Abstract
This review considers the regional scale of impacts arising from disturbance to desert soil ecosystems. Deserts occupy over one-third of the Earth’s terrestrial surface, and biological soil covers are critical to stabilization of desert soils. Disturbance to these can contribute to massive destabilization and mobilization of dust. This results in dust storms that are transported across inter-continental distances where they have profound negative impacts. Dust deposition at high altitudes causes radiative forcing of snowpack that leads directly to altered hydrological regimes and changes to freshwater biogeochemistry. In marine environments dust deposition impacts phytoplankton diazotrophy, and causes coral reef senescence. Increasingly dust is also recognized as a threat to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction: the desert soil ecosystem
Deserts constitute the most abundant and persistent terrestrial biome on Earth (Peel and Finlayson 2007; Thomas 2011; UNEP 1992). The term desert is used here interchangeably with drylands, and used to delineate regions in moisture deficit as indicated by a precipitation to potential evapotranspiration ratio of <0.65 (precipitation supplies less than 65 % of the moisture needed to sustain optimum plant growth) (UNEP 1992). The environmental stresses imposed by moisture limitation, plus thermal, UV, and oligotrophic pressures, have resulted in landscapes that support relatively sparse vascular plant and animal life. Instead, drylands are dominated biologically by soil-rock-surface communities (SRSCs) comprising microorganisms, mosses and lichens (Pointing and Belnap 2012).
Desert soils are dominated by biological soil crusts (biocrusts). These are cohesive soil surface structures; morphologically variable with climate (Belnap et al. 2003). They are dominated by cyanobacteria (particularly Microcoleus sp. (Garcia-Pichel et al. 2013) plus heterotrophic bacteria, algae, fungi, mosses and lichens. They are ubiquitous in dryland soils of all but the most extreme deserts worldwide (Belnap et al. 2003). Desert surfaces in extreme hyper-arid locations are often dominated by desert pavement, where pebbles are embedded in the surface soil matrix. These surfaces are characterized by hypolithic communities (Chan et al. 2012; Pointing et al. 2007; Warren-Rhodes et al. 2006, 2007; Wong et al. 2010). Hypoliths support extensive cyanobacteria-dominated (notably Chroococcidiopsis sp.) biofilms that colonize the ventral surface of translucent stones (mainly quartz), and may facilitate extensive microbial and moss colonization in surrounding soils (Chan et al. 2012). Open soils in drylands support a largely heterotrophic community dominated by Actinobacteria (Pointing and Belnap 2012). These poikilohydric communities extend over soils and rock surfaces, covering 70 % or more in dryland landscapes (Belnap et al. 2003). They comprise ancient phylogenetic lineages (Bahl et al. 2011) and may be very long lived. Estimates of longevity ranging into hundreds of years for lichens (Lange 1990) and thousands of years for hypoliths in extreme arid landscapes (Warren-Rhodes et al. 2006).
Globally SRSCs account for ~7 % of terrestrial productivity and almost 50 % of terrestrial nitrogen fixation (Elbert et al. 2012). They perform many critical ecosystem roles and can define the ‘critical zone’ of biological interaction in the ecosystem (Pointing and Belnap 2012). There is mounting evidence that biological soil crusts are important in regulating multi-functionality in dryland ecosystems (Bowker et al. 2013, 2011; Maestre et al. 2012). Local hydrological cycles are strongly influenced by biocrusts as they mediate the transport of water, gases, nutrients, heat and light down into underlying soil as well as movement outwards from the soil (Delgado-Baquerizo et al. 2012; Delgado-Baquerizo et al. 2013; Maestre et al. 2013) and to vascular plants (Harper and Belnap 2001; Harper and Pendleton 1993). Biocrusts and hypoliths trap incoming dust, increasing soil fertility (Chan et al. 2012; Field et al. 2009). Most importantly, biocrusts and hypolithic communities promote soil stability; indeed, where they are well-developed, soils can be almost totally resistant to wind and water erosion (Belnap and Gillette 1997; Chan et al. 2012; Zhang et al. 2006).
Here we highlight the regional scale of impacts that arise from disturbance to desert soils. We identify the causes of disturbance, and emphasize the mobilization of dust to the atmosphere as a major driver of impacts that manifest remotely. Examples of negative environmental consequences are given for terrestrial and marine ecosystems, including potential threats to biotic and human health.
