Abstract
Niche conservatism explains biological invasions worldwide. However, a plethora of ecological processes may lead invasive species to occupy environments that are different from those found within native ranges. Here, we assess the potential invadable areas of the world’s most pervasive invasive amphibians: the cane toad, Rhinella marina + R. horribilis, and the North American bullfrog, Lithobates catesbeianus. The uncontrolled spread of such voracious, large-bodied, and disease-tolerant anurans has been documented to impact native faunas worldwide. To disentangle their invasion-related niche dynamics, we compared the predictive ability and distributional forecasts of ecological niche models calibrated with information from native, invaded and pooled (native + invaded) ranges. We found that including occurrences from invaded ranges improved model accuracy for both studied species. Non-native occurrences also accounted for 54% and 61% increase in the total area of potential distribution of the cane toad and bullfrog, respectively. Besides, the latter species occupied locations with climatic conditions that are more extreme than those found within its native range. Our results indicate that the occupancy of environments different from those found in native ranges increases the overall potential distribution of the studied invasive anuran species. Therefore, climate information on native ranges alone is insufficient to explain and anticipate the distributional patterns of invasion of cane toads and bullfrogs, underestimating predictions of potential invadable distribution. Moreover, such an observed expansion of realized niches towards occupancy of climates not found within native ranges also has clear implications for invasion risk assessments based on climate modelling worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The origins and mechanisms of the spread of non-native species have long interested ecologists. The past couple of decades, however, have experienced a renewed interest in the general causes of biological invasion from the geographical perspective of species range dynamics and climatic niches shifts (Broennimann et al. 2012; Guisan et al. 2014; Sales et al. 2017). The tendency of several invasive species to retain their fundamental climatic niches (i.e., niche conservatism hypothesis; Wiens and Graham 2005; Peterson 2011), suggests that climate-related evolutionary constraints pose long-term and stable constraints to species geographic distributions (Peterson 2011; Pyron et al. 2015), which may be preserved during biological invasions.
Occupancy of environments that are different from native ranges, however, can arise from different processes, such as competitive release, artificial selection or even the truncated nature of the realized niche itself (Guisan et al., 2014). Interaction with superior competitors, for example, can prevent the occupancy of potentially suitable regions (Soberón and Peterson 2005). Therefore, the absence of competitive exclusion on invaded ranges can cause an observed expansion of realized niches (Bolnick et al. 2010). Niche expansion can also be the outcome of eco-evolutionary dynamics that drive changes in species’ fundamental niches (Tingley et al. 2014), a process that usually does not happen within the timespan of anthropogenic biological invasions (Peterson 2011). Furthermore, domestication of wild species and the selection of individuals possessing traits on the extremes of phenotype distributions may also expand observed realized niches (Yanar et al. 2019). Regardless of the mechanism, not accounting for potential niche shifts during biological invasions often leads to underestimated invasion risk assessments (Jiménez-Valverde et al. 2011).
In this work, we compared the niche dynamics related to the invasion of two voracious, large-bodied, and disease-tolerant amphibians: the cane toad, the species complex formed by Rhinella marina (Linnaeus, 1758) + R. horribilis (Wiegmann, 1833) and the North American bullfrog (hereafter, bullfrog), Lithobates catesbeianus (Shaw, 1802). These species are included among the most pervasive invaders among all taxa (Lowe et al. 2000), due to the well-documented impact they pose on native faunas (Nori et al. 2011; Barbosa et al. 2017). Effects of cane toads and bullfrogs on native faunas span from increased predation and competitive exclusion (e.g., Snow and Witmer 2010), interference with the communication of other frog species (Both and Grant 2012; Forti et al. 2017), and induced morpho-physiological change of native predators (Phillips and Shine 2006a, b). In addition, their invasion has been associated with the co-introduction of lethal pathogens, such as chytrid fungus and ranavirus (Schloegel et al. 2009; O’Hanlon et al. 2018; Brunner et al. 2019), which have been linked to amphibian population declines and extinctions around the world (Scheele et al. 2019, 2020).
