Abstract
Long distance migrants are declining more rapidly than residents, with birds that breed in Europe and winter in tropical Africa providing particularly clear examples. Causal mechanisms may include climate change, but are poorly understood partly because carry-over effects from non-breeding ranges can influence breeding performance. Using long-term data spanning four decades we assess how climatic variation in migrants’ winter, passage and breeding ranges determine timing of breeding and reproductive success. We do so for three Afro-European avian migrants of regional conservation concern (redstart, spotted flycatcher and wood warbler). We find that carry-over effects from passage regions consistently had stronger impacts on breeding phenology than breeding climate. Warm Mediterranean passage conditions promoted earlier breeding in all species, and redstarts also bred earlier following higher Sahel rainfall. Warmer springs on the breeding grounds promoted slightly earlier breeding in redstart and wood warbler, but not spotted flycatcher. Carry-over effects also typically influenced breeding performance to a greater extent than weather on the breeding grounds. Greater rainfall in the Sahel increased redstart brood size, warmer Mediterranean passage conditions increased spotted flycatcher brood size and, to a lesser extent, the number of wood warbler fledglings. In contrast to the concern regarding climate change impacts on migrants’ breeding grounds we found no evidence that warmer temperatures on the breeding grounds were associated with reduced reproductive performance. We thus find that climatic variation on the non-breeding grounds, especially passage regions, typically influenced migrants’ breeding phenology and demography more strongly than equivalent variation on the breeding sites. Such carry-over effects should be considered when assessing the causes of migrants’ marked population declines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A new global phenomenon appears to be emerging in the extinction crisis; migratory species, from diverse taxonomic groups, eco-regions and habitats, are declining much more rapidly than residents (Wilcove and Wikelski 2008). These trends are particularly pronounced in the northern hemisphere and some of the strongest evidence for declines comes from long-distance avian migrants, such as those that breed in Europe and winter in sub-Saharan Africa (Sanderson et al. 2006). These migratory populations may be affected by environmental change on the breeding, passage or wintering grounds, making them particularly susceptible to anthropogenic pressures (Newton 2004). This makes diagnosing causes of decline particularly challenging, partly because environmental conditions in one season may not only affect demographic parameters in that season, but can also carry-over to influence fitness in another season (Norris and Marra 2007; Harrison et al. 2011). For example, red-backed shrikes Lanius collurio breeding in south-west Germany produce more fledglings in years following higher primary productivity in the Sahel autumn passage region (Schaub et al. 2011). Similarly, barn swallow Hirundo rustica populations in Italy produce more fledglings following higher rainfall in the African wintering grounds (Saino et al. 2004a). These species winter in fairly arid ecosystems, thus wetter conditions increase plant growth and insect abundance, enabling insectivorous migrants to migrate faster and thus commence breeding earlier; birds may also arrive in better body condition promoting greater reproductive investment (Saino et al. 2004a; Schaub et al. 2008; Studds and Marra 2011; Altwegg et al. 2012). Recent work suggests that, amongst Afro-European migrants, carry-over effects from the winter grounds may only be important for a relatively small number of species (Ockendon et al. 2013), but more work is required to assess carry-over effects across species’ entire geographic ranges and the factors driving inter-specific variation in their magnitude (Harrison et al. 2011; Saino et al. 2012).
Environmental conditions on passage or wintering grounds that determine timing of breeding may interact with climatic changes on the breeding grounds in a manner that determines migrant breeding success and population trends. Reduced synchrony, i.e. increased mismatch, between the timing of avian breeding and the spring peak in food availability can lead to population decline through reduced reproductive success (Post et al. 2001). For example, in pied flycatcher Ficedula hypoleuca warmer passage temperatures are associated with earlier arrival which can increase reproductive output (Ahola et al. 2004; Both et al. 2006). Migrants may be more vulnerable to these mismatches than residents because the onset of migration can be constrained by climatic cues on the non-breeding grounds that are at best weakly associated with weather on the breeding grounds (Sanderson et al. 2006).
Weather on migrants’ breeding grounds can also directly determine breeding success. In northern temperate regions cool and wet conditions during the breeding season reduce the breeding success of numerous bird species, including migrants due to increased risk of nests flooding, offspring chilling and reduced foraging opportunities that limit the quantity of food provided to nestlings (Crick 2004; Summers et al. 2004; Tyler and Green 2004; Jørgensen et al. 2013).