Disturbance to desert soil surface ecosystems
Desert SRSCs are at risk from human activities (e.g., agriculture [cropping and livestock grazing], human habitation, energy and mineral extraction, fire, and recreational use), environmental stochasticity (particularly drought), and predicted long-term climate change (Belnap 2003; Pointing and Belnap 2012). The increase in disturbance by human activity is of special concern, as it is potentially catastrophic. Subsistence and large-scale agricultural use are ironically both threatened by and a causal agent of desertification. Approximately 1 billion people rely on drylands for their livelihood, and it is estimated that of the 5.2 billion hectares of dryland used for agriculture, 69 % is degraded or undergoing desertification (Gilbert 2011). Clear differences in the level of subsistence usage, large-scale exploitation, and the level of conservation effort are evident between under-developed and developed regions (Anon 2005).
Disturbance to SRSCs at a local scale results in substrate degradation and loss of soil stability and fertility, plus changes to hydrological and heat regimens in soils that can be irreversible (Pointing and Belnap 2012). This occurs naturally in stochastic ‘pulses’ due to desert storms (Kellogg and Griffin 2006), and increasingly from human disturbance. When this severely impacts local SRSC and plant covers, biological feedbacks may be activated towards increased desertification (Rietkerk et al. 2004; Schlesinger et al. 1990). A major factor that compounds the issue of SRSC disturbance is that recovery of communities is extremely slow (Belnap and Eldridge 2003). In addition, large soil losses can lead to fundamental changes to soil properties that impede re-colonization and succession of these organisms (Bowker et al. 2013; Pointing and Belnap 2012; Requena et al. 2001).
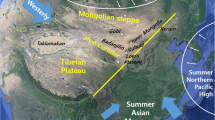
A major outcome is mobilization of soil previously bound in biocrusts and associated with hypolith communities of desert pavements (Chan et al. 2012; Field et al. 2009; Middleton 1989). This may manifest as massive dust storms or chronic smaller events (Fig. 1a) (Bullard et al. 2011; McTainsh and Strong 2007). The trajectory of transport for desert dust is well established (Fig. 1b) (Kellogg and Griffin 2006; Lawrence and Neff 2009; Smith et al. 2013) and includes dispersal across trans-continental trajectories (Collyer et al. 1984; De Deckker et al. 2008; Derimian et al. 2006; Lim 2011; Reynolds 2001) and trans-oceanic distances (Collyer et al. 1984; Marx et al. 2005; Prospero et al. 2005; Prospero and Lamb 2003). There is growing concern that the frequency and magnitude of such dust events is increasing (McTainsh et al. 1998; Middleton 1989; Neff et al. 2008; Safriel 2006).
Impacts of aerosolized desert dust
Biodiversity
Wind-blown dust may physically damage organisms, for example by scratching the cuticles of plants and insects, leading to increased water loss and thus mortality (Belnap 2003). Dust deposited on plant leaves can clog stomata, reducing photosynthetic rates, and in sufficient amounts, bury young seedlings or low stature plants (Collyer et al. 1984; Field et al. 2009). Dust is also a vector for airborne microbial and plant propagule dispersal (Womack et al. 2010), and so threats to local endemic biodiversity may arise due to invasive species and pathogen introduction. Microorganisms have been recovered from desert dust and estimated to increase microbial loading in aerosols by an order of magnitude (Favet et al. 2013; Griffin et al. 2006; Griffin 2007; Jeon et al. 2011; Kellogg et al. 2004; Lim 2011; Prospero et al. 2005; Schlesinger et al. 2006). Diversity assessments have indicated a likely desert origin for many of the microorganisms deposited in remote dust sinks (Abed et al. 2012; De Deckker et al. 2008; Favet et al. 2013; Griffin et al. 2006; Jeon et al. 2011; Lim 2011; Prospero et al. 2005; Woo et al. 2013).
