Abstract
Diatoms, microscopic unicellular algae, have been widely sampled from streams around the world because of their use in biological assessments, but as a result they are often only collected from epilithic habitats in riffles, which may lead to a substantial underreporting of their diversity and distributional patterns. The goals of this study were to identify how sampling methods and water chemistry affect the characterization of local and regional diversity and distributions. Species richness, Shannon diversity, and evenness were significantly greater in multiple habitat (MH) than epilithic habitat (EH) samples (paired t-test; P < 0.01). At the local scale, MH sampling yielded 76% of the total species collected (42% unique), EH sampling captured 58% (24% unique), and 34% were shared. From MH samples, 3, 28, and 60 regionally rare species were collected from >75, >50, and >25% of sites, respectively, whereas 1, 14, and 34 were collected from EH samples, respectively. Regional abundance more strongly predicted site frequency than mean local abundance. Smaller drainage basins tended to have fewer species, likely because of species-area relationships, but local factors (% EH and alkalinity) influenced the taxonomic complexity of assemblages. Diversity was greatest at intermediate % EH, but low alkalinity reduced diversity, which is potentially important for ecosystems affected by anthropogenic acidification, as in our study. Multiple habitats need to be sampled for better documenting diatom diversity and distributions, which could improve conservation efforts at local and regional scales along with the characterization of ecological patterns and processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Documenting species diversity and distributions is both a practical and philosophical issue. Species diversity can be important to ecosystem processes, integrity, and management of natural resources (Tilman et al. 1997; Cottingham et al. 2001; France and Duffy 2006), although its significance can vary among ecosystems and functional diversity may be as crucial, if not more so (Hooper and Vitousek 1997; Schwartz et al. 2000; Hooper et al. 2005). Efforts to characterize species diversity and distributions are becoming increasingly important and urgent as many ecological systems face escalating pressures from human induced environmental change (Tilman 1999; Hooper et al. 2005). The diversity and function of lotic ecosystems are especially sensitive to changes in climate and land use because they rely on hydrologic patterns affected by precipitation, hydro-modifications, and land use patterns in the upstream watershed (Allan 2004; Palmer et al. 2004). In addition, non-glacial freshwater comprises less than 1% of water worldwide, with less than 1% of that being streams and rivers (Wetzel 2001), thus the global distribution of many freshwater organisms is restricted to a relatively small percentage of the global land surface. Nevertheless, organisms living in streams contribute disproportionate ecosystem services, such as nutrient and biogeochemical cycling, energy flow through food webs, and processing various types of pollution, which have important terrestrial and downstream linkages (Vitousek et al. 1997; Finlay 2001; Baron et al. 2002).
Diatoms, unicellular algae with global distributions in nearly all aquatic environments (Round et al. 1990), are important structural and functional components in stream ecosystems because they are the predominant members of biofilms and primary producers (Lowe and Pan 1996; Battin et al. 2003). They have been used widely as aquatic bioindicators for decades, and their rich diversity and wide distribution make them an ideal group of organisms to study constraints on diversity and distributional patterns. Diatoms are particularly strong responders to water chemistry, such as nutrients (Winter and Duthie 2000a; Potapova and Charles 2007; Leira et al. 2009), conductivity or ionic composition (Potapova and Charles 2003), and alkalinity (Soininen 2007). However, characterizing patterns of diatom diversity has been difficult because they often show inconsistent responses to environmental gradients and pollution (Jüttner et al. 1996; Stevenson and Pan 1999). Many diatom species also display strong physical habitat specificities (Winter and Duthie 2000b; Soininen and Eloranta 2004; Townsend and Gell 2005), and diversity can differ considerably among substrata (Cattaneo et al. 1997; Potapova and Charles 2005). Distinct substrata and habitats have different constraints on the development and structure of diatom communities (Snoeijs 1991; Burkholder 1996; Besse-Lototskaya et al. 2006; Passy and Legendre 2006), so it should be expected that documenting diversity and distributional patterns would depend on the type of sampling at the local scale (i.e., within a stream reach).
Diatoms have been widely sampled from streams around the world because of their use in biological assessments, but as a result they are often only collected from epilithic habitats in riffles (Kelly et al. 1998; Stevenson and Bahls 1999; AFNOR 2000), which may lead to a substantial underreporting of their diversity and distributional patterns. A large portion of diversity could be missed when conducting single habitat sampling because only one component of the species pool is collected (Jüttner et al. 1996; Potapova and Charles 2005), but this has rarely been directly studied. This potential underestimation would likely have considerable implications for characterizing species distributional patterns, ecological patterns and processes, monitoring species gains and losses, and conservation efforts. The objectives of this research were (1) to identify how substrata sampling and chemistry affect diatom diversity in streams using regression techniques, (2) to document any diversity potentially missed by single habitat samples (i.e., epilithic habitats in riffles) using simple descriptive statistics, species accumulation curves, bootstrapping, and paired t-tests for significant differences, and (3) to evaluate the subsequent implications for conserving biodiversity and characterizing species distributional patterns by investigating site frequency distributions and abundance-frequency relationships.
