Abstract
Protected areas are crucial for Amazonian nature conservation. Many Amazonian reserves have been selected systematically to achieve biodiversity representativeness. We review the role natural-scientific understanding has played in reserve selection, and evaluate the theoretical potential of the existing reserves to cover a complete sample of the species diversity of the Amazonian rainforest biome. In total, 108 reserves (604,832 km2) are treated as strictly protected and Amazonian; 87 of these can be seen as systematically selected to sample species diversity (75.3% of total area). Because direct knowledge on all species distributions is unavailable, surrogates have been used to select reserves: direct information on some species distributions (15 reserves, 14.8% of total area); species distribution patterns predicted on the basis of conceptual models, mainly the Pleistocene refuge hypothesis, (5/10.3%); environmental units (46/27.3%); or a combination of distribution patterns and environmental units (21/22.9%). None of these surrogates are reliable: direct information on species distributions is inadequate; the Pleistocene refuge hypothesis is highly controversial; and environmental classifications do not capture all relevant ecological variation, and their relevance for species distribution patterns is undocumented. Hence, Amazonian reserves cannot be safely assumed to capture all Amazonian species. To improve the situation, transparency and an active dialogue with the scientific community should be integral to conservation planning. We suggest that the best currently available approach for sampling Amazonian species diversity in reserve selection is to simultaneously inventory indicator plant species and climatic and geological conditions, and to combine field studies with remote sensing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous Amazonian development programmes based on land conversion have had adverse environmental and social impacts (Dourojeanni 1990; Foresta 1991; Kohlhepp 2001). This has accentuated the importance of sustainable utilisation and conservation of the region’s natural ecosystems. There is now considerable international and domestic political pressure to retain the rainforest biome as a provider of a variety of goods and ecological services, as a biodiversity reservoir, and as a wilderness area attracting public appreciation and nature-based tourism. However, on-going deforestation, logging, burning, mining, and unsustainable hunting (Laurance 1998; Nepstad et al. 1999; Steininger et al. 2001; Achard et al. 2002; Cochrane and Laurance 2002; Cochrane 2003; Whitfield 2003; Asner et al. 2005) result in a diminishing potential of Amazonia to produce these benefits. Although serious efforts to achieve environmentally and socially sustainable progress are under way (e.g., Anonymous 1997a, 1998; BIODAMAZ 2001; Kohlhepp 2001; Comunidad Andina 2002; WWF 2006), major development programmes that threaten to accelerate habitat destruction in Amazonia are also still on the agenda (Kohlhepp 2001; Laurance et al. 2001; Peres 2001; Nepstad et al. 2002; IIRSA 2006). Therefore, continuous activities are needed to ensure the preservation of the Amazonian nature.
Nature can be protected in many ways and, to be successful, Amazonian conservation must certainly include many different approaches (cf. Redford and Stearman 1993; Pardo 1994; Fearnside 1999; Schwartzman et al. 2000; Ricardo 2001a; Veríssimo et al. 2002a, b; Soares-Filho et al. 2006). In Amazonia, as elsewhere, political, economic, and social issues ultimately determine which conservation measures are realised (Foresta 1991; Pardo 1994; Peres and Terborgh 1995; Harcourt and Sayer 1996; Fearnside 1999; Laurance et al. 2001; Nepstad et al. 2002). However, the concept of protected areas is well established in Amazonian countries and, therefore, comparatively straightforward to handle in socio-political and administrative terms (McNeely and MacKinnon 1990; Rojas and Castaño 1990; Foresta 1991; Pardo 1994; Rodríguez 1996; Capobianco et al. 2001). Protected areas have also proven effective in conserving tropical biodiversity (Sánchez-Azofeifa et al. 1999; Bruner et al. 2001). Hence, a network of protected areas will likely continue to form the core of biodiversity conservation in Amazonia, whereby it is imperative to ensure this network is as effectively designed as possible.
Margules and Pressey (2000) outlined an ideal procedure of reserve selection, which they termed ‘Systematic Conservation Planning’. They recognised representativeness, i.e., the need to represent the full variety of a region’s biodiversity, as the primary objective of a reserve network. To achieve representativeness reserves should be selected starting with three steps as follows: (1) review existing biodiversity data and make a clear choice of features to be used as surrogates for overall biodiversity; (2) determine explicit conservation goals; and (3) evaluate existing reserves in relation to the goals.
In comparison to other major biomes, the history of biological conservation in Amazonia is unique in the sense that reserve selection has largely followed the initial steps of Systematic Conservation Planning. Hardly any conservation areas existed in Amazonia in the 1970s, when several national processes resulted in proposals for extensive reserve networks (Wetterberg et al. 1976; Dourojeanni 1990; Foresta 1991), and most of Amazonia was little altered by human activities. Conservation planners thus had real choices, and reserve networks were constructed on the basis of the best available scientific knowledge with the aim to achieve representativeness. Indeed, Foresta (1991) suggested that perhaps nowhere else on the globe has contemporary scientific understanding affected conservation planning as directly as in Amazonia.
Conservation based on solid scientific argumentation is relatively value-free and, accordingly, even in the diverse and often rapidly changing political climate of the nine Amazonian countries, successful conservation efforts have been taken (Wetterberg et al. 1981; Foresta 1991; Anonymous 1997b). The traditionally strong connection between conservation planning and state-of-the-art science should, therefore, be maintained. However, as Margules and Pressey (2000) pointed out, the systematic planning process should not be unidirectional: initial decisions should be reviewed periodically and the planning process redirected according to the reviews. In Amazonia, research has continued and the natural-scientific paradigm has changed considerably since the 1970s, when the foundation of the present reserve network was laid. At the same time, the reserve network has been developed in pulses following new sets of recommendations. Gradually Amazonian conservation planning has moved further from the international scientific community. Conservation priorities have been defined in cooperation between non-governmental organisations and Amazonian conservation agencies. Many leading scientists have participated in the work, but the scientific justification of conservation recommendations has often not been thoroughly documented in international scientific fora and, hence, not been rigorously tested through standard scientific procedure. The biodiversity data and the different surrogate measures for overall biodiversity applied in the construction of representative Amazonian reserve networks have not been summarised and critically evaluated against the most recent scientific understanding.
These developments have two undesirable consequences. First, the choice of features used as surrogates for overall biodiversity in Amazonian conservation, which is the first step of Systematic Conservation Planning, is no longer clearly justified by contemporary scientific understanding. Second, it has become difficult to fulfil the third step of the systematic procedure, i.e., to asses how well the present Amazonian reserve network meets the goal of representativeness.
The aim of this review is to evaluate the theoretical potential of the existing Amazonian reserves to cover a complete sample of the region’s biodiversity. For the purpose of this paper, we equate biodiversity with species diversity, and set the ideal goal that Amazonian reserves should protect every species of the region. We answer the following questions:
-
1.
How has natural-scientific information been applied in reserve selection in Amazonia?
-
2.
Which surrogate measures have been chosen to represent overall biodiversity?
-
3.
What is the current scientific understanding on the surrogates used?
-
4.
On the basis of the current understanding, can the natural-scientific information and surrogates employed be considered appropriate, or should Amazonian reserve selection criteria be redirected?
Definitions and premises
Amazonia
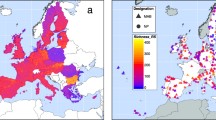
This review focuses on the Amazonian rainforest biome (Fig. 1). It does not entirely coincide with the watershed of the Amazon: the dryer southern and high-Andean western parts of the watershed are excluded, while Guayana and the Orinoco delta are included. This delimitation follows Fittkau (1971), Wetterberg et al. (1976), and Daly and Prance (1989), and others, and it corresponds with Amazonia sensu latissimo of Eva and Huber (2005). Depending on the exact delimitation, the size of the rainforest area is between 6.25 million km2 (Daly and Prance 1989) and 8.12 million km2 (Eva and Huber 2005). The region is predominantly forest-covered, but includes restricted tracts of non-forest vegetation types as well, such as savannas and flooded grasslands. The definition of the area is somewhat vague, especially as regards the upper altitudinal limit in the Andes. We apply a rough, biogeographically justifiable limit of 1,000 m a.s.l. (Daly and Prance 1989).
Amazonia, as defined here, with locations and relative size of strictly protected areas analysed. Numbering corresponds with Appendices 1 and 2 in which the name of and further information on each reserve is given. Note that some reserves appear to lie outside Amazonia because they are plotted on the map on the basis of their centre point
Amazonian countries
Parts of Amazonia are situated in the following countries: Bolivia, Brazil, Colombia, Ecuador, Guyana, Peru, Surinam, and Venezuela, and in French Guiana. By far the largest area is in Brazil (4.1 million km2; Ferreira et al. 2001), followed by Peru (750,000 km2; Dourojeanni 1990). In this review we therefore emphasise these two countries, which together harbour roughly three quarters of Amazonia, although we briefly treat the others as well.
Scale and resolution
We deal with an area the size of Europe, and with over 100 reserves, which cover more than 600,000 km2 in total. The available data are scarce and inaccurate in comparison to most areas at higher latitudes. A general and somewhat superficial treatment is, hence, unavoidable. However, we aim to convey the broad picture. It is our judgement that this is not significantly affected by possible errors and gaps in details of the information on the different reserves, which we have had available, and the interpretations we have made thereof.
Literature search
Much of the literature relevant for this review is not readily obtainable. We paid due attention to getting hold of the original texts even in the case of ‘grey literature’, e.g., administrative reports on planning and implementation exercises. We believe we managed to study the great majority of relevant texts. Naturally we also benefited from the information available on governmental Internet pages, and those of non-governmental organisations. These were queried repeatedly, and the last inspections were made in May 2006.
Reserves
Different protected areas have been established for different purposes. Most importantly, they differ in the degree to which utilisation of natural resources is allowed. Hence, not all areas conserve biodiversity equally well. We have restricted our analysis to areas whose degree of protection conforms to categories I–IV in the six-grade scale of the IUCN (1994) and WDPA (2005), and refer to such protected areas as ‘reserves’ and as ‘strictly protected area’. These types of conservation areas can be assumed to fully protect the biological diversity found within their limits. We acknowledge that this is a theoretical assumption, which is dependent on how well decrees and regulations are implemented on the ground (the ‘paper park’ problem, see e.g. Peres and Terborgh 1995; Bruner et al. 2001), but that dependence is beyond the scope of the present review. Furthermore, the size of a protected area affects its ability to protect species, regardless of the degree of protection implemented (e.g., Lovejoy et al. 1986), but this question has to be treated separately for different organism groups and is therefore too intricate to be treated here.
Selecting reserves to be analysed and collating their base data
In some cases the a priori decision to restrict the analysis to strictly protected areas did not solve the inclusion or exclusion of a particular reserve because the national authorities may have classified the reserve differently from international observers. In these cases we made ad hoc decisions on the inclusion.
Some reserves include parts below and above the upper altitudinal limit of Amazonia, and we were unable to subtract areas above the limit from the total area of such reserves. Hence, the figures we provide for total strictly protected area within Amazonia are slightly overestimated.
Exact limits, areas, and locations of reserves are sometimes given differently in different sources. In some governmental databases on the Internet, figures given had been changed from one inspection to another with no apparent reason. Hence, the information given here may differ from figures in another source, but we believe the differences are insignificant as regards the overall picture.
The reserves selected for analysis under these premises, and some basic information on them, are listed in Appendices 1 and 2, and their approximate locations are shown in Fig. 1.
Defining original selection criteria of reserves
We primarily looked for explicit statements on the reasons for the delimitation of a certain reserve. In some cases, however, we had to deduce the a priori criteria from a posteriori descriptions of the conservation values of the reserve in question. For a few reserves we were unable to find any clear information on the selection criteria.
For Brazil, we also overlaid maps of established reserves (SIUC/CGEUC/DIREC/IBAMA 2000a, b, c, d, e, f, 2001) with maps of areas recommended for conservation in different prioritisation schemes (Wetterberg et al. 1976; Workshop 90 1991; Seminário de Consulta Macapá 2001) to see where these match.