Our primary goal is to test for carry-over effects on annual variation in the breeding phenology and performance of long-distance Afro-European migrants. We build on recent analyses of carry-over effects from wintering grounds on breeding phenology and clutch size of 19 Afro-European migrants (Ockendon et al. 2013) by considering for three of these carry over-effects from all sections of the species’ geographic ranges, i.e. Mediterranean and Sahelian passage regions, wintering and breeding areas. In addition, we assess carry-over effects on a wider range of demographic traits, i.e. brood size and number of fledglings in addition to clutch size. When we detect carry-over effects on annual variation in demographic traits we also test the hypothesis that these effects are mediated through the influence of non-breeding ground conditions on lay date. Our focal species (redstart Phoenicurus phoenicurus, spotted flycatcher Muscicapa striata and wood warbler Phylloscopus sibilatrix) were selected due to data availability and because they are all of European conservation concern (Burfield and van Bommel 2004). Spotted flycatcher and wood warbler have exhibited severe population declines in the UK (respectively −50 and −65 % between 1995 and 2009); the redstart’s UK population has also exhibited marked population declines during this period, although its recent trend is relatively stable (Risely et al. 2012). We use data from one of the world’s most comprehensive long-term datasets of breeding phenology and productivity, the Nest Record Scheme run in the UK by the British Trust for Ornithology. The scheme provides an accurate assessment of annual variation in phenology and productivity across the country, and therefore provides a unique resource for examining the importance of drivers of national-scale temporal variation in these parameters (Crick et al. 2003).
Materials and methods
Breeding phenology and fecundity
Annual mean first egg date, clutch size, brood size and fledglings per breeding attempt (FPBA) were calculated for nesting attempts across the 36 year period 1971–2006 using data from the British Trust for Ornithology’s (BTO) Nest Record Scheme (NRS, http://www.bto.org/ nrs). Whilst the NRS spans a wider temporal period appropriate weather data from all sections of species’ geographic ranges were not available at the time of data analysis. First egg dates were calculated as the mid-point between the minimum and maximum estimates; those in which these estimates differed by more than 10 days were discarded, and thus data were accurate to within 5 days (Crick et al. 2003). We used clutch size as a measure of reproductive investment, and two alternative measures of productivity, brood size and fledglings per breeding attempt. Brood size is taken as the maximum number of chicks observed in the nest across all visits during the nesting cycle. Annual means were calculated including zeros and thus include total failures (e.g. nests where at least one egg was laid but this did not result in chicks being recorded in the nest). As nests are typically visited at a minimum of 5-day intervals, these data are likely to incorporate partial brood losses that occur early in the nestling period. Nest visits by volunteers are typically insufficiently frequent to enable direct observation of the number of fledglings produced per breeding attempt (FPBA). To provide an additional indicator of breeding success, we therefore calculate FPBA for each year, as FPBA = CS × HS × (1 − EF)EP × (1 − YF)YP where CS represents clutch size, HS represents hatching success (based on brood size observations), EF and YF respectively represent egg and chick stage daily failure rates and EP and YP respectively represent the length of the egg and nestling periods (Crick et al. 2003). These data do not fully take partial brood losses into account but do include complete reproductive failures up to the time of fledging. All estimates used standardized procedures developed by the BTO and only use data from nests visited more than once (Crick et al. 2003). Mean annual sample sizes are provided in Supplementary Table S1, and comprise a total of over 6,000 records of lay date, 5,000 records of clutch size, and 13,500 records of brood size over a 36 year time period for our three focal species.
Weather data
To test for carry-over effects of non-breeding conditions, whilst controlling for the influence of breeding ground conditions, we extracted annual broad-scale weather variables from sub-Saharan wintering grounds, Sahelian and Mediterranean spring passage grounds, and British breeding grounds. We use mean monthly average temperature and precipitation data, which follows the methods of many other studies assessing migrant phenology and carry-over effects (e.g. Gordo and Sanz 2008; Schaub et al. 2011). We do not use a moving window approach to select weather variables calculated over finer temporal periods as given the marked annual variation in phenology documented in this study using finer temporal windows will result in the use of time periods that do not capture the focal activity in early and late years.
Breeding weather variables were calculated by pairing the grid reference of each breeding attempt, which contributed to the annual mean of each response variable, to the nearest 5 km2 grid centroid of the UKCIP monthly climate dataset (http://metoffice.gov.uk). We then calculated, across all nests mean monthly (i) temperature during February to April, which controls bud burst and invertebrate phenology (Buse et al. 1999); hereafter termed early spring temperature, (ii) temperature and precipitation during the nest building and egg laying period (May for redstart and wood warbler, June for spotted flycatcher), termed nesting weather, and (iii) temperature and precipitation during the chick rearing period (June for redstart and wood warbler, July for spotted flycatcher); termed chick rearing weather (Cramp and Perrins 1988–1993; Ferguson-Lees et al. 2009).