Methods
Study sites and sampling
Diatoms were collected from 61 streams throughout the Western Allegheny Plateau of southeast Ohio, USA (30,828 km2) during base flow conditions, August through September, 2005–2006. This region is predominantly forested with dissected topography, principally sandstone, shale, limestone and coal geology, and has a history of extensive coal mining, logging, and some agricultural activities (Omernik 1987; further details in Smucker and Vis 2009). At each site, two samples were collected, one from the epilithic habitat (EH) within a riffle (e.g., rocks and cobble), and a separate multi-habitat (MH) composite sample collected proportionately from available habitats (e.g., riffles and pools) and substrata (e.g., rock, sand, silt, etc.) in a 50 m reach encompassing the riffle sampled. Plankton and macrophytes were not sampled because this study only included wadeable streams in which the abundance of diatoms in the plankton is almost non-existent and macrophytes were absent in nearly all streams in our study region and comprised much less than 5% of the available habitat when present. Epilithic diatoms were removed by firmly holding a 7.1 cm2 rubber O-ring on the upper surface of the rock while scraping with a firm-bristled toothbrush. Diatoms growing on sediments were sampled by gently placing the O-ring on the substratum while suctioning the upper surface with a pipette (Stevenson and Bahls 1999). For single substratum samples, epilithic habitats (EH) were sampled (10 O-ring scrapings) equidistantly along a zigzag path from the downstream end to the upstream end of the riffle. Multi-habitat (MH) samples were proportionately comprised of 10 O-ring scrapings of epilithic habitats or suctions of sediments after visually estimating habitats and substrata within the 50 m reach. Each protocol sampled 71 cm2 of substratum. Samples were kept on ice until returning to the lab where 10-ml subsamples were preserved in 2.5% CaCO3-buffered glutaraldehyde after homogenization.
Diatom samples were cleaned using 30% H2O2 and 50% HNO3. After repeatedly rinsing samples with distilled water to increase pH to 7, samples were settled onto cover slips in a chamber used to randomly disperse diatom valves (Battarbee 1973), and mounted on slides with NAPHRAX™ (Brunel Microscopes Ltd., Hazelbrook, Wiltshire, UK). A total of 500 valves were counted and identified per sample in a light microscope at 1000× (Olympus BX40™, Center Valley, Pennsylvania, USA). The keys used for species identification were primarily by Kramer and Lange-Bertalot (1986), 1988, 1991a, b), but primary literature was also used and taxonomy was updated accordingly to account for recently erected genera, described species, and transferred species, which allow for the current recognition of genus and species status. Just as one example of many, genera, such as Luticola, Geissleria, Placoneis, Hippodonta, and others have been split from the genus Navicula.
At each site, pH, conductivity, and temperature were measured in situ using handheld probes (Waterproof ECTestr® and pHTestr 30®, Oakton, Vernon Hills, Illinois, USA). Stream water was filtered (0.45 μm pores) and stored on ice until returning to the lab for subsequent chemical analysis. Protocols accepted by the United States Environmental Protection Agency were used for measuring PO4-P, NO3-N, SO4 2−, alkalinity, and acidity with powder pills from Hach Company (Anonymous 1997), and Cl− with an ion selective electrode. The cations, Ca2+, Mg2+, K+, and Na+, were measured using an inductively coupled plasma atomic emission spectrometer (Varian Vista, Palo Alta, California, USA).
Statistical analyses
Diatom species richness (S), Shannon diversity (H′), evenness (E), and percent dominant taxon (D) were calculated for each sample. Regressions were conducted with the % epilithic substratum at each site to explore relationships with diatom diversity measures. Percent epilithic substratum was used as a simplified measure of substrata composition at each site because substrata at sites varied only in proportion of sediment to rock habitat, and diatom communities are similar among soft substrata (e.g., sand, mud, silt, etc.) in streams (Potapova and Charles 2005). After preliminary analyses, the taxonomic measures indicated nonlinear relationships with the % epilithic substratum, and subsequent nonlinear peak regressions were conducted in SigmaPlot v. 8.0 (SYSTAT Software Inc., Chicago, Illinois, USA). Patterns in the residuals from nonlinear regressions were examined in forward stepwise multiple regressions with water chemistry variables and drainage area to explore non-habitat related variation in S, H′, E, and D using Number Cruncher Statistical Systems (NCSS 2004, Kaysville, UT, USA).
Only 58 sites were used for comparing diversity and distribution patterns from EH and MH samples because three sites had no epilithic habitat in riffles. Shannon diversity, species accumulation curves, and bootstrapped species richness accumulation generated from EH and MH samples were calculated and compared using EstimateS v. 8.0 with 250 randomizations (Colwell 2005). Bootstrapped species richness is a resampling statistical method, which provides estimates of the potential richness and species accumulation curves from each protocol (Smith and Van Belle 1984; Colwell 2005). Pearson correlations were conducted to examine the relationship of S, H′, E, and D between EH and MH. Paired t-tests were conducted to test for significant differences in diversity measured between EH and MH samples. Pearson correlations were used to examine relationships of shared species and species unique to each sampling method with % epilithic substratum and water chemistry variables. Rank abundance curves of species were plotted to compare species distributional patterns produced by EH and MH sampling protocols. Occupancy-frequency distributions were compared between the two methods to explore relationships of regional abundance with distributions and shared species. The term “regional distribution,” as used in this study implies site frequency or the number of sites occupied by species (i.e., widely distributed species are species found at many sites), and the term “local scale” means at a sampling site.