Terrestrial ecosystems
Dust transport can lead to regional impacts on hydrology, with far-reaching consequences for hydrological regimes of distant catchments (Fig. 2). Wind-blown soils from desert sources have been traced to deposits on snowpack in Colorado in the USA (Painter et al. 2010). Similarly Australian desert dust has frequently crossed the Tasman Sea and deposited on New Zealand’s South Island (Marx et al. 2005; Marx and McGowan 2005; McGowan et al. 2005). The deposition of dust on snow causes radiative forcing due to enhanced absorption of solar radiation, and results in disturbance to the magnitude and timing of runoff from snowpack (Painter et al. 2010). The increased snowmelt enhances evapo-transpiration from exposed soils and plants and this in turn reduces freshwater input to lower catchments. For example, reduced input to the Colorado River has been estimated at up to 5 % of the annual average (Painter et al. 2010). This can have direct economic consequences at dust sinks (e.g., ski resorts) and where the water is used for agriculture and domestic supplies. Dust can also introduce toxic compounds and into water catchments. For example mercury contamination of a Florida lake has been explained as a consequence of desert dust originating in West Africa (Holmes and Miller 2004). Nutrient input from dust to soils (Okin et al. 2004) and lakes (Mahowald 2003) has been linked to altered aquatic biogeochemical function. Invasive microorganisms may also be introduced with dust, as postulated for African dust in European alpine lakes (Hervas et al. 2009).
Schematic illustration of local and regional impacts from desert dust mobilization: 1 at local scales disturbance removes soil crust and hypolith cover and impedes re-colonization of soils, 2 local disturbance mobilizes soils that may become aerosolized or enter waterways, 3 dust deposition on snowpack impacts regional hydrology and reduces freshwater input to lowland catchments, 4 dust input to marine systems impacts nitrogen fixation by phytoplankton and is detrimental to coral reefs, 5 desert dust storms have major impacts on human infrastructure, and dust is linked to respiratory allergies and pathogen dispersal. Yellow arrows denote airborne transport, whilst red arrows denote soil/aquatic transport.
Marine ecosystems
Mobilization of desert dust results in significant deposition to ocean basins (Mackie et al. 2008) (Fig. 2). Here the massive increases in iron input from dust has directly contributed to significant impacts on global patterns of marine nitrogen fixation by phytoplankton, due to localized removal of iron limitation to diazotrophy (Jickells et al. 2005; Sohm et al. 2011). Additional impacts on marine ecosystems arise from deposition of dust to coral reefs, where physical coverage of coral by dust reduces zooxanthellae symbioses (Garrison 2003). In addition to this physical disturbance, dust-borne microorganisms have also been implicated as causal agents in coral diseases and death (Rypien 2008; Weir-Brush et al. 2004).
Human health
Dust deposition on human settlements has clear impacts on infrastructure and safety (Fig. 2) (Bener et al. 1996; Griffin 2007; Pöschl 2005). The fine particulate nature of desert dust easily clogs machinery and impaired visibility can lead to fatal highway accidents during large dust storms. Globally, chronic exposure to desert dust is estimated to cause 1.7 % of deaths due to lung cancer and cardio-pulmonary disease, although in latitudes across Africa, the middle East and Asia that support extensive deserts this figure is estimated to be between 15 and 50 % (Giannadaki et al. 2013). Even short-term exposure may be harmful, a survey of Afghan and gulf war veterans deployed during 2003–2004 revealed almost 70 % reported respiratory illness that was attributed to dust (Sanders et al. 2005). African desert dust falling on Caribbean islands after trans-Atlantic transport has also been implicated in respiratory illness among local populations (Gyan et al. 2005).
Human pathogenic bacteria and fungi are transmitted via aerosols and concern is rising that they may travel vast distances with a desert dust vector (Griffin 2007). Under normal conditions the outdoor aerosols of urban settlements support very low levels of pathogenic microorganisms (Brodie et al. 2007; Woo et al. 2013). Putative bacterial and fungal pathogens have been identified in desert dust worldwide (reviewed in Griffin 2007), commonly encountered taxa were gram-negative bacteria and the fungal genus Aspergillus. Desert dust is unlikely to support harmful levels of human pathogens under normal circumstances, although dust sources are often in regions where living standards are low and outbreaks of highly contagious disease are not uncommon. The potential role of dust as a long distance microbial vector warrants more research attention.