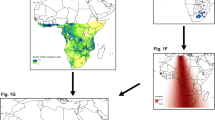
Mediterranean passage areas north of the Sahara desert (Fig. 1) were defined for each species as the north African and southern European spring stopover region used by populations of each species migrating to western Europe (as defined by references (Curry-Lindahl 1981; Cramp and Perrins 1988–1993; Wernham et al. 2002), with additional information from spring ringing recoveries of birds breeding in Britain (http://www.bto.org/volunteer-surveys/ringing/publications/online-ringing-reports). The southern limit to these passage regions was defined as 30°N (following Saino et al. 2007; Gordo and Sanz 2008). Mean monthly temperature and precipitation for each species’ passage distribution were extracted from 0.5° × 0.5° global monthly climate grids (http://badc.nerc.ac.uk) using ArcGIS 9.3 for the core passage months (March for redstart and wood warbler, March to April for spotted flycatcher; (Wernham et al. 2002).
Breeding (green), spring passage (orange), the Sahel zone (yellow) and winter distributions (blue) of British breeding a redstart, b spotted flycatcher and c wood warbler used in this analysis. Multiple potential winter distributions are illustrated, i.e. the maximum potential range of birds breeding in western Europe (all blue areas), that restricted by distributions of preferred wintering habitat type and, when available, the location of British ringing recoveries (hatched blue areas) which were further sub-divided into quartiles (numbered sections). Winter distributions used in analyses are outlined in red. See text for more details
The Sahel zone is located immediately south of the Sahara desert (Fig. 1) and is used by a wide range of migrants as a re-fuelling zone prior to commencing north bound desert crossings (Zwarts et al. 2009). Additionally, the wintering area of redstart largely overlaps with the Sahel. The rainy season in this region occurs from June to October and exhibits marked inter-annual variation in its intensity that can influence migrant population size (Peach et al. 1991; Baillie and Peach 1992; Zwarts et al. 2009). We thus used the Sahel rainfall anomaly index (obtained from http://jisao.washington.edu/data/sahel/) in the year prior to breeding as an indicator of habitat quality that migrants experienced in this region.
British breeding populations of each of our focal species winter in sub-Saharan Africa, although precise wintering locations are poorly known. This uncertainty was taken into account by using multiple potential wintering distributions defined as (i) the maximum range, i.e. the reported winter occurrence of birds breeding in western Europe, (ii) using vegetation maps (http://esp.cr.usgs.gov) to restrict the maximum range to the distribution of each species’ optimum wintering habitat type and further restricted (when sufficient data were available) to within the range of British ringing recoveries in sub-Saharan Africa (http://www.bto.org/volunteer-surveys/ringing/publications), and (iii) dividing the resultant restricted range into quartiles (Fig. 1). This was achieved using data on geographic ranges and habitat preferences from Curry-Lindahl (1981), Cramp and Perrins (1988–1993), and Wernham et al. (2002). This process generated six different wintering ranges for redstart and wood warbler, and five for spotted flycatcher in which the maximum and restricted range were identical, due to few ring recoveries and limited habitat specificity. Mean monthly temperature and precipitation over the winter period (November-February) were extracted for each wintering distribution from 0.5° × 0.5° global monthly climate grids (http://badc.nerc.ac.uk). Transformations were conducted when required to ensure that predictors did not exhibit significant skew (Supplementary Table S2). All transformations were selected and conducted prior to statistical analyses being conducted and were thus performed with no knowledge of the effects of transformation on the analyses.
Weather conditions varied between potential wintering distributions suggesting that the choice of range estimate may influence temporal patterns in the weather variables analysed. To determine which estimate of winter distribution was likely to be the most accurate, linear models were constructed using winter temperature and precipitation as predictors of each response variable (first egg date, clutch size, brood size and FPBA). The objective was to select the wintering range which consistently produced the highest r 2. However, within each species no winter distribution consistently generated the highest r 2 across all response variables (Supplementary Table S3). The restricted winter range was thus selected as the most appropriate estimate for all further analyses, as none of the alternative ranges had consistently higher predictive capacity, and it avoided the disadvantage of using a range estimate which was likely to be much smaller, or much larger, than the actual winter range.
Statistical analyses
For each species, four global models were built, one for each response variable, i.e. first egg date, clutch size, brood size and FPBA. Temporal autocorrelation in the data may lead to Type I errors. To assess if this could be a problem, we constructed mixed models with first-order autoregressive terms to assess the extent to which data from consecutive years may be more similar than other years. For all response variables (first egg date, clutch size, brood size and FPBA) the residual covariance associated with this term was zero, indicating that there was no evidence for temporal autocorrelation in the data. We therefore conducted our analyses using a simple linear model framework.