Results
Environmental relationships with diatom diversity from multiple habitats
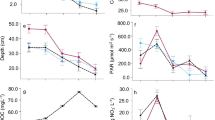
Percent epilithic habitat present in the study streams ranged from 0 to 90% (mean = 55%, 25th, 75th percentile = 40, 70%). Diatom S, H′, E, and D had unimodal relationships with % epilithic substratum present at sites, as indicated by non-linear peak regressions [R 2 = 0.13, 0.24, 0.25, and 0.25, respectively (Fig. 1)]. The more homogeneous the physical habitat was at a site, the more diatom communities were dominated by a single taxon while being less species rich, diverse, and even. Greatest S, H′, and E, and subsequently least D, existed at sites with intermediate % epilithic substratum (30–70%). Diatom S was positively correlated with drainage area (r = 0.31, P = 0.02), but H′, E, and D were not (P > 0.05). H′, E, and D were correlated with alkalinity (r = 0.41, 0.47, and −0.42, respectively, P < 0.01 for each).
Forward stepwise multiple regressions of residuals from nonlinear regressions and environmental variables were subsequently conducted. Of the 14 variables used in stepwise multiple regressions, only drainage area was selected as a correlate with S and alkalinity for H′, E, and D. Residuals of S were increasingly positive with sites having larger drainage areas (R 2 = 0.10, P = 0.02), indicating that S was potentially overestimated in smaller drainage basins, underestimated in larger basins, and more accurately estimated in mid-sized basins by the % EH present at a site (Fig. 2a). Residuals of H′, E, and D were associated with alkalinity in the stepwise multiple regressions (Fig 2b–d). H’ and E residuals were increasingly positive with greater alkalinity (R 2 = 0.16, P < 0.01 and R 2 = 0.21, P < 0.01, respectively), indicating that H′ and E were potentially overestimated at low alkalinities [i.e., more negative residuals in the EH regressions (Fig. 1)], underestimated at high alkalinities (i.e., more positive residuals in the EH regressions), and more accurately estimated at intermediate alkalinities by the % EH present at a site (Fig 2b, c). D showed the opposite pattern, with greater dominance than predicted by % EH at lower alkalinities (Fig. 2d). Percent epilithic substratum was an important predictor of H′, E, and D at intermediate alkalinity, but extremes in alkalinity can override or reduce the effect of intermediate amounts of EH on diatom diversity measures.
Regressions showing relationships between a residual species richness [S] and drainage area, and b residual Shannon diversity [H′], c evenness [E], and d relative abundance of the dominant diatom taxon with alkalinity. Residuals are from peak-regressions of diversity measures of multiple habitat samples with % epilithic substratum
Single habitat versus multi-habitat diversity
Regionally, a total of 416 diatom species were identified, with 300 of these species collected in epilithic habitat samples, and 327 in multi-habitat samples. Mean total S (i.e., all species collected by the two methods combined) at a site was 73.8 ± 2.1 (1 SE). Multi-habitat mean species richness was 56.1 ± 1.7 (1 SE), and epilithic habitat mean species richness was 42.5 ± 1.4 (1 SE). At the local scale, 76.2 ± 0.9% (1 SE) of species collected by the two methods were present in MH [42.1 ± 1.2% (1 SE) unique to MH], 57.9 ± 1.2% (1 SE) were in EH [23.8 ± 0.9% (1 SE) unique to EH], and 34.1 ± 1.0% (1 SE) shared (Fig. 3a). At the regional scale, 79% of species collected by the two methods were present in MH (28% unique), 72% in EH (21% unique), and 51% shared (Fig. 3b). The % shared species between MH and EH at a site was weakly correlated with % EH (r = 0.28, P = 0.04) and alkalinity (r = 0.35, P = 0.01). The % species unique to MH was negatively correlated with % EH (r = −0.35, P = 0.01).
a Mean local species richness attributed exclusively to multi-habitat samples (black), epilithic habitat samples (white), and species shared between the two protocols (gray) at each site. b Regional species richness attributed exclusively to multi-habitat samples (black), epilithic samples (white), and species shared between the two protocols (gray)
Diatoms with high total relative abundances at the regional scale were often collected in both EH and MH samples (Fig. 4). The 15 most regionally abundant species in EH and MH samples are listed in Table 1, with nine species being common in both EH and MH samples. The six abundant species unique to MH were all highly motile biraphid species (Hill et al. 2000), whereas five of the six abundant species unique to EH were not characterized as highly motile, with the exception of Navicula schroeterii, and had modes of attachment or adnate growth forms (Table 1).
