Abstract
The amount of large and old trees has decreased in the boreal forests during the last centuries of forestry. Such trees are important habitats for epiphytic lichens and there is a growing concern for lichen species that are associated with large and old trees. However, only little is known about the relative importance of tree size versus age as determinants of lichen diversity. Here we have determined the size, age and growth rate of 157 Norway spruce trees and recorded the occurrence of epiphytic lichen species on their branches and lower stems. The study includes crustose lichens and was done in two old-growth forests in SE Norway. Tree age and tree size were the parameters that explained the largest part of epiphytic lichen diversity. Only the growth rate of the most recent time period, i.e. 1984–2004, showed a statistically significant relationship to diversity. There was no indication of a stabilising species number with increasing tree age. Slow-growing and old trees were, however, mainly of importance to the lichen species growing on stems, and this set of species were in general adversely affected by a large amount of branches. The opposite was the case for the species that were confined to branches as their diversity increased when the amount of branches increased. Our study adds empirical data to support the importance of large and old trees as bearers of biodiversity in boreal forests. Site preservation and patch retention of groups of old and large trees is recommended as measures to maintain epiphytic lichen diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epiphytic lichens are sensitive to forestry and the interest and concern for this biodiversity component have grown steadily during the last two decades. Diversity patterns for epiphytic lichen have been described on different spatial scales ranging from the single tree scale (Kruys and Jonsson 1997), via the forest stand- and landscape scales (Jovan and McCune 2004; Dettki and Esseen 1998), up to the regional scale (Halonen et al. 1991; McCune et al. 1997). Which factors that best explains diversity patterns is scale-dependent. On large spatial scales, i.e. from the landscape scale and upwards, it has recently been shown that lichen diversity is correlated with climate, pollution and geographically related variables such as latitude and elevation (Will-Wolf et al. 2006). On finer spatial scales, forest stand variables such as stand age and tree species composition become more important (Holien 1998; Hyvärinen et al. 1992; Will-Wolf et al. 2006; Neitlich and McCune 1997). On the scale of the single tree, variables like tree species, branch quality, bark texture, tree size and tree age have been shown to significantly explain variation in epiphytic species composition and diversity (Holien 1997; Kuusinen 1996; Gauslaa et al. 1998; Löbel et al. 2006; Fritz et al. 2009).
Trees can be considered as discrete ecological units with fixed borders surrounded by a different environment (Southwood and Kennedy 1983). From species-area relationships it is expected that large trees have more species than small trees. Epiphyte diversity is also influenced by temporal processes like tree ageing. Several studies have examined the importance of tree age in relation to epiphytic lichen diversity (Hilmo and Såstad 2001; Hyvärinen et al. 1992; Kuusinen 1996; Johansson et al. 2007). Tree age may affect diversity in various ways. If time needed for colonisation is a limiting factor, the number of lichen species would increase with tree age (Flores-Palacios and García-Franco 2006). It is also possible that the physical and chemical quality of the trees changes through time and that an old tree provides a different substrate than the young tree.
As trees get older they often grow larger. When a tree reaches the age of maturity the growth normally decreases (Thomas 1996; Koch et al. 2004). Tree size and tree growth rate might thus often represent confounded variables with tree age, complicating the interpretation of their individual effects on epiphytic lichens. This study aims to explore the relationship between tree age, tree size, growth rate and epiphytic lichen diversity. In addition we wanted to study the basic patterns of epiphytic lichen diversity at a tree scale. To do this we studied trees differing in age within old-growth forests where tree size and tree age had been found to be uncorrelated. Such a situation offers unique possibilities to separate effects of tree size and tree age.
Some studies indicate that lichen growth forms respond in specific ways to various environmental impacts (Berryman and McCune 2006; Gauslaa et al. 2007; Hilmo 1994; Hyvärinen et al. 1992; Kivisto and Kuusinen 2000). In addition tree branches and the stems, might represent two distinct habitats with different species composition (Holien 1997) and the two respective epiphytic assemblages may respond differently to the tree characteristics. Our study has therefore two main aims. First, to examine the relationship between lichen diversity patterns and tree characteristics for different lichen growth forms. Second, to investigate if the lichen assemblages on stems and on branches show different relationships between diversity and tree characteristics. As regard lichen growth form, we have given the crustose lichens special attention because they are much less known than spectacular foliose macro lichens such as, e.g. Lobaria pulmonaria (see Gauslaa et al. 2006; Coxson and Stevenson 2007).