First egg date was regressed against mean temperature and precipitation on the winter and Mediterranean passage grounds, Sahel rainfall index, early spring temperature and nesting temperature and precipitation. Clutch size was regressed against the same eight variables as first egg date. Brood size and FPBA were regressed against chick-rearing temperature and precipitation in addition to the eight predictors used in models of first egg date and clutch size. Linear multiple regression analyses were conducted in R (using the ‘MuMIn’ package) using an information theoretic approach to model selection in which, for each global model, all possible main effect models were constructed. We used Akaike Information Criteria (AICc to correct for small sample sizes) to calculate each model’s weight, i.e. the probability that it provides the most parsimonious fit to the data. The smallest number of models whose cumulative weights summed to 0.95 was included in the 95 % confidence set of models, and AICc-weighted model averaging was conducted across these models to assess the influence of each predictor. This enabled us to meet our primary goal of testing for carry-over effects on phenology and three measures of breeding performance, whilst taking into account the influence of breeding weather. Correlation coefficients between the vast majority of predictor variables were not statistically significant (P > 0.05). In the few cases when correlation coefficients were significant, they were consistently below the threshold (r > 0.7) at which co-linearity prevents reliable model estimation (Quinn and Keough 2002; Dormann et al. 2013; Supplementary Table S4).
When the above analyses detected carry-over effects on breeding performance we tested the additional hypothesis that carry-over effects were mediated through the influence of non-breeding ground conditions on mean population lay date (Saino et al. 2004b). To achieve this we added lay date to the carry-over effect model (defined as the top model, in the 95 % confidence set of models, which contained the carry-over effects). The hypothesis that carry-over effects arise because non-breeding conditions determine lay-date would be supported if incorporating lay-date as an additional predictor reduced the explanatory capacity of the carry-over effect.
Results
Breeding phenology
Weather in the non-breeding areas explained more of the annual variation in population mean lay date than weather in the breeding areas, with the combined weather effects explaining between one-third and half of the variation in lay date (Table 1). All three species bred earlier following higher spring passage temperatures in the Mediterranean; a relationship which explained 28 % of the variation in spotted flycatcher lay date, 10 % for redstart and 11 % for wood warbler (Table 1). The observed range in Mediterranean temperatures advanced lay dates by 4.6, 6.3 and 7.6 days respectively in the wood warbler, redstart and spotted flycatcher (estimated from the model averaged parameter estimates; Fig. 2a–c). Additionally, redstarts bred earlier following years with wetter Sahelian conditions; the Sahel rainfall index explains 15 % of the variation in lay date (Table 1) and the observed variation in the index altered breeding phenology by 9.1 days (Fig. 2d). There was also a weak relationship between winter precipitation and wood warbler first egg date (partial r 2 = 0.06), with wetter conditions tending to delay breeding with the variation in rainfall altering breeding dates by 2.6 days.
Carry over effects from passage regions influence the breeding phenology of three avian long-distance migrants. Breeding occurs earlier following passage through a warmer Mediterranean in a wood warbler, b spotted flycatcher, and c redstart; and d redstarts also breed earlier following passage through a wetter Sahel. Solid grey lines represent the best fit line obtained from model averaged parameter estimates in multiple regressions that take climatic conditions throughout the rest of the species’ geographic distributions into account and holds these at their mean value
Breeding ground weather explained some of the annual variation in redstart and wood warbler mean lay dates, but not that of spotted flycatcher lay date (Table 2). Both of the former species bred earlier following higher early spring temperatures (redstart: partial r 2 = 0.06; wood warbler: partial r 2 = 0.11). The observed variation in early spring temperature altered breeding dates by 5.3 and 4.9 days respectively in redstart and wood warbler (Fig. 3). Redstarts also tended to breed earlier in years with higher nesting temperatures (partial r 2 = 0.05), and higher precipitation during the nesting period (partial r 2 = 0.05).
Annual mean first egg dates of a redstart and c wood warbler advance in years with warmer early spring temperatures on the breeding grounds; b spotted flycatcher breeding phenology is not associated with such temperatures. Solid grey lines represent the best fit line obtained from model averaged parameter estimates in multiple regressions that take climatic conditions throughout the rest of the species’ geographic distributions into account and holds these at their mean value
Breeding success
Clutch size
Weather variables explained little variation in redstarts’ (model averaged r 2 = 0.07) or wood warblers’ mean clutch size (model averaged r 2 = 0.04). Weather was a stronger predictor of spotted flycatcher clutch size (model averaged r 2 = 0.25), which surprisingly produced smaller clutches following passage through a wetter Sahel (partial r 2 = 0.09; Table 2). The magnitude of this effect was, however, very small; the observed range of Sahel rainfall anomalies reduced clutch size by just one tenth of an egg (mean clutch size = 4.2; Fig. 4a); taking lay date into account had little influence on its explanatory capacity or parameter estimate (Table 3).