S, H’, E, and D correlations between EH and MH were significant (r = 0.65, 0.61, 0.58, and 0.46, respectively, all P < 0.01) (Fig 5a–d). Species richness, H′, and E were significantly greater in MH samples than EH samples (paired t-test, P < 0.01), and D was significantly greater in EH samples than MH samples (paired t-test, P < 0.01). The mean species accumulation curve for MH samples was steeper than that for EH samples, but the lower 95% confidence interval of MH and the upper 95% confidence interval of EH overlapped (Fig. 6a). Bootstrapped species richness was significantly greater in MH [366 ± 1.9 (1 SD)] than EH [343 ± 1.8 (1 SD)], and the bootstrapped species accumulation curve was steeper for MH samples than EH samples (Fig. 6a). After six sampled sites, or approximately 3,000 counted individuals, one standard deviations of bootstrapped species richness means no longer overlapped between EH and MH, giving strong statistical support for greater S, H′, and E in MH samples (Fig. 6a). The Shannon diversity accumulation curve was steeper for MH samples than EH, and regional H’ was 4.25 ± 0.01 (1 SD) in MH samples and 3.90 ± 0.01 (1 SD) in EH samples (Fig. 6b). After four sampled sites, or approximately 2,000 counted individuals, one standard deviations of Shannon diversity means no longer overlapped between EH and MH (Fig. 6b).
Black circles or lines = multi-habitat (MH) dataset, gray circles or lines = epilithic habitat (EH) dataset. a Species accumulation curves (solid lines) of MH and EH samples with 95% confidence intervals (dashed lines), and bootstrapped species richness (dotted lines, error bars = ± 1SD). b Relationship of Shannon diversity with increased number of sampled individuals ± 1 SD
Species distributions
The majority of species from both sampling methods were infrequently found at a large number of sites (MH median = 4 sites, 75th, 25th % = 13, 1, EH median = 3 sites, 75th, 25th% = 10, 1). The site frequency distribution of MH species had a shallower curve than that for EH species, which indicated more frequent regional occurrences of species in MH samples than species in EH samples (Fig. 7). Of the 300 species collected from EH samples, 7, 26, and 48 species were present at >75, >50, and >25% of sites, respectively, whereas 11, 40, and 70 of the 327 species collected from MH samples were present at >75, >50, and >25% of sites, respectively (Fig. 7).
Rare species (regional relative abundance <1.5%, determined from the vertex of species rank abundance curves) had greater regional site occurrences in MH samples than EH samples. From MH samples, 3, 28, and 60 rare species were collected from >75, >50, and >25% of sites, respectively, whereas only 1, 14, and 34 rare species were collected from EH samples at >75, >50, and >25% of sites, respectively. In MH samples, 4.3% (14/327) of diatom species considered non-rare comprised 50% of all individuals counted, and in EH samples, 4.3% (13/300) of diatom species comprised 60% of all individuals counted. The rare diatom species seldom had wide distributions (Fig. 7). Only Navicula cryptocephala was collected in >75% of sites with <1.5% regional abundance in both EH and MH. Synedra ulna and Cyclotella meneghiniana also fit this condition in MH samples. Brachysira vitrea was the only species with >1.5% regional relative abundance but collected in less than 50% of sites, which further indicated the widespread distribution of regionally abundant species (e.g., 13/14 MH and 12/13 EH non-rare species were found at >50% of sites).
The MH and EH sampling methods produced similar sigmoidal occupancy-frequency distributions with regionally abundant species being most widely distributed and species with low regional abundances being more narrowly distributed (Fig. 8). Mean local species abundances, calculated using only sites where each species was present, were correlated with regional site distributions for both EH and MH samples (r = 0.37, 0.47 and P < 0.01, respectively), but total regional abundance was more strongly correlated with site distributions (r = 0.73, 0.89 and P < 0.01, respectively). Species with observed site frequencies outside the 5 and 95% confidence intervals were considered extremely under- or over-dispersed, respectively. Under-dispersed species in EH and MH samples were not as widely distributed as predicted by regional abundance, tended to have high local relative abundances, but were regionally rare, with the exception of Achnanthidium deflexum from epilithic habitats (Table 2). Over-dispersed species had wider regional distributions than predicted, had low local relative abundances, and low regional relative abundances, with the exception of Amphipleura pellucida and Amphora montana in multi-habitat samples (Table 3).
