Abstract
Soils are incredibly biodiverse habitats, yet soil-dwelling organisms have received little attention within the field of conservation biology. Due to difficulties involved in studying soil biota, and to taxonomic biases in conservation research, the full extent of soil biodiversity is not well understood, and soil-dwelling organisms are rarely candidates for conservation. The biogeography of soil biota differs significantly from that of plants or animals aboveground, and the taxonomic and functional diversity of soil-dwellers allows them to have a multitude of ecological effects on aboveground organisms. Soil organisms exhibit levels of biodiversity several orders of magnitude greater than those found in their aboveground counterparts on a per-area basis. The biodiversity of soils underpins many crucial ecosystem services which support the plants and animals typically targeted by conservation efforts. Strategies detailed in this paper provide practitioners with the ability to address many of the challenges related to incorporating soils and soil organisms into conservation planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When an endangered southern sea otter (Enhydra lutris nereis) is killed along the California coast, one might not expect the culprit to be a pathogenic soil fungus from the arid Southwestern United States. But Coccidioides immitis, the same soil-dwelling microbe that causes a human illness known as San Joaquin Valley Fever, has contributed to the deaths of several otters in California waters (Thomas and Cole 1996). Spores of C. immitis become wind-borne through soil disturbance, and can cause respiratory infections when inhaled by otters, humans, or other mammals. If the infection spreads through the body it can lead to tissue damage and even death. Without sea otters, biodiverse kelp forests can change into sea urchin barrens. For this reason, aggressive conservation management strategies have been employed to protect this keystone species. While a soil-based fungus capable of harming marine life may appear unusual or extreme, it is one of many examples of the far-reaching effects of soil biota on biodiversity conservation.
Belowground biodiversity has been understudied because of the formidable difficulties involved in sampling soils in a representative way, and because of the taxonomic intractability or uncultivable nature of many of the groups it typically contains. Consequently, the reticence to address soil ecology is apparent in conservation biology literature. The conservation of microscopic soil biota in particular has been very limited (Clark and May 2002; Klironomos 2002). However, as significant links continue to materialize between the soil biota we overlook and the larger conservation targets (such as sea otters) that we tend to study, we must begin to focus more of our attention on soil organisms and recognize that belowground biodiversity and the services it provides are an essential component of the health of ecosystems (Bever et al. 2001) upon which all species depend.
Inherent biodiversity of soils
Soils are the earth’s only meeting place for four crucial life-supporting realms: the atmosphere, lithosphere, hydrosphere, and biosphere. These come together to form the pedosphere, a complex micro-landscape that provides a multitude of unique niches supporting soil-dwelling life-forms. From bacteria, fungi, algae, and amoebas to centipedes, nematodes, mites, and moles, the taxonomic range of soil biota reaches into many branches on the tree of life, with representatives in all three domains: archaea, eubacteria, and eucarya (sensu Woese et al. 1990). The variance in body size of soil-dwelling organisms spans over five orders of magnitude. The spores of actinomycetes, which produce a familiar smell when rains first hit the earth, can be less than 1 μm in diameter (Reponen et al. 1998). In contrast, the Cape Dune mole rat (Bathyergus suillus), one of the largest completely subterranean mammals, measures 350 mm in length (Mills and Hes 1997). The functional group diversity of soil biota is also high. These functional groups cut across taxa and include ecosystem engineers, litter transformers, decomposers, and root herbivores, among others (Brussaard 1998). Given the large number of organisms that constitute each functional group, belowground activities and phenomena may be conceptually organized into various “spheres” related to their origin and significance in regulating major soil processes (Beare et al. 1995; Lavelle 1984). For example, the rhizosphere refers to the portion of the soil that is adjacent to and influenced by plant root exudation and associated microbial activity.
Phenomenal belowground biodiversity has led to soils being described as “the poor man’s tropical rainforest” (Usher et al. 1979). Swift et al. (2008) estimated overall soil biodiversity at approximately 1.6 million species. Soil organisms contribute the majority of genetic diversity to terrestrial ecosystems, with levels of taxonomic diversity several orders of magnitude greater than those found in their aboveground counterparts on a per-area basis (Bardgett 2005). Species are more densely packed in soil communities than in any other environment on earth (Hågvar 1998); a single gram of soil can contain millions of individuals and can harbor in excess of 10,000 unique taxa representing a dozen different phyla (Fierer et al. 2007). While the majority of belowground organisms are microscopic, even macroscopic animal species living in soil can be numerous (Wolters 2001). For example, Dymond et al. (1997) found that earthworm density in aspen forests of the Canadian Rocky Mountains could exceed 3,000 individuals per square meter of topsoil.
In some cases it is difficult to categorize species unequivocally as soil organisms. For example some decomposers live at the edge of the soil and are partially responsible for its genesis, yet do not necessarily spend their entire lives dwelling within the soil. If we define “soil” broadly to include associated elements such as dead and decaying plants or animals, soil diversity likely encompasses the majority of terrestrial animal species on earth (Decaens et al. 2006). In 1997, the global monetary value of soil biodiversity was estimated to be US $13 trillion (European Soil Bureau Network 2005), which would be nearly $17 trillion today.