In most cases, several criteria have affected the delimitation of a certain reserve. These include, and often start with, political, economic and social motivations. However, we recorded only natural-scientific selection criteria. Where such criteria were absent, the selection criterion of a reserve was defined as ‘other’.
Often also several natural-scientific criteria have been applied simultaneously in reserve delimitation. For clarity’s sake we decided to condense the often multifaceted selection process into three classes of natural-scientific criteria, even if this admittedly may oversimplify some cases: (1) direct information on species distributions verified by empirical field studies; (2) species distribution patterns predicted on the basis of conceptual models, mainly the Pleistocene refuge hypothesis; and (3) distribution of environmental types or biotic units, such as ecosystems or biogeographical provinces. Two more classes were formed by the combination of (3) with either (1) or (2).
Which biodiversity data and surrogates have been applied in reserve selection in Amazonia?
The scientific paradigm in Amazonia at the advent of conservation planning
Science was largely ignorant of environmental and biological variation within Amazonia until the late 1960s. Despite its vast extent, Amazonia harbours remarkably little topographical and climatic variation. Much of the rainforest is structurally relatively uniform, and the extreme diversity of the flora renders it difficult to intuitively detect distinct community types. The paradox of having very high species numbers in a homogeneous environment was explained by the so-called stability hypothesis, which held that overall favourable climatic conditions had prevailed for millions of years and allowed the biota to diversify (Wallace 1878; Fischer 1960).
The conception of biotic homogeneity and historical stability was first challenged by the Pleistocene refuge hypothesis, which strived to explain distribution patterns of birds (Haffer 1969). This hypothesis holds that the Amazonian rainforest would have turned into fragments separated by savanna during periodic drying of the climate in the Pleistocene. The forest fragments would have functioned as species repositories, and as speciation and repopulation centres. Current concentrations of species richness and endemism would reveal the locations of those centres. Subsequent to Haffer’s ornithological studies, similar patterns were reported for other organism groups (Vanzolini and Williams 1970; Prance 1973; Brown 1975). The existence of biotic differences was eventually established by the division of Amazonia into eight phytogeographic provinces on the basis of distribution patterns in five plant families (Prance 1977).
The notion of ecological homogeneity was disputed around the same time. In Peru, an ecological map of the country was published that recognised 26 ecosystem types in Amazonia (Tosi 1960). Discontinuities in the structure of the forests in Brazilian Amazonia led to the separation of 22 vegetation types (Pires 1973; Pires and Prance 1985).
In summary, the former view of high numbers of evenly distributed species changed into a model embracing some areas of particular species richness and endemism, and biotic differences on a geographic scale. The preferred explanation for the observed patterns changed from long-lasting stability to climatic instability in recent geological history. Environmental heterogeneity was observed to cause some biological variation, but its role was seen as secondary in comparison to the historical factors.
It was against this scientific paradigm that the first plans for systematic reserve selection in Amazonia emerged. It has strongly influenced later priority setting too, although other scientific approaches have also been used to design representative reserve networks, as explained below in the country analyses.
Reserve selection in the Amazonian countries
Brazil
Brazilian Amazonia harbours 35 strictly protected areas established by federal institutions: 14 national parks, 9 biological reserves, 11 ecological stations, 1 ecological reserve (the category of two ecological reserves was changed to ecological station in 2001, see Appendix 1), which together cover 244,771 km2 (DIREC/IBAMA 2004, 2006; Mattos 2006). In addition, Amazonian states have established their own strictly protected areas, but we have not included them here because of difficulties in acquiring up-to-date information and in classifying them according to IUCN categories of degree protection. Our judgment is that their omission does not alter the overall picture significantly. According to our interpretation of the available information, the scientific background and criteria that contributed to the delimitation of the Brazilian Amazonian federal reserves are as follows.
The first systematic conservation plan for Amazonia was the so-called Amazon Analysis (Wetterberg et al. 1976), which was designed by a relatively small project team by analysing available biological information and through consulting leading scientists (Wetterberg et al. 1976, 1981; Foresta 1991). It focused on the non-inundated forests (ca. 90% of Amazonia), and recommended that a reserve network should include sufficient representation of each of Prance’s phytogeographical provinces. Recommendations on the exact placement of reserves were primarily based on the refuge hypothesis. First priority was given to areas that two or more authors had identified as Pleistocene forest refuges. Second priority was assigned to locations where, in addition to a possible refuge, vegetation heterogeneity had been recognised on the map by Pires (1973). A third priority class included areas proposed for conservation by various authorities for various reasons, but often on the basis of ecological significance (Wetterberg et al. 1976; Câmara 1983). In other words, the primary surrogate measure for overall biodiversity employed in the Amazon Analysis was species distribution patterns. These were inferred from a general historical hypothesis or model, which was based on distribution data of a small subset of all Amazonian species (birds, butterflies, five flowering plant families, and some lizard species). The species distribution patterns predicted on the basis of the model were complemented by a higher order surrogate, vegetation, and a vaguely defined ‘ecological significance’.
The Amazon Analysis was influential in Brazil. In 1979–1983 reserves were established according to this scheme as follows (DIREC/IBAMA 2004): two national parks, one biological reserve, and one ecological reserve totalling 49,316 km2 in first priority areas; and two national parks and two biological reserves totalling 28,270 km2 in second priority areas (DIREC/IBAMA 2004, 2006). We calculated that of the current total strictly protected area in Brazilian Amazonia, 31.7% has been selected on the basis of the Amazon Analysis.
In parallel with the scheme to establish reserves in line with the Amazon Analysis, a programme of establishing Ecological Stations and Ecological Reserves was initiated in 1976 (the SEMA programme). These were selected as representative samples of Brazilian ecosystems defined by geological, climatological, and biogeographical aspects (Nogueira-Neto and de Melo Carvalho 1979). Hence, the SEMA programme can be viewed as reserve selection using ecosystems as a surrogate for overall biodiversity, although several of the areas established were selected more on an ad hoc basis (Foresta 1991). In this programme, nine reserves (20,224 km2), were founded in Amazonia in 1981–1985 (DIREC/IBAMA 2004, 2006). This represents 8.3% of the strictly protected area in Brazilian Amazonia today.
Following re-organisation of the environmental administration in Brazil, the role of the Amazon Analysis as a key criterion for reserve selection diminished. In 1990, more than 100 scientists convened in Manaus (the so-called Workshop 90) to produce a map depicting priority areas for conservation (Kuliopulos 1990; Prance 1990; Rylands 1990; Workshop 90 1991). Prioritisation was primarily based on centres of species endemism and richness, but also on occurrence of endangered species, fragile soils, unusual geological features, and degree of anthropogenic pressure (Rylands 1990). Areas of highest priority were those where the most priority areas of different disciplines overlapped (Rylands 1990; Prance 1990).
Workshop 90 identified 94 priority areas for conservation. Five ranks of priority were identified (1 lowest, 5 highest). A far greater portion of Amazonia was prioritised for conservation in the Workshop 90 map than in the Amazon Analysis, but the first priority areas of the two schemes largely overlapped. Two national parks (5,939 km2; DIREC/IBAMA 2006) were established in 1998 following the recommendations of Workshop 90 (priority rank 2). This represents 2.4% of the strictly protected area in Brazilian Amazonia.
The ‘workshop approach’ to establish conservation priorities was continued with a meeting held in 1999 in Macapá (Seminário de Consulta Macapá 2001; henceforth ‘Workshop 99’), where 226 participants drew together various kinds of information to form a basis for reserve selection. A hierarchical spatial approach was applied, i.e., Brazilian Amazonia was first divided into seven subregions taking into account the ecoregion delimitation of Dinerstein et al. (1995), interfluvial areas, planned economic development schemes, and present human impact. Priority areas for conservation were identified separately for each subregion, if at least two areas regarded by experts as biologically important coincided. The Macapá meeting was preceded by data compilation, production of thematic maps, and consultation of specialists. Several types of data contributed to the definition of conservation priorities, including demographics, anthropogenic pressure, and other socio-economic indicators, but biological value was a central criterion. This was evaluated using both direct information on species distributions, approximations of species richness and endemism, and distribution of ecosystems. A wide array of organisms was considered: birds, mammals, reptiles, amphibians, many invertebrate groups, and plants. The data were collated from existing publications and reports, but new data were not collected specifically for this workshop. Ecosystem distribution was assessed on the basis of a vegetation map of Brazilian Amazonia adapted from IBGE (1997). Following the approach of Fearnside and Ferraz (1995), vegetation maps and geographic distance (‘ecoregions’) were used as a means to maximise biotic dissimilarity between reserves (de Oliveira and Nelson 2001).
Workshop 99 identified 385 priority areas for conservation. Three priority grades (‘a’ highest–‘c’ lowest) were defined, and areas prioritised by local experts were also treated (as category ‘n’). In 2001–2006, three new national parks and two ecological stations were established in areas of ‘a’, ‘b’, and ‘n’ priority, totalling 83,392 km2 of new strictly protected area (DIREC/IBAMA 2004, 2006; Mattos 2006). This is 34.1% of the Brazilian Amazonian total.
In 2002 the Brazilian government announced the Amazon Region Protected Area Programme ARPA, a partnership with the Brazilian Biodiversity Fund (FUNBIO), the German Development Bank (KfW), the Global Environment Facility, the World Bank, and WWF. Its principal aim is “to protect for future generations the full range of biological and ecological features found in the Brazilian Amazon” (WWF 2006) by increasing the amount of protected area in Brazilian Amazonia to 500,000 km2 or 12% of the total area (WWF-Brazil 2006). ARPA also aims to improve the administration and management of the reserve network. The programme has already resulted in the establishment of several protected areas, including strictly protected ones (see Appendix 1).
WWF (2006) states: “The creation of the Amazon parks system hinges on the application of the best available science. ARPA’s scientific design is based on the results of a two-year planning process involving hundreds of experts representing a wide range of perspectives, including biologists ... This process resulted in the identification of a set of priority zones strung throughout the Amazon in which ... reserves would be ideally situated. An independent panel of scientific experts guides the park selection process to ensure that sound science remains a hallmark of the program.” From WWF (2006) or WWF-Brazil (2006) we have not, however, been able to identify the exact natural-scientific theories and data applied in the selection of areas to protect.
In addition to the reserves selected on the basis of clearly defined natural-scientific criteria, five national parks, six biological reserves, and one ecological reserve have been selected on an ad hoc basis in the sense that their establishment has not been explicitly justified with reference to a prioritisation scheme. The whole area selected for protection in this way does, however, coincide with areas recommended for protection by either the Amazon Analysis, Workshop 90, or Workshop 99 (21,215 km2 only by either of the two earlier schemes, not by Workshop 99).
Peru
Peruvian Amazonia harbours five strictly protected areas (national parks; Appendix 2). They cover a total area of ca. 69,461 km2, which, however, includes an undefined area of extra-Amazonian biotopes of the Andes. Scientific criteria have contributed to reserve delimitation as follows.
The first protected areas in Peru were established in the1960s to protect certain species only (Anonymous 1997b). Soon thereafter it was proposed that a Peruvian protected area network should include samples of the three macroregions of the country (coastal, Andean, and Amazonian) and be complemented by reserves for certain animal species (Grimwood 1968 according to Rodríguez 1996). The National Park of Manu was established in 1973 to represent the Amazonian macroregion. The park covers ca. 17,160 km2, which is ca. 25% of all strictly protected area in Peruvian Amazonia.