An overall aim is to discuss the implications of our results in a forest management perspective. The amount of old trees in the boreal forests has decreased during the last centuries of forestry. New forestry practices now often involve efforts to compensate for the adverse effects of the on-going removing of old trees from the forest. Green tree retention or patch retention are management practices frequently used in the boreal forest landscape, ensuring that the forest has a mixture of old and young trees as well as providing the possibilities for such trees to die and decay supporting additional species dependent on decaying wood (Junninen et al. 2007; Nascimbene et al. 2008). Several studies indicate that a number of epiphytic lichen species can survive on retained trees (Hazell and Gustafsson 1999; Lõhmus et al. 2006; Peck 1997). The survival success seem to vary between functional groups of lichens (Peck 1997). Lõhmus et al. (2006) recommend that tree species, tree size and bark texture should be considered for green tree retention to be successful. Our study aims at giving further information about useful tree characteristics to look for when selecting trees or patches for retention as a preservation measure for epiphytic lichens in boreal forests.
Methods
Study area
The study area, located in Siljan, Telemark county, SE Norway (59°22′N9°45′E, 500–600 m a.s.l.), comprised of two sites, (Årum and Kapteinstjern) approximately 15 km apart. Both sites were located in a forest landscape dominated by Picea abies with scattered Betula pubescens and Pinus sylvestris. The ground vegetation was dominated by Vaccinium myrtillus, Pleurozium schreberi, Hylocomium splendens, Polytrichum commune and Sphagnum girgensohnii. Plants like Vaccinium vitis-idaea, Calluna vulgaris, Cornus suecica, Avenella flexuosa and Oxalis acetosella were also abundant.
There have been no logging activities in the study sites the last approximately 100 years and Molinari et al. (2005) gives detailed information about the long-term Holocene forest history of the study area. The present forest was mainly multi-layered with even-layered forest in smaller areas and with varying tree density. In the study sites, the number of trees larger than 5 cm diameter in breast height (dbh) ranged from 265 to 750 trees per hectare, and the average tree age was 153 ± 65 years (±1 SD). The youngest trees were sprouts and the oldest tree identified was 450 years old. The largest tree was 63 cm dbh and the average tree size was 21 ± 14 cm dbh (±1 SD). The average basal area was 24.1 m2 ha−1and varied from 6.8 to 31.7 m2 ha−1 throughout the study area. There was no correlation between tree size and tree age (Table 2).
Sampling design and recording of lichens
Four circular 2,000 m2 plots were placed in each of the two sites that had an area of approximately 200 ha, respectively. The distance between the plots were ca. 500–1,000 m in each site. In each plot 19 P. abies trees >15 cm in diameter at breast height were selected. Thirteen trees were selected at random and six trees were selected to represent the oldest ones. Six plots were randomly selected and two plots at Kapteinstjern were selected due to the presence of the lichen Usnea longissima, which is a spectacular alectorioid species that is very rare in NW Europe and red-listed all over its European distribution range (see Esseen et al. 1981; Kålås et al. 2006; Tønsberg et al. 1996). At present, Usnea longissima is restricted to these two plots within the study area and the plots were selected to generate information about host tree characteristics and associated lichen diversity for a lichen of special concern. All the trees with Usnea longissima were included, i.e. one and seven trees per plot, respectively.
Lichens were recorded in 2004 and 2005 on stem and branches separately up to ca. 2 m. Each tree was examined for 30–60 min, depending on the amount of branches. Epiphytic diversity was recorded as total number of lichens per tree as well as per set of branches per tree and stem, respectively. In a strict sense, our recordings are a measure of species richness since abundance was not recorded. For simplicity, however, the term diversity is used throughout the paper. Difficult specimens were collected and examined by microscopy and subjected to spot tests and or/TLC analyses in the lab to identify secondary compound assisting the species identification. Voucher specimens are available by request to Ulf Arup. The nomenclature follows Santesson et al. (2004). Red-listed species follows the Norwegian Red List (Kålås et al. 2006). The classification of old-growth species is based partly upon the experience of Ulf Arup and partly upon literature (Hallingbäck 1995; Tibell 1999).
Lichens were grouped in the following life form groups: foliose, alectorioid, crustose and squamulose lichens.
Tree characteristics
Tree size was assessed by measuring the stem diameter at breast height (dbh, 1.3 m above ground). The amount of branches examined for lichens on each tree was rated visually according to a 3-degree scale, i.e. low, medium and high, which corresponds roughly to 1–3, 4–8 and >8 branches, respectively. The rating was done separately for dead and living branches and was not solely based on the number of branches, (e.g. if a tree had few but large branches it was assigned to “high”, and to “low” if the branches were many but small).
Trees were cored with an increment borer 20–50 cm above ground. The height above ground for each coring position was measured in the field and site-specific data on tree growth rate was used to calculate the numbers of years needed to reach a given coring position. These years were then added to the number years found by counting tree rings from the increment core to obtain the actual tree age. Tree-rings were counted and measured with an Addo micrometer (precision of 0.01 mm). To determine the age of trees in which increment cores failed to reach the pith, we estimated the length of the missing radius by matching the curvature of the inner rings to concentric circles drawn on a clear plastic sheet. The mean ring width of the 10 innermost rings of the cores with piths of each plot was used to estimate the number of rings in the missing radius (for further details on tree rings see Schweingruber 1988).