Recognition of the soil as biodiverse
Significant effort has gone into documenting and categorizing both the taxonomic and functional diversity of soil organisms in recent years (e.g. Swift et al. 2008). Parameters used to assess diversity in soils vary significantly depending on the scale of assessment; given the minute size of most soil organisms, direct identification of soil organisms and measurement of the function of particular soil biota are both difficult tasks. In addition, soil microbial taxa are not easy to describe using physical characteristics. The complexity and challenges related to inventorying soils mean that the true extent of soil biodiversity is not fully known. It is estimated that only 10% of protozoa and 5% of mite species in soils have been taxonomically described (Brussaard et al. 1997), and smaller organisms are even less well-catalogued. Fewer than 1% of soil bacteria, and perhaps just as few as just 0.1%, can be successfully cultured in the lab (Clardy 2005). Historically, this has made it difficult for scientists to capture and record the identity of individuals within the microbial community. Only recently have the first steps been taken to develop molecular probes that will catalogue soil biota, and the challenges here remain formidable.
Some microbiologists assume that all soil microbial life is distributed worldwide, but that within a given soil habitat much of the microbial biodiversity would be undetectable because many species would occur at densities below our ability to observe them (de Wit and Bouvier 2006). These ideas were developed empirically by Beijerinck (1913), and distilled by Baas Becking (1934), who wrote “alles is overall: maar het milieu selecteert” (italics following the original text), which translates to “everything is everywhere but the environment selects”. Now that molecular techniques exist to test these assumptions, researchers have a renewed focus on understanding patterns in soil microbial biodiversity. Evidence of single-celled soil organisms with a less-than-world-wide distribution (Foissner 2008) has called into question the idea that “everything is everywhere”, and demonstrated that some groups of soil microbes have distinct biogeographical patterns.
In addition to being historically understudied due to technological constraints, soil biodiversity has also been understudied due to the intractability of some groups. Researchers prefer to study larger species, resulting in severe underrepresentation in the number of studies conducted on microfauna (Pawar 2003). Taxonomic bias within the field of conservation biology has lead to macroscopic invertebrates being overlooked in studies of biodiversity conservation: while they constitute 79% of known species, only 11% of research articles in the journals Conservation Biology and Biological Conservation focus on invertebrates (Clark and May 2002). Soil microorganisms in particular are overlooked; for example, protists are rarely considered as candidates for conservation in their own right (Cotterill et al. 2008). Klironomos (2002) pointed out that the underrepresentation of microbial research in a conservation context is so severe that microbes were ignored even in the assessment of taxonomic bias in conservation biology conducted by Clark and May (2002). Klironomos (2002) also found that in the biodiversity conservation literature (including issues of Conservation Biology, Biodiversity and Conservation, and Biodiversity and Distribution published from 1997 to 2001) studies of fungi/lichens constituted only 0.024 as a proportion of all articles, and protists and bacteria/viruses made up 0.007 and 0.006, respectively. To combat this demonstrated bias, conservation biologists committed to evaluating and protecting the earth’s genetic diversity should consider earmarking more resources for assessments of soil biota.
The unique biogeography of soil organisms
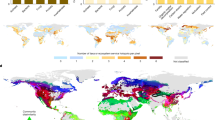
The geographic distribution of genetic diversity for soil biota is considerably different than that of above-ground organisms. Soil bacterial diversity peaks in soils that have a neutral pH (Fierer and Jackson 2006), so, while the tropics are considered hotspots of biodiversity for plants and animals (Myers et al. 2000), the acidic soil found in tropical regions makes these areas more like deserts for soil bacteria (Fig. 1). On average, grassland and desert soils have much higher bacterial diversity than tropical rainforest soils, and soil nematodes appear to reach maximum diversity in temperate zones (Bernard 1992). Soil microbial diversity also differs from that of animals and plants along elevation gradients, perhaps because the forces and factors structuring microorganism communities differ from those that structure communities of macroorganisms (Bryant et al. 2008).
A global comparison of biogeography of: (a) soil pH as a predictor of soil bacterial diversity and (b) terrestrial vertebrate species diversity based on the total number of amphibian, reptile, bird, and mammal species with each terrestrial ecoregion. Spatial data for soil pH data obtained from the International Geosphere-Biosphere Programme (IGBP-DIS 1998). Soil bacterial diversity is highest in soils with neutral pH, and lower in acidic and alkali soils, as reported by Fierer and Jackson (2006). Vertebrate species diversity data obtained from the World Wildlife Fund (2006)
Soils are three-dimensional and heterogeneous; the species that inhabit them vary not only horizontally across the landscape, but vertically with depth as well. In this way, soils are similar to tropical rainforests or oceans in terms of the spatial “layering” of species that occurs down the soil profile. Soil texture, chemistry, moisture, and a multitude of other factors vary with depth, providing unique, vertically-stratified habitats for soil-dwelling organisms. Several studies have shown that soil organisms vary with soil depth, from microfauna such as fungi or bacteria (Fierer et al. 2003), to macrofauna such as earthworms, gophers, or plant roots (Canadell et al. 1996). All of these examples demonstrate that biogeographical patterns in soil microbial diversity differ from those of plant and animal diversity. Given that microorganisms, including soil biota, constitute the majority of life on earth (Whitman et al. 1998), these findings should give conservation biologists pause. If protected, will designated hotspots really capture most of the earth’s living organisms? Or are we risking the loss of a multitude of species and genetic diversity that, due to a variety of factors, has not yet been adequately assessed?