Discussion
Impacts to diatom diversity
Habitat sampling and alkalinity were important to the diversity in MH samples. More homogeneous substrata had lower diversity and greater dominance by a single taxon. This importance of physical substrata composition is similar to what has been documented for other aquatic (Gorman and Karr 1978; Townsend et al. 1997; Palmer et al. 2000) and terrestrial organisms (MacArthur and MacArthur 1961; Rosenzweig and Winakur 1969; Otte 1976). We feel that percent EH is meaningful for describing patterns associated with MH sampling, but we also acknowledge that the percent EH is a very simplified measure of habitat and is a measure that inextricably interacts with other biotic and abiotic variables. For example, increased heterogeneity of nutrient concentrations within a stream reach increases algal diversity (Pringle 1990), and habitat type is associated with nutrient availability (e.g., greater accumulation of nutrients in depositional habitats than in erosional, and different diffusion rates associated with boundary layers) (Cattaneo et al. 1997; Dodds 2003). Diversity of macroinvertebrates and feeding guilds in heterogeneous stream habitats (Death and Winterbourn 1995; Downes et al. 1998), current velocity and heterogeneity (Stevenson 1983), and flood disturbances interacting with algal biomass (Cardinale et al. 2006) and habitat variety (Soininen and Eloranta 2004) also affect algal diversity in streams. In addition, channelization, agriculture, and urbanization impact the habitat of stream ecosystems by reducing habitat complexity, which would be expected to decrease diversity (USEPA (United States Environmental Protection Agency) 2000a; Paul and Meyer 2001; Walsh et al. 2005). Despite the many variables that interact with habitats, percent EH, which can be affected by human impacts, was a useful proxy that was related to diatom diversity. Beyond effects on diversity, sediment loads attributed to agriculture can cause a subsequent increase in the relative abundance and number of motile and high nutrient diatom species (Bahls et al. 1992; Fore and Grafe 2002; Smucker and Vis 2009), which is a relationship that could potentially be missed when only collecting EH samples (Smucker and Vis 2010).
Low and high alkalinity reduced or negated the effect of intermediate amounts of EH on diatom diversity, and increased diversity was directly and significantly correlated with greater alkalinity. Stream alkalinity is likely a predominant filter controlling assemblage structure at sites because diatoms are very sensitive to pH and acidification (Charles et al. 1991; Battarbee et al. 1999; DeNicola 2000). Alkalinity is important to diatom diversity in regions with anthropogenic acidification (Battarbee et al. 1999), but might not be as crucial in naturally low alkalinity systems (Passy 2001). Acid mine drainage (AMD) pollution, with acidic pH and high concentrations of metals, from abandoned coal waste especially threatens the diversity of lotic diatom communities (Verb and Vis 2000). AMD is a common environmental stressor that severely impacts aquatic biota in >7000 stream kilometers throughout southeast Ohio and Appalachia (OEPA (Ohio Environmental Protection Agency) 2000; USEPA (United States Environmental Protection Agency) 2000b). In the current study, AMD is suspected of reducing alkalinity at some sites and being associated with reduced diversity and evenness of diatom communities, which is a pattern that has also been observed in streams impacted by acid precipitation (Passy 2001). Extremely high alkalinities and pH could likely reduce diatom diversity (Pither and Aarssen 2005), but a monotonic relationship between increasing diversity and greater alkalinity was observed, likely because the maximum pH in the current study was 8.3 and no highly alkaline streams were present. Beyond the effects of habitat and alkalinity, intermediate levels of water quality impairment, particularly by nutrients, can also be associated with the greatest amounts of diatom diversity (Jüttner et al. 1996; Stevenson and Pan 1999).
Drainage area was only important for the number of species at a site and not for Shannon diversity, evenness, or dominance by a taxon. This finding could indicate that dispersal throughout the watershed limits the number of species at a site, and local factors, such as substrata composition and alkalinity, constrain taxonomic complexity of diatom assemblages (e.g., Shannon diversity, evenness, and dominance by a taxon). Sites with larger drainage areas upstream likely have greater species pools than sites located closer to headwater sources, which coincides with the frequently observed trend that larger areas are capable of supporting a greater number of species than smaller areas (Broenmark et al. 1984; Rosenzweig 1995). While streams of all sizes need protection from environmental impacts, this finding suggests that smaller headwater streams are of even more conservation value because of their greater sensitivity to loss of species than sites farther downstream. In addition, promoting conservation of diversity in headwater streams could help maintain ecosystem structure and services that would benefit downstream community dynamics (Allan 2004). Further research on the role of algal species richness and species loss in ecosystem processes could improve conservation strategies.
Diversity missed by sampling only one habitat
Not surprisingly, local species richness, Shannon diversity, and evenness were significantly less in EH than MH samples, and EH samples potentially missed 42% of the species at a site, whereas MH samples potentially missed only 24%. This finding complicates the documentation of species distributions throughout the region and has subsequent implications for characterizing ecological patterns. The most regionally abundant diatom species were typically the most widely distributed and tended to be collected from both protocols, indicating potentially wide tolerances to environmental conditions and non-specificity to substratum type, which is a pattern that has been observed in many other species and ecosystems (Brown 1984). Species found in both samples at a site could be a result of some MH samples containing epilithic diatoms collected from a riffle where EH samples were also collected, which was supported by a weak, but significant, correlation of shared species with the percent EH sampled in MH samples.
Steep Shannon diversity accumulation curves indicated a large number of rare species in both EH and MH samples, which was also evident in that only ~4.3% of the species accounted for 50 and 60% of the individuals counted in MH and EH samples, respectively. The distribution of rare species was underestimated by EH samples as indicated by a much steeper rank abundance curve when compared to MH samples (Fig. 7), and the number of rare species found in MH samples at >25, >50, and >75% of sites was almost double that for EH samples. Species accumulation curves followed similar trajectories, but the curve for MH samples was much steeper, further indicating greater richness and evenness than in EH samples. Bootstrapping showed much greater estimation of regional richness and diversity in samples collected from MH than from EH. These findings could have substantial implications for documenting species distributions and for conservation efforts because of the large underestimation of species diversity in EH samples.