Carry over effects from the spring passage regions influence a redstart brood size, which increases following passage through a wetter Sahel, and b spotted flycatcher brood size, which increases following passage through a warmer Mediterranean. Solid grey lines represent the best fit line obtained from model averaged parameter estimates in multiple regressions that take climatic conditions throughout the rest of the species’ geographic distributions into account and holds these at their mean value
Brood size
Weather conditions on the non-breeding grounds explained more of the variation in redstart and spotted flycatcher brood size than weather conditions on the breeding grounds; wood warbler brood size exhibited little relationship with any weather variables (Table 2). Redstart brood size increased following passage through a wetter Sahel (partial r 2 = 0.07) with brood size increasing by 0.5 over the observed range of Sahel rainfall anomalies (mean brood size = 3.8; Fig. 4b). This effect became negligible when taking lay date into account (Table 3). Spotted flycatcher brood size increased with Mediterranean passage temperature, explaining a fifth of the variation in brood size, which increased by 0.5 over the observed range of passage temperatures (mean brood size = 2.4; Fig. 4c). Taking lay date into account did not reduce the explanatory capacity of this carry-over effect or its parameter estimate (Table 3).
Fledglings per breeding attempt (FPBA)
Weather on the breeding grounds explained more of the variation in redstart FPBA than weather on the non-breeding grounds. Warmer conditions during the chick rearing period (partial r 2 = 0.11) and, to a lesser extent, early spring increased the number of redstart fledglings by 0.7 and 0.4 respectively over the observed temperature range in these periods (mean FPBA = 4.7). Wood warbler FPBA increased following passage through a warmer Mediterranean (partial r 2 = 0.05), with the number of fledglings increasing by 0.5 over the observed temperature range (mean FPBA = 3.5; Fig. 4d). The explanatory capacity and parameter estimate for this carry-over effect increased when taking lay-date into account (Table 3). Weather had little influence on spotted flycatcher FPBA (model averaged r 2 = 0.06; Table 2).
Discussion
Carry-over effects and phenology
There was strong evidence for carry-over effects in all three species, with weather on the breeding grounds explaining more of the variation in breeding dates than weather on the breeding grounds. As found by Ockendon et al. (2013) weather effects on the wintering grounds were relatively unimportant, with the exception of redstart. Instead, we identified that the most important carry-over effect was spring temperature in the Mediterranean passage region which explained over a quarter of variation in spotted flycatcher lay date, and approximately a tenth of the variation in wood warbler and redstart lay date. The observed range of passage temperatures is predicted to shift mean lay dates by a considerable number of days relative to the range in observed lay dates over our 36 year time period (wood warbler: 4.6 cf to 15.6 days; redstart 6.3 cf to 18.2 days; spotted flycatcher 7.6 cf. to 12.4 days). The effect of en route temperature on arrival to the breeding grounds is well documented (Gordo and Sanz 2008), but studies establishing a link between passage conditions and breeding date are much rarer (Both et al. 2006), and passage conditions are often not considered in analyses of carry-over effects (cf. Ockendon et al. 2013). Such associations are likely to arise due to the influence of passage conditions on arrival date, but warmer spring temperatures at Mediterranean stopover sites may also increase the availability of invertebrate food sources (as found in North America; Dunn 2002; Smith and Moore 2005), potentially improving adult body condition and enabling earlier breeding.
Sahel rainfall exerted a large carry-over effect on redstart breeding date, with earlier breeding following wetter years, and had higher explanatory capacity than Mediterranean temperature. The observed variation in Sahel rainfall was predicted to advance mean lay dates by 9 days, which is a substantial proportion of the observed variation in mean lay date of 18.2 days over the same time period. Our finding is consistent with previous studies showing that redstarts are sensitive to Sahelian rainfall (Zwarts et al. 2009, Ockendon et al. 2013). A small number of other studies on temperate breeding passerines that winter in low latitudes also suggest that arid overwinter conditions delay breeding (Saino et al. 2004b; Norris et al. 2004; Rockwell et al. 2012), which appears to be a general finding in terms of statistical significance across passerines that winter in the Sahel, albeit one that has a relatively low magnitude impact on breeding phenology overall (Ockendon et al. 2013). This carry-over effect on phenology is presumably driven by increased abundance of invertebrate prey following wet years, which improves migrant body condition (Bearhop et al. 2004), leading to an advance in migration phenology (Schaub et al. 2008; Bridge et al. 2010) and possibly also to improved physiological condition on arrival to the breeding grounds, further enabling advancement of clutch initiation. Spotted flycatcher and wood warbler winter south of the Sahel, but their phenologies were not associated with Sahel rainfall which is the expected pattern if the British breeding populations of these species do not typically rely on this area as a major spring stop-over location.
The only other carry-over effect on lay date from the African sectors of the focal species’ geographic ranges was a tendency for wood warblers to initiate clutches slightly earlier following years with lower rainfall on the wintering grounds. Explanatory power was limited and this result may at first seem paradoxical given the more typically observed phenological advances in response to higher rainfall in arid regions (see above). Wood warblers, however, use much more mesic habitats during the winter than many other Afro-European migrants (Evans et al. 2012). Thus food availability may exhibit different associations with rainfall in the habitats occupied by wood warblers than those that have been documented in drier habitats (Zwarts et al. 2009; Studds and Marra 2011). Relatively few studies have assessed effects of winter temperature on migrant phenology. Gordo et al. (2005) found that high winter temperatures delayed nightingale Luscinia megarhynchos arrival dates, but we find no evidence that temperatures on the wintering grounds influenced breeding dates in our focal species.