Threats to soil biodiversity
Human activities threaten soils around the globe. An estimated 1% of topsoil is lost every year to erosion, largely due to deforestation and agricultural practices involving tillage and other forms of soil disturbance (Montgomery 2007). Urban development and pollution also contribute to soil disturbance and alter the fundamental properties of soils. These activities degrade “soil integrity”, a concept that is generally understood as the capacity of soil to fulfill all of its various functions in an ecosystem (Doran and Parkin 1994). Soil integrity can be thought of as the degree to which a soil is protected from anthropogenic degradation such that the natural factors of climate, organisms, relief, parent material, and time (Jenny 1941) constitute the dominant controls over both its genesis and present state. Soil integrity controls the resident biodiversity of soils, determines how well soil biodiversity serves as the basis for terrestrial food webs, and dictates how soils control a variety of ecosystem functions discussed in detail below. Therefore, the activities that threaten to pollute, disturb, and destroy soil integrity may also have strong impacts on soil biodiversity and soil function.
In many places, soil degradation has led to certain soil types becoming threatened enough that researchers have alerted the scientific community to their probable demise (Pimentel and Sparks 2000), and called for them to be included in formal conservation planning (Drohan and Farnham 2006; Ibanez et al. 1998). There are 508 endangered soil series in the US, equivalent to about 0.3% of the nation’s total land area, and many of these are in agricultural and urbanized areas (Amundson et al. 2003). Distinct, naturally-occurring, intact soils are quantitatively important to society for many reasons, not the least of which is that unique soils may harbor a unique assemblage of soil biota with novel taxa or metabolisms. Undiscovered natural resources of this kind can strengthen arguments for the conservation of soils where unique plant or animal species may be lacking. Unfortunately, while a case is easy to make for the protection of a unique plant or animal species, soil conservationists may require a more compelling argument for why certain soil series may be important to preserve. Amundson et al. (2003) argue that “a fundamental institutional shift in [soil science and agronomy] is required to quantify and derive societal value from remaining natural soils and ecosystems and to provide the scientific basis to argue for their preservation”.
Ecosystem services provided by soils and soil biota
Soils and soil biota have not gone completely unrecognized in conservation circles, but much of the attention has focused on the indirect effects of soils on ecosystem processes, and how these processes or “ecosystem services” (Chan et al. 2006) support familiar species targeted for conservation. Wall (2004) provides a thorough review of both the ecosystem processes and the services provided by soils and soil biota. These include, but are not limited to: providing the medium for growth of terrestrial plants (including the crops humans grow for food and fiber), recycling energy and nutrients from waste materials and dead organisms, storing and retaining organic matter and water in ecosystems, regulating air and water quality, providing the physical environment required by burrowing animals to dig, preventing erosion by wind and rain, sequestering carbon for millennia, and providing humans with a variety of organisms and compounds used on a daily basis for food, medicine, and raw materials. Although conservation practitioners may be unaware that the biological diversity of soils underpins these ecosystem services, some in the conservation community have recognized that ecosystem services such as natural water storage and filtration require intact soils (Altieri 1999). The water stored and filtered by soil eventually flows into streams, and these streams are crucial for the survival of aquatic species, such as salmon, which conservationists often target for protection. In this indirect way, soil integrity (and therefore, soil biodiversity) is demonstrably crucial to conservation success.
The natural services provided by soils and soil biota can underpin the biodiversity of ecosystems. For example, fragile soil biological crusts constitute the living floor of some desert and other arid terrestrial ecosystems. These crusts include mosses, lichens, liverworts, cyanobacteria, and other organisms which bind particles of mineral soil together to create a thin, cohesive horizontal layer along the surface of the ground (Bowker 2007). By holding soil particles together and modifying water run-off, fertility, and soil temperatures, biological soil crusts enhance soil quality (Evans and Ehleringer 1993; Lange et al. 1994; Kidron and Yair 1997). Without these crusts, soils are prone to erosion and to invasion by non-native species, resulting in particulate air pollution and declines in native plant and animal abundance and diversity. Researchers have also attributed measured annual rates of net ecosystem CO2 uptake in the Mojave Desert rivaling those of forest or grassland to the carbon fixation activity of biological soil crusts (Wohlfahrt et al. 2008). Given that deserts and arid lands cover 40% of the earth’s land surface, this finding suggests the crusts are crucial components of ecosystem health that could have global importance. In addition to the services they provide, soil biological crust organisms contribute towards global biodiversity; new crust-affiliated species with limited geographical ranges have been discovered in recent years, highlighting the potential for localized endemism across their range (Rehakova et al. 2007).