To understand the mechanisms that underlie the biological invasions of the cane toad and the bullfrog, we disentangled potential niche dynamics, comparing the environment occupied in native and invaded ranges, while mapping invadable areas. Specifically, we test for (i) differences in the niches of native vs non-native populations, while also (ii) looking for their mechanistic drivers. Also, we evaluate (iii) if models calibrated with native occurrences can accurately predict non-native occurrences, and (iv) the effect of including non-native records in models of species’ potential distribution. Using such an approach, we aim to bring light not only to observed patterns but also to the mechanisms that control and facilitate the spread of invasive species.
Methods
Study species
Cane toads (Rhinella horribilis + R. marina) belongs to the family Bufonidae and are large-sized (snout-vent length of adult females ~ 18 cm), lowland nocturnal species, which occur from sea level up to 1000 m of elevation. Cane toads are the most widely distributed and abundant New World amphibians around the world (Lever 2001), whose native populations are essentially Neotropical, occurring from Mexico to Brazil (Frost 2021). Non-native populations can be found in the Caribbean and Pacific Islands and southeastern Asia and Australia (Mayer et al. 2015; Pikacha et al. 2015; Tingley et al. 2017; Fig. 1a). Cane toads’ generalist diet includes invertebrates and small vertebrates (Meshaka Jr and Powell 2000; Heise-Pavlov and Longway 2011; Pamintuan and Starr 2016), with recorded cannibalism in experimental conditions (Crossland et al. 2011). Reproductive rates are high and clutch sizes comprise up to 30,000 eggs (Zug and Zug 1979; Lever 2001).
Cane toads (upper panel) and bullfrogs (lower panel) native and non-native distribution around the world. Dark-green-colored silhouettes indicate occurrences within species’ native distribution, defined according to polygons of extent of occurrence provided by the International Union for the Conservation of Nature, while pale-pink silhouettes represent non-native occurrences. This figure represents a subset of the total occurrence dataset, to differentiate global invasion patterns
Recent evidence suggests the existence of two distinct species (Rhinella horribilis and R. marina) throughout the geographical distribution originally attributed to Rhinella marina (Acevedo et al. 2016). Such taxonomic rearrangement hinders the precise definition of the origin of translocated populations. Furthermore, all the literature on the recently revalidated R. horribilis can be found under R. marina so that it is not possible, using the methods we have in hand, to differentiate which populations were used for each translocation event. Therefore, we consider the monophyletic Rhinella marina + R. horribilis species complex as a single lineage of cane toads. We caution that our methods and results must be evaluated acknowledging that we are referring to this species complex.
Bullfrogs (Lithobates catesbeianus) belong to the family Ranidae and are also large-sized and nocturnal species (snout-vent length of adult females ~ 16 cm; Howard 1981), considered the largest frog in North America and among the largest true frogs in the world (D’Amore 2012). Native bullfrog populations occupy vast areas of the Nearctic region, being recorded in the eastern United States, Canada, and Mexico (Fig. 1b). Non-native populations are also found worldwide once the species was introduced in all continents except Antarctica (D’Amore 2012). Bullfrogs’ feeding habits are generalist and opportunistic; most of the diet is composed of invertebrates and small vertebrates (Simon and Toft 1991; Jacowski and Orchard 2013), including other frogs and cannibalistic behaviour (Stuart and Painter, 1993; Toledo et al. 2007; Leivas et al. 2012). Offspring production is also abundant, where females can lay more than 20,000 eggs per clutch (Howard 1978).
