Abstract
Remnant isolation following fragmentation is considered to be one of the main drivers of ecological decline in modified landscapes. Thereby, connecting remnants using ecological corridors has been increasingly suggested as being important for conservation. Our objectives were to test isolation effects on extinction, colonization and turnover rates of a litter-dwelling ant assemblage, and to evaluate effectiveness of ecological corridors to mitigate habitat fragmentation consequences. We used a mesocosm manipulative design, with three different isolation treatments: far, near and near connected by a corridor, sampled fortnightly for 60 days. Fragmentation caused an ongoing decrease in ant species richness, probably due to a negative balance between extinction and colonization. Extinction was steady with time, while colonization decreased over the same period. This outcome highlights the key role that colonization plays in the persistence of populations of litter-dwelling ants. Turnover rates increased with time in the treatment with a corridor, but remained steady in the other treatments. We conclude that corridors are important for not only immigration to remnants, but also emigration, acting as a two way traffic route. Thus, it may be an important “leakage” point for threatened species, with implications for their conservation status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation is the division of a landscape into two or more isolated and smaller remnants (DeSouza et al. 2001; Laurance 2008), and is one of the main threats to biodiversity globally (Burkey and Reed 2006; Gonzalez and Chaneton 2002; Hanski 1998). Higher extinction rates are expected to occur in smaller remnants, because these can only support smaller populations, which are then more prone to stochastic and inbreeding events (DeSouza et al. 2001). Additionally, more isolated and smaller remnants are also less likely to receive colonizers compared to more connected and larger ones, thereby diminishing the rescue effect (Brown and Kodric-Brown 1977; MacArthur and Wilson 1963, 1967). Even in cases where species richness is unaffected by fragmentation, community composition may change (Kadmon and Pulliam 1993; Sobrinho et al. 2003; Sodhi et al. 2005) due to non-random extinction and colonization rates among species (Schoereder et al. 2004b).
The use of ecological corridors between remnants has been broadly suggested as a means of reducing the negative effects of isolation among remnants (Beier and Noss 1998). Several studies have concluded that connected patches are able to support more persistent populations and higher species richness (e.g. Debinski and Holt 2000; Gonzalez et al. 1998; Gonzalez and Chaneton 2002; Holzschuh et al. 2008, 2009; Noss 1987; Staddon et al. 2010), as well as mitigate ecosystem effects of habitat fragmentation (Staddon et al. 2010). Nevertheless, the usefulness of corridors is not universal, as several studies have also found null and negative effects of corridors on populations (e.g. Boswell et al. 1998; Burkey 1997; Collinge 2000; Rantalainen et al. 2005; Weldon 2006) and that corridors may facilitate the spread of some invasive species (Alofs and Fowler 2010; Hulme 2006).
Since the development of the island biogeography theory (IBT) (MacArthur and Wilson 1963, 1967), numerous studies on habitat fragmentation have presumed that terrestrial remnants will follow islands dynamics. But the validity of this assumption is being increasingly contested, as terrestrial systems are repeatedly shown to be subject to different processes and constraints (Laurance 2008; Watson 2002). This debate remains, as despite the many tests of IBT’s predictions on terrestrial habitats (e.g. Brose 2003; Pellet et al. 2007; Simberloff and Wilson 1969; Steffan-Dewenter and Tscharntke 2000), aspects such as accelerated turnover in fragments remain surprisingly understudied and therefore controversial (Laurance 2008).
Much of the controversy associated with habitat fragmentation studies is due to methodological drawbacks. For example, most study designs assessing corridor efficacy utilise post-built corridors, but it may be difficult, or too slow, for conditions in the corridors to become similar to the pre-existent remnant, impairing fauna spreading. Other studies do not assess unconnected (control) and connected remnants with the same distance from a species source (Gilbert-Norton et al. 2010; Laurance 2008), thereby impairing comparisons. Most fragmentation studies are only conducted long after the fragmentation event, preventing comparisons between the fragmented and pristine states. In addition, the lack of areas with appropriate features to build true replicates may lead to pseudoreplication and single snapshots experiments, which might mislead parameter estimates (ter Braak et al. 1998). These issues can only be avoided by using well designed manipulative experiments.