Carry-over effects and breeding performance
We find little evidence that carry-over effects influence clutch size in our focal species. This is compatible with the few previous studies of clutch size in small bodied long distance passerine migrants (Laaksonen et al. 2006; Ockendon et al. 2013). It contrasts with the carry-over effects on clutch size, including those arising from winter weather, documented in migratory waterbirds (e.g. Bêty et al. 2003; Guillemain et al. 2008), which are more likely to be capital breeders and thus directly use resources gained in non-breeding areas in egg production (Klaassen 2002).
Carry-over effects of non-breeding season weather had a stronger impact on redstart and spotted flycatcher brood size than breeding season weather conditions. Redstart brood size increased with Sahel rainfall by half a chick over the observed variation in rainfall intensity. The mechanism driving this carry-over effect appears to be earlier breeding following greater Sahel rainfall as models that took lay date into account did not detect an effect of Sahel rainfall on brood size. This concurs with other studies which conclude that wetter Sahel improves habitat quality for redstarts (Zwarts et al. 2009), and that birds which breed earlier invest more in reproduction (Crick et al. 1993). Indeed, most demonstrated carry-over effects of non-breeding conditions on songbird productivity appear to be mediated by timing of breeding (Norris et al. 2004; Saino et al. 2004a; Rockwell et al. 2012).
Warmer Mediterranean passage temperatures have a positive effect on spotted flycatcher brood size, offspring production increasing by approximately half a chick over the observed variation in Mediterranean passage temperatures. This is quite marked relative to the documented variation in spotted flycatcher brood size (1.9–2.9 chicks). Remarkably, this carry-over effect remained when taking lay date into account. The mechanism driving this is thus unclear, but it is plausible that higher quality passage environments enable greater investment in egg quality that increases hatching success, or improves female body condition and thus parental care during the incubation and brooding periods. As an example, egg hatching rates in shore larks Eremophila alpestris can be predicted by environmental conditions that determine parental body condition and resultant incubation investment (MacDonald et al. 2013).
The number of wood warbler fledglings increased following passage through a warmer Mediterranean, although explanatory capacity was somewhat limited and the increase in number of fledglings over the observed range in passage temperature (0.5 fledglings) was small compared to the range in annual mean FPBA (1.4–5.0 fledglings). No other species exhibited carry-over effects on fledgling production, which is perhaps not surprising given the role of nest predation in determining fledging rates.
Breeding weather effects on phenology and reproductive performance
We found no evidence that spotted flycatcher mean breeding date was associated with weather conditions on the breeding grounds. This is compatible with the lack of a long-term trend in timing of breeding (Baillie et al. 2012). Redstart and wood warbler advanced their breeding dates in response to warmer temperatures during early spring on their breeding grounds despite spending the winter over 5,000 km away. This is highly unlikely to arise through correlations between weather conditions on the breeding grounds and those elsewhere in the species’ geographic ranges as such associations are almost invariably insignificant and always limited in magnitude (Supplementary Table S4). Moreover, the statistical analyses took passage and winter weather into account. We therefore suspect that redstarts and wood warblers are partially able to adjust breeding dates to conditions on the breeding grounds by altering the interval between arrival and onset of egg laying (Both and Visser 2001; Weidinger and Kral 2007). Redstarts and wood warblers have advanced their breeding dates by fewer than 1.5 days per 1 °C increase in spring temperature. This seems unlikely to be sufficient to track fully changes in the timing of peak caterpillar abundance (an important nestling food source in these species; Cramp and Perrins 1988–1993) arising from spring warming (Visser et al. 2006; Vatka et al. 2011). In all our focal species neither the annual mean brood size or FPBA are adversely influenced by warmer breeding conditions. Carry-over effects from the passage or wintering grounds also invariably have a stronger influence on brood size than weather at the breeding sites.
Conclusions
We find strong evidence of carry-over effects on the breeding dates of three Afro-European migrants of regional conservation concern (redstart, wood warbler and spotted flycatcher). These carry-over effects explained more of the annual variation in lay dates than breeding ground climate, and primarily arose from earlier breeding following passage through a warmer Mediterranean. In addition redstarts bred earlier following higher Sahel rainfall, and this effect was stronger than that of passage temperature. Carry-over effects from weather on the non-breeding grounds also influenced migrant’s breeding performance. Their explanatory power was rather limited, as expected due to the influence of other factors such as predation on breeding success. Notably, carry-over effects typically explained more of the variation in breeding performance, particularly brood size, than weather on the breeding grounds. Indeed, there was no evidence that warmer breeding conditions were associated with reduced reproductive performance, contrasting with recent concerns regarding the impacts of climate change on migrants’ breeding grounds on their reproductive success. The carry-over effects that we detect were not always mediated by lay-date; the mechanisms driving these carry-over effects thus remain unclear, although they could arise from impacts on physiological condition. Our results demonstrate that climatic variation on migrants’ wintering and passage geographic ranges can determine their breeding phenology and performance, thus complicating assessment of the drivers of their marked population declines.