The average tree ring width per year of each tree for the 100-year period 1904–2004 was used as one measure of tree growth. In addition the average ring width was computed for two shorter periods, 1954–2004 and 1984–2004, respectively. The different time periods were tested as predictors in statistical analyses with lichen species number as response variable.
Analyses and statistical methods
Forest structure and history for the last century were alike for the two sites and data from the sites were combined in the analyses and presentation of results. To illustrate the difference in epiphytic lichen diversity between old and young trees, a sample-based rarefaction curve (Gotelli and Colwell 2001) was made by repeating the re-sampling of trees 1,000 times selecting the appropriate number of trees from one of two pools. The first pool consists of relatively young trees (<200 years) and the second pool consists of old trees (≥200 years). In addition a rarefaction curve was made based on all trees.
Regression analyses were performed to investigate the relationship between epiphytic lichen diversity and tree characteristics. Since the response variable is counts (number of species) we evaluated Generalised Linear Model (GLM) assuming Poisson distribution of the response variable against a model assuming a normal distribution. We found no indication of heteroscedasticity and that the model assuming normal distribution performed equally well as the GLM with Poisson distribution when inspecting diagnostic plots that were produce by use of the open access R statistical package (standardized residuals versus fitted values, see http://www.r-project.org/). We therefore used Ordinary Least Square (OLS) regression assuming a normal distribution for all regression analyses performed.
We first performed simple regressions relating each tree characteristic to lichen diversity. The variance between plots was accounted for by adding a factorial variable indicating plot identity before testing the tree characteristic variables. Reported R 2 values were found after accounting for plot identity, i.e. R 2 = 1−(residual error after accounting for both plot identity and tree characteristic)/(residual error after accounting for plot identity). We also performed a multiple regression for each lichen growth form and a forward selection using Akaikes Information Criterion (Hastie et al. 2001) to select an optimal model explaining diversity for each lichen growth form.
A randomization test was used to check if species occurred on branches, stems, or both substrates combined more often than should be expected by chance. For each species we randomly assigned the species to one of the three groups (branches, stem, both) as many times as the trees the species were found on. For each randomization for each species the maximum number of occurrence in the three groups were counted and compared to the maximum number of occurrence in the observed data set. The randomizations were repeated 999 times and a P-value derived (Manly 1997).
Results
Lichen species diversity
In total 108 lichen species were recorded on P. abies. Twenty of the species were squamulose (Cladonia), eight alectorioid, 14 foliose and 66 crustose species (Appendix Table 6). Only green-algal lichens were found in the study plots. The average number of species per tree was 24 species, total range 12–41. The distribution of species occurrences was highly skewed (Fig. 1). Fifty-seven of the species occurred only on 10 trees or less. Only 19 species occurred on more than 100 of the 157 registered trees. Hypogymnia physodes was the only lichen that occurred on all study trees.
The rarefaction curve based on trees showed that 50% of the sampled trees supported approximately 90% of all recorded lichens (Fig. 2). The cumulative species number increased rapidly up to the point where about 30% of the trees were included. The curve then increased more slowly, but it never stabilised. Comparing the accumulation of species on young trees (<200 years) with the accumulation of species on old trees (>200 years) showed that the number of species on old trees increased faster than on young trees. When inspecting the 95% confidence interval of the difference between the two rarefaction curves, the confidence interval contained zero difference up to 67 of 76 trees. With higher number of trees the confidence interval becomes very narrow because almost all trees were included in the rarefaction. We therefore conclude that there is an indication that trees older than 200 years have a steeper accumulation curve for species than trees younger than 200 years, but this difference is not statistically significant.
The analyses of habitat preference showed that 58 of the species occurred mainly on either stem or branches (Table 1). Only 8 species were more frequent than expected on both stems and branches on the same tree. The remaining 43 species showed no specific preferences or had too few recordings to allow detection of statistically significant trends. For more information about each species see Appendix Table 6.
The correlation between number of species growing on branches and species growing on stems was positive, but weak (r = 0.264; n = 157; P < 0.01). A tree with many species on the branches would thus not necessarily have many species on the stem and vice versa. Moreover, the correlation between total number of species and number of species on branches were considerably higher (r = 0.857; n = 157; P < 0.01 than the correlation between total number of species and species on stems (r = 0.653; n = 157; P < 0.01) mainly because of the higher number of species on branches compared to stems.