Distributional patterns and conservation implications
Local and regional extinction rates are influenced by both the distribution and abundance of species (Hanski 1982). The most abundant and widely dispersed species are less likely to become locally or regionally extinct because of their greater tolerance of environmental variability, many propagules for dispersal, and wide distributions. In the current study, high mean local abundance of species did not necessarily signify greater geographic distributions, although this is a common finding with other organisms (Hanski 1982; Brown 1984; Hartley 1998) and in diatom datasets from larger spatial scales (Passy 2008). Regional abundance was much more strongly associated with the site frequency of species. Many species were capable of dominating the local community, but did not have wide distributions (i.e., under-dispersed species) (Table 2, Fig. 8). Many species with wide regional distributions also tended to have high local abundance means as previously reported in other studies, but some had wide geographic distributions and never reached high local abundances (i.e., over-dispersed species) (Table 3, Fig. 8). Under-dispersed species are at greatest risk for regional extinction because they were collected at a few sites and likely thrived under less common regional conditions. Over-dispersed species are likely at greater risk for local extinction because of their inability to be abundant at any of our sites, but their wide distributions make them less likely to become regionally extinct.
The notion that some species are under-dispersed does not necessarily mean they represent unique habitats of high conservation value, because they could represent highly impacted streams or upstream conditions, such as reservoirs, that are less common in the study region. Examples include Eunotia exigua, Surirella roba, and Brachysira vitrea, which are species typical of low alkalinity and AMD impacted conditions in the region (Verb and Vis 2000), Pleurosigma salinarum, Nitzschia reversa, Pleurosira laevis, which are species typical of anthropogenic impacted waters with extremely high conductivities (van Dam et al. 1994; Leland et al. 2001), and Stephanodiscus hantzschii and Aulacoseira granulata which are common planktonic species (Heinsalu et al. 2008) that likely washed down from upstream reservoirs and settled more readily in depositional habitats. Species such as Rhopalodia gibba, Epithemia sorex, and Nitzschia sinuata v. tabellaria, may indicate habitats of greater conservation value because they are typical of very low nitrogen conditions (Potapova and Charles 2007). Collectively considering habitat and water chemistry along with the diversity, abundance, and types of diatom species present (i.e., autecologies) is important for improving management efforts designed to effectively restore and conserve streams and biodiversity.
Recently, diatom assessments generated from epilithic habitat and multi-habitat sampling methods have shown similar conclusions or slightly stronger relationships of multi-habitat samples with environmental and landuse variables (Winter and Duthie 2000b; Smucker and Vis 2010). In addition, algal communities on hard and soft substrata may indicate similar environmental gradients, although species composition can differ (Cattaneo et al. 1997; Potapova and Charles 2005). With mounting evidence that diatoms from MH samples can be as effective as EH samples in biomonitoring studies, MH sampling for diatoms would greatly benefit the future documentation and characterization of diatom diversity and distribution patterns, along with species losses and gains. Reducing anthropogenic contributions of nutrients and sediments to streams, conscientious land use practices, and conserving habitats are obviously imperative to sustaining stream ecosystems, but MH sampling could help guide future efforts and monitoring trends in lost or gained diversity and richness in the face of escalating human pressures on streams.
Abbreviations
- AMD:
-
Acid mine drainage
- D:
-
Dominant taxon
- E:
-
Evenness
- EH:
-
Epilithic habitats
- H:
-
Shannon diversity
- MH:
-
Multiple habitats
- S:
-
Species richness
References
AFNOR (2000) Norme Française NF T 90-354. Détermination de l’Indice Biologique Diatomées (IBD)
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol S 35:257–284
Anonymous (1997) Data logging colorimeter handbook. Hach Company, Loveland, CO
Bahls LL, Burkantis R, Tralles S (1992) Benchmark biology of Montana reference streams. Department of Health and Environmental Science, Water Quality Bureau, Helena, Montana
Baron JS, Poff NL, Angermeier PL et al (2002) Meeting ecological and societal needs for freshwater. Ecol Appl 12:1247–1260
Battarbee RW (1973) A new method for estimating absolute microfossil numbers with special reference to diatoms. Limnol Oceanogr 18:647–653
Battarbee RW, Charles DF, Dixit SS, Renberg I (1999) Diatoms as indicators of surface water acidity. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 85–127
Battin TJ, Kaplan LA, Newbold JD, Hansen CME (2003) Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426:439–442