In the 1970s, hypotheses predicting spatial distribution of biodiversity were advocated as guidelines for reserve selection also in Peru. The refuge hypothesis was promoted by scientists (Lamas 1979 according to Rodríguez 1996) and Peruvian planning authorities recognised the merits of the Amazon Analysis (Jorge Pádua and Bernardes Qintão 1982; Dourojeanni 1990; Foresta 1991). The natural-scientific criteria for establishing Río Abiseo National Park in 1983 were the desire to protect a representative sample of cloud forest, and endangered animal species, but it was also noted that this area included the Pleistocene refuge of Huallaga. It covers 2,745 km2 (much of which is Andean, not Amazonian vegetation), i.e., ca. 4% of the Peruvian Amazonian strictly protected area considered here.
Since the foundation of the National System of Conservation Units (SINUC) in 1975 the aim has been to create a reserve network which would include samples of all Peruvian ecosystem types. These were first defined on an ecological map (Tosi 1960) based on the Life Zone system of Holdridge (1947), and later identified separately for all biogeographical regions on Udvardy’s (1975) map (Dourojeanni 1990; CDC-UNALM 1991). The current guidelines for protected area establishment in Peru (INRENA 1999) continue to emphasise representativeness of different ecosystems or life zones as one of the main criteria for selecting future areas for conservation, although other criteria are considered as well (e.g., endemism and evolution centres, connectivity, presence of interesting geomorphological or physiographical features, and importance for biological cycles of characteristic species). Revision of the guidelines is currently underway in Peru. The revision corresponds to decentralisation efforts in Peru. The revision is done through an expert group and consultations in various parts of the country. It seems that representativeness of ecosystems continues to be a central tool in reserve establishment (see www.inrena.gob.pe and www.plandirectoranp.com).
In the early 1990s, a first attempt was made to analyse whether the different biogeographical provinces were represented within the protected area network of Peru. This work included a workshop, which was organised in 1994 to determine priority areas for conservation in Peru. Most of the specialists participating in the workshop used species richness and endemism of certain groups of organisms to locate priority areas. However, the process was summarised as a listing of biogeographical provinces that were under-represented in the protected area network, including those in Amazonia (Appendix III in Rodríguez 1996). Hence, the national parks of Bahuaja Sonene (established in 1996), Cordillera Azul (2001), and Alto Purus (2004) created as a result of the prioritisation made in this workshop were justified by direct information on species occurrences and by using environmental types as surrogates for overall biodiversity. These reserves together cover ca. 49,550 km2, which is about 71% of the Peruvian Amazonian strictly protected area considered here.
Other Amazonian countries
Bolivia, Colombia, Ecuador, French Guiana, Guyana, Surinam, and Venezuela harbour a total of 68 reserves, which cover a total area of 291,553 km2 (Appendix 2). This number includes an undefined area of extra-Amazonian biotopes of the Andes plus coastal biotopes in the north. As regards the scientific reserve selection criteria in these countries, we have not had available as much information as for Brazil and Peru. The below analysis therefore contains parts that are preliminary, but these are insignificant as regards the overall numbers of reserves and protected area.
In Bolivia the first national park was established in 1939 and the first Amazonian strictly protected reserve in 1965 (Suárez 1985; UNEP-WCMC 2006). In the 70s, two more national parks were created. These were all selected without systematically applying scientific criteria or planning methods (Ibisch 2004). However, we have deduced the natural-scientific rationale behind the selection of these areas from a posteriori descriptions of their values (Pacheco et al. 1994; UNEP-WCMC 2006; www.sernap.gov.bo, May 2006). It seems like ecosystems have been used as a surrogate for overall biodiversity, although the occurrence of endemic species has also been taken into account. Since the 1970s, the coverage of strictly protected area in Bolivian Amazonia has increased considerably (see summary in Ibisch and Mérida 2004). Today, there are seven reserves, which together cover 53,465 km2 (includes a sizeable but undefined area of Andean biotopes). In 1992, regional land-use planning was initiated, and several new protected areas were established, however, none of these in the categories studied in this article. The role of IUCN and other non-governmental organisations has been fundamental in the development of conservation criteria and management plans. The Rapid Assessment Program of Conservation International (CI) has been applied in identifying conservation priorities (Ibisch and Mérida 2004).
The representativeness of the Bolivian reserve network has been judged inadequate (Pacheco et al. 1994; Pardo 1994; Harcourt and Sayer 1996). In the first gap analysis of the Bolivian reserve network (Pacheco et al. 1994) the representation of phytogeographic regions was emphasised, but abiotic factors, such as topography, soils, and rivers, were also included. The gap analysis has since been further developed by including diversity and endemism centres, critical and minimum viable habitats, threatened species, and data on selected taxa (Csuti and Crist 2000; Ibisch and Mérida 2004). In general, the Bolivian strategy has largely followed the National Gap Analysis Program of USA (Zorn and Quirouette 2002). The Bolivian National Service for Protected Areas (SERNAP) is currently carrying out an analysis concerning gaps in protection of Bolivia (www.sernap.gov.bo, May 2006).
The first protected area in Colombian Amazonia was founded in 1948. In the 70s and 80s, at least 10 new reserves, or enlargements to existing ones, were established in the Amazonian region. Colombian Amazonia now harbours 11 reserves with a total area of 68,702 km2 (includes an undefined area of Andean biotopes). Most of this area has been selected using ecosystems as a biodiversity surrogate, although species distribution patterns have contributed as an additional criterion in the selection of some reserves (www.parquesnacionales.gov.co, April 2005).
The first fully Amazonian national park in Ecuador, Yasuní, was established in 1979. The Pleistocene refuge hypothesis affected its selection as a reserve (Wetterberg et al. 1981). The other three areas have been selected on the basis of high ecosystem diversity, species richness, and endemism (CIAM 2004; UNEP-WCMC 2006; www.ambiente.gov.ec/April 2005). Together the four reserves in Ecuadorian Amazonia cover an area of 17,096 km2, of which a considerable part is Andean ecosystems.
The protected area network (12 reserves) of French Guiana covers 5,154 km2 (UNEP-WCMC 2006). The first of these was created in 1992 and the rest in mid-1990s and one in 2000. We have not managed to form a clear picture on the scientific selection criteria of these reserves.
There is only one protected area in Guyana. It was established in 1929, and covers an area of at least 630 km2 (UNEP-WCMC 2006). A protected area strategy has been proposed (Ter Steege 1998, 2000). It emphasises the need to protect samples of seven different regions within the country. These have been identified primarily using forest inventory data, and are defined by geological differences. Variation in soils has been found to explain variation in floristic diversity, whereby reserve delimitation to include as much soil heterogeneity as possible has been advocated (Ter Steege 1998).
In Surinam, 11 reserves were established between 1961 and 1986, and a large one was added in 1998 (Harcourt and Sayer 1996; UNEP-WCMC 2006). They cover a total area of 18,979 km2 (includes some coastal biotopes). Most of the areas are small: the largest reserve, Central Suriname nature reserve, accounts for more than 80% of the total area. However, in comparison with other Amazonian countries the reserve network of Surinam has a good coverage (Baal et al. 1988; Harcourt and Sayer 1996). The development of the reserve network has been based on a desire to protect diverse and scenic landscapes, unique geological formations, different ecosystem types, and particular emblematic species. Species richness and endemism has been a central selection criterion for some reserves (Harcourt and Sayer 1996; UNEP-WCMC 2006).
In Venezuela, the large (30,000 km2) Canaima national park, which lies in the outskirts of Amazonia as defined here, was established in 1968. In the late 70s and early 90s many new protected areas were created to protect Venezuelan Amazonia. There are currently 21 reserves (7 national parks, 14 natural monuments), which cover a total area of 126,573 km2. Selection criteria for the reserves can be roughly classified into three categories (Huber 2001): scenic beauty, direct information on species distributions, ecosystem types, and the latter two criteria combined. For a number of reserves we have been unable to form a clear picture on their scientific selection criteria.
Summary: two main routes towards representativeness
The relative importance of different surrogate measures applied in justifying reserve selection in Amazonian countries is summarised in Table 1. See Appendices 1 and 2 for lists of included reserves and for selection criteria reserve by reserve.
Our analysis shows that the great majority of the Amazonian reserves can be seen as systematically selected to sample the region’s biodiversity (87 reserves or 80.6% out of a total of 108 reserves; 455,163 km2 or 75.3% out of 604,832 km2). The surrogate for overall biodiversity has been ecosystem types or other environmental units (42.6% of reserves, 27.3% of area), direct information on species distributions (13.9%/14.8%), estimated or predicted species distribution patterns (4.6%/10.3%), or a combination of distribution patterns and environmental units (19.4%/22.9%).
The ‘environmental units approach’ relies on the assumption that in Amazonia, as in other biomes, distinct biotopes exist, and species diversity can be captured by full representation of such higher-order surrogates. A number of different environmental classifications have been used (biogeographical provinces, ecological maps, vegetation maps). Species distributions have been sampled either using the refuge hypothesis (Haffer 1969) as a model, or through summarising available specialist knowledge, often in specifically convened workshops.
Reserve selection schemes realised in practice, as well as studies evaluating the Amazonian reserve network, have applied a hierarchical approach to representativeness. The chosen biodiversity surrogate has been considered separately for subunits within the Amazonian region with the aim to take into account gamma-diversity. In the Amazon Analysis, these subunits were the phytogeographic provinces of Prance (1977). Fearnside and Ferraz (1995) considered Brazilian states separately. Workshop 99 divided Brazilian Amazonia on the basis of ecoregions (which were mainly based on interfluvial regions), principal catchment areas, and policies for regional development (Seminário de Consulta Macapá 2001). The Peruvian system uses biogeographic provinces within the country. In practice, reserve networks have often been designed nationally, i.e., without estimating representativeness of the complete, trans-national reserve network.
What is the current scientific understanding on the data and surrogate measures applied in Amazonian reserve selection?
The theoretical potential of the Amazonian reserve network to protect a complete sample of the region’s biodiversity hinges on the appropriateness of the surrogate measures and data on which systematic reserve selection has been based (of lesser importance is the coincidental inclusion of biodiversity values in reserves selected using other criteria because these are clearly fewer than the systematically chosen ones). We therefore discuss the development in natural-scientific understanding of Amazonia since the 1970s to evaluate the appropriateness of the applied selection criteria.
The refuge hypothesis in trouble
Although the refuge hypothesis was formulated as an explanation for observed biotic patterns, it was soon backed by interpretations of Amazonian geomorphology, sedimentology and fossil records (for reviews see Hooghiemstra and van der Hammen 1998; van der Hammen and Hooghiemstra 2000; Haffer and Prance 2001). However, a parallel theme has been the refutation of all purported evidence from these fields on the basis of inadequacy, irrelevancy, or plain faultiness (for reviews see Colinvaux 1987, 1996; Salo 1987; Bush 1994, 1996; Colinvaux and De Oliveira 2000; Colinvaux et al. 2000, 2001; Bush and De Oliveira 2006). Palynological data gathered since the mid-1980s, and re-interpretations of earlier data, have made a strong case against widespread fragmentation of the Amazonian forest block (Liu and Colinvaux 1985; Bush et al. 1990; Bush 1994; Colinvaux 1996; Colinvaux et al. 1996, 1997, 2000; Ledru et al. 2001). These studies suggest that the forest was continuous throughout the Pleistocene, although somewhat smaller and compositionally different due to climatic cooling, but only modest drying, during the glacial maxima. Paleoecological data from present-day forest-savanna ecotones also suggest migration of forest borders, in the Pleistocene as well as the Holocene (Mayle et al. 2000).
From a conservationist’s point of view, the invalidity of the assumptions of the refuge hypothesis is all but irrelevant: what counts are the predictions, i.e., where, if anywhere, universal species richness and endemism centres are located. However, it has been shown that the predictions cannot be confirmed either. First, the locations of species richness and endemism centres, given by different authors for different organism groups, overlap as little as if they were randomly placed (Beven et al. 1984). Second, different growth forms of vascular plants (e.g., trees vs. herbs) have different diversity and endemism centres, and endemism is only partly correlated with species richness, being concentrated in patches of unusual habitat and in topographically diverse montane areas (Gentry 1992). Moritz et al. (2000) reached similar conclusions for mammals, which suggests that current ecological factors, rather than historical climate changes, determine Amazonian species diversity patterns.