Red-listed species and “old growth species”
There were recorded five red-listed species (Biatora fallax, Chaenothecopsis viridialba, Alectoria sarmentosa, Bryoria nadvornikiana and Usnea longissima) and six additional species commonly associated with old growth forest (Arthonia leucopella, Hypocenomyce friesii, Lecidea albofuscencens, Microcalicium disseminatum, Ochrolechia alboflavescens and Hypogymnia vittata). The endangered U. longissima was recorded on eight trees in the research area. Since two research plots were located to include U. longissima, the frequency of U. longissima is a pure artifact. However, U. longissima was not observed in any other location within the study area. Trees with U. longissima were large and old. Their diameter in breast height was 36.4 ± 6.8 cm (mean ± 1 SD) (compared to a mean of 21 cm for all trees in the study area) and their age was 233 ± 75 years (mean ± 1 SD) (compared to a mean of 153 years for the study area). All U. longissima trees had many branches on the lower stem. The number of lichen species per U. longissima tree was 34.6 ± 3.8 (mean ± 1 SD), which was far higher than the mean for the total study area (21 species). Biatora fallax, characterised as vulnerable, was recorded on two trees. These two trees were 277 and 447 years old. The red-listed species Alectoria sarmentosa (near threatened) was recorded on 108 trees. Most red-listed and old-growth species were crustose or alectorioid species. Hypogymnia vittata was the only foliose lichen. Ochrolechia alboflavescens was the most frequent old-growth species with 14 recordings. The number of red-listed and old growth species per tree was positively correlated (r = 0.54, P < 0.001) with the total number of species per tree (after subtracting the red-listed and old-growth species from the total).
Trees
The age of the trees selected for lichen sampling was 204 ± 68 years (mean ± 1 SD) with a median of 197 years. The youngest tree was 53 years and the oldest was 447 years old. The diameter (dbh) of the trees was 32 ± 11 cm (mean ± 1 SD), the smallest measured tree was 15 cm and the biggest 63 cm. The tree ring width per year of the 20 last years was 0.89 ± 0.68 mm (mean ± 1 SD). There was no correlation between tree age and tree size measured as dbh (Table 2). Growth rate was negatively correlated with age in all three studied time intervals (Table 2). The correlation between growth rate and dbh increased with the length of the time period used to calculate the growth rate, with no significant relationship for the last 20 years period. The number of living or dead branches showed no significant relationships with the other variables used to predict lichen diversity (Table 2).
Lichen species diversity in relation to tree age, tree size and growth rate
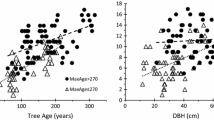
All measured tree variables were significant predictors for the total species diversity when tested one by one in simple linear regressions (Table 3). Tree age and tree size were the parameters that explained the largest part of the variation (Fig. 3 and Table 3). Only the growth rate of the most recent time period, i.e. 1984–2004, showed a statistically significant relationship to lichen diversity and is therefore the only growth rate presented in the results. In the multiple regression model all predictors of epiphytic lichen diversity except growth rate turned out significant (R 2 = 0.33; n = 157). The model selection procedure for the red-listed and old-growth species resulted in a model containing dbh, growth rate and living branches (R 2 = 0.08; n = 157) but, when each variable was tested one by one only the variable living branches was significant (Table 3).
Scatter plots of epiphytic lichen species per tree versus diameter at breast height and tree age. The fitted line is made on species number directly without accounting for plot identity. R-squared = 0.045 (F = 7.3, P = 0.008) for diameter at breast height and R-squared = 0.143 (F = 25.8, P = <0.001)
Habitat; branches and stems
The epiphytic communities on stems and on branches, respectively, were associated with different variables (Table 4). The number of branches (both living and dead) was the only important single factor for the lichen diversity on branches. When all predictors were tested combined in a multiple regression, tree age became significant in addition to the amount of branches (R 2 = 0.29; n = 157). For the lichen diversity on stems, all predictors except the amount of dead branches were significant when tested one by one. Tree age alone explained nearly 19% of the variation in species number (Table 4). An increasing amount of living branches on the stem reduced the number of lichens on the stem. When all predictors were combined in the model selection procedure, growth rate was the only variable that was not included in the model for the number of species growing on the stem (R 2 = 0.31; n = 157).
Growth form
The studied growth forms of lichens responded differently to measured variables. The total number of crustose and squamulose lichen species increased with tree age (Table 5). The diversity of crustose lichens showed the strongest relationship with tree age, whereas squamulose lichens responded most significantly with stem diameter. Neither foliose nor alectorioid lichens showed any relationship with tree age, and no or weak correlation with tree size. All lichen groups except squamulose species showed positive relationships with the amount of branches. Growth rate was negatively correlated with species diversity of foliose and squamulose species. The best multiple model was found for the most species rich group, crustose lichens; the weakest model for the small group of alectoiroid lichens.
