Ehrlich PR, Fay JP (2001) Countryside biogeography of moths in a fragmented landscape: biodiversity in native and agricultural habitats. Conserv Biol 15:378–388
Rickman JK, Connor EF (2003) The effect of urbanization on the quality of remnant habitats for leaf-mining Lepidoptera on Quercus agrifolia. Ecography 26:777–787
Rodwell J S (2006) National vegetation classification: user’s handbook, http://jncc.defra.gov.uk/pdf/pub06_NVCusershandbook2006.pdf
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/
Sadler J, Small E, Fiszpan H, Telfer M, Niemelä J (2006) Investigating environmental variation and landscape characteristics of an urban–rural gradient using woodland carabid assemblages. J Biogeogr 33:1126–1138
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci 109:16083–16088
Slade EM, Merckx T, Riutta T, Bebber DP, Redhead D, Riordan P et al (2013) Life-history traits and landscape characteristics predict macro-moth responses to forest fragmentation. Ecology 94:1519–1530
Smith DA, Gehrt SD (2010) Bat response to woodland restoration within urban forest fragments. Restor Ecol 18:914–923
Smith RM, Warren PH, Thompson K, Gaston KJ (2006) Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodivers Conserv 15:2415–2438
Summerville KS, Crist TO (2003) Determinants of lepidopteran community composition and species diversity in eastern deciduous forests: roles of season, eco-region and patch size. Oikos 100:134–148
Summerville KS, Crist TO (2004) Contrasting effects of habitat quantity and quality on moth communities in fragmented landscapes. Ecography 27:3–12
Summerville KS, Crist TO (2008) Structure and conservation of lepidopteran communities in managed forests of northeastern North America: a review. Can Entomol 140:475–494
Takano T, Nakamura K, Watanabe M (2002) Urban residential environments and senior citizens’ longevity in megacity areas: the importance of walkable green spaces. J Epidemiol Community Health 56:913–918
Truxa C, Fiedler K (2012) Attraction to light-from how far do moths (Lepidoptera) return to weak artificial sources of light?. European Journal of Entomology, 109(1)
Usher MB, Keiller SW (1998) The macrolepidoptera of farm woodlands: determinants of diversity and community structure. Biodivers Conserv 7:725–748
Vallet J, Daniel H, Beaujouan V, Roze F, Pavoine S (2010) Using biological traits to assess how urbanization filters plant species of small woodlands. Appl Veg Sci 13:412–424
Vaughan N (1997) The diets of British bats (Chiroptera). Mamm Rev 22:77–94
Waring P, Townsend M (2003) Field guide to the moths of Great Britain and Ireland. British Wildlife Publishing, Dorset
Warren MS, Bourn NA (2011) Ten challenges for 2010 and beyond to conserve Lepidoptera in Europe. J Insect Conserv 15:321–326
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wilson JD, Morris AJ, Arroyo BE, Clark SC, Bradbury RB (1999) A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric Ecosyst Environ 85:13–30
Wood BC, Pullin AS (2002) Persistence of species in a fragmented urban landscape: the importance of dispersal ability and habitat availability for grassland butterflies. Biodivers Conserv 11:1451–1468
Yela JL, Holyoak M (1997) Effects of moonlight and meteorological factors on light and bait trap catches of noctuid moths (Lepidoptera: Noctuidae). Environ Entomol 26(6):1283–1290
Zuur AF (2009) Mixed effects models and extensions in ecology with R. Springer
Acknowledgments
We would like thank Rebekah Mayhew, Lena Olley, Catherine Gibson-Poole and the numerous volunteers who helped with the data collection. We would like to offer special thanks to Keith Bland (National Museum of Scotland) for identifying micromoth species and Robert Dawson for macromoth identification. We also thank Keith Summerville, Thomas Merckx and one anonymous reviewer for their valuable comments on the manuscript. This project was supported by the People’s Trust for Endangered Species and Nuffield Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jari Niemela.
Appendices
Appendix 1
See Table 3.
Appendix 2
See Table 4.
Appendix 3
See Table 5.
Appendix 4
See Fig. 4.
Predicted measurements of moth assemblages plotted against woodland type; the strongest categorical predictor in each model. Error bars represent 95 % confidence intervals around the predictions. The prediction plot is calculated by setting all other parameters at their median observed values in the model
Appendix 5
See Fig. 5.
Rights and permissions
About this article
Cite this article
Lintott, P.R., Bunnefeld, N., Fuentes-Montemayor, E. et al. Moth species richness, abundance and diversity in fragmented urban woodlands: implications for conservation and management strategies. Biodivers Conserv 23, 2875–2901 (2014). https://doi.org/10.1007/s10531-014-0753-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0753-z