The original refuge hypothesis, which concentrated on the last glacial maximum, has later been modified and temporally extended (Haffer 1997; Haffer and Prance 2001). The modified hypothesis postulates that climatic cycles have acted as a species pump and moulded species distribution patterns in Amazonia repeatedly throughout the Pleistocene and also in pre-Pleistocene epochs. The refined refuge hypothesis thus accommodates the findings that high plant diversity of South American rainforests by far predates the Pleistocene (Wilf et al. 2003; Jaramillo et al. 2006) and that many animal speciations seem to be older than the Pleistocene (Cracraft and Prum 1988; Fjeldså and Rahbek 1997; Patton et al. 1997; Moritz et al. 2000). A cyclic history could perhaps also explain the inconsistency of endemism centres of different organism groups noted above, but it is hard to envisage how present-day distribution patterns could be accurately predicted from such a model.
Direct information on species distributions
The precise data on which specialist workshops for conservation recommendations have based their consensus views on Amazonian areas of special biological value have not been published, which renders their evaluation difficult. However, evaluations of the quantity and spatial distribution of species occurrence data suggest that considerable parts of Amazonia are both floristically and faunistically unexplored, that is, the floristic and faunistic understanding suffers from a severe sampling bias (Nelson et al. 1990; Oren and Albuquerque 1991; Voss and Emmons 1996; Patton et al. 1997; L. Schulman et al., submitted manuscript). Additionally, species occurrence data have a taxonomical bias, i.e., those species that are scientifically named have on average wider ranges than species that are still unnamed (Ruokolainen et al. 2002; Diniz-Filho et al. 2005). This means that there probably still are considerable numbers of narrow-range endemics to be found in Amazonia.
Formal analyses, in the context of conservation planning, of reliably documented occurrence data of Amazonian species (Williams 1996; Kress et al. 1998; Funk et al. 1999; Lim et al. 2002) give a near-uniform signal: the current level of knowledge on species distributions is inadequate for giving well-justified recommendations for conservation planning. Exceptions have been works analysing bird species distributions (Fjeldså and Rahbek 1997, 1998) as they have considered the quality of species distribution data sufficient for identifying priority areas for conservation at a rather coarse resolution of 1° squared. According to Fjeldså and Rahbek (1997, 1998), the existing reserves are sufficient to conserve nearly all bird species occurring in Amazonian lowlands, whereas in Andean submontane and montane areas there are several unprotected areas with endemic species.
Birds evidently are the biogeographically best known group of organisms, but even their distributions are still far from satisfactorily known. In the last decade, for example, field work in the Allpahuayo-Mishana reserve in northern Peru has produced four new bird species and 13 range extensions of over 300 km (Alvarez Alonso and Whitney 2003). This increase in biogeographical knowledge is particularly notable for the location of the reserve: it lies only 25 km from the centre of the city of Iquitos, which has for long been one of the main ports of entry for biological exploration in lowland Amazonia. It is also noteworthy that the Amazonian areas of bird endemism (Cracraft 1985, cited in Eberhard and Bermingham 2005; Haffer 1985) were delimited subjectively using very sparse occurrence data available at that time (Oren and Albuquerque 1991). It may be that the areas of endemism and their limits do indeed reflect biogeographical reality in a useful way, but one should also realise that these areas have not been evaluated against possible geographical bias in records of occurrence data as has been done with plants (Nelson et al. 1990). Furthermore, there are no studies using the much improved current knowledge on bird species distributions (Ridgely et al. 2005) to test the appropriateness of the delimitations of the areas of endemism.
Environmental heterogeneity and beta-diversity
Lowland Amazonia has traditionally been divided into three principal types of environment – inundated land; tierra firme (non-inundated land) with loamy to clayey soil; and tierra firme with white sand. There is ample documentation of the significance of these divisions for the distribution of plant and animal species (e.g., Anderson 1981; Balslev et al. 1987; Kubitzki 1989; Borges 2004; Sääksjärvi et al. 2006). A variety of more elaborated schemes of vegetation classification (Pires 1973; Pires and Prance 1985; Huber and Alarcón 1988; IBGE and IBDF 1988; IBGE 1989, 1997; Rojas and Castaño 1990) and other environmental typification (Tosi 1960; ONERN 1976; Anonymous 1994, 1995; Dinerstein et al. 1995; Zamora 1996; BIODAMAZ 2004a, b) have been presented over the years.
The multitude of classification criteria and terminology applied renders it difficult to compare different classifications across national borders, but it is clear that the amount of environmental heterogeneity recognised has increased steadily. For instance, fairly recent GIS maps (IBGE 1997) distinguish 161 vegetation types in Brazilian Amazonia (Nelson and de Oliveira 2001). However, it is important to notice that the most abundant vegetation types typically occupy vast areas. For example, the four most widespread of those forest types recognised in recent classifications in Brazilian Amazonia cover 67% of all forest area in the Brazilian legal Amazonia (Fearnside and Ferraz 1995). Furthermore, even the refined classifications leave out variation known to exist. For instance, the Projeto RADAMBRASIL (1973–1983) surveys, on which Brazilian vegetation maps have been based, excluded peatlands (Schulman et al. 1999), which have been estimated to cover 55,000 km2 in Brazilian Amazonia (Ruokolainen et al. 2001). Tuomisto et al. (1995) concluded that Peruvian lowland Amazonia alone harbours over 100 biotope types, which is an order of magnitude more than in existing vegetation classifications.
The development of the Amazonian reserve network has not managed to keep pace with the advance in the recognition of environmental heterogeneity. Twelve years ago the Peruvian reserve system covered 21 of the 40 upland Amazonian life zones and 7 of the 22 lowland ones (CDC-UNALM 1991). The situation has since improved slightly, but the coverage is still far from the national goal (Rodríguez and Young 2000). In Brazil, similar gaps in the coverage of vegetation types of the national classification schemes have been noted (Fearnside and Ferraz 1995; Capobianco 2001).
Contrary to the focus on environmental variation, some recent studies have postulated extensive homogeneity for Amazonian forests by assuming that either competitive interactions (Pitman et al. 2001) or spatially restricted dispersal and regeneration dynamics (Condit et al. 2002) determine plant species distributions. Terborgh et al. (2002) even concluded that western Amazonian forests are so homogeneous that “randomly situated conservation areas will capture most tree species inhabiting the region”. These studies have not included measured soil characteristics in their analyses and therefore possible environmental control on species distributions has not been tested. In fact, all studies that have tested this control have found that it exists and that it is relevant within the recognised vegetation classes (Lescure and Boulet 1985; Ter Steege et al. 1993; Tuomisto et al. 1995, 2003a, b, c; Duivenvoorden 1995; Tuomisto and Poulsen 1996, 2000; Ruokolainen et al. 1997; Sabatier et al. 1997; Ruokolainen and Tuomisto 1998; Ter Steege 1998; Phillips et al. 2003; Vormisto et al. 2004; Duque et al. 2005).
Four central conclusions can be drawn regarding the use of environmental units, such as vegetation types, as surrogates for species diversity in Amazonian reserve selection. First, the early vegetation classification applied in the Amazon Analysis clearly did not recognise all existing variation. Second, all existing environmental variation is still not covered by available classification schemes. Third, maps of vegetation or other environmental types enable a relatively extensive analysis of conservation priorities, unlike species distribution data, because they do not to the same extent suffer from spatially biased sampling. This enables rapid and repeated analyses of the coverage of reserve networks. However, for a conservationist, the most critical is the fourth conclusion: the real problem with existing formal environmental classifications of Amazonia is neither their incompleteness nor their poor representation in reserves, but the fact that their significance for species distribution patterns is not documented. Even though environmental variation has been shown to reflect biotic variation, the proposed and partly applied formal classification systems have not been tested for this. For example, we are not aware studies which would have tested thoroughly if species composition in any plant or animal group changes as one moves between the ‘dense’, ‘non-dense’ and ‘submontane’ types of forest which together cover two thirds of the Brazilian Amazonia according to the classification applied by Fearnside and Ferraz (1995).
The temporal dimension: dynamic landscape evolution
Even though the humidity and vegetation changes in Pleistocene Amazonia evidently were not as dramatic as held by the refuge hypothesis, our understanding of Amazonia’s past has far from reverted to the historical idea of long-term stability. The geological history of the Andean forelands includes phases when interior seaways (Räsänen et al. 1995; Hovikoski et al. 2005) and large lake complexes (Wesselingh et al. 2002) separated the west from central and eastern parts of Amazonia. In the east, past sea level changes have apparently created extensive embayments along the present Amazon Basin (see Haffer 1997). Such landscape modifications have important historical-biogeographical implications.
In addition, plate tectonics, which drive the Andean uplift, have shaped the western Amazonian lowlands from the Tertiary through to the Quaternary resulting in widespread fluvial perturbance (Räsänen et al. 1987). These landscape dynamics have resulted in a temporally structured patchwork of fossil and current floodplains with the forests growing on a lithologic mosaic as a patchwork of stands in different successional stages (Salo et al. 1986).
There is also a considerable array of geological histories among Amazonian soils. Tectonically tranquil areas in the Guayanan and Brazilian shield areas have had ample time for a long and continuous soil-forming process (Irion 1978), whereas in the more active western parts of Amazonia the ages of soils can vary from recent to some millions of years (Räsänen et al. 1990, 1992; Salo and Räsänen 1989; Jacques 2003, 2004). At least in the west, the soils are also derived from quite distinct parent materials from thoroughly leached quartzitic sands to semimarine or lacustrine clays and sands, as well as volcanoclastic sediments (Räsänen et al. 1992, 1998; Hoorn 1995; Wesselingh 2006). The clarification of a possible causal chain from the geological setting to certain soil characteristics and up to predictable plant species composition appears as a high priority research question. If such a chain exists, it will provide a powerful tool for ecologically and biogeographically relevant vegetation mapping because then also various existent and developing geological maps could be utilised for constructing vegetation maps and targeting biological field studies.
Differences between subregions: gamma-diversity within Amazonia
There is plenty of evidence showing that the species composition of many organism groups is different in geographically distant parts of Amazonia (e.g., Mori 1991; Ek 1997; Terborgh and Andresen 1998; Ron 2000; Tuomisto et al. 2003c; Racheli and Racheli 2004; Clarke and Funk 2005). However, data are insufficient for testing if the limits of Amazonian biogeographic regions really are as they have been drawn (Prance 1977; Haffer 1985); we simply do not know where borders between biogeographic provinces could rightly be drawn.
While national borders certainly are pragmatic when defining the highest hierarchical level on which to apply systematic conservation planning, they do not make much sense from a natural-scientific point of view. Some biological criteria, or geographic or geological criteria correlating with biological changes, should be found and used for drawing limits between sub-regions. A large-scale attempt of this kind is the assessment of Central and South American ecoregions by Dinerstein et al. (1995). A problem with this study is the inadequacy and poor documentation of the data applied in ecoregion delimitation in Amazonia. This was also noted by the authors: “...the large ecoregion units used in this study for Amazonia should be subdivided in future studies to more accurately reflect patterns of biodiversity...” (Dinerstein et al. 1995, p. 84). Several authors have noted the potential of large rivers and their floodplains as dispersal barriers (reviewed by Haffer 1997), and promoted their use as a template for a biogeographic division of Amazonia. The separation of the western Amazonian landscape into tectonically uplifted areas dissected by erosion, and subsiding basins with younger fluvial sediments, has also been suggested to cause biogeographic barriers, which would result in speciation and accumulation of biotic differences (Räsänen et al. 1987). However, we are not aware of any studies that would have tested the usefulness of either landscape features as limits reflecting gamma-diversity patterns.