Here we present a manipulative experiment that measures three basic IBT parameters, extinction, colonization and turnover, to assess isolation effects and corridor effectiveness for litter-dwelling ant assemblages. We first tested the assumption that ant species richness would decrease with time after fragmentation, and then assessed which ecological processes were involved, by testing the following hypotheses: (i) extinction rate does not change with time after fragmentation, increasing with remnant isolation; and (ii) colonization rate does not change with time after fragmentation, decreasing with remnant isolation. Further, we aimed to evaluate the influence of isolation on turnover rates, testing the hypothesis (iii) species turnover increases over time and with remnant isolation.
Materials and methods
Sampling area
The study was conducted in an approximately 300 ha forest remnant in Viçosa, Minas Gerais, Brazil (20°48′08 S, 42°51′31 W). The local climate is moderate subtropical moist, with a rainy season from September to April, and a dry season from May to August (Golfari 1975). The annual mean temperature is 20 °C, relative humidity of 80 % and with an annual rainfall of 1,800 mm (Castro et al. 1983). Altitude ranges from 600 to 800 m, and the dominant vegetation is secondary seasonal semi deciduous montane forest (Veloso et al. 1991). Until 1966, the studied area was subject to selective logging and conversion into coffee plantations. Logging has since ceased, but pastures, coffee and Eucalyptus plantations, and urban areas now surround the area. The experiment was conducted from January to March 2008 during the rainy summer. Conducting the experiment during this particular period decreases the chance of a seasonal effect because there is little variation in temperature and rainfall in this tropical climate during this period.
Sampling design
This study utilised natural mesocosms, which are as versatile as artificial micro/mesocosms and as complex and biologically real as any natural system. This approach potentially offers a way to circumvent the trade-off between artificiality and tractability (Srivastava et al. 2004). We used ants as the study organism because they are easily collected, and are commonly used in fragmentation studies (e.g. Carvalho and Vasconcelos 1999; Gascon et al. 1999; Leal et al. 2012; Majer et al. 1997; Schoereder et al. 2004b; Sobrinho et al. 2003; Suarez et al. 1998).
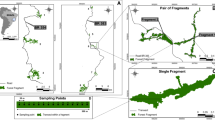
Our experimental design consisted of forest floor portions, naturally covered with litter, that were experimentally isolated from the remaining forest floor by the removal of the surrounding litter. We conducted the experiment in five 11 × 29 m rectangles, hereafter called mesocosm landscapes, positioned at least 10 m apart from each other. In each of these mesocosm landscapes, we removed litter to create twelve 1 m plots, hereafter called mesocosm remnants, divided into the following three treatments (Fig. 1): positioned 5 m from the outside forest floor (“far”), positioned 1 m from the outside forest floor (“near”) and positioned 1 m from the outside forest floor, connected to it by a 0.3 m wide litter corridor (“corridor”). We set up all mesocosm remnants with the same area and shape to standardise area, shape and edge effects. Each of the five mesocosm landscapes was therefore a true replicate, representing independent fragmented landscapes on a micro-scale.
Before creating the experimental fragmentation, we haphazardly collected five 1 m2 samples from each mesocosm landscape to access the pre-fragmentation ant species richness and composition. The experiment was sampled fortnightly for 60 days. In each sampling event, 15 mesocosm remnants, being one mesocosm remnant of each treatment category within each mesocosm landscape, were randomly removed from the site. This staggered design enabled us to track temporal variation of the three isolation treatments after manipulative fragmentation.
We used ant workers from each species as a surrogate for the presence of its nest in the mesocosm remnant to calculate colonization and extinction rates. We believe that this approach is justified because in the tropics the density of ant nests at the 1 m2 spatial scale is commonly high (Byrne 1994; Kaspari 1996b; Soares and Schoereder 2001), and because ants naturally move their nests every 35–146 days (Byrne 1994), probably due to disturbance (Campos et al. 2007; Soares and Schoereder 2001). Thus, we could calculate colonization, extinction and turnover rates, even within a small time span, because the disturbance caused by us may have accelerated movements of colonies and consequently caused changes in colonization, extinction and turnover patterns.