Conclusions
In this short review we have identified the connection between disturbance of desert soils and negative environmental consequences at remote dust deposition sites. The worldwide airborne dispersal of desert dust makes this a truly global problem that affects both terrestrial and aquatic biomes. A major concern is that the magnitude and frequency of dust storms appear to be increasing due to climate change (Prospero and Lamb 2003) and anthropogenic causes (Neff et al. 2008). Indeed the very nature of dust mobilization into the atmosphere may also create a positive feedback where transfer of CO2 from soils to atmosphere may exacerbate global warming and its impacts on desertification, in addition to regional carbon stock reductions (Reichstein et al. 2013) and increased inputs to downwind carbon sinks (Chappell et al. 2013). A greater focus on understanding and monitoring the causes, trajectory and impacts of dust events is therefore critical to understanding global ecology. We highlight the need for greater understanding of microbial dispersal in airborne dust as an area in need of urgent research attention, since this likely impacts ecosystem health and biogeography on a global scale.
References
Abed RMM, Ramette A, Hübner V, De Deckker P, de Beer D (2012) Microbial diversity of eolian dust sources from saline lake sediments and biological soil crusts in arid Southern Australia. FEMS Microbiol Ecol 80:294–304
Anon. (2005) Millenium Ecosystem Assessment. World Resources Institute, Washington, DC
Bahl J et al (2011) Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nat Commun 2:163. doi:10.1038/ncomms1167
Belnap J (2003) The world at your feet: desert biological soil crusts. Front Ecol Environ 1:181–189
Belnap J, Eldridge D (2003) Disturbance and recovery of biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer, Berlin, pp 363–384
Belnap J, Gillette DA (1997) Disturbance of biological soil crusts: impacts on potential wind erodibility of sandy desert soils in southeastern Utah. Land Degr Devel 8:355–362
Belnap J, Büdel B, Lange OL (2003) Biological soil crusts: characteristics and distribution. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer, Berlin, pp 3–30
Bener A, Abdulrazzaq YM, Al-Mutawwa J, Debuse P (1996) Genetic and environmental factors associated with asthma. Hum Biol 68:405–414
Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP (2011) Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct Ecol 25:787–795
Bowker MA, Maestre FT, Mau RL (2013) Diversity and patch-size distributions of biological soil crusts regulate dryland ecosystem multifunctionality. Ecosystems 16:923–933
Brodie EL, DeSantis TZ, Parker JPM, Zubietta IX, Piceno YM, Andersen GL (2007) Urban aerosols harbor diverse and dynamic bacterial populations. PNAS 104:299–304
Bullard JE et al. (2011) Preferential dust sources: a geomorphological classification designed for use in global dust-cycle models. J Geophys Res 116(F4) doi:10.1029/201JF002061
Chan Y et al (2012) Hypolithic microbial communities: between a rock and a hard place. Environ Microbiol 14:2272–2282
Chappell A et al (2013) Soil organic carbon dust emission: an omitted global source of atmospheric CO2. Glob Change Biol 19:3238–3244
Collyer FX, Barnes BG, Churchman GJ, Clarkson TS, Steiner JT (1984) A trans-Tasman dust transport event. Weather Clim 4:42–46
De Deckker P et al. (2008) Geochemical and microbiological fingerprinting of airborne dust that fell in Canberra, Australia, in October 2002. Geochem Geophys Geosys G3, v9(12). doi:10.1029/2008GC002091
Delgado-Baquerizo M, Maestre FT, Gallardo A (2012) Biological soil crusts increase the resistance of soil nitrogen dynamics to changes in temperatures in a semi-arid ecosystem. Plant Soil 366:35–47
Delgado-Baquerizo M, Maestre FT, Rodríguez JGP, Gallardo A (2013) Biological soil crusts promote N accumulation in response to dew events in dryland soils. Soil Biol Biochem 62:22–27
Derimian Y et al. (2006) Dust and pollution aerosols over the Negev desert, Israel: properties, transport, and radiative effect. J Geophys Res D05205. doi:10.1029/2005JD006549
Elbert W et al (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462