Conclusions: the theoretical representativeness of the Amazonian reserve network
With currently available knowledge, it is impossible to accurately assess the representativeness of the Amazonian reserve network. We do not have complete species lists of existing reserves and we do not know nearly all Amazonian species from any sizeable taxonomic group. Environmental classifications of Amazonia are not satisfactory yet, and information on biotope types present in current reserves is incomplete. Hence, the question we pose in the title of this review cannot yet be answered. We simply do not know nearly enough about the biodiversity of Amazonia. Before we can evaluate the real representativeness of the Amazonian reserve network a tremendous amount of further information on the region’s biota has to be collected.
It is, however, possible to evaluate the scientific criteria on which reserves have been selected, such as the distribution of environmental types of a chosen classification, or perceived species distribution patterns, against the current state of knowledge on these criteria. Such a comparison can shed light on the potential representativeness of the reserves, i.e., on the probability that they could cover a complete sample of the species of Amazonia.
Environmental classifications, direct information on the distribution of some species, and estimated locations of supposed Pleistocene forest refuges have been the three most important natural-scientific selection criteria for Amazonian reserves (Table 1; Appendices 1 and 2). Current scientific understanding is that both the assumptions and the predictions of the refuge hypothesis are ill-founded: the Amazonian forest biome apparently never was fragmented and universal centres of endemism do not exist (or at least the available data do not enable their identification). Hence, a reserve network based on the refuge hypothesis and purported endemism centres does not sample Amazonian species diversity systematically, but rather in an arbitrary manner. The severe lack and uneven quality of verified distribution data on Amazonian biota renders precarious their use in designing a representative reserve network. As with purported Pleistocene refuges, the areas identified as biologically valuable on the basis of such data are certainly rich in diversity, but there is no scientific evidence proving that they are unique, or even more valuable, than the extensive unstudied regions.
There is now ample evidence that the role of environmental heterogeneity in determining spatial and temporal biodiversity patterns in Amazonia is central, which justifies the use of environmental surrogates in reserve selection. However, none of the existing formal classifications for Amazonia have documented the degree of species turnover, i.e., beta-diversity, between the classes separated. Hence, there is little scientific evidence promoting any particular classification as a reliable higher order surrogate for species diversity. There is, however, evidence that Amazonia harbours much beta-diversity that has not been captured by existing formal vegetation classifications. Taken together, this means that a reserve network based on existing environmental classifications cannot be safely assumed to evenly represent beta-diversity.
Current scientific understanding is that gamma-diversity must be taken into account in Amazonian reserve network design, but there is no scientifically satisfactory division scheme, which should be used as a template in systematic conservation planning. Due to the extensive unprotected tracts left by the current reserve system, gamma-diversity probably is not adequately covered.
The historical development of the western Amazonian landscape, and its current dynamics, do not seem to have affected reserve selection in the region. It is therefore likely that ecologically, biogeographically, and evolutionarily influential abiotic processes are inadequately protected by the existing reserves. In contrast to the refuge hypothesis, the geohistory of western Amazonia provides testable hypotheses on patterns of species distribution and differentiation. According to the results of such tests, it may turn out to be necessary to include habitat history as one criterion in conservation planning (Cowling and Pressey 2001; Graham et al. 2006).
Guidelines for the future: how to improve representativeness using natural-scientific understanding?
Maddock and Du Plessis (1999) promoted a hierarchical approach to conservation priority setting such that higher order biodiversity surrogates should be applied first, and a coarse system thus obtained refined through the application of species distribution data and socio-economic considerations. This is close to what has happened in reality in many cases of Amazonian reserve selection. However, since there is no evidence of the existing Amazonian environmental classifications correlating with species turnover, using them even as a higher level starting point is risky. Instead, we would emphasise the need for new approaches of making use of the existing scanty data on species distributions and their abiotic determinants.
One promising approach is to base the selection of conservation areas on observed and modelled patterns of floral or faunal communities instead of individual species (Faith and Walker 1996; Ferrier 2002). Heuristically this is reminiscent of the strategy of optimising the conservation of vegetation classes, ecoregions, or other surrogates for species. However, in the approach of community patterns, the region of interest (Amazonia in this case) is not divided into classes. Instead, environmental and observed biotic data are used to build up a model, which allows one to estimate the relative species compositional distance even of biologically undocumented points to every other point in the region. Accordingly, the position of each point will be defined in a multidimensional species compositional space, and the task of conservationists becomes to ensure as complete representation as possible of this multidimensional space in reserves.
It is naturally impossible to use the whole biota for making the model between biotic and environmental data. Therefore a critical question is if it were possible to select a representative and easily recordable part of the flora and fauna for making the model. Higgins and Ruokolainen (2004) have shown that the most effective way to get a representative idea of floristic patterns is to choose a taxonomically defined part (e.g., a certain plant family) and not a structurally defined part (e.g., big trees) as is the prevailing custom in floristic inventories. Pteridophytes, and the flowering-plant families Melastomataceae and Arecaceae, have been shown to be possible indicator species groups for Amazonian floristic patterns (Ruokolainen et al. 1997; Ruokolainen and Tuomisto 1998; Vormisto et al. 2000; Duque et al. 2005). For the fauna, similar studies have not been carried out, and the eventual correspondence between floristic and faunistic patterns is almost completely unknown in Amazonia. The study by Sääksjärvi et al. (2006), however, suggests that correlation exists even over at least one trophic level.
For environmental data to be useful for modelling biotic patterns, they must cover the whole Amazonia and be as accurate as possible. Landsat satellite data fulfil this requirement, and they have also been shown to co-vary with floristic data (Ruokolainen and Tuomisto 1998; Tuomisto et al. 2003a, b; Salovaara et al. 2005; Thessler et al. 2005). Unfortunately the Landsat data have a systematic East-West bias (Toivonen et al. 2006), which appears to be an irremovable property of the technology. The only certain way to cope with this problem is to have so dense network of species inventories that East-West interpolations need not be made over distances greater than ca. 30 km. This means that if an Amazonian-wide application of the approach is to be applied seriously, data of perhaps some 10,000 species inventories would be needed. This is hardly achievable through the most commonly applied inventory method of recording big trees within one hectare. However, it is a realistically achievable number when using indicator species groups, which are of suitable size and sufficiently well known taxonomically.
Even though we believe that a focus on similarity patterns would be very much needed in Amazonian conservation planning, information and estimations on individual species ranges should not be neglected. Natural history museums and other depositories exist from which it would be possible to retrieve information on species occurrences that, in turn, can be used together with environmental information for modelling species distribution areas (Guisan and Zimmermann 2000). At the moment a serious problem in these species data is that georeferencing often is not sufficiently exact for modelling. However, this situation is rapidly improving with the common use of GPS-receivers for accurate locating of observations. Recent development in algorithms (Moilanen et al. 2005) has also made it possible to handle a practically unlimited number of species and size of area in the search for an optimal solution of saving areas for the persistence of species. These algorithms also offer the possibility to explicitly include any other possible criteria, such as political and economical considerations, into the optimisation procedure. Hence, possibilities for using observations and estimations of species distributions in Amazonian conservation planning are improving. It must nevertheless be kept in mind that ranges of a great majority of the species will remain unknown for a long time, and the species ranges that will be first resolved are biased towards widespread species whereas local endemics are likely to remain unknown (Ruokolainen et al. 2002; Diniz-Filho et al. 2005).
Systematic conservation planning in Amazonia to date and tomorrow
Amazonian reserve networks have repeatedly been developed with the implicit goal of protecting all Amazonian species. We find this a reasonable target: unachievable in practice it may be, but the reserve system should continuously be complemented to approach this ideal. The work done to date, and that still in progress, is laudable. Great effort has been put into selecting reserves efficiently by applying scientific criteria, and there is no doubt that the actions taken so far have significantly contributed to biodiversity protection. Despite this, the above review leads us to conclude that the criteria used in the selection of Amazonian reserves cannot result in a reserve network that could safely be assumed to reach full representativeness of the region’s biodiversity. Even if presently implemented prioritisation schemes were carried out completely, the result would be a system whose potential to sample Amazonian species diversity is undermined by deficiencies and uncertainties in the scientific constructs and data used as selection criteria.
We see the central role of the questionable refuge hypothesis, and spatially and taxonomically biased observed species distributions, as well as the continued application of untested environmental surrogates in reserve selection as manifestations of a divergence between conservation planning and the development in natural-scientific understanding on Amazonia. More scientific information on Amazonia is continuously arising. Those who carry out conservation in practice should therefore constantly be alert, and ensure that planning processes are transparent and susceptible to new scientific insight. On the other hand, scientists should accept their responsibility to disseminate scientific information to decision-makers in such a form that it can readily be applied in conservation planning. In the meantime, the principle of caution is the best safeguard against irreversible bad decisions.