We extracted ants nesting within litter by placing the litter of each mesocosm remnant in Berlese funnels for seven days under 40 watt light bulbs. In some instances foraging ants not nesting within the litter may also have been collected, thereby giving a false colonization result, but this error was anticipated to be minor and not different among treatments. Following extraction, the litter was oven dried at 55 °C for 72 h, and its weight was recorded. We identified ants to morphospecies, with genus-level classifications according to Bolton (1994) and Palacio and Fernández (2003). The classification was checked and, whenever possible, identified to species level by a specialist. Only ant workers were considered in our analyses, because ergatoid and alate queens could represent colonizers that would not necessarily succeed in nest founding.
Following Schoereder et al. (2004b) and Didham et al. (1998), we defined extinction to be when a species found in a sampling event was not found in the subsequent sampling event. Importantly, we always compared remnants from the same isolation treatment within the same mesocosm landscape. We calculated the extinction rate of each sampling event by dividing its number of extinct species by the total number of species sampled in the previous sampling event. We considered as colonizers those species that did not occur in one sampling event but were sampled in the subsequent sampling event in the same mesocosm landscape. We also calculated the colonization rate by dividing the number of colonizers in a given sampling event by the total number of species sampled in the previous sampling event.
Statistical analyses
To determine whether the manipulative fragmentation decreased ant species richness, we adjusted generalized linear models (ANCOVA) with Poisson errors, corrected for under-dispersion (Logan 2010). We used ant species richness as the response variable, time as the explanatory variable and isolation and litter weight as co-variables, the latter in order to avoid sampling artifacts. To estimate the pre-fragmentation ant species richness, we randomly chose 15 samples within the 25 initially collected. It was necessary to standardise treatments samples because each sampling time had 15 points (i.e. three isolation treatments from five replicates). We additionally randomly allocated these pre-fragmentation samples into isolation treatments, so that each of the three isolation treatments was represented by five samples.
To test our three hypotheses we performed ANCOVAs with binomial errors, corrected for overdispersion. On hypotheses (i) and (ii) we respectively used extinction and colonization rates as response variables, time as the explanatory variable and isolation treatment as a co-variable. To calculate colonization and extinction rates for the first sampling event after fragmentation, we randomly selected one of the pre-fragmentation samples to act as a zero state. This procedure was necessary to keep sampling effort homogenous between pre- and post-fragmentation calculations.
To test hypothesis (iii) we used turnover as the response variable, and time, treatments and interaction between time and treatments as explanatory variables. We defined turnover as that in Anderson et al. (2011), being the measure of community structural change from one sampling unit to another with time. The points were obtained in the following way: a sample of each isolation treatment, at each time, was compared using a Jaccard (J) similarity index with the sum of the five initial samples, always from the same mesocosm landscape. We used the sum of the five initial samples because they may well contain most of the species composition from each mesocosm landscape. As we wanted to compare changes in composition between a fragmented habitat and its previous continuous habitat, the sampling effort had to be different (Schoereder et al. 2004a). Turnover was defined as 1–J. As suggested by Anderson et al. (2011), we used the Jaccard index to compare two different communities because it excludes joint absences.
We performed all analyses using R (R Core Team 2011). The validity of all analyses was assessed by residual analyses to check for suitability of error distribution and adequacy of the adjusted models (Crawley 2007). In all analyses, we lumped the categorical variables together when they were not significantly different (Crawley 2007).
Results
We collected 85 ant species from nine subfamilies. Ant species richness decreased with time after fragmentation (F 1,71 = 10.9, P = 0.0015) and was positively correlated with litter weight (F 1,72 = 6.13, P = 0.015). There was no significant relationship between ant species richness and distance of isolation (F 1,70 = 2.73, P = 0.10), but there was a significant interaction between time after fragmentation and isolation (F 1,69 = 7.54, P = 0.007), as ant species richness in the “near” treatment did not vary with time after fragmentation (F 1,24 = 0.01, P = 0.91; Fig. 2).