Discussion
Epiphytic lichen diversity
Most species found in our study had low incidence rate as more than 50% were recorded on only ten trees or less and only nine species occurred on more than 100 of the trees. The sample-based rarefaction curve showed that a rather large number of the species could be found by sampling fewer trees. Still, the curve did not cease to increase and further sampling effort would most probably have contributed more species. This shows that a large number of trees are needed to support the epiphytic lichen diversity on P. abies. There was also a tendency for trees older than 200 years to show a faster increase in species number than trees younger than 200 years. This difference was however not statistically significant. To our knowledge, not many attempts in constructing cumulative species-area curves for epiphytic lichens have been made, there is however one other study reporting species/area curves that indicate that 90% of the species could be found in 50% of the area (Holien 1998).
Tree age and growth rate are important
Old trees had more species than young trees. The relationship between tree age and species diversity was highly significant. Inspection of the scatterplots showed that there was no indication of the species number stabilising with increasing tree age, and no quadratic term could be significantly added to the model. On the other hand, there was a large variation in the dataset not explained by tree age, blurring the relationship between tree age and species number. The positive relationship between lichen diversity and tree age supports the ideas that either time for colonisation or age-dependent changes in substratum qualities, or possibly a combination of both, might be crucial for epiphytic lichen diversity. Several studies suggest that many lichens are slow colonisers (Armstrong 1987; Dettki et al. 2000; Sillett et al. 2000; Hilmo and Såstad 2001). If time was the limiting factor we would expect older trees to have more species on both branches and stems. We see only small positive effects of age on species growing on branches. On the other hand, by studying epiphytic lichen species on branches, Hilmo (1994) found that tree age could explain a large part of the variation in species composition. Tree age was far more successful in explaining the number of species growing on stems than on branches in our study. The substratum qualities of the stem of the old tree might become more suitable to a larger number of species by time (Fritz et al. 2008). Bark stability might be one such quality that is changing with the age of the tree. Bark stability is normally linked to tree growth rate, on a slow growing tree the bark is expected to be more stable, and in this study tree age and growth rate were negatively correlated. There was a significant negative relationship between diversity of species mainly growing on stems and tree growth rate. In fact, the number of foliose lichens also showed a negative relationship to growth rate. This might support the idea of bark stability as an important quality of old or slow growing trees. The different growth forms followed a similar pattern as species growing on stems and branches in general; the number of squamulose and crustose species was significantly related to tree age and growth rate, and for foliose and alectorioid species the amount of branches was the most important factor.
Tree size and amount of branches are also important
Tree size explained approximately the same amount of variation in species number as tree age. There are several other studies looking at the relationships between tree size and epiphytic diversity patterns (Flores-Palacios and García-Franco 2006; Holien 1997; Hyvärinen et al. 1992; Löbel et al. 2006) One of the problems with these studies is that tree size and tree age are mostly positively correlated, which might blur the interpretation of the separate effects of tree size and tree age. Tree size and tree age are uncorrelated in this study and can thus be studied separately. There could be several possible explanations for the observed positive relationship between tree size and number of species. Testing area-effects per se was beyond the scope of this study and we could thus only discuss possible effects of area on the epiphytic lichen diversity. The most obvious explanation might be that by sampling lichens from a larger area, more individuals are sampled and the probability for sampling more species increases. The observed relationship might then just be a result of the sampling method. The theories of island biogeography and habitat diversity are among the most widely discussed and recognized in ecology (Cox and Moore 1993; Huston 1994). In island biogeography theory it is assumed that populations on large islands are larger than on small islands, and therefore less likely to go extinct (MacArthur and Wilson 1963). By increasing the sample area the number of habitat types with their associated species might also increase (Huston 1994). Another aspect of size is that the bigger the interception area, the greater the probability that it will be encountered by a potential colonist.
Tree size explained nothing of the variation in species number of species growing on branches. The significant effect was mainly on species growing on the stem. In a study looking at the relationship between epiphytic lichen diversity and tree size of broad leaved trees there were no relationship between tree size and number of epiphytic lichens (Löbel et al. 2006). Holien (1997), on the other hand, found that the number of stem base lichen species was positively correlated with tree size in Norway spruce. He also found that there was no relationship between branch species and tree size, which is in agreement with our results.
An aspect of branches is that they impact on the light availability inside the tree crown. There was a negative relationship between lichens growing on the stem and amount of branches. The positive correlation between the number of lichens growing on stems and lichens growing on branches was weak, indicating that what was positive for one group was not necessarily positive for the other. The interpretation of light availability in relation to the size of the tree crown is, however, not straightforward. A tree with a large lower crown will usually be growing in a rather open spot, and trees with few branches might be standing in a dense cluster with other trees. A large crown might also function as a barrier to diaspores of stem species and might thus have a negative impact on the diversity of the tree stem.