References
Achard F, Eva HD, Stibig H-J, Mayaux P, Gallego J, Richards T, Malingreau J-P (2002) Determination of deforestation rates of the world’s humid tropical forests. Science 297:999–1002
Alvarez Alonso J, Whitney B (2003) New distributional records of birds from white-sand forests of the northern Peruvian Amazon, with implications for biogeography of northern South America. The Condor 105:552–566
Anderson AB (1981) White-sand vegetation of Brazilian Amazonia. Biotropica 13:199–210
Anonymous (1994) Mapa ecológico del Perú. Ministerio de Agricultura and Instituto Nacional de Recursos Naturales, Lima
Anonymous (1995) Mapa ecológico del Perú. Guía Explicativa. Ministerio de Agricultura and Instituto Nacional de Recursos Naturales, Lima
Anonymous (1997a) Propuesta metodológica para la zonificación ecológica-económica para la Amazonía. Memorias del seminario taller, Santafé de Bogotá, Colombia, 9–12 diciembre 1996. Tratado de Cooperación Amazónica, Secretaría Pro-Tempore, Lima
Anonymous (1997b) Estudio Nacional de la Diversidad Biológica. Volumen III: Sistema Nacional de Áreas Protegidas por el Estado – SINANPE. Ministerio de Agricultura and Instituto Nacional de Recursos Naturales, Lima
Anonymous (1998) Manual de zonificación ecológica-económica para la Amazonía Peruana. Ministerio de Relaciones Exteriores, Comisión Nacional Permanente Peruana del Tratado de Cooperación Amazónica, Lima
Asner GP, Knapp DE, Broadbent EN, Oliveira PJC, Keller M, Silva JN (2005) Selective logging in the Brazilian Amazon. Science 310:480–482
Baal FLJ, Mittermeier RA, van Roosmalen MGM (1988) Primates and protected areas in Suriname. Oryx 22:7–14
Balslev H, Luteyn J, Øllgaard B, Holm-Nielsen LB (1987) Composition and structure of adjacent unflooded and floodplain forest in Amazonian Ecuador. Opera Bot 92:37–57
Beven S, Connor EF, Beven K (1984) Avian biogeography in the Amazon basin and the biological model of diversification. J Biogeogr 11:383–399
BIODAMAZ (2001) Estrategia Regional de la Diversidad Biológica Amazónica. Documento Técnico No 1. Serie BIODAMAZ-IIAP, Iquitos
BIODAMAZ (2004a) Diversidad de vegetación de la Amazonía peruana expresada en un mosaico de imágenes de satélite. Serie IIAP-BIODAMAZ, Documento Técnico 12, Iquitos
BIODAMAZ (2004b) Macrounidades ambientales en la Amazonía peruana con énfasis en la Selva Baja: Primera approximación a manera de hipótesis de trabajo. Serie IIAP-BIODAMAZ, Documento Técnico 13, Iquitos
Borges SH (2004) Species poor but distinct: bird species assemblages in white sand vegetation in Jaú National Park, Brazilian Amazon. Ibis 146:114–124
Brown KS (1975) Geographical patterns of evolution in neotropical Lepidoptera. Systematics and derivation of known and new Heliconiini (Nymphalidae: Nymphalinae). J Entomol 44:201–242
Bruner AG, Gullison RE, Rice RE, da Fonseca GAB (2001) Effectiveness of parks in protecting tropical biodiversity. Science 291:125–128
Bush MB (1994) Amazonian speciation: a necessarily complex model. J Biogeogr 21:5–17
Bush MB (1996) Amazonian conservation in a changing world. Biol Conserv 76:219–228
Bush MB, De Oliveira PE (2006) The rise and fall of the refugial hypothesis of Amazonian speciation: a paleoecological perspective. Biota Neotropica, http://www.biotaneotropica.org.br/v6n1/pt/abstract?point-of-view+bn00106012006
Bush MB, Weimann M, Piperno DR, Liu K-B, Colinvaux PA (1990) Pleistocene temperature depression and vegetation change in Ecuadorian Amazonia. Quatern Res 34:330–345
Câmara IdeG (1983) Tropical moist forest conservation in Brazil. In: Sutton SL, Whitmore TC, Chadwich AC (eds) Tropical rain forest: ecology and management. Blackwell Scientific Publications, Oxford, pp 413–421
Capobianco JPR (2001) Representividade da unidades de conservação e terras indígenas em relação às fitofisionomias da Amazônia Legal. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 263–267
CDC-UNALM (1991) Plan director del Sistema Nacional de Unidades de Conservación (SINUC), una aproximación desde la diversidad biológica. Centro de Datos para la Conservación, Universidad Nacional Agraria La Molina, Lima
CIAM (2004) Sistema Nacional de Areas Protegidas del Ecuador SNAP – Areas Protegidas Actuales. Información Base y Temática, Ministerio del Ambiente 1:250.000. Dirección Nacional de Biodiversidad/Laboratorio SIG del CIAM, Ecuador
Clarke HD, Funk VA (2005) Using checklists and collections data to investigate plant diversity: II. An analysis of five florulas from northeastern South America. Proc Acad Natl Sci Philadelphia 154: 29–37
Cochrane MA (2003) Fire science for rainforests. Nature 421:913–919
Cochrane MA, Laurance WF (2002) Fire as a large-scale edge effect in Amazonian forests. J Trop Ecol 18:311–325
Colinvaux PA (1987) Amazon diversity in the light of the paleoecological record. Quatern Sci Rev 6:93–114
Colinvaux PA (1996) Quaternary environmental history and forest diversity in the Neotropics. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. University of Chicago Press, Chicago, pp 359–405
Colinvaux PA, De Oliveira PE (2000) Palaeoecology and climate of the Amazon basin during the last glacial cycle. J Quatern Sci 15:347–356
Colinvaux PA, De Oliveira PE, Moreno JE, Miller MC, Bush MB (1996) A long pollen record from lowland Amazonia: forest and cooling in glacial times. Science 274:85–88
Colinvaux PA, Bush MB, Steinitz-Kannan M, Miller MC (1997) Glacial and postglacial pollen records from the Ecuadorian Andes and Amazon. Quatern Res 48:69–78
Colinvaux PA, De Oliveira PE, Bush MB (2000) Amazonian and neotropical plant communities on glacial time-scales: the failure of the aridity and refuge hypothesis. Quatern Sci Rev 19:141–169
Colinvaux PA, Irion G, Räsänen ME, Bush MB, Nunes de Mello JAS (2001) A paradigm to be discarded: geological and paleoecological data falsify the Haffer & Prance refuge hypothesis of Amazonian speciation. Amazoniana 16(3/4):609–646
Comunidad Andina (2002) Decision 523 and the Annex. Regional biodiversity strategy for the tropical Andean Countries. Available at: http://www.comunidadandina.org
Condit R, Pitman N, Leigh EG Jr, Chave J, Terborgh J, Foster RB, Nuñez VP, Aguilar S, Valencia R, Villa G, Muller-Landau HC, Losos E, Hubbel SP (2002) Beta-diversity in tropical forest trees. Science 295:666–669
Cowling RM, Pressey RL (2001) Rapid plant diversification: planning for an evolutionary future. Proc Natl Acad Sci 98:5452–5457
Cracraft J (1985) Historical biogeography and patterns of differentiation within the South American areas of endemism. In: Buckley PA, Foster MS, Morton ES, Ridgely RS, Buckley FG (eds) Neotropical ornithology. American Ornithologists’ Union, Washington D.C., pp 49–84
Cracraft J, Prum RO (1988) Patterns and processes of diversification: speciation and historical congruence in some neotropical birds. Evolution 42:603–620
Csuti B, Crist P (2000) Methods for assessing accuracy of animal distribution maps Version 2.0.0. US Geological Survey, Biological Resources Division
Daly CD, Prance GT (1989) Brazilian Amazon. In: Campbell DG, Hammond HD (eds) Floristic inventory of tropical countries: the status of plant systematics, collections, and vegetation, plus recommendations for the future. New York Botanical Garden, Bronx, pp 401–426
Dinerstein E, Olson DM, Graham DJ, Webster AL, Primm SA, Bookbinder MP, Ledec G (1995) A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean. The World Bank, Washington DC
Diniz-Filho JAF, Pereira Bastos R, Rangel TFLVB, Bini LM, Carvalho P, Silva RJ (2005) Macroecological correlates and spatial patterns of anuran description dates in the Brazilian Cerrado. Global Ecol Biogeogr 14:469–477
DIREC/IBAMA (2004) Lista das Unidades de Conservação Federais. Available at: www2.ibama.gov.br/unidades/geralucs/fr_tabl.htm; updated on 17 Nov 2004
DIREC/IBAMA (2006) Lista das Unidades de Conservação Federais. Available at: www.ibama.gov.br/; updated on February 2006 (erroneously dated 31/12/2006 when queried in May 2006)
Dourojeanni MJ (1990) Amazonia Peruana, que hacer? Centro de Estudios Teológicos de la Amazonía, Iquitos
Duivenvoorden JF (1995) Tree species composition and rain forest-environment relationships in the middle Caquetá area, Colombia, NW Amazonia. Vegetatio 120:91–113
Duque AJ, Duivenvoorden JF, Cavelier J, Sánchez M, Polanía C, León A (2005) Ferns and Melastomataceae as indicators of vascular plant composition in rain forests of Colombian Amazonia. Plant Ecol 178:1–13
Eberhard JR, Bermingham E (2005) Phylogeny and comparative biogeography of Pionopsitta parrots and Pteroglossus toucans. Mol Phylogenet Evol 36:288–304
Ek RC (ed) (1997) Botanical diversity in the tropical rainforest of Guyana. Tropenbos-Guyana Series 4, Wageningen
Eva HD, Huber O (eds) (2005) A proposal for defining the geographical boundaries of Amazonia. Synthesis of the results from an expert consultation workshop organized by the European Commission in collaboration with the Amazon Cooperation Treaty Organization – JRC Ispra, 7–8 June 2005. Office for Official Publications of the European Communities, Luxemburg
Faith DP, Walker PA (1996) How do indicator groups provide information about the relative biodiversity of different sets of areas?: on hotspots, complementarity and pattern-based approaches. Biodivers Lett 3:18–25
Fearnside PM (1999) Biodiversity as an environmental service in Brazil’s Amazonian forests: risks, value and conservation. Environ Conserv 26:305–321
Fearnside PM, Ferraz J (1995) A conservation gap analysis of Brazil’s Amazonian vegetation. Conserv Biol 9:1134–1147
Ferreira LV, Lemos de Sá R, Buschbacher R, Batmanian G, Cardoso da Silva JM, Arruda MB, Moretti E, Fernando L, de Sá SN, Falcomer J, Bampi MI (2001) Identificação de áreas prioritárias para a conservação da biodiversidade por meio da representatividade das Unidades de Conservação e tipos de vegetação nas ecorregiões da Amazônia brasileira. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 268–286
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51:331–363
Fischer AG (1960) Latitudinal variations in organic evolution. Evolution 14:64–81
Fittkau EJ (1971) Ökologische Gliederung des Amazonas-Gebiets auf geochemischer Grundlage. Münsters Forsch Geol Paläontol 20/21:35–50
Fjeldså J, Rahbek K (1997) Species richness and endemism in South American birds: implications for the design of networks of nature reserves. In: Laurance WF, Bierregaard RO Jr (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. The University of Chicago Press, Chicago, pp 466–482
Fjeldså J, Rahbek C (1998) Priorities for conservation in Bolivia, illustrated by a continent-wide analysis of bird distributions. In: Barthlott W, Winiger M (eds) Biodiversity – a challenge for development research and policy. Springer Verlag, Heidelberg, pp 313–327
Foresta RA (1991) Amazon conservation in the age of development, the limits of providence. University of Florida Press, Gainesville
Funk VA, Fernanda Zermoglio M, Nasir N (1999) Testing the use of specimen collection data and GIS in biodiversity exploration and conservation decision making in Guyana. Biodivers Conserv 8:727–751
Gentry AH (1992) Tropical forest biodiversity: distributional patterns and their conservational significance. Oikos 63:19–28
Graham CH, Moritz C, Williams SE (2006) Habitat history improves prediction of biodiversity in rainforest fauna. Proc Natl Acad Sci 103:632–636
Grimwood IR (1968) Notes on the distribution and status of some Peruvian mammals. IUCN & NYZS Special Publication No. 21:1–86
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Haffer J (1969) Speciation in Amazonian forest birds. Science 165:131–137