As soils disappear, biodiversity declines
Given the distinct biogeographical patterns in soil biota, degradation in soil integrity or the loss of soil series will have both direct and indirect consequences for the earth’s total biodiversity. Degradation of soil integrity may harm biodiversity directly by causing local extirpation or global extinction of certain soil organisms. As strongly as this loss may impact the earth’s total biodiversity, extinction or extirpation of soil biota is likely to go unnoticed by conservationists for the same reasons that these organisms are not well-studied in the first place. Soil-dwelling species will not always disappear without notice, however. The loss of these organisms could have indirect consequences for the biodiversity of larger, more obvious species targeted by conservationists through two mechanisms: (1) alteration of species-specific interactions between soil-dwellers and other organisms, or (2) alteration of ecosystem functions upon which species depend.
Interactions between soil organisms and other species
The potentially deadly Coccidioides immitis infection in sea otters, mentioned at the beginning of this article, highlights just one effect of a single soil microbe on a long-standing conservation effort. However, given their taxonomic and functional biodiversity, soil-dwelling organisms can have a multitude of effects on ecosystems, and there are many cases where soil biota can exert strong control over aboveground forms of biodiversity.
Soil biota can strongly influence the success of different plant species, leading to consequences for plant conservation. Soil biota influence plants through a variety of mechanisms and the impacts of soil microbial communities on plant growth are complex and highly site-dependent. Native vs. exotic plants can differ in their interactions with belowground biota (Wolfe and Klironomos 2005). A meta-analysis of the effect of soil communities on exotic species shows that both the direction (positive vs. negative) and magnitude of such effects differs from one location to another (Levine et al. 2004). For example, the invasive species spotted knapweed (Centaurea maculosa) can benefit from the services of soil microbial communities even when found beyond its native range (van der Putten et al. 2007), while other invasive plants may not.
Mycorrhizal fungi provide another example of how soil biota affect plant success. These nearly ubiquitous fungi infect plant roots and can either enhance or hinder plant growth. Mycorrhizal species can outnumber plant species within a given area and their interactions with plants can be species-specific. Their ecological functions, which include nutrient and water uptake, are not fully understood but are more numerous and varied than previously thought (Bever et al. 2001).
These examples of interactivity between soil biota and plants support the idea that geographical differences in soil communities can strongly influence the success of different plant species, leading to consequences for plant conservation. A review by van der Heijden et al. (2008) concluded that soil microbes, in particular, are important drivers of plant diversity in terrestrial systems, suggesting that management and restoration plans may need to be tailored to account for geographic differences in soil communities.
In addition to their interactions with plants, soil biota can also interact with animals that dwell primarily aboveground, leading to ecologically significant changes within an ecosystem. Invasive Argentine ants (Linepithema humile), which were inadvertently transported from their native range in South America to ecosystems worldwide (Tsutsui et al. 2001) provide an excellent case study of the wide-ranging effects of subterranean organisms. As non-native invaders, these ants displace ecologically important native ant species (Human and Gordon 1996) and disrupt local arthropod communities (Cole et al. 1992). In California, Argentine ants have been linked to population declines of coast horned lizards (Phrynosoma coronatum). The lizards, which are a candidate species for state and federal listing under the Endangered Species Act, prefer to eat California harvester ants (Pogonomyrmex californicus) over Argentine ants, and will switch to eating other, less abundant arthropods if the native ants are displaced by invasive ants (Suarez et al. 2000). In the fynbos of South Africa, Argentine ants outcompete native seed-dispersing ants, threatening the long-term viability of Mimetes cucullatus, a member of the highly-diverse native plant family Proteacea and mutualist with native ants (Bond and Slingsby 1984). These examples illustrate how changes in the identity of soil-dwelling organisms can lead a broad array of ecological changes that impact biodiversity.
Problems with the “insurance hypothesis”
Despite the recognition that soils as a whole provide valuable ecosystem services, little work has been done to identify which soil dwelling organisms are needed to maintain specific ecosystem functions. Some ecosystem processes, such as decomposition, are controlled by several interacting climatic, edaphic, and chemical factors, while being actively performed by a broad suite of soil organisms. For example, in a temperate forest, macrofauna such as earthworms are ecosystem engineers; they mix and aerate the soil and create macropores which allow for the movement of water down the soil profile, thereby influencing decomposition. Mesofauna and larger microfauna (springtails, nematodes, and protists) play a direct role in decomposition by breaking down leaf litter, and also by regulating smaller microfauna through predation. Finally, fungi and bacteria (the microfauna) participate in decomposition by breaking down organic particles and molecules into mineral form. In this example, three distinct functional groups of soil fauna (macrofauna, mesofauna, and microfauna) each contribute to the larger process of decomposition. Each functional group includes a multitude of species that simultaneously carry out similar tasks (Swift et al. 2008).