Besse-Lototskaya A, Verdonschot PFM, Sinkeldam JA (2006) Uncertainty in diatom assessment: sampling, identification and counting variation. Hydrobiologia 566:247–260
Broenmark C, Herrmann J, Malmqvist B et al (1984) Animal community structure as a function of stream size. Hydrobiologia 112:73–79
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Burkholder JM (1996) Interactions of benthic algae with their substrata. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Academic Press, New York, pp 253–298
Cardinale BJ, Hillebrand H, Charles DF (2006) Geographic patterns of diversity in streams are predicted by a multivariate model of disturbance and productivity. J Ecol 94:609–618
Cattaneo A, Kerimian T, Roberge M, Marty J (1997) Periphyton distribution and abundance on substrata of different size along a gradient of stream trophy. Hydrobiologia 354:101–110
Charles DF, Dixit SS, Cumming BF, Smol JP (1991) Variability in diatom and chrysophyte assemblages and inferred pH: paleolimnological studies of Big Moose Lake, New York (USA). J Paleolimnol 5:267–284
Colwell RK (2005) EstimateS: statistical estimation of species richness and shared species from samples. In: Version 8.0. User’s guide and application. http://purl.oclc.org/estimates
Cottingham KL, Brown BL, Lennon JT (2001) Biodiversity may regulate the temporal variability of ecological systems. Ecol Lett 4:72–85
Death RG, Winterbourn MJ (1995) Diversity patterns in stream benthic invertebrate communities: the influence of habitat stability. Ecology 76:1446–1469
Denicola DM (2000) A review of duiatoms found in highly acidic environments. Hydrobiologia 433:111–122
Dodds WK (2003) The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. J Phycol 39:840–849
Downes BJ, Lake PS, Schreiber ESG, Glaister A (1998) Habitat structure and regulation of local species diversity in a stony, upland stream. Ecol Monogr 62:237–257
Finlay JC (2001) Stable-carbon-isotope ratios of river biota: implications for energy flow in lotic food webs. Ecology 82:1052–1064
Fore LS, Grafe C (2002) Using diatoms to assess the biological condition of large rivers in Idaho (USA). Freshwater Biol 47:2015–2037
France KE, Duffy JE (2006) Diversity and dispersal interactively affect predictability of ecosystem function. Nature 441:1139–1143
Gorman OT, Karr JR (1978) Habitat structure and stream fish communities. Ecology 59:507–515
Hanski I (1982) Dynamics of regional distribution: the core and satellite species hypothesis. Oikos 38:210–221
Hartley S (1998) A positive relationship between local abundance and regional occupancy is almost inevitable (but not all positive relationships are the same). J Anim Ecol 67:992–994
Heinsalu A, Luup H, Alliksaar T et al (2008) Water level changes in a large shallow lake as reflected by the plankton:periphyton-ratio of sedimentary diatoms. Hydrobiologia 599:23–30
Hill BH, Herlihy PR, Kaufman PR et al (2000) Use of periphyton assemblage data as an index of biotic integrity. J N Am Benthol Soc 14:451–457
Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277:1302–1305
Hooper DU, Chapin FS III, Ewel JJ et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Jüttner I, Rothfritz H, Ormerod SJ (1996) Diatoms as indicators of river quality in the Nepalese Middle Hills with consideration of the effects of habitat specific sampling. Freshwater Biol 36:475–486
Kelly MG, Cazaubon A, Coring E et al (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224
Kramer K, Lange-Bertalot H (1986) Bacillariophyceae, Teil 1: Naviculaceae. Gustav Fischer Verlag, Stuttgart, Germany
Kramer K, Lange-Bertalot H (1988) Bacillariophyceae, Teil 2: Bacillariaceae, Epithemiaceae and Surirellaceae. Gustav Fischer Verlag, Stuttgart, Germany
Kramer K, Lange-Bertalot H (1991a) Bacillariaphyceae, Teil 3: Centrales, Fragilariaceae and Eunotiaceae. Gustav Fischer Verlag, Stuttgart, Germany
Kramer K, Lange-Bertalot H (1991b) Bacillariophyceae, Teil 1: Achnanthaceae. Gustav Fischer Verlag, Stuttgart, Germany
Leira M, Chen G, Dalton C et al (2009) Patterns in freshwater diatom taxonomic distinctness along an eutrophication gradient. Freshwater Biol 54:1–14
Leland HV, Brown LR, Mueller DK (2001) Distribution of algae in the San Joaquin River, California, in relation to nutrient supply, salinity and other factors. Freshwater Biol 46:1139–1167
Lowe RL, Pan Y (1996) Benthic algal communities as biological monitors. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Academic Press, New York, pp 705–739
MacArthur RH, MacArthur JW (1961) On bird diversity. Ecology 42:594–598
OEPA (Ohio Environmental Protection Agency) (2000) Year 2000 Ohio water resource inventory. Ohio Environmental Protection Agency, Columbus, OH. http://www.epa.state.oh.us/dsq/document_index/305b.html
Omernik JM (1987) Ecoregions of the coterminous United States. Ann Assoc Am Geogr 77:118–125
Otte D (1976) Species richness patterns of New World desert grasshoppers in relation to plant diversity. J Biogeogr 3:197–209
Palmer MA, Swan CM, Nelson K et al (2000) Streambed landscapes: evidence that stream invertebrates respond to the type and spatial arrangement of patches. Landscape Ecol 15:563–576
Palmer M, Bernhardt E, Chornesky E et al (2004) Ecology for a crowded planet. Science 304:1251–1252