Favet J et al (2013) Microbial hitchhikers on intercontinental dust: catching a lift in Chad. ISME J 7:850–867
Field JP et al (2009) The ecology of dust. Front Ecol Environ 8:423–430
Garcia-Pichel F, Loza V, Marusenko Y, Mateo P, Potrafka RM (2013) Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science 340:1574–1577
Garrison VH (2003) African and Asian dust: from desert soils to coral reefs. Bioscience 53:469
Giannadaki D, Pozzer A, Lelieveld J (2013) Modeled global effects of airborne desert dust on air quality and premature mortality. Atmos Chem Phys Disc 13:24023–24050
Gilbert N (2011) Science enters desert debate. Nature 477:262
Griffin DW (2007) Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin Microbiol Rev 20:459–477
Griffin DW, Westphal DL, Gray MA (2006) Airborne microorganisms in the African desert dust corridor over the mid-Atlantic ridge, Ocean Drilling Program, Leg 209. Aerobiologia 22:221–226
Gyan K et al (2005) African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. Int J Biometeorol 49:371–376
Harper KT, Belnap J (2001) The influence of biological soil crusts on mineral uptake by associated vascular plants. J Arid Environ 47:347–357
Harper KT, Pendleton RL (1993) Cyanobacteria and cyanolichens: can they enhance availability of essential minerals for higher plants? Great Basin Nat 53:59–72
Hervas A, Camarero L, Reche I, Cassamayor F (2009) Viability and potential for immigration of airborne bacteria from African that reach high mountain lakes in Europe. Environ Microbiol 11:1612–1623
Holmes CW, Miller R (2004) Atmospherically transported elements and deposition in the southeastern United States: local or transoceanic? Appl Geochem 19:1189–1200
Jeon EM et al (2011) Impact of Asian dust events on airborne bacterial community assessed by molecular analyses. Atmos Environ 45:4313–4321
Jickells TD et al (2005) Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308:67–71
Kellogg CA, Griffin DW (2006) Aerobiology and the global transport of desert dust. Trends Ecol Evol 21:638–644
Kellogg CA et al (2004) Characterization of aerosolized bacteria and fungi from desert dust events in West Africa. Aerobiologia 20:99–110
Lange OL (1990) Twenty-three years of growth measurements on the crustose lichen Caloplaca aurantia in the central Negev desert. Israel J Bot 39:883–894
Lawrence CR, Neff JC (2009) The contemporary physical and chemical flux of aeolian dust: a synthesis of direct measurements of dust deposition. Chem Geol 267:46–63
Lim N (2011) Microbiological and meteorological analysis of two Australian dust storms in April 2009. Sci Total Environ 412–413:223–231
Mackie DS et al. (2008) Biogeochemistry of iron in Australian dust: from eolian uplift to marine uptake. Geochem Geophys Geosys 9(3) doi:10.1029/2007GC001813
Maestre FT et al (2012) Plant species richness and ecosystem multifunctionality in global drylands. Science 335:214–218
Maestre FT et al (2013) Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob Change Biol 19:3835–3847
Mahowald NM (2003) Ephemeral lakes and desert dust sources. Geophys Res Lett. doi:10.1029/2002GL016041
Marx SK, McGowan HA (2005) Dust transportation and deposition in a superhumid environment, West Coast, South Island, New Zealand. Catena 59:147–171
Marx SK, Kamber BS, McGowan HA (2005) Estimates of Australian dust flux into New Zealand: quantifying the eastern Australian dust plume pathway using trace element calibrated 210Pb as a monitor. Earth Plan Sci Lett 239:336–351
McGowan HA, Kamber B, McTainsh GH, Marx SK (2005) High resolution provenancing of long travelled dust deposited on the Southern Alps, New Zealand. Geomorphology 69:208–221
McTainsh G, Strong C (2007) The role of aeolian dust in ecosystems. Geomorphology 89:39–54
McTainsh GH, Lynch AW, Tews EK (1998) Climatic controls upon dust storm occurrence in eastern Australia. J Arid Environ 39:457–466
Middleton NJ (1989) Climatic controls on the frequency, magnitude and distribution of dust storms: examples from India/Pakistan, Mauritania and Mongolia. In: Leinen M, Sarnthein M (eds) Paleoclimatology andpaleometeorology: modern and past patterns of global atmospheric transport. Kluwer, Boston, pp 97–132
Neff JC et al (2008) Increasing aeolian dust deposition in the western United States linked to human activity. Nat Geosci 1:189–195