Proponents of the “insurance hypothesis” (Yachi and Loreau 1999) argue that the loss of any one species within a functional group responsible for a given process is unlikely to cause changes in the process. This assumes that the other members of the functional group would provide an equivalent substitute for the work of the missing species. The applicability of this hypothesis to soil microfauna has been called into question by Wohl et al. (2004), who found that a system with several functionally-redundant species of cellulose decomposer bacteria can support rates of cellulose decomposition that are twice as fast as a system with only one species of the bacteria. The rate of cellulose decomposition is critical in forest ecosystems, where the break-down of cellulose-rich wood has consequences for soil fertility, carbon sequestration, growth of seedlings, and habitat availability for a variety of species. While the findings of Wohl et al. (2004) may have little bearing on the true rates of cellulose decomposition in temperate forests, where decomposition is largely carried out by fungi (Swift et al. 1979), the study does demonstrate that that an important ecosystem function can be closely and directly linked to soil microbial diversity, and it suggests that the identity and diversity of the soil microbial community could make a big difference for other ecosystem functions.
Understanding the relationship between soil microbial diversity and ecosystem function becomes even more significant when we consider that some functions are performed by only a few soil taxa. Processes such as nitrification (the oxidation of ammonium to create nitrate) are carried out by a narrow range of microbes. Nitrate is a much more labile form of nitrogen, and is subject to being lost from soil through leaching or conversion to N2 gas. A loss in nitrifier species could result in lower rates of nitrification, leading to changes in soil fertility, which is known to affect the distribution of plant species and can influence the success of rare endemic plants over time. Orchids provide another example of the dependence of plant species on a narrow range of microbes: some orchids require a single, specific fungal species in order to grow. Given the lack of microbial redundancy in these cases, loss of either the fungus species required by an orchid, or the bacterial nitrifier capable of converting ammonium to nitrate in soils of a given type, could have large consequences for ecosystem function and/or biodiversity.
Incorporating soils and soil biota into conservation planning
Information about soils and soil fauna is not typically included in biodiversity assessments or other similar conservation plans. This may be in part because conservationists have yet to fully and directly embrace soil integrity as crucial to the mission of conserving the earth’s biodiversity. The divergent disciplinary histories of soil ecology and conservation biology may be responsible for the rift in the two fields; while soil ecology is rooted in the field of agronomy, a discipline that grew out of a need to increase food production (Wagener et al. 1998), conservation biology emerged from the human desire to preserve threatened species of plants and animals. These disparate histories have prevented soil ecologists from addressing questions that are relevant to conservation. As a result, information on soils or soil biota at a scale that is meaningful to conservation planners is often lacking, a fact that contrasts sharply with the abundance of useful natural heritage information for plants and animals. In addition, conservationists sometimes take soil for granted and assume that it will be of high enough quality to support the organisms they care about. Given human activities that threaten soil integrity, this assumption is faulty.
Examples that demonstrate how soils have been incorporated into conservation planning typically come from management and recovery plans where there is an obvious association between a special soil type and a particular species (or suite of species) aboveground. In these cases, further disturbance of the soils must be prevented to stop the species from going extinct. For example, to save native California bunchgrasses, The Nature Conservancy (TNC) has focused on protecting unplowed sites where remnant stands of the native grasses are most likely to occur (Robin Cox, pers. comm.). In addition, vernal pools within grasslands are threatened by agriculture and development which disturb and destroy the unique physical structure of soils required for the pools to form. As a result, land managers have delineated and protected vernal pools in various locations using information about the location of these unique soil types (Holland 1978; Bainbridge 2002; TAIC and EDAW 2005). Rare edaphic factors such as serpentine, limestone, or carbonate outcrops have also been incorporated into recovery plans that aim to protect the plants that specialize on them by limiting mining and other activities that disturb soil (U.S. Fish and Wildlife Service 1997). In all of these examples, it is the particular physical and chemical attributes of soils themselves, rather than the biota contained by soils, which have a strong and obvious effect on the plant or animal species of conservation interest. Because measuring these soil characteristics is fairly simple, an easy case can be made for the inclusion of this type of soil information in conservation planning.
Where certain soil characteristics are crucial for the long-term protection of species targeted for conservation, spatially-explicit information about soils should be incorporated into planning documents. This may require mapping and delineation of soil characteristics at a finer scale than what currently exists, which generates a challenge: aligning real-time field-based studies of soil with conservation planning. If the data do not already exist at the needed scale, a serious attempt to include information about soils in a conservation plan for a given area must include time for field collections, sample processing, and data compilation and analysis. In locations where soils are already known to play a strong role in driving patterns of diversity, conservation planners would be well-advised to encourage interest in soil surveys several years prior to the point at which the data will be used to plan for conservation.