Data collection
We collected geo-referenced occurrence data for cane toads (R. marina + R. horribilis species complex) and bullfrogs (L. catesbeianus) from different online repositories, namely: the Global Biodiversity Information Facility (GBIF; www.gbif.org), the VertNet (www.vertnet.org), the Biodiversity Information Serving Our Nation (BISON; www.bison.usgs.ornl.gov), the Berkeley Ecoinformatics Engine (Ecoengine; www.ecoengine.berkeley.edu), and the iNaturalist (www.inaturalist.org). Records were downloaded (date of search: February 3rd 2020, no DOI was generated) using the function occ(), then collapsed into the same string using the function fixnames(), both from the R package spocc (Scott et al. 2016). Additional data for L. catesbeianus were extracted from Both et al. (2011) and Hanselmann et al. (2004). All geographic coordinates positioned exactly in centroids of political polygons were ignored, as well as duplicates, incomplete coordinates, and those that were georeferenced to museum locations. Data cleaning was based on the suite of coord_ functions from R package scrubr (Chamberlain 2016). In addition, all records were individually quality-checked, according to available literature (Hanselmann et al. 2004; Both et al. 2011) and to our own experience.
Species’ native ranges were defined according to polygons of extent of occurrence considered “native” by the International Union for the Conservation of Nature [henceforth, IUCN range maps] (IUCN 2020). Such polygons are useful to identify current distributional limits of species (Sales et al. 2017), as well as to inform conservation planning, wildlife management and monitoring (Rodrigues et al. 2006). To account for border uncertainty, we added a 200 km buffer to IUCN range maps. This 200 km threshold is above the known dispersal abilities of most amphibians and was chosen as a conservative attempt to distinguish invasion from climate niche tracking (Urban 2020). By doing so, invasion is considered here as a process in which a species occupies territories that are widely distant from native ranges, most likely intentionally introduced or otherwise anthropogenically-driven. In contrast, climate tracking occurs when a species colonizes areas that are adjacent to the species’ native distribution and that became available due to recent environmental change, as defined by Urban (2020). This buffer around the species’ current extent of occurrence, therefore, intends to accommodate the uncertainty related to potential recent range expansion due to climate niche tracking. All occurrence records located outside this expanded polygon were considered non-native. To minimize problems related to spatial autocorrelation (Broennimann et al. 2012; Strubbe et al. 2015), we randomly removed occurrences less than 0.5 degrees from each other from the full dataset (both native and non-native ranges), using the function ecospat.occ.desaggregation() from R package ecospat (Broennimann et al., 2016). A total of 495 occurrence records were included for the cane toad (231 native and 264 non-native), while the bullfrog dataset comprised 1164 records (830 native and 334 non-native) (Fig. 1, Supporting occurrences Ocorr_supp.csv).
Ecological niche models
We predicted the potential invadable areas for cane toads and bullfrogs using climate-calibrated ecological niche models (Broennimann and Guisan 2008; Jiménez-Valverde et al. 2011). Climate information for the contemporary conditions encompassed a time range of 30 years (1960–1990) and was downloaded from WorldClim version 1.4 (www.worldclim.org/data/v1.4), using the function getData() from the raster R package (Hijmans and van Etten 2016), as 10′ resolution gridded files. Here, we used bioclimatic variables, which are derived from monthly temperature and precipitation values but adjusted to express situations that likely influence species’ ranges (Hijmans et al. 2005), such as annual trends, seasonality and extreme climatic events. Prior to calibrating ecological niche models, we tested for multicollinearity among predictors using a stepwise approach (Naimi et al. 2014). At first, we calculated the variance inflation factor (VIF) for all variables. Then, a pairwise correlation matrix identified highly correlated variable pairs. Correlation values above 0.7 were highlighted and the variable with the highest VIF was then excluded from the pair, using the function vifstep() from usdm R package (Naimi et al. 2014). This procedure was repeated until all the strongly correlated variables were excluded, which resulted in eight predictors for bullfrogs’ models (BIO2, BIO7, BIO8, BIO9, BIO10, BIO13, BIO15, BIO18) and nine for cane toads (BIO2, BIO3, BIO4, BIO8, BIO13, BIO14, BIO15, BIO18, BIO19).