Haffer J (1985) Avian zoogeography of the Neotropical lowlands. Ornithol Monogr 36:113–147
Haffer J (1997) Alternative models of vertebrate speciation in Amazonia: an overview. Biodivers Conserv 6:451–476
Haffer J, Prance GT (2001) Climatic forcing of evolution in Amazonia during the Cenozoic: on the refuge theory of biotic differentiation. Amazoniana 16(3/4):579–607
Harcourt CS, Sayer JA (eds) (1996) The conservation atlas of tropical forests: the Americas. The World Conservation Union, Simon & Schuster, New York
Higgins MA, Ruokolainen K (2004) Rapid tropical forest inventory: a comparison of techniques based on inventory data from western Amazonia. Conserv Biol 18:799–811
Holdridge LS (1947) Determination of world plant formations from simple climatic data. Science 105:367–368
Hooghiemstra H, van der Hammen T (1998) Neogene and Quaternary development of the Neotropical rain forest: the refugia hypothesis, and a literature overview. Earth-Sci Rev 44:147–183
Hoorn C (1995) Fluvial palaeoenvironments in the intracratonic Amazonas Basin (Early Miocene – early Middle Miocene, Colombia). Palaeogeogr Palaeoclimatol Palaeoecol 109:1–54
Hovikoski J, Räsänen M, Gingras M, Roddaz M, Brusset S, Hermoza W, Romero Pittman L, Lertola K (2005) Miocene semidiurnal tidal rhythmites in Madre de Dios, Peru. Geology 33:177–180
Huber O (2001) Conservation and environmental concerns in the Venezuelan Amazon. Biodivers Conserv 10:1627–1643
Huber O, Alarcón C (1988) Mapa de Vegetación de Venezuela. Ministerio del Ambiente y de los Recursos Naturales Renovables. The Nature Conservancy
IBGE (1989) Mapa de vegetação da Amazônia Legal. Scale 1:2,500,000. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA), Brasília
IBGE (1997) Diagnóstico Ambiental da Amazônia Legal. CD-ROM. SAE/IBGE, Rio de Janeiro
IBGE and IBDF (1988) Mapa de vegetação do Brasil. Scale 1:5,000,000. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA), Brasília
Ibisch PL (2004) History of biodiversity conservation in Bolivia. In: Ibisch PL, Mérida G (eds) Biodiversity: the richness of Bolivia. State of knowledge and conservation. Ministry of Sustainable Development. Editorial FAN, Santa Cruz de la Sierra, pp 338–347
Ibisch PL, Mérida G (eds) (2004) Biodiversity: the richness of Bolivia. State of knowledge and conservation. Ministry of Sustainable Development. Editorial FAN, Santa Cruz de la Sierra
IIRSA (2006) La Iniciativa para la Integración de la Infraestructura Regional Suramericana. Available at www.iirsa.org
INRENA (1999) Plan director. Estrategia Nacional para las Áreas Naturales Protegidas. Instituto Nacional de Recursos Naturales, Lima
INRENA (2000) Perú. Áreas Naturales Protegidas. Instituto Nacional de Recursos Naturales, Lima
Irion G (1978) Soil infertility in the Amazonian rain forest. Naturwissenschaften 65:515–519
IUCN (1994) Guidelines for protected area management categories. World Conservation Union IUCN, Gland
Jacques JM (2003) A tectonostratigraphic synthesis of the Sub-Andean Basins: implications for the geotectonic segmentation of the Andean Belt. J Geol Soc London 160:687–701
Jacques JM (2004) The effect of intraplate structurate accommodation zones on delineating petroleum provinces of the Sub-Andean foreland basins. Petrol Geosci 10:1–19
Jaramillo C, Rueda MJ, Mora G (2006) Cenozoic plant diversity in the Neotropics. Science 311:1893–1896
Jorge Pádua MT, Bernardes Qintão AT (1982) Parks and biological reserves in the Brazilian Amazon. Ambio 11:309–314
Kohlhepp G (2001) Amazonia 2000: an evaluation of three decades of regional planning and development programmes in the Brazilian Amazon region. Amazoniana 16(3/4):363–395
Kress WJ, Heyer WR, Acevedo P, Coddington J, Cole D, Erwin TL, Meggers BJ, Pogue M, Thorington RW, Vari RP, Weitzman MJ, Weitzman SH (1998) Amazonian biodiversity: assessing conservation priorities with taxonomic data. Biodivers Conserv 7:1577–1587
Kubitzki K (1989) The ecogeographical differentiation of Amazonian inundated forests. Plant Syst Evol 162:285–304
Kuliopulos H (1990) Amazonian biodiversity. Science 248:1305
Lamas G (1979) Algunas reflexiones y sugerencias sobre la creación de Parques Nacionales en el Perú. Rev Ciencias UNMSM 71(1):101–114
Laurance WF (1998) A crisis in the making: responses of Amazonian forests to land use and climate change. Trends Ecol Evol 13:411–415
Laurance WF, Cochrane MA, Bergen S, Fearnside PM, Delamônica P, Barber C, d’Angelo S, Fernandes T (2001) The future of the Brazilian Amazon: development trends and deforestation. Science 291:438–439
Ledru MP, Cordeiro RC, Landim JM, Martin L, Mourguiart P, Sifeddine A, Turcq B (2001) Late-glacial cooling in Amazonia inferred from pollen at Lagoa do Caçó, Northern Brazil. Quatern Res 55:47–56
Lescure J-P, Boulet R (1985) Relationships between soil and vegetation in a tropical rain forest in French Guiana. Biotropica 17:155–164
Lim BK, Peterson AT, Engstrom MD (2002) Robustness of ecological niche modeling algorithms for mammals in Guyana. Biodivers Conserv 11:1237–1246
Liu K-B, Colinvaux PA (1985) Forest changes in the Amazon basin during the last glacial maximum. Nature 318:556–557
Lovejoy TE, Bierregaard RO, Rylands AB, Malcolm JR, Quintela CE, Harper LH, Brown KS, Powell AH, Powell GVN, Schubart HOR, Hays MB (1986) Edge and other effects of isolation on Amazonian forest fragments. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer Assoc., Sunderland, Massachusetts, pp 257–285
Maddock A, Du Plessis MA (1999) Can species data only be appropriately used to conserve biodiversity? Biodivers Conserv 8:603–615
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253
Mattos M (2006) Amazônia ganha Unidades de Conservação e 1o. Distrito Florestal Sustentável. Available at: www.mma.gov.br/ascom/impressao.cfm?id=2257
Mayle FE, Burbridge R, Killeen TJ (2000) Millennial-scale dynamics of southern Amazonian rain forests. Science 290:2291–2294
McNeely JA, MacKinnon JR (1990) Protected areas, development, and land use in the tropics. In: Furtado JI, Morgan WB, Pfafflin JR, Ruddle K (eds) Tropical resources: ecology and development. Hardwood Academic Publishers, Chur, pp 191–208
Moilanen A, Franco AMA, Early RI, Fox R, Wintle B, Thomas CD (2005) Prioritizing multiple-use landscapes for conservation: methods for large multi-species planning problems. Proc Roy Soc B 272:1885–1891
Mori SA (1991) The Guyana lowland floristic province. Comp Rendu Séan Soc Biogéogr 67:67–75
Moritz C, Patton JL, Schneider CJ, Smith TB (2000) Diversification of rainforest faunas: an integrated molecular approach. Annu Rev Ecol Evol Syst 31:533–563
Nelson BW, de Oliveira AA (2001) Área botânica. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 132–176
Nelson BW, Ferreira CA, Da Silva MF, Kawasaki ML (1990) Refugia, endemism centers and collecting density in Brazilian Amazonia. Nature 345:714–716
Nepstad DC, Veríssimo A, Alencar A, Nobre C, Lima E, Lefebvre P, Schlesinger P, Potter C, Moutinho P, Mendoza E, Cochrane M, Brooks V (1999) Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398:505–508
Nepstad DC, McGarth D, Alencar A, Barros AC, Carvalho G, Santilli M, Vera Diaz MdelC (2002) Frontier governance in Amazonia. Science 295:629–631
Nogueira-Neto P, de Melo Carvalho JC (1979) A programme of ecological stations for Brazil. Environ Conserv 6:95–104
de Oliveira AA, Nelson BW (2001) Floristic relationships of terra firme forest in Brazilian Amazon. For Ecol Manage 146:169–179
ONERN (1976) Mapa Ecológico del Perú: Guía Explicativa. Oficina Nacional de Evaluación de Recursos Naturales, Lima
Oren DC, Albuquerque HG (1991) Priority areas for new avian collections in Brazilian Amazonia. Goeldiana Zool 6:1–11
Pacheco LF, Simonetti JA, Moraes M (1994) Conservation of Bolivian flora: representation of phytogeographic zones in the national system of protected areas. Biodivers Conserv 3:751–756
Pardo C (1994) South America. In: McNeely JA, Harrison J, Dingwall P (eds) Protecting nature: regional reviews of protected areas. IUCN, Gland, pp 351–371
Patton JL, da Silva MNF, Lara MC, Mustrangi MA (1997) Diversity, differentiation, and the historical biogeography of nonvolant small mammals of the neotropical forests. In: Laurance WF, Bierregaard RO Jr (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. The University of Chicago Press, Chicago, pp 455–465
Peres CA (2001) Paving the way to the future of Amazonia. Trends Ecol Evol 16:217–219
Peres CA, Terborgh JW (1995) Amazonian nature reserves: an analysis of the defensibility status of existing conservation units and design criteria for the future. Conserv Biol 9:34–46
Phillips OL, Núñez P, Lorenzo A, Peña A, Chuspe ME, Galiano W, Yli-Halla M, Rose S (2003) Habitat association among Amazonian tree species: a landscape-scale approach. J Ecol 91:757–775
Pires JM (1973) Tipos de vegetação da Amazonia. Publicações Avulsas Museu Paraense Emilio Goeldi 20:179–202
Pires JM, Prance GT (1985) The vegetation types of the Brazilian Amazon. In: Prance GT, Lovejoy TE (eds) Amazonia. IUCN Key Environments series, Pergamon Press, Oxford, pp 109–145
Pitman NCA, Terborgh JW, Silman MR, Nuñez VP, Neill DA, Cerón CE, Palacios WA, Aulestia M (2001) Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology 82:2101–2117
Prance GT (1973) Phytogeographic support for the theory of Pleistocene forest refuges in the Amazon Basin, based on evidence from distribution patterns in Caryocaraceae, Chrysobalanaceae, Dichapetalaceae and Lechytidaceae. Acta Amazon 3:5–25
Prance GT (1977) The phytogeographic subdivisions of Amazonia and their influence on the selection of biological reserves. In: Prance GT, Elias TS (eds) Extinction is forever. The New York Botanical Garden, Bronx, pp 195–213
Prance GT (1990) Consensus for conservation. Nature 345:384
Projeto RADAMBRASIL (1973–1983) Levantamento de recursos naturais, vols 1–23. Ministério das Minas e Energia, Departamento Nacional de Produção Mineral, Rio de Janeiro
Racheli L, Racheli T (2004) Patterns of Amazonian area relationships based on raw distributions of papilionid butterflies (Lepidoptera: Papilioninae). Biol J Linn Soc 82:345–357
Räsänen ME, Salo JS, Kalliola RJ (1987) Fluvial perturbance in the Western Amazon basin: regulation by long-term sub-andean tectonics. Science 238:1398–1401
Räsänen M, Salo J, Jungner H, Romero PL (1990) Evolution of the western Amazon lowland relief: impact of Andean foreland dynamics. Terra Nova 2:320–332
Räsänen M, Neller R, Salo J, Jungner H (1992) Recent and ancient fluvial depositional systems in the Amazonian foreland basin, Peru. Geol Mag 129:293–306
Räsänen ME, Linna AM, Santos JCR, Negri FR (1995) Late Miocene tidal deposits in the Amazonian foreland basin. Science 269:386–390
Räsänen M, Linna A, Irion G, Rebata HL, Vargas HR, Wesselingh F (1998) Geología y geoformas de la zona de Iquitos. In: Kalliola R, Flores PS (eds) Geoecología y desarrollo amazónico: estudio integrado en la zona de Iquitos, Peru. Annales Universitatis Turkuensis Ser A II 114, pp 59–137
Redford KH, Stearman AM (1993) Forest-dwelling native Amazonians and the conservation of biodiversity: interests in common or in collision? Conserv Biol 7:248–255
Ricardo B (2001a) A sociodiversidade nativa contemporânea no Brasil e a biodiversidade na Amazônia. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 194–204
Ricardo F (2001b) Sobreposições entre Unidades de Conservação (Ucs) federais, estaduais, terras indígenas, terras militares e reservas garimpeiras na Amazônia Legal. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 259–262
Ridgely RS, Allnutt TF, Brooks T, McNicol DK, Mehlman DW, Young BE, Zook JR (2005) Digital distribution maps of the birds of the Western Hemisphere. Version 2.1. NatureServe, Arlington, Virginia, USA