References
Ahola M, Laakonsen T, Sippola K, Eeva T, Rainio K, Lehikoinen E (2004) Variation in climate warming along the migration route uncouples arrival and breeding dates. Glob Change Biol 10:1610–1617
Altwegg R, Broms K, Erni B, Barnard P, Midgley GF, Underhill LG (2012) Novel methods reveal shifts in migration phenology of barn swallows in South Africa. Proc R Soc Lond B 279:1485–1490
Baillie SR, Peach WJ (1992) Population limitation in Palaearctic-African migrant passerines. Ibis 134:120–132
Baillie SR, Marchant JH, Leech DI, Renwick AR, Eglington SM, Joys AC, Noble DG, Barimore C, Conway GJ, Downie IS, Risely K, Robinson RA (2012) BirdTrends 2011 BTO research report no 609. BTO, Thetford
Bearhop S, Hilton GM, Votier SC, Waldron S (2004) Stable isotope ratios indicate that body condition in migrating passerines is influenced by winter habitat. Proc R Soc Lond B 271:S215–S218
Bêty J, Gauthier G, Jean-François G (2003) Body condition, migration, and timing of reproduction in snow geese: a test of the condition-dependent model of optimal clutch size. Am Nat 162:110–121
Both C, Visser ME (2001) Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411:296–298
Both C, Sanz JJ, Artemyev AV, Blaauw B, Cowie RJ, Dekhuizen AJ, Enemar A, Järvinen A, Nyholm NEI, Potti J, Ravussin P-A, Silverin B, Slater FM, Sokolov LV, Visser ME, Winkel W, Wright J, Zang H (2006) Pied flycatchers Ficedula hypoleuca travelling from Africa to breed in Europe: differential effects of winter and migration conditions on breeding date. Ardea 94:511–525
Bridge ES, Kelly JF, Bjornen PE, Curry CM, Crawford PHC, Paritte JM (2010) Effects of nutritional condition on spring migration: do migrants use resource availability to keep pace with a changing world? J Exp Biol 213:2424–2429
Burfield I, van Bommel F (2004) Birds in Europe: population estimates, trends and conservation status. BirdLife International, Cambridge
Buse A, Dury SJ, Woodburn RJW, Perrins CM, Good JEG (1999) Effects of elevated temperature on multi-species interactions: the case of pedunculate oak, winter moth and tits. Funct Ecol 13:S74–S82
Cramp S, Perrins CM (1988–1993) The birds of the Western Palearctic, vol 5–7. Oxford University Press, Oxford
Crick HQP (2004) The impact of climate change on birds. Ibis 146:S48–S56
Crick HQP, Gibbons DW, Magrath RD (1993) Seasonal changes in clutch size in British Birds. J Anim Ecol 62:263–273
Crick HQP, Baillie SR, Leech DI (2003) The UK nest record scheme: its value for science and conservation. Bird Study 50:254–270
Curry-Lindahl K (1981) Bird migration in Africa. Academic Press Inc., London
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, Mcclean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Dunn EH (2002) Mass change during migration stopover: a comparison of species groups and sites. J Field Ornithol 72:419–432
Evans KL, Newton J, Mallord JW, Markman S (2012) Stable isotope analysis provides new information on winter habitat use of declining avian migrants that is relevant to their conservation. PLoS One 7:e34542
Ferguson-Lees IJ, Castell R, Leech DI (2009) A field guide to monitoring nests. British Trust for Ornithology, Thetford
Gordo O, Sanz JJ (2008) The relative importance of conditions in wintering and passage areas on spring arrival dates: the case of long-distance Iberian migrants. J Ornithol 149:199–210
Gordo O, Brotons L, Ferrer X, Comas P (2005) Do changes in climate patterns in wintering areas affect the timing of the spring arrival of trans-Saharan migrant birds? Glob Change Biol 11:12–21
Guillemain M, Elmberg J, Arzel C, Johnson AR, Simon G (2008) The income–capital breeding dichotomy revisited: late winter body condition is related to breeding success in an income breeder. Ibis 150:172–176
Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18
Jørgensen PS, Tøttrup AP, Rahbek C, Geertsma M (2013) Effects of summer weather on reproductive success of the Red-backed Shrike (Lanius collurio). Bird Study 60:1–10
Klaassen M (2002) Relationships between migration and breeding strategies in arctic breeding birds. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer-Verlag, Berlin, pp 237–249
Laaksonen T, Ahola M, Eeva T, Vaisanen RA, Lehikoinen EA (2006) Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos 114:277–290
MacDonald EC, Camfield AF, Jankowski JE, Martin K (2013) Extended incubation recesses by alpine-breeding Horned Larks: a strategy for dealing with inclement weather? J Field Ornithol 84:58–68