Relationship between ant species richness and time after fragmentation. Richness decreases through time after the fragmentation event (F 1,71 = 10.9, P = 0.0015), despite the absence of relationship between the near treatment and time after fragmentation (F 1,24 = 0.01, P = 0.91). Day zero is the pre-fragmentation state
Extinction rates did not vary with time (F 1,55 = 0.07, P = 0.78) or isolation (F 2,56 = 0.93, P = 0.40). Colonization rates decreased with time (F 1,56 = 3.87, P = 0.054; Fig. 3), but did not vary with isolation (F 2,56 = 0.81, P = 0.45; Fig. 3).
Relationships between colonization and extinction rates with time after fragmentation. Extinction rates did not vary with time (F 1,55 = 0.07, P = 0.78) or isolation (F 2,56 = 0.93, P = 0.40). Colonization rates decreased through time (F 1,56 = 3.87, P = 0.054), but did not vary with isolation (F 2,56 = 0.81, P = 0.45)
Turnover did not vary with time (F 1,57 = 2.27, P = 0.14) or isolation (F 1,56 = 0.008, P = 0.93) independently, but varied with the interaction between them (F 1,55 = 13.33, P = 0.0005). The turnover in the corridor treatment increased with time after fragmentation (F 1,18 = 29.19, P = 0.0001), but in the near and far treatments it remained steady (F 1,37 = 0.66, P = 0.42) and equal among them (F 2,55 = 1.59, P = 0.21; Fig. 4).
Relationship of turnover rate with time after fragmentation. Interaction between time and isolation was significant (F 1,55 = 13.33, P = 0.0005). The turnover in corridor treatment increased over time after fragmentation (F 1,18 = 29.186, P = 0.0001), while in near and far treatments it remained steady (F 1,37 = 0.6562, P = 0.4231) and statistically equal among them (F 2,55 = 1.59, P = 0.21)
Discussion
Extinction and colonization
As expected, the fragmentation event caused an ongoing decrease of ant species richness, indicating a negative balance between extinction and colonization. Extinction rates were steady over time, though higher than zero, and did not vary with isolation, whereas colonization rates decreased with time, also not varying with isolation (Fig. 3). We suggest that extinction rates were steady and non-variable among treatments because it is a local process. Local extinction can be driven by mechanisms such as resource depletion, inbreeding and stochastic effects (DeSouza et al. 2001). Smaller populations are more prone to these last two effects, but in the time scale of this study inbreeding effects were unlikely to have occurred. Stochastic effects, however, may have contributed to extinction rates, but due to their random nature they are not expected to vary with time after fragmentation or with remnant isolation. Consequently, stochastic effects could have diluted eventual treatment effects. Resources in the litter, notably nest sites, may limit local patchiness (Kaspari 1996b) and therefore be a driver of extinction (or emigration) of ants in our experiment. As mentioned above, litter ants naturally change nest location every 35–146 days (Byrne 1994), probably due to natural disturbance such as litter decomposition and litter-fall (Campos et al. 2007; Soares and Schoereder 2001). These conditions, which possibly occurred in our experiment, are thought to shape litter ant patchiness at fine (1 m2) spatial scales (Kaspari 1996a). A fragmentation event merely results in greater disturbance and resource depletion than a natural disturbance alone.
Another possible explanation for the lack of a relationship between extinction and isolation may be that colonization rates were also independent of isolation. This may have caused a lack of differences in the “rescue effect” among isolation treatments, which we term here the “differential rescue effect”. Thereby, extinction rates would be dependent only on local processes. As an example, Schoereder et al. (2004b), surveying the same region, also did not find an effect of isolation on colonization rate. Although they did not measure extinction as response variable to isolation, it is expected that it also would not vary, due to a lack of the differential rescue effect. According to this hypothesis, when there is a differential rescue effect, extinction rates are expected to vary with isolation.
Unlike extinction, which occurred at the level of each remnant, colonization is a regional process, influenced by the landscape structure. Colonization rate did not differ among isolation treatments, which indicates that the main colonizers source was not the forest, but the surrounding matrix. All mesocosm remnants were within the same surrounding matrix, but at different distances to continuous litter habitat. This scenario would also explain the reduction in colonization rates, as the matrix colonizers’ pool might have shrunk with time, thereby reducing colonization capacity. In our experiment we isolated remnants, and consequently the bare soil surrounding them became an artificial matrix within which the litter-dwelling ant diversity would have been dramatically reduced. Nevertheless, the area relationship between matrix and mesocosm remnants was high, enabling this vast matrix area to harbour some individuals. This habitat is thought to be an important colonizers resource (Cook et al. 2002), especially for some ant species, which are able to perennially survive in matrix habitat (Perfecto and Vandermeer 2002).