What about situations where no obvious connection exists between soils and the conservation targets of interest? For many conservation projects, a connection is more attenuated than it is for the examples indicated above. In these cases, the scope and goals of the biodiversity assessment, conservation plan, or land management document crafted by a planning team for a given area will determine the level of soils information required. Conservation planners have many options for addressing the absence of information about soils and soil biota in planning documents. The degree to which they tackle this topic will depend on their interest and resources. An easy way to start when one wishes to incorporate soil into an assessment of biodiversity, rather than a management or recovery plan, is to look for landscape-level target elements such as sand dunes, volcanic outcroppings, or soil biological crusts which constitute unique soil substrates (TNC 2001). Soil maps produced by U.S. Geological Survey (USGS) can provide a good coarse filter when doing conservation planning, and may highlight areas with rare soil types in a given geography of interest. Areas with unique soil types that are also highly threatened by agriculture, urban development, or other disturbances are likely candidates as conservation priorities.
Where resources and interest allow, conservationists may choose to enhance the quantitative rigor of one of their most fundamental tools—the biodiversity assessment—by explicitly including soils and soil biota. When a team chooses to include soils and/or soil biota in an assessment of regional or local biodiversity, several challenges must be overcome before this goal can be successfully met (Fig. 2).
The first challenge is to bring together an assessment team that includes expertise in the field of soil ecology. This may be achieved through a variety of means, but would typically necessitate the inclusion of at least one soil ecologist when assembling a team for a given area. Soil ecologists are few and far between in the conservation community, so the building of new partnerships within academia and agencies may be required. In the United States, likely agency partners may include the USGS, the U.S. Department of Agriculture, and the Natural Resources Conservation Service (NRCS). The second challenge relates to the training of soil ecologists. A soil ecologist may require supplemental information regarding the history, culture, aims, and techniques used by conservation planners. In order to be successful, the inclusion of a soil ecologist must be fully supported by the other assessment team members, with the assumption that the extra effort involved will result in a more complete biodiversity assessment and better conservation outcomes.
Once the assessment team is assembled, the next hurdle is likely to be lack of knowledge. Expert knowledge regarding the presence and status of soil biota at any given location is likely to be slim, given the factors discussed throughout this article. The biogeographical study of soil biota is in its infancy, and cannot compare to the wealth of knowledge about plants and animals that has been collected and compiled by natural heritage programs. These gaps in knowledge may be addressed in two ways. The first is to comb through the scant information regarding soil biota that has been collected by soil ecologists within the landscape of interest. Much of this information is likely to be unpublished, and therefore difficult to access. The second way to address gaps in knowledge is to conduct original field work, assuming that the timeframe for the biological assessment can accommodate this. This brings us to the challenge discussed above of aligning real-time field-based studies with conservation planning.
Another challenge along the path to creating a complete biodiversity assessment is the well-recognized fact that assessing soil biodiversity can be very difficult. Despite this difficulty, Swift and Bignell (2001) standardized methods for doing so nearly a decade ago. These methods, which involve the selection of target taxa based on three criteria: (1) significance to soil fertility; (2) ease of simultaneous sampling; and (3) ease of sampling across the landscape, have been employed around the world in order to monitor the effects of certain land use practices on soil biodiversity.
Instead of assessing soil biodiversity directly, conservation planners may pull information from soil maps and use these to create conservation plans. Embedded in this tactic is the assumption that the biodiversity of soil biota is directly related to mapped soil type. However, soil types are often mapped based on extrapolations that involve boundaries between plant communities. As discussed previously, soil biota differ from plants in terms of their spatial patterns in biodiversity. Therefore, soil type may or may not influence patterns in soil biodiversity.
Finally, the assessment team faces the challenge of incorporating their soil biogeographical data into the overall picture of biodiversity for their planning area, and using the data they have gathered to guide conservation planning. When employing any evaluation of soil biodiversity, scaling up must be dealt with carefully. It may be difficult to get an adequate picture of soil biodiversity across a landscape, which is the unit of scale commonly used by conservation planners today. In addition, the assessment team must grapple with how to determine where there are areas of high conservation value when soil microbial diversity does not align well with plant and animal diversity. Questions such as this present novel challenges for conservation planners.
In addition to revealing, counting, and cataloguing the vast majoring of biodiversity at a site, the inclusion of soil biota as part of a total biodiversity assessment is essential when establishing a baseline understanding of the biodiversity of a given location. Only by monitoring changes in this baseline can conservationists determine how their conservation actions and management techniques are influencing biodiversity over time. If soil biota are not included, we will never know how they contribute to the overall picture of biodiversity in a given location, nor will we be able to thoughtfully pursue management actions such as microbial re-introduction which may enhance the health and survival of other conservation targets and overall ecosystem function.
Conclusions
Soils are treasure-troves of biodiversity but have received limited attention within the field of conservation biology. Despite the knowledge that soils are brimming with a miniature menagerie of life forms, little has been done to assess which soils or soil-dwelling organisms are most worthy of conservation action. A variety of human activities such as agriculture and urban development threaten soil integrity. If these threats are left unabated, and conservation biologists continue to ignore the importance of soil biodiversity in conservation efforts, unique soil-dwelling organisms may continue to disappear, and the ecological functions supported by these organisms will be threatened. Experts have argued that conservation of soil through the NRCS has explicit benefits for biodiversity conservation (Dunn et al. 1993). Without an explicit focus on preserving soil integrity, human efforts to protect and conserve natural landscapes will be threatened by an ever-increasing loss of undisturbed lands and the intact soils they contain.