Rodríguez LO (ed) (1996) Diversidad biológica del Perú: zonas prioritarias para su conservación. Proyecto Fanpe GTZ-INRENA, Lima
Rodríguez LO, Young KR (2000) Biological diversity of Peru: determining priority areas for conservation. Ambio 29:329–337
Rojas UM, Castaño UC (eds) (1990) Areas Protegidas de la Cuenca del Amazonas: Diagnóstico preliminar de su Estado Actual y Revisión de las Políticas Formuladas para su Manejo. INDERENA, Bogotá
Ron SR (2000) Biogeographic area relationships of lowland Neotropical rainforest based on raw distributions of vertebrate groups. Biol J Linn Soc 71:379–402
Ruokolainen K, Tuomisto H (1998) Vegetación natural de la zona de Iquitos. In: Kalliola R, Flores PS (eds) Geoecología y desarrollo amazónico: estudio integrado en la zona de Iquitos, Perú. Annales Universitatis Turkuensis Ser A II 114, pp 253–365
Ruokolainen K, Linna A, Tuomisto H (1997) Use of Melastomataceae and pteridophytes for revealing phytogeographic patterns in Amazonian rain forests. J Trop Ecol 13:243–256
Ruokolainen K, Schulman L, Tuomisto H (2001) On Amazonian peatlands. IMCG Newslett 4:8–10
Ruokolainen K, Tuomisto H, Vormisto J, Pitman N (2002) Two biases in estimating range sizes of Amazonian plant species. J Trop Ecol 18:935–942
Rylands AB (1990) Priority areas for conservation in the Amazon. Trends Ecol Evol 5:240–241
Rylands AB, de S. Pinto LP (1998) Conservação da Biodiversidade na Amazônia Brasileira: uma Análise do Sistema de Unidades de Conservaçãõ. Cadernos FBDS No. 1, Rio de Janeiro
Sääksjärvi IE, Ruokolainen K, Tuomisto H, Haataja S, Fine P, Cárdenas G, Mesones I, Vargas V (2006) Comparing composition and diversity of parasitoid wasps and plants in an Amazonian rain forest mosaic. J Trop Ecol 22:167–176
Sabatier D, Grimaldi M, Prévost M-F, Guillaume J, Godron M, Dosso M, Curmi P (1997) The influence of soil cover organization on the floristic and structural heterogeneity of a Guianan rain forest. Plant Ecol 131:81–108
Salo J (1987) Pleistocene forest refuges in the Amazon: evaluation of the biostratigraphical, lithostratigraphical and geomorphological data. Ann Zool Fenn 24:203–211
Salo J, Räsänen M (1989) Hierarchy of landscape patterns in Western Amazon. In: Holm-Nielsen LB, Nielsen I, Balslev H (eds) Tropical forests: botanical dynamics, speciation and diversity. Academic Press, London, pp 35–45
Salo J, Kalliola R, Häkkinen I, Mäkinen Y, Niemelä P, Puhakka M, Coley PD (1986) River dynamics and the diversity of Amazon lowland forest. Nature 322:254–258
Salovaara K, Thessler S, Malik RN, Tuomisto H (2005) Classification of Amazonian primary rain forest vegetation using Landsat ETM+ satellite imagery. Remote Sens Environ 97:39–51
Sánchez-Azofeifa GA, Quesada-Mateo C, Gonzales-Quesada P, Dayanandan S, Bawa KS (1999) Protected areas and conservation of biodiversity in the tropics. Conserv Biol 13:407–411
Schulman L, Ruokolainen K, Tuomisto H (1999) Parameters for global ecosystem models. Nature 399:535–536
Schwartzman S, Moreira A, Nepstad D (2000) Rethinking tropical forest conservation: perils in parks. Conserv Biol 14:1351–1357
Seminário de Consulta, Macapá (2001) Áreas prioritárias para a biodiversidade da Amazônia Brasileira. In: Capobianco JPR, Veríssimo A, Moreira A, Sawyer D, dos Santos I, Pinto LP (organizadores) Biodiversidade na Amazônia Brasileira. Estação Liberdade & Instituto Socioambiente, São Paulo, pp 387–447
SIUC/CGEUC/DIREC/IBAMA (2000a) Distribuição das Unidades de Conservação no Estado do Amazonas. Map 1:4,000,000
SIUC/CGEUC/DIREC/IBAMA (2000b) Distribuição das Unidades de Conservação no Estado do Acre. Map 1:2,200,000
SIUC/CGEUC/DIREC/IBAMA (2000c) Distribuição das Unidades de Conservação no Estado do Amapá. Map 1:3,000,000
SIUC/CGEUC/DIREC/IBAMA (2000d) Distribuição das Unidades de Conservação no Estado de Rondônia. Map 1:2,800,000
SIUC/CGEUC/DIREC/IBAMA (2000e) Distribuição das Unidades de Conservação no Estado de Roraima. Map 1:3,000,000
SIUC/CGEUC/DIREC/IBAMA (2000f) Distribuição das Unidades de Conservação no Estado do Maranhão. Map 1:2,500,000
SIUC/CGEUC/DIREC/IBAMA (2001) Distribuição das Unidades de Conservação no Estado do Pará. Map 1:4,200,000
Soares-Filho BS, Nepstad DC, Curran LM, Cerqueira GC, Garcia RA, Ramos CA, Voll E, McDonald A, Lefebvre P, Schlesinger P (2006) Modelling conservation in the Amazon basin. Nature 440:520–523
Steininger MK, Tucker CJ, Townshend JRG, Killeen TJ, Desch A, Bell V, Ersts P (2001) Tropical deforestation in the Bolivian Amazon. Environ Conserv 28:127–134
Steyermark JA (1986) Speciation and endemism in the flora of Venezuelan tepuis. In: Vuilleumier F, Monasterio M (eds) High altitude tropical biogeography. Oxford University Press, New York, pp 317–373
Suárez MO (1985) Parques nacionales y afines de Bolivia. La Paz
Terborgh J, Andresen E (1998) The composition of Amazonian forests: patterns at local and regional scales. J Trop Ecol 14:645–664
Terborgh J, Pitman N, Silman M, Schichter H, Núñez VP (2002) Maintenance of tree diversity in tropical forests. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CABI Publishing, Wallingford, pp 1–17
Ter Steege H (1998) The use of forest inventory data for a national protected area strategy in Guyana. Biodivers Conserv 7:1457–1483
Ter Steege H (2000) Plant diversity in Guyana, with recommendations for a National Protected Area Strategy. Tropenbos series 18, Wageningen
Ter Steege H, Jetten VG, Polak AM, Werger MJA (1993) Tropical rain forest types and soil factors in a watershed area in Guyana. J Veget Sci 4:705–716
Thessler S, Ruokolainen K, Tuomisto H, Tomppo E (2005) Mapping gradual landscape-scale floristic changes in Amazonian primary rain forests by combining ordination and remote sensing. Global Ecol Biogeogr 14:315–325
Toivonen T, Kalliola R, Ruokolainen K, Malik RN (2006) Across-path DN gradient in Landsat TM imagery of Amazonian forests: a challenge for image interpretation and mosaicking. Remote Sens Environ 100:550–562
Tosi JA (1960) Zonas de vida natural en el Perú: memoria explicativa sobre el mapa ecológico del Perú. Boletín Técnico no. 5. Proyecto de Cooperación Técnica de la OFA/IIC, Zona Andina, Lima
Tuomisto H, Poulsen AD (1996) Influence of edaphic specialization on pteridophyte distribution in neotropical rain forests. J Biogeogr 23:283–293
Tuomisto H, Poulsen AD (2000) Pteridophyte diversity and species composition in four Amazonian rain forests. J Veget Sci 11:383–396
Tuomisto H, Ruokolainen K, Kalliola R, Linna A, Danjoy W, Rodriguez Z (1995) Dissecting Amazonian biodiversity. Science 269:63–66
Tuomisto H, Poulsen AD, Ruokolainen K, Moran RC, Quintana C, Celi J, Cañas G (2003a) Linking floristic patterns with soil heterogeneity and satellite imagery in Ecuadorian Amazonia. Ecol Appl 13:352–371
Tuomisto H, Ruokolainen K, Aguilar M, Sarmiento A (2003b) Floristic patterns along a 43-km-long transect in an Amazonian rain forest. J Ecol 91:743–756
Tuomisto H, Ruokolainen K, Yli-Halla M (2003c) Dispersal, environment, and floristic variation of western Amazonian forests. Science 299:241–244
Udvardy MDF (1975) A classification of the biogeographical provinces of the world. IUCN Occasional Paper No. 18. IUCN, Gland
UNEP-WCMC (2006) Nationally designated protected areas database (prototype). UNEP-WCMC Protected Areas Programme, URL: http://www.unep-wcmc.org/protected_areas/data/nat2.htm. Queried in May 2006
van der Hammen T, Hooghiemstra H (2000) Neogene and Quaternary history of vegetation, climate, and plant diversity in Amazonia. Quatern Sci Rev 19:725–742
Vanzolini PE, Williams EE (1970) South American anoles: the geographic differentiation and evolution of the Anolis chrysolepis species group (Sauria, Iguanidae). Arq Zool São Paulo 19:1–298
Veríssimo A, Cochrane MA, Souza Jr C (2002a) National forests in the Amazon. Science 297:1478
Veríssimo A, Cochrane MA, Souza Jr C, Salomão R (2002b) Priority areas for establishing national forests in the Brazilian Amazon. Conserv Ecol 6(1):4, [online] URL: http://www.consecol.org/vol6/iss1/art4
Vormisto J, Phillips OL, Ruokolainen K, Tuomisto H, Vásquez R (2000) A comparison of fine-scale distribution patterns of four plant groups in an Amazonian rainforest. Ecography 23:349–359
Vormisto J, Svenning J-C, Hall P, Balslev H (2004) Diversity and dominance in palm (Arecaceae) communities in terra firme forests in the western Amazon basin. J Ecol 92:577–588
Voss RS, Emmons LH (1996) Mammalian diversity in Neotropical lowland rainforests: a preliminary assessment. Bull Am Mus Nat Hist 230:1–115
Wallace AR (1878) Tropical nature and other essays. MacMillan, London
WDPA (2005) World Database of Protected Areas. UNEP-WCMC, World Comission on Protected Areas (WCPA) and World Database on Protected Areas Consortium. Available at: http://sea.unep-wcmc.org/wdbpa/search.cfm?.Query date:28/01/05
Wesselingh FP (2006) Molluscs from the Miocene Pebas formation of Peruvian and Colombian Amazonia. Scripta Geol 133:19–290
Wesselingh FP, Räsänen ME, Irion G, Vonhof HB, Kaandorp R, Renema W, Romero Pittman L, Gingras M (2002) Lake Pebas: a palaeoecological reconstruction of a Miocene, long-lived lake complex in western Amazonia. Cainozoic Res 1:35–81
Wetterberg GB, Jorge Pádua MT, de Castro CS, de Vasconcellos JMC (1976) Uma análise de prioridades em conservação da natureza na Amazônia. Série Técnica No. 8. PNUD/FAO/IBDF/BRA - 45, Brasília
Wetterberg GB, Prance GT, Lovejoy TE (1981) Conservation progress in Amazonia: a structural review. Parks 6:5–10
Whitfield J (2003) The law of the jungle. Nature 421:8–9
Wilf P, Rubén Cuneo N, Johnson KR, Hicks JF, Wing SL, Obradovich JD (2003) High plant diversity in Eocene South America: evidence from Patagonia. Science 300:122–125
Williams PH, Prance GT, Humphries CJ, Edwards KS (1996) Promise and problems in applying quantitative complementary areas for representing the diversity of some Neotropical plants (families Dichapetalaceae, Lecythidaceae, Caryocaraceae, Chrysobalanaceae and Proteaceae). Biol J Linn Soc 58:125–157
Workshop 90 (1991) Biological priorities for conservation in Amazonia. Map, scale 1:5,000,000. Conservation International, Washington, DC
WWF (2006) The Amazon Region Protected Areas Program (ARPA). Available at www.worldwildlife.org/wildplaces/amazon/projects/arpa.cfm#factsheets
WWF-Brasil (2006) Programa Áreas Protegidas da Amazônia (Arpa). Available at: www.wwf.org.br/wwf/opencms/site/list_subchannels.jsp?channelId=591&parentChannelId=600
Zamora JC (1996) Las regiones ecologicas del Perú. In: Rodríguez LO (ed) Diversidad Biologica del Perú: Zonas Proritarias para su Conservación. Proyecto FANPE GTZ-INRENA, Lima, pp 137–141
Zorn P, Quirouette J (2002) Towards the design of a core protected areas network in the Eastern Georgian Bay Region. In: Bondrup-Nielsen S, Munro N, Nelson G, Willison JHM, Herman TB, Eagles P (eds) Managing protected areas in a changing world. Proceedings of the fourth international conference of science and the management of the protected areas. SAMPAA, Canada, pp 622–638
Acknowledgements
We thank Matti Leisti who laid the foundation to this work in his Master’s thesis and Tuuli Toivonen for preparing Fig. 1. We also gratefully acknowledge constructive comments on an earlier version of the manuscript by Wilfried Morawetz and an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulman, L., Ruokolainen, K., Junikka, L. et al. Amazonian biodiversity and protected areas: do they meet?. Biodivers Conserv 16, 3011–3051 (2007). https://doi.org/10.1007/s10531-007-9158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-007-9158-6