Although a flux of individuals among mesocosm remnants might have occurred, this was overcome in our study by the independence of established nests within each remnant. Furthermore, a non-independence among remnants is inherent in the fragmentation process, which generates a matrix that allows a flux of individuals, greater or smaller depending on its harshness (Gascon et al. 1999).
Turnover
By the end of the experiment, the mesocosm remnants connected with corridors had the highest turnover rates, indicating that corridors greatly influenced colonization and emigration. Given that species richness decreased with time in this treatment, it is plausible to assume that our mesocosm remnants were lower-quality compared with the original habitat, due to habitat fragmentation secondary effects and smaller size (DeSouza et al. 2001). Therefore, the corridor might have been used more frequently as an escape route from habitat impacted by fragmentation, supporting the idea that high-quality remnants may attract more immigrants or facilitate settlement (Jaquiéry et al. 2008).
According to IBT, weakly isolated fragments should have higher turnover rates. Nevertheless, Brown and Kodric-Brown (1977) incorporated the rescue effect into the model and, accordingly, turnover rates should increase with distance to a source until reaching a maximum, and then decrease. Our result supports IBT’s assumption, probably because our system did not experience a significant rescue effect, as colonization was lower than extinction with time. In addition, the presence of a corridor boosted the turnover on this treatment. Besides colonizers from surrounding matrix, this treatment also had the forest as a source of different species, and consequently a higher colonizers pool.
Conclusions
Steady and higher than zero extinction rates, associated with decreasing colonization rates by the depletion of the colonizer source, may lead a community to an extinction vortex. Remnants embedded in a harsh matrix, incapable of supplying colonizers, may experience gradually decreasing species richness by this mechanism, which in long term can generate “empty forest” remnants. Hence, a secondary decrease in species richness can be caused by regional effects (colonization) and not by local effects (extinction). Our outcomes corroborate Byrne’s (1994) conclusion that twig-dwelling ant species richness is maintained by external immigrants, which highlights the key role that colonization plays on the persistence of litter ant populations.
In our study, ecological corridors seem to have been used by species to arrive and leave remnants, therefore acting as a “two way traffic route”. Typically, literature on ecological corridors has measured their effects only on the isolated habitat, discussing corridors role as a biotic immigration route (e.g. Collinge 2000; Gonzalez and Chaneton 2002; Rantalainen et al. 2006; Schmiegelow et al. 1997; Staddon et al. 2010). If species richness in a connected remnant is lower than in a remnant without connectivity, for example, most studies conclude that corridors had a negative or at least null effect (e.g. Burkey 1997; Collinge 2000; Rantalainen et al. 2005). But species that would not survive in remnants could have just emigrated to a higher quality habitat through the corridor, which we argue is a positive effect. Thus even when a habitat connected by corridors has higher species richness than another one not connected, there may be additional unseen benefits. A change between capable and incapable surviving species appears to be easier with the presence of a corridor. We believe that this different point of view needs to be considered in future ecological corridor research, as it might have important implications for conservation.