The only significant variables explaining alectorioid lichen diversity were amount of branches and tree size. However, alectorioid lichens are more abundant in the upper canopy than in the lower (Lyon et al. 2000) and we recorded only eight alectorioid species that tended to be either very frequent or very rare, which makes the results vulnerable to random events.
Red-listed species and old-growth species
It was unexpected that we did not find a significant relationship between tree age and the number of red-listed lichens and lichen species supposed to be indicative for old forests. Tree size, amount of branches and growth rate turned out significant in the full model, but when the variables were analysed one by one, only the amount of living branches was significant. The most frequent red-listed and old-growth species were species mainly found on branches and this probably affected the outcome of the analyses. The naturally low number of red-listed and old-growth species and the low number of occurrences would also possibly affect the outcome of the statistical analyses (see also Hottola and Siitonen 2008). With a larger sample of red-listed and old-growth species the results may have given different results. The mean tree age was also quite high in this study, 204 years, if more young trees had been included in the study, the effect of age on species diversity might have been more pronounced, see (Fritz et al. 2008, 2009). Two of the research plots were laid out to encompass the species Usnea longissima, but the number of recorded trees with the species was not sufficient to make conclusions on the host tree characteristics. There was, however, a positive correlation between red-listed and old-growth species and the total lichen species diversity which might indicate that some trees were more favourable than others, either with regards to the habitat that the trees offered or as a result of the history of the trees and their surroundings. In this context it should also be noted that there is much conjecture as regard species supposed to be indicative for old forest stand continuity. For example, when examining the long-term disturbance dynamics in species rich spruce forest in Sweden, Ohlson et al. (1997) found no relationship between the diversity of epiphytic lichens and the continuity of a forest stand.
Conclusions and implications for management
The richest diversity of epiphytic lichens was found on old, large and slow-growing trees with many branches. Interestingly, there was no indication of a stabilising species number with increasing tree age, which is a solid argument for the importance of old trees as bearer of biodiversity in boreal forests. Slow-growing and old trees were, however, mainly of importance to the lichen species growing on stems, and this set of species were in general adversely affected by a large amount of branches. The opposite was the case for the species that were confined to branches as their diversity increased when the amount of branches increased. Taken together, this indicates that there is no single and straight-forward relationship between epiphytic lichen diversity and host tree characteristics.
This study clearly shows the importance of old and large trees as bearers of biodiversity in boreal forests. When transforming our results into forest management practices, the first recommendation is to preserve sites with old trees and species-rich lichen communities. The second recommendation is to care for old and large trees in general and make sure that the oldest trees of a forest stand are not being cut. We suggest that old and large trees should be retained in patches in order to be a functionally effective conservation measure. Secondly, trees with many branches should also be left as retention trees. To benefit the species growing on stems, slow growing trees with few branches should be included as retention trees as well. Moreover, trees with large crowns are rare in the managed forest and we recommend that foresters should adjust their practices to incorporate more slow growing trees and trees with large crowns in the managed forests.
References
Armstrong RA (1987) Dispersal in a population of the lichen Hypogymna physodes. Environ Exp Bot 27:357–363. doi:10.1016/0098-8472(87)90046-3
Berryman S, McCune B (2006) Estimating epiphytic macrolichen biomass from topography, stand structure and lichen community data. J Veg Sci 17:157–170
Cox CB, Moore PD (1993) Biogeography. An ecological and evolutionary approach. Blackwell Scientific Ltd, London