We estimated potential invadable areas using ecological niche models (ENMs) calibrated with climate data associated with the species’ occurrence data, following acknowledged standards (Araújo et al. 2019). Species-specific study areas were built by limiting our background to a bounding box of species environmental points, plus an additional 10 degrees to each bound, thus only encompassing regions that are probably accessible to species via migration (Barve et al. 2011). Environmental layers were, then, cropped to adjust to the study extent and 10,000 background/pseudo-absence points were sampled one per cell, with no replacement (Barbet-Massin et al. 2012). To assess the environmental conditions associated with species’ occurrences, we used Maxlike, a widely used presence-only method known to be well-suited in estimating species’ potential distribution (Jiménez-Valverde et al. 2011). The output parameters of Maxlike have formal relationships with the classical Maxent software (Fitzpatrick et al. 2013; Merow and Silander Jr, 2014), plus the added benefit of directly estimating the probability of occurrence using presence-only data (Royle et al. 2012). We chose to use such a presence-only method because invasion risk assessments must not rely on true absence information, which is often biased by sampling effort (Guillera-Arroita et al. 2015).
Model accuracy was evaluated using a repeated sub-sampling procedure where 25% of occurrence records were randomly selected for model validation and the remaining 75% were used for model training. This procedure was repeated 30 times for each modelling method per species. Two metrics were used to assess model accuracy: (i) the true skill statistic (TSS) (Allouche et al. 2006), and (ii) the area under the receiver operating characteristic curve (AUC) (Fielding and Bell 1997). While AUC is a threshold-independent metric, measuring TSS relies on selecting an environmental threshold where presences and absences are discriminated from each other. By using multiple evaluation metrics, we also explored the sensitivity of our results to the uncertainty that arises from threshold-based metrics (Leroy et al. 2018). Then, we selected a threshold that maximized TSS (Liu et al. 2005) to convert continuous predictions of climate suitability into binary maps. Finally, we used an ensemble forecasting procedure to generate a consensus prediction for each species by combining individual model outcomes, by weighting averaged suitability predictions by the accuracy of the model.
To test for the effect of the inclusion of non-native occurrences on model outcome, we created different combinations of dataset sub-sampling, namely (i) Native-native models trained and validated with occurrences from native ranges; (ii) Native-invaded models trained with native occurrences and validated with non-native (invaded) occurrences; and (iii) Pooled models trained and validated with both native and non-native (invaded) occurrences to predict the global potential distribution. Using native ranges as the reference, we, thus, consider that environments similar to those found within species’ native distributions are climate analogs. Likewise, no-analog climates are, here, considered those conditions not found within species’ native distributions. We caution that this definition may differ from those defined in previous work, in which no-analog climates referred to conditions found in different times (Williams et al. 2007), especially future climate change scenarios (Fitzpatrick and Hargrove, 2009). Therefore, we rely on the conditions that currently exist in geographic space and within which species occur, were detected, and are attributed to the native range limits, as defined by the IUCN, as a baseline for the definition of analog.
Occupancy of no-analog environments was defined by areas whose suitability depends on the inclusion of non-native occurrences, i.e., are not considered suitable by native-based models. It was calculated as follows:
where analog indicates the number of cells predicted as suitable from native-based models, and noanalog indicates cells that are only predicted as suitable when non-native occurrences are included. Results referred to total potential distribution are given in km2, considering a WGS84 projection.
Niche equivalence and similarity tests
To test for the equivalence and similarity of the niches of native vs non-native populations, we extracted the climate information referred to species occurrences. In Broennimman et al. (2012), the authors compared several ordination methods for quantifying niche overlap and found that, among the methods they evaluated, the PCA-env calibrated on the pooled background most accurately estimated the simulated niche overlap. Therefore, we used the PCA-env, via function dudi.pca() from R package ecospat, to reduce the dimensionality of environmental data (all 19 bioclimatic predictors) from the pooled background, i.e., native plus non-native ranges (a definition also based on IUCN polygons of extent of occurrence classified as “native”), to the first two axes of the principal component analysis (PCA), which accounted for 75% of the variation in the data. Then, we compared the climate spaces of areas that are occupied by populations (native vs non-native) to the pooled background of environmental conditions, using the functions ecospat.grid.clim.dyn