Integration of the fields of soil ecology and conservation biology will fulfill needs within each field. As human impacts on natural systems continue to increase, the need to conserve soils as part of natural systems grows as well. We must begin studying soil properties and processes and soil biota within a conservation context in order to understand how we can best manage soil integrity for future ecosystem function. This will allow us to meet the current goals of biodiversity conservation, and provide for the future health of both humans and natural ecosystems.
References
Altieri M (1999) The ecological role of biodiversity in agroecosystems. Agric Ecosyst Environ 74:19–31
Amundson R, Guo Y, Gong P (2003) Soil diversity and land use in the United States. Ecosystems 6:470–482
Baas Becking L (1934) Geobiologie of inleiding tot de milieukunde. W.P. van Stockum and Zoon, The Hague
Bainbridge S (2002) San Joaquin and adjacent Sierra foothills vernal pool geomorphic classification and conservation prioritization. California Department of Fish and Game, Fresno
Bardgett R (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, Oxford
Beare M, Coleman D, Crossley D et al (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170:1–18
Beijerinck M (1913) Oxydation des Manganocarbonates durch Bakterien und Schimmelpilze. Folia Microbiol (Delft) 2:123–134
Bernard E (1992) Soil nematode biodiversity. Biol Fertil Soils 14:99–103
Bever J, Schultz P, Pringle A et al (2001) Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why. Bioscience 51:923–931
Bond W, Slingsby P (1984) Collapse of an ant-plant mutualism—the Argentine ant (Iridomyrmex humilis) and Myrmecochorous Proteaceae. Ecology 65:1031–1037
Bowker M (2007) Biological soil crust rehabilitation in theory and practice: an underexploited opportunity. Restor Ecol 15:13–23
Brussaard L (1998) Soil fauna, guilds, functional groups and ecosystem processes. Appl Soil Ecol 9:123–135
Brussaard L, Behan-Pelletier V, Bignell D (1997) Biodiversity and ecosystem functioning in soil. Ambio 26:563–570
Bryant J, Lamanna C, Morlon H et al (2008) Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci USA 105:11505–11511
Canadell J, Jackson R, Ehleringer J et al (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Chan K, Shaw M, Cameron D et al (2006) Conservation planning for ecosystem services. PLoS Biol 4:2138–2152
Clardy J (2005) Discovery of new compounds in nature. Proc Am Philos Soc 151:201–210
Clark J, May R (2002) Taxonomic bias in conservation research. Science 297:191–192
Cole F, Medeiros A, Loope L et al (1992) Effects of the argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 73:1313–1322
Cotterill F, Al-Rasheid K, Foissner W (2008) Conservation of protists: is it needed at all? Biodivers Conserv 17:427–443
de Wit R, Bouvier T (2006) ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8:755–758
Decaens T, Jimenez J, Gioia C et al (2006) The values of soil animals for conservation biology. Eur J Soil Biol 42:S23–S38
Doran J, Parkin T et al (1994) Defining and assessing soil quality. In: Doran J et al (eds) Defining soil quality for a sustainable environment. Soil Science Society of America Special Publication no 35, Madison, WI, pp 3–21
Drohan P, Farnham T (2006) Protecting life’s foundation: a proposal for recognizing rare and threatened soils. Soil Sci Soc Am J 70:2086–2096
Dunn C, Stearns F, Guntenspergen G et al (1993) Ecological benefits of the conservation reserve program. Conserv Biol 7:132–139
Dymond P, Scheu S, Parkinson D (1997) Density and distribution of Dendrobaena octaedra (Lumbricidae) in aspen and pine forests in the Canadian Rocky Mountains (Alberta). Soil Biol Biochem 29:265–273
European Soil Bureau Network (2005) Soil atlas of Europe. European Commission, Ispra, Italy
Evans R, Ehleringer J (1993) A break in the nitrogen-cycle in aridlands—evidence from delta-N-15 of soils. Oecologia 94:314–317
Fierer N, Jackson R (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Fierer N, Schimel J, Holden P (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Fierer N, Breitbart M, Nulton J et al (2007) Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of bacteria, archaea, fungi, and viruses in soil. Appl Environ Microbiol 73:7059–7066
Foissner W (2008) Protist diversity and distribution: some basic considerations. Biodivers Conserv 17:235–242
Hågvar S (1998) The relevance of the Rio-Convention on biodiversity to conserving the biodiversity of soils. Appl Soil Ecol 9:1–7
Holland R (1978) The geographic and edaphic distribution of vernal pools in the Great Central Valley. California Native Plant Society, Sacramento, California
Human K, Gordon D (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105:405–412
Ibanez J, De-Alba S, Lobo A et al (1998) Pedodiversity and global soil patterns at coarse scales. Geoderma 83:171–192
IGBP-DIS (1998) SoilData(V.0) a program for creating global soil-property databases. IGBP Global Soils Data Task, France