Coxson DS, Stevenson SK (2007) Growth rate responses of Lobaria pulmonaria to canopy structure in even-aged and old-growth cedar–hemlock forests of central-interior British Columbia. For Ecol Manage 242:5–16. doi:10.1016/j.foreco.2007.01.031
Dettki H, Esseen P-A (1998) Epiphytic macrolichens in managed and natural forest landscapes: a comparison at two spatial scales. Ecography 21:613–624. doi:10.1111/j.1600-0587.1998.tb00554.x
Dettki H, Klintberg P, Esseen P-A (2000) Are epiphytic lichens in young forests limited by local dispersal? Ecoscience 7:317–325
Esseen P-A, Ericson L, Lindström H, Zackrisson O (1981) Occurrence and ecology of Usnea longissima in Central Sweden. Lichenologist 13:177–190. doi:10.1017/S0024282981000224
Flores-Palacios A, García-Franco J (2006) The relationship between tree size and epiphyte species richness: testing four different hypotheses. J Biogeogr 33:323–333. doi:10.1111/j.1365-2699.2005.01382.x
Fritz Ö, Gustafsson L, Larsson K (2008) Does forest continuity matter in conservation?–a study of epiphytic kichens and bryophytes in beech forests of southern Sweden. Biol Conserv 141:655–668. doi:10.1016/j.biocon.2007.12.006
Fritz Ö, Niklasson M, Churski M (2009) Tree age as a key factor for the conservation of epiphytic lichens and bryophytes in beech forests. Appl Veg Sci 12:93–106. doi:10.1111/j.1654-109X.2009.01007.x
Gauslaa Y, Ohlson M, Rolstad J (1998) Fine-scale distribution of the epiphytic lichen Usnea longissima on two even-aged neighbouring Picea abies trees. J Veg Sci 9:95–102. doi:10.2307/3237227
Gauslaa Y, Lie M, Solhaug KA, Ohlson M (2006) Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climate. Oecologia 147:406–416. doi:10.1007/s00442-005-0283-1
Gauslaa Y, Palmqvist K, Solhaug KA, Holien H, Hilmo O, Nybakken L, Myhre LC, Ohlson M (2007) Growth of epiphytic lichens across climatic and successional gradients. Can J For Res 37:1832–1845. doi:10.1139/X07-048
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. doi:10.1046/j.1461-0248.2001.00230.x
Hallingbäck T (1995) Ekologisk katalog över lavar. ArtDatabanken SLU, Uppsala
Halonen P, Hyvärinen M, Kauppi M (1991) The epiphytic lichen flora on conifers in relation to climate in the Finnish middle boreal subzone. Lichenologist 23:61–72
Hastie T, Tibshirani R, Friedman J (2001) The elements of statistical learning. Springer, Berlin
Hazell P, Gustafsson L (1999) Retention of trees at final harvest—evaluation of a conservation technique using epiphytic bryophyte and lichen transplants. Biol Conserv 90:133–142. doi:10.1016/S0006-3207(99)00024-5
Hilmo O (1994) Distribution and succession of epiphytic lichens on Picea abies branches in a boreal forest, central Norway. Lichenologist 26:149–169. doi:10.1006/lich.1994.1013
Hilmo O, Såstad SM (2001) Colonization of old-forest lichens in a young and an old boreal Picea abies forest: an experimental approach. Biol Conserv 102:251–259. doi:10.1016/S0006-3207(01)00100-8
Holien H (1997) The lichen flora on Picea abies in a suboceanic spruce forest area in Central Norway with emphasis on the relationship to site and stand parameters. Nord J Bot 17:55–76. doi:10.1111/j.1756-1051.1997.tb00290.x
Holien H (1998) Lichens in spruce forest stands of different successional stages in central Norway with emphasis on diversity and old growth species. Nova Hedwigia 66:283–324
Hottola J, Siitonen J (2008) Significance of woodland key habitats for polypore diversity and red-listed species in boreal forests. Biodivers Conserv 17:2559–2577. doi:10.1007/s10531-008-9317-4
Huston MA (1994) Biological Diversity. The coexistence of species on changing landscapes. Cambridge Univeristy Press, Cambridge
Hyvärinen M, Halonen P, Kauppi M (1992) Influence of stand age and structure on the epiphytic vegetation in the middle-boreal forests of Finland. Lichenologist 24:165–180
Johansson P, Rydin H, Thor G (2007) Tree age relationships with epiphytic lichen diversity and lichen life history traits on ash in southern Sweden. Ecoscience 14:81–91. doi:10.2980/1195-6860(2007)14[81:TARWEL]2.0.CO;2
Jovan S, McCune B (2004) Regional variation in epiphytic macrolichen communities in northern and central Californian forests. Bryologist 107:328–339. doi:10.1639/0007-2745(2004)107[0328:RVIEMC]2.0.CO;2
Junninen K, Penttila R, Martikainen P (2007) Fallen retention aspen trees on clear-cuts can be important habitats for red-listed polypores: a case study in Finland. Biodivers Conserv 16:475–490. doi:10.1007/s10531-005-6227-6
Kålås JA, Viken Å, Bakken T (2006) Norsk Rødliste 2006–2006 Norwegian Red List. Artsdatabanken, Norway
Kivisto L, Kuusinen M (2000) Edge effects on the epiphytic lichen flora of Picea abies in middle boreal Finland. Lichenologist 32:387–389. doi:10.1006/lich.2000.0282