References
Alofs KM, Fowler NL (2010) Habitat fragmentation caused by woody plant encroachment inhibits the spread of an invasive grass. J Appl Ecol 47:338–347
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28
Beier P, Noss R (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Bolton B (1994) Identification guide to the ant genera of the world. Harvard University Press, Cambridge
Boswell GP, Britton NF, Franks NR (1998) Habitat fragmentation, percolation theory and the conservation of a keystone species. Proc R Soc B Biol Sci 265:1921–1925
Brose U (2003) Island biogeography of temporary wetland carabid beetle communities. J Biogeogr 30:879–888
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58:445–449
Burkey TV (1997) Metapopulation extinction in fragmented landscapes: using bacteria and protozoa communities as model ecosystems. Am Nat 150:568–591
Burkey TV, Reed DH (2006) The effects of habitat fragmentation on extinction risk: mechanisms and synthesis. Songk J Sci Technol 28:9–37
Byrne MM (1994) Ecology of twig-dwelling ants in a wet lowland tropical forest. Biotropica 26:61–72
Campos RBF, Schoereder JH, Sperber CF (2007) Small-scale patch dynamics after disturbance in litter ant communities. Basic Appl Ecol 8:36–43
Carvalho KS, Vasconcelos HL (1999) Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biol Conserv 91:151–157
Castro PS, Valente OF, Coelho DT, Ramalho RS (1983) Interceptação da chuva por mata natural secundária na região de Viçosa, MG. Rev Árvore 7:76–88
Collinge SK (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns. Ecology 81:2211–2226
Cook WM, Lane KT, Foster BL, Holt RD (2002) Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett 5:619–623
Crawley MJ (2007) The R book. Wiley, Chichester
Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355
DeSouza O, Schoereder JH, Brown V, Bierregaard RO Jr (2001) A theoretical overview of the processes determining species richness in forest fragments. In: Bierregaard RO Jr, Gascon C, Lovejoy TE, Mesquita RCG (eds) Lessons from Amazonia: the ecology and conservation of a fragmented forest. Yale University Press, New Haven, pp 13–20
Didham RK, Hammond PM, Lawton JH, Eggleton P, Stork NE (1998) Beetle species responses to tropical forest fragmentation. Ecol Monogr 68:295–323
Gascon C, Lovejoy TE, Bierregaard RO Jr, Malcolm JR, Stouffer PC, Vasconcelos HL, Laurance WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Gilbert-Norton L, Wilson R, Stevens JR, Beard KH (2010) A meta-analytic review of corridor effectiveness. Conserv Biol 24:660–668
Golfari L (1975) Zoneamento ecológico do estado de Minas Gerais para reflorestamento. PRODEPEF. Série Técnica 3, Belo Horizonte
Gonzalez A, Chaneton EJ (2002) Heterotroph species extinction, abundance and biomass dynamics in an experimentally fragmented microecosystem. J Anim Ecol 71:594–602
Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I (1998) Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281:2045–2047
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Holzschuh A, Steffan-Dewenter I, Tscharntke T (2008) Agricultural landscapes with organic crops support higher pollinator diversity. Oikos 117:354–361
Holzschuh A, Steffan-Dewenter I, Tscharntke T (2009) Grass strip corridors in agricultural landscapes enhance nest-site colonization by solitary wasps. Ecol Appl 19:123–132
Hulme PE (2006) Beyond control: wider implications for the management of biological invasions. J Appl Ecol 43:835–847
Jaquiéry J, Guelat J, Broquet T, Berset-Brandli L, Pellegrini E, Moresi R, Hirzel AH, Perrin N (2008) Habitat-quality effects on metapopulation dynamics in greater white-toothed shrews, Crocidura russula. Ecology 89:2777–2785
Kadmon R, Pulliam HR (1993) Island biogeography: effect of geographical isolation on species composition. Ecology 74:977–981
Kaspari M (1996a) Litter ant patchiness at the 1 m2 scale: disturbance dynamics in three Neotropical forests. Oecologia 107:265–273
Kaspari M (1996b) Testing resource-based models of patchiness in four Neotropical litter ant assemblages. Oikos 76:443–454
Laurance WF (2008) Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biol Conserv 141:1731–1744
Leal IR, Filgueiras BKC, Gomes JP, Iannuzzi L, Andersen AN (2012) Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Biodivers Conserv 21:1687–1701
Logan M (2010) Biostatistical design and analysis using R: a practical guide. Wiley-Blackwell, Chichester