Jenny H (1941) Factors of soil formation. A system of quantitative pedology. McGraw Hill Book Company, New York
Kidron G, Yair A (1997) Rainfall-runoff relationship over encrusted dune surfaces, Nizzana, Western Negev, Israel. Earth Surf Proc Land 22:1169–1184
Klironomos J (2002) Another form of bias in conservation research. Science 298:749–750
Lange O, Meyer A, Zellner H et al (1994) Photosynthesis and water relations of lichen soil crusts—field-measurements in the coastal fog zone of the Namib Desert. Funct Ecol 8:253–264
Lavelle P (1984) The soil system in the humid tropics. Biol Int 9:2–15
Levine J, Adler P, Yelenik S (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Mills G, Hes L (1997) The complete book of southern African mammals. Struik Winchester, Cape Town
Montgomery D (2007) Dirt: the erosion of civilizations. University of California Press, Berkeley
Myers N, Mittermeier R, Mittermeier C et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Pawar S (2003) Taxonomic chauvinism and the methodologically challenged. Bioscience 53:861–864
Pimentel D, Sparks D (2000) Soil as an endangered ecosystem. Bioscience 50:947–950
Rehakova K, Johansen J, Casamatta D et al (2007) Morphological and molecular characterization of selected desert soil cyanobacteria: three species new to science including Mojavia pulchra gen. et sp. nov. Phycologia 46:481–502
Reponen T, Gazenko S, Grinshpun S et al (1998) Characteristics of airborne actinomycete spores. Appl Environ Microbiol 64:3807–3812
Suarez A, Richmond J, Case T (2000) Prey selection in horned lizards following the invasion of Argentine ants in southern California. Ecol Appl 10:711–725
Swift M, Bignell D (2001) Standard methods for assessment of soil biodiversity and land use practice. International Centre for Research in Agroforestry, Bogor, Indonesia
Swift M, Heal O, Anderson J (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley, CA
Swift M, Bignell D, Moreira F et al (2008) The inventory of soil biological diversity: concepts and general guidelines. In: Moreira F, Huising E, Bignell D (eds) A handbook of tropical soil biology: sampling and characterization of below-ground biodiversity. Earthscan, London
TAIC and EDAW (2005) Ramona vernal pool conservation study. TAIC, San Diego
Thomas N, Cole R (1996) The risk of disease and threats to the wild population. Endanger Species Update 13:24–28
TNC (2001) Ecoregion based conservation in the Mojave Desert. The Nature Conservancy, Las Vegas
Tsutsui N, Suarez A, Holway D et al (2001) Relationships among native and introduced populations of the Argentine ant (Linepithema humile) and the source of introduced populations. Mol Ecol 10:2151–2161
U.S. Fish and Wildlife Service (1997) San Bernardino Mountains carbonate plants draft recovery plan. U.S. Fish and Wildlife Service, Portland, OR
Usher M, Davis P, Harris J et al (1979) A profusion of species? Approaches towards understanding the dynamics of the populations of microarthropods in decomposer communities. In: Anderson R, Turner B, Taylor L (eds) Population dynamics. Blackwell Scientific Publications, Oxford
van der Heijden M, Bardgett R, van Straalen N (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
van der Putten W, Klironomos J, Wardle D (2007) Microbial ecology of biological invasions. ISME J 1:28–37
Wagener S, Oswood M, Schimel J (1998) Rivers and soils: parallels in carbon and nutrient processing. Bioscience 48:104–108
Wall D (2004) Sustaining biodiversity and ecosystem services in soils and sediments. SCOPE Series. Island Press, Washington, DC
Whitman W, Coleman D, Wiebe W (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95:6578–6583
Woese C, Kandler O, Wheelis M (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Wohl D, Arora S, Gladstone J (2004) Functional redundancy supports biodiversity and ecosystem function in a closed and constant environment. Ecology 85:1534–1540
Wohlfahrt G, Fenstermaker L, Arnone J (2008) Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob Change Biol 14:1475–1487
Wolfe B, Klironomos J (2005) Breaking new ground: soil communities and exotic plant invasion. Bioscience 55:477–487
Wolters V (2001) Biodiversity of soil animals and its function. Eur J Soil Biol 37:221–227
World Wildlife Fund (2006) WildFinder: online database of species distributions. http://www.worldwildlife.org/WildFinder. Cited Jan 2006
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA 96:1463–1468
Acknowledgements
The author wishes to thank Kirk Klausmeyer for technical help in creating Figure 1, Noah Fierer, Lynn Lozier, Robin Cox, and Stacey Solie for review of early versions of the manuscript, and The Nature Conservancy for support during the writing process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parker, S.S. Buried treasure: soil biodiversity and conservation. Biodivers Conserv 19, 3743–3756 (2010). https://doi.org/10.1007/s10531-010-9924-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9924-8