Passy SI (2001) Diatom community dynamics in streams of chronic and episodic acidification: the roles of environment and time. J Phycol 42:312–323
Passy SI (2008) Species size and distribution jointly and differentially determine diatom densities in US streams. Ecology 89:475–480
Passy SI, Legendre P (2006) Power law relationships among hierarchical taxonomic categories in algae reveal a new paradox of the plankton. Global Ecol Biogeogr 15:528–535
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol Syst 32:333–365
Pither J, Aarssen LW (2005) The evolutionary species pool hypothesis and patterns of freshwater diatom diversity along a pH gradient. J Biogeogr 32:503–513
Potapova MG, Charles DF (2003) Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshwater Biol 48:1311–1328
Potapova MG, Charles DF (2005) Choice of substrate in algae-based water-quality assessment. J N Am Benthol Soc 24:415–427
Potapova MG, Charles DF (2007) Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol Indic 7:48–70
Pringle CM (1990) Nutrient spatial heterogeneity: effects on community structure, physiognomy, and diversity of stream algae. Ecology 71:905–920
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press, Cambridge
Rosenzweig ML, Winakur J (1969) Population ecology of desert rodent communities: habitats and environmental complexity. Ecology 50:558–572
Round FE, Crawford RM, Mann DG (1990) The diatoms: biology and morphology of the genera. Cambridge University Press, Cambridge
Schwartz MW, Brigham CA, Hoeksema JD et al (2000) Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122:297–305
Smith EP, van Belle G (1984) Nonparametric estimation of species richness. Biometrics 40:119–129
Smucker NJ, Vis ML (2009) Use of diatoms to assess agricultural and coal mining impacts on streams and a multiassemblage case study. J N Am Benthol Soc 28:659–675
Smucker NJ, Vis ML (2010) Using diatoms to assess human impacts on streams benefits from multiple-habitat sampling. Hydrobiologia 654:93–109
Snoeijs PJM (1991) Monitoring pollution effects by diatom community composition: a comparison of sampling methods. Archiv für Hydrobiologie 121:497–510
Soininen J (2007) Environmental and spatial control of freshwater diatoms—a review. Diatom Res 22:473–490
Soininen J, Eloranta P (2004) Seasonal persistence and stability of diatom communities in rivers: are there habitat specific differences? Eur J Phycol 39:153–160
Stevenson RJ (1983) Effects of current and conditions simulating autogenically changing microhabitats on benthic algal immigration. Ecology 64:1514–1524
Stevenson RJ, Bahls LL (1999) Periphyton protocols. In: Barbour MT, Gerritsen J, Snyder BD, Stribling JB (eds) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, EPA 841-B-99–002, 2nd edn. Office of Water, US Environmental Protection Agency, Washington DC, pp 6-1–6-22
Stevenson RJ, Pan Y (1999) Assessing environmental condition in rivers and streams with diatoms. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 11–40
Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci USA 96:5995–6000
Tilman D, Knops J, Wedin D et al (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Townsend SA, Gell PA (2005) The role of substrate type on benthic diatom assemblages in the Dalp and Toper Rivers of the Australian wet/dry tropics. Hydrobiologia 548:101–115
Townsend CR, Scarsbrook MR, Dolédec S (1997) The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol and Oceanogr 42:938–949
USEPA (United States Environmental Protection Agency) (2000a) The quality of our nation’s waters. EPA 841-S-00-001
USEPA (United States Environmental Protection Agency) (2000b) Mid-Atlantic highlands streams assessment. EPA/903/R00/015, US Environmental Protection Agency Region 3, Philadelphia, PA
van Dam H, Mertens A, Sinkeldam J (1994) A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth J Aquat Ecol 28:117–133
Verb RG, Vis ML (2000) Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. J N Am Benthol Soc 19:274–288
Vitousek PM, Aber JD, Howarth RW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Walsh CJ, Roy AH, Feminella JW et al (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol 24:706–723
Wetzel RG (2001) Limnology: lake and river systems. Academic Press, San Diego
Winter JG, Duthie HC (2000a) Epilithic diatoms as indicators of stream total N and total P concentration. J N Am Benthol Soc 19:32–49
Winter JG, Duthie HC (2000b) Stream epilithic, epipelic and epiphytic diatoms: habitat fidelity and use in biomonitoring. Aquat Ecol 34:345–353
Acknowledgments
Funding was provided by a USEPA Science to Achieve Results grant (R831365) and an Ohio University Clippinger Fellowship. Emily Hollingsworth and Jason Zalack are thanked for sampling assistance. Dina Lopez and Prosper Gbolo analyzed water chemistry. Suggestions for manuscript improvement by Jared DeForest, Kelly Johnson, and Brian McCarthy are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smucker, N.J., Vis, M.L. Contributions of habitat sampling and alkalinity to diatom diversity and distributional patterns in streams: implications for conservation. Biodivers Conserv 20, 643–661 (2011). https://doi.org/10.1007/s10531-010-9972-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9972-0