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution 17:373–387
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Majer JD, Delabie JHC, McKenzie NL (1997) Ant litter fauna of forest, forest edges and adjacent grassland in the Atlantic rain forest region of Bahia, Brazil. Insectes Sociaux 44:255–266
Noss RF (1987) Corridors in real landscapes: a reply to Simberloff and Cox. Conserv Biol 1:159–164
Palacio EE, Fernández F (2003) Claves para las subfamilias y géneros. In: Fernández F (ed) Introducción a las Hormigas de La región Neotropical. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá pp 233–260
Pellet J, Fleishman E, Dobkin DS, Gander A, Murphy DD (2007) An empirical evaluation of the area and isolation paradigm of metapopulation dynamics. Biol Conserv 136:483–495
Perfecto I, Vandermeer J (2002) Quality of agro ecological matrix in a tropical montane landscape: ants in coffee plantations in southern Mexico. Conserv Biol 16:174–182
Rantalainen ML, Fritze H, Haimi J, Pennanen T, Setala H (2005) Species richness and food web structure of soil decomposer community as affected by the size of habitat fragment and habitat corridors. Glob Chang Biol 11:1614–1627
Rantalainen ML, Haimi J, Fritze H, Setälä H (2006) Effects of small-scale habitat fragmentation, habitat corridors and mainland dispersal on soil decomposer organisms. Appl Soil Ecol 34:152–159
R Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org/. Accessed 29 Aug 2011
Schmiegelow FKA, Machtans CS, Hannon SJ (1997) Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology 78:1914–1932
Schoereder JH, Galbiati C, Ribas CR, Sobrinho TG, Sperber CF, DeSouza O, Lopes-Andrade C (2004a) Should we use proportional sampling for species–area studies? J Biogeogr 31:1219–1226
Schoereder JH, Sobrinho TG, Ribas CR, Campos RBF (2004b) Colonization and extinction of ant communities in a fragmented landscape. Aust Ecol 29:391–398
Simberloff DS, Wilson EO (1969) Experimental zoogeography of islands: the colonization of empty islands. Ecology 50:278–296
Soares SM, Schoereder JH (2001) Ant-nest distribution in a remnant of tropical rainforest in southeastern Brazil. Insectes Sociaux 48:280–286
Sobrinho TG, Schoereder JH, Sperber CF, Madureira MS (2003) Does fragmentation alter species composition in ant communities (Hymenoptera: formicidae)? Sociobiology 42:329–342
Sodhi NS, Lee TM, Koh LP, Dunn RR (2005) A century of avifaunal turnover in a small tropical rainforest fragment. Anim Conserv 8:217–222
Srivastava DS, Kolasa J, Bengtsson J, Gonzalez A, Lawler SP, Miller TE, Munguia P, Romanuk T, Schneider DC, Trzcinski MK (2004) Are natural microcosms useful model systems for ecology? TREE 19:379–384
Staddon P, Lindo Z, Crittenden PD, Gilbert F, Gonzalez A (2010) Connectivity, non-random extinction and ecosystem function in experimental metacommunities. Ecol Lett 13:543–552
Steffan-Dewenter I, Tscharntke T (2000) Butterfly community structure in fragmented habitats. Ecol Lett 3:449–456
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology 79:2041–2056
ter Braak CJF, Hanski I, Verboom J (1998) The incidence function approach to modeling of metapopulation dynamics. In: Bascompte J, Solé RV (eds) Modeling spatiotemporal dynamics in ecology. Springer, New York, pp 167–188
Veloso HP, Rangel Filho ALR, Lima JCA (1991) Classificação da vegetação brasileira adaptada a um sistema universal. Fundação Instituto Brasileiro de Geografia e Estatística(IBGE), Rio de Janeiro
Watson DM (2002) A conceptual framework for studying species composition in fragments, islands and other patchy ecosystems. J Biogeogr 29:823–834
Weldon AJ (2006) How corridors reduce indigo bunting nest success. Conserv Biol 20:1300–1305
Acknowledgments
We acknowledge the help from Vitor D. Fernandes and Raquel G. Loreto for fieldwork, and Rodrigo M. Feitosa and Vivian E. Sandoval for ant identification. Comments from Ben Hoffmann and three anonymous referees improved the manuscript. The authors were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) grants. The study was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paolucci, L.N., Solar, R.R.C., Sobrinho, T.G. et al. How does small-scale fragmentation affect litter-dwelling ants? The role of isolation. Biodivers Conserv 21, 3095–3105 (2012). https://doi.org/10.1007/s10531-012-0356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0356-5