Koch GW, Sillett SC, Jennings GM, Davis SD (2004) The limits to tree height. Nature 428:851–854. doi:10.1038/nature02417
Kruys N, Jonsson BG (1997) Insular patterns of calicoid lichens in a boreal old-growth forest-wetland mosaic. Ecography 20:605–613. doi:10.1111/j.1600-0587.1997.tb00429.x
Kuusinen M (1996) Epiphyte flora and diversity on basal trunks of six old-growth forest tree species in southern and middle-boreal Finland. Lichenologist 28:443–463. doi:10.1006/lich.1996.0043
Löbel S, Snäll T, Rydin H (2006) Species richness patterns and metapopulation processes—evidence from epiphyte communities in boreo-nemoral forests. Ecography 29:169–182. doi:10.1111/j.2006.0906-7590.04348.x
Lõhmus P, Rosenwald P, Lõhmus A (2006) Effectiveness of solitary retention trees for conserving epiphytes: differential short-term responses of bryophytes and lichens. Can J For Res 36:1319–1330. doi:10.1139/X06-032
Lyon B, Nadkarni NM, North MP (2000) Spatial distribution and succession of epiphytes on Tsuga heterophylla (western hemlock) in an old-growth Douglas-fir forest. Can J Bot 78:957–958. doi:10.1139/cjb-78-7-957
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution Int J Org Evolution 17:373–387. doi:10.2307/2407089
Manly BFJ (1997) Randomisations, bootstrap, and Monte Carlo methods in biology. Chapman & Hall, London
McCune B, Dey J, Peck J, Heiman K, Will-Wolf S (1997) Regional gradients in lichen communities of the Southeast United States. Bryologist 100:145–158
Molinari C, Bradshaw RHW, Risbøl O, Lie M, Ohlson M (2005) Long-term vegetational history of a Picea abies stand in south-eastern Norway: implications for the conservation of biological values. Biol Conserv 126:155–165. doi:10.1016/j.biocon.2005.05.007
Nascimbene J, Marini L, Caniglia G, Cester D, Nimis PL (2008) Lichen diversity on stumps in relation to wood decay in subalpine forests of Northern Italy. Biodivers Conserv 17:2661–2670. doi:10.1007/s10531-008-9344-1
Neitlich PN, McCune B (1997) Hotspots of epiphytic lichen diversity in two young managed forests. Conserv Biol 11:172–182. doi:10.1046/j.1523-1739.1997.95492.x
Ohlson M, Söderström L, Hörnberg G, Zackrisson O, Hermansson J (1997) Habitat qualities versus long-term continuity as determinants of biodiversity in boreal old-growth swamp forests. Biol Conserv 81:221–231. doi:10.1016/S0006-3207(97)00001-3
Peck JE (1997) Remnant trees and canopy lichen communities in western Oregon: a retrospective approach. Ecol Appl 7:1181–1187. doi:10.1890/1051-0761(1997)007[1181:RTACLC]2.0.CO;2
Santesson R, Moberg R, Nordin A, Tønsberg T, Vitikainen O (2004) Lichen-forming and lichenicolous fungi of Fennoscandia. Museum of Evolution. Uppsala University, Uppsala
Schweingruber FH (1988) Tree rings. Basics and applications of dendrochronology. Kluwer, Dordrecht
Sillett SC, McCune B, Peck JE, Rambo TR, Ruchty A (2000) Dispersal limitations of epiphytic lichens result in species dependent on old-growth forests. Ecol Appl 10:789–799. doi:10.1890/1051-0761(2000)010[0789:DLOELR]2.0.CO;2
Southwood TRE, Kennedy CEJ (1983) Trees as islands. Oikos 41:359–371. doi:10.2307/3544094
Thomas SC (1996) Asymptotic height as a predictor of growth and allometric characteristics Malaysian rain forest trees. Am J Bot 83:556–566. doi:10.2307/2445913
Tibell L (1999) Calicioid lichens and fungi. In: Ahti T, Jørgensen PM, Kristinsson H, Moberg R, Søchting U, Thor G (eds) Nordic lichen Flora. Volume 1. Introductory Parts. Calicioid Lichens and Fungi. Nordic Lichen Society, Uddevalla, pp 20–94
Tønsberg T, Gauslaa Y, Haugan R, Holien H, Timdal E (1996) The threatened macrolichens of Norway—1995. Sommerfeltia 23:1–283
Will-Wolf S, Geiser L, Neitlich P, Reis A (2006) Forest lichen communities and environment—how consistent are relationships across scales? J Veg Sci 17:171–184
Acknowledgments
This research was financed by the Research Council of Norway (NFR). Yngvar Gauslaa and an anonymous reviewer have contributed valuable comments on the manuscript. We thank Fritzøe skoger for giving access to the study sites and for all kind help with organising practicalities related to the field work. Thanks to the research assistant Steen Ravn Andersen for persistence in coring trees and Kari-Anne Taije for volunteering as a field assistant and providing good company in many long hours in the forest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Lie, M.H., Arup, U., Grytnes, JA. et al. The importance of host tree age, size and growth rate as determinants of epiphytic lichen diversity in boreal spruce forests. Biodivers Conserv 18, 3579–3596 (2009). https://doi.org/10.1007/s10531-009-9661-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-009-9661-z