Newton I (2004) Population limitation in migrants. Ibis 146:197–226
Norris DR, Marra PP (2007) Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109:535–547
Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc R Soc Lond B 271:59–64
Ockendon N, Leech DI, Pearce-Higgins JW (2013) Climatic effects on breeding grounds are more important drivers of breeding phenology in migrant birds than carry-over effects from wintering grounds. Biol Lett 9:20130669
Peach W, Baillie SR, Underhill L (1991) Survival of British sedge warblers Acrocephalus schoenobaenus in relation to West African rainfall. Ibis 133:300–305
Post E, Forchhammer MC, Stenseth NC, Callaghan TV (2001) The timing of life-history events in a changing climate. Proc R Soc Lond B 268:15–23
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Risely K, Massimino D, Johnston A, Newson SE, Eaton MA, Musgrove AJ, Noble DG, Procter D, Baillie SR (2012) The breeding bird survey 2011 BTO research report 624. British Trust for Ornithology, Thetford
Rockwell SM, Bocetti CI, Marra PP (2012) Carry-over effects of winter climate on spring arrival date and reproductive success in an endangered migratory bird, Kirtland’s Warbler (Setophaga kirtlandii). Auk 129:744–752
Saino N, Szep T, Ambrosini R, Romano MR, Møller AP (2004a) Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proc R Soc Lond B 271:681–686
Saino N, Szep T, Romano MR, Rubolini D, Spina F, Møller AP (2004b) Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol Lett 7:21–25
Saino N, Rubolini D, Jonzen N, Ergon T, Montemaggiori A, Stenseth NC, Spina F (2007) Temperature and rainfall anomalies in Africa predict timing of spring migration in trans-Saharan migratory birds. Clim Res 35:123–134
Saino N, Romano MR, Caprioli M, Ambrosini R, Rubolini D, Scandolara C, Romano A (2012) A ptilochronological study of carry-over effects of conditions during wintering on breeding performance in the barn swallow Hirundo rustica. J Avian Biol 43:513–524
Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ (2006) Long-term population declines in Afro-Palearctic migrant birds. Biol Conserv 131:93–105
Schaub M, Jenni L, Bairlein F (2008) Fuel stores, fuel accumulation, and the decision to depart from a migration stopover site. Behav Ecol 19:657–666
Schaub M, Jakober H, Stauber W (2011) Demographic response to environmental variation in breeding, stopover and non-breeding areas in a migratory passerine. Oecologia 167:445–459
Smith RJ, Moore FR (2005) Fat stores of American redstarts Setophaga ruticilla arriving at northerly breeding grounds. J Avian Biol 36:117–126
Studds CE, Marra PP (2011) Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proc R Soc Lond B 278:3437–3443
Summers RW, Green RE, Proctor R, Dugan D, Lambie D, Moncrieff R, Moss R, Baines D (2004) An experimental study of the effects of predation on the breeding productivity of capercaillie and black grouse. J Appl Ecol 41:513–525
Tyler GA, Green RE (2004) Effects of weather on the survival and growth of Corncrake Crex crex chicks. Ibis 146:69–76
Vatka E, Orell MI, Rytkonen SO (2011) Warming climate advances breeding and improves synchrony of food demand and food availability in a boreal passerine. Glob Change Biol 17:3002–3009
Visser ME, Holleman LJM, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–172
Weidinger K, Kral M (2007) Climatic effects on arrival and laying dates in a long-distance migrant, the Collared Flycatcher Ficedula albicollis. Ibis 149:836–847
Wernham CV, Siriwardena GM, Toms MP, Marchant JW, Clark JA, Baillie SR (2002) The Migration Atlas: movements of the birds of Britain and Ireland. T & AD Poyser, London
Wilcove DS, Wikelski MC (2008) Going, going, gone: is animal migration disappearing? PLoS Biol 6:e188
Zwarts L, Bijlsma RG, van der Kamp J, Wymenga E (2009) Living on the edge: wetlands and birds in a changing Sahel. KNNV Publishing, Zeist
Acknowledgments
We thank all those volunteers who contribute to the BTO’s Nest Record Scheme, and the Joint Nature Conservancy Council for helping to fund the scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stuart Pimm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Finch, T., Pearce-Higgins, J.W., Leech, D.I. et al. Carry-over effects from passage regions are more important than breeding climate in determining the breeding phenology and performance of three avian migrants of conservation concern. Biodivers Conserv 23, 2427–2444 (2014). https://doi.org/10.1007/s10531-014-0731-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0731-5