Okin GS, Mahowald N, Chadwick OA and Artaxo P (2004) Impact of desert dust on the biogeochemistry of phosphorus in terrestrial ecosystems. Glob Biogeochem Cycl 18(2) doi:10.1029/2003GB002145
Painter TH et al (2010) Response of Colorado river runoff to dust radiative forcing in snow. PNAS 107:17125–17130
Peel MC, Finlayson BL (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11:1633–1644
Pointing SB, Belnap J (2012) Microbial colonization and controls in dryland systems. Nat Rev Microbiol 10:551–562
Pointing SB et al (2007) Hypolithic community shifts occur as a result of liquid water availability along environmental gradients in China’s hot and cold hyperarid deserts. Environ Microbiol 9:414–424
Pöschl U (2005) Atmospheric aerosols: composition, transformation, climate and health effects. Angew Chem Int Ed Engl 44:7520–7540
Prospero JM, Lamb PJ (2003) African droughts and dust transport to the Caribbean: climate change implications. Science 302:1024–1027
Prospero JM, Blades E, Mathison G, Naidu R (2005) Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 21:1–19
Reichstein M et al (2013) Climate extremes and the carbon cycle. Nature 500:287–295
Requena N et al (2001) Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Reynolds R (2001) Aeolian dust in Colorado plateau soils: nutrient inputs and recent change in source. PNAS 98:7123–7127
Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J (2004) Self-organized patchiness and catastrophic shifts in ecosystems. Science 305:1926–1929
Rypien K (2008) African dust is an unlikely source of Aspergillus sydowii, the causative agent of sea fan disease. Mar Ecol Prog Ser 367:125–131
Safriel L (2006) Deserts and the planet—linkages between deserts and non-deserts. In: Ezcurra E (ed) Global desert outlook. Scanprint, Denmark, pp 49–72
Sanders JW et al (2005) Impact of illness and non-combat injury during operations Iraqi freedom and enduring freedom (Afghanistan). Am J Trop Med Hyg 73:713–719
Schlesinger Wh et al (1990) Biological feedbacks in global desertification. Science 247:1043–1048
Schlesinger P, Mamane Y, Grishkan I (2006) Transport of microorganisms to Israel during Saharan dust events. Aerobiologia 22:259–273
Smith DJ et al (2013) Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl Environ Microbiol 79:1134–1139
Sohm JA, Webb EA, Capone DG (2011) Emerging patterns of marine nitrogen fixation. Nat Rev Microbiol 9:499–508
Thomas DSG (2011) Arid environments: their nature and extent. In Thomas DSG (Ed) Arid zone geomorphology: process, form and change in drylands, 3rd edn. Wiley-Balckwell, London, pp 3–16
UNEP (1992) World atlas of desertification. Edward Arnold, London
Warren-Rhodes KA et al (2006) Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama desert. Microb Ecol 52:389–398
Warren-Rhodes KA et al (2007) Cyanobacterial ecology across environmental gradients and spatial scales in China’s hot and cold deserts. FEMS Microbiol Ecol 61:470–482
Weir-Brush JR, Garrison VH, Smith GW, Shinn EA (2004) The relationship between gorgonian coral (Cnidaria: Gorgonacea) diseases and African dust storms. Aerobiologia 20:119–126
Womack AM, Bohannan BJM, Green JL (2010) Biodiversity and biogeography of the atmosphere. Phil Trans R Soc B 365:3645–3653
Wong FKY et al (2010) Hypolithic microbial community of quartz pavement in the high-altitude tundra of central Tibet. Microb Ecol 60:730–739
Woo AC et al (2013) Temporal variation in airborne microbial populations and microbially-derived allergens in a tropical urban landscape. Atmos Environ 74:291–300
Zhang YM, Wang HL, Wang XQ, Yang WK, Zhang DY (2006) The microstructure of microbiotic crust and its influence on wind erosion for a sandy soil surface in the Gurbantunggut desert of northwestern China. Geoderma 132:441–449
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Guest Editors of S.I.: Biocrust.
Rights and permissions
About this article
Cite this article
Pointing, S.B., Belnap, J. Disturbance to desert soil ecosystems contributes to dust-mediated impacts at regional scales. Biodivers Conserv 23, 1659–1667 (2014). https://doi.org/10.1007/s10531-014-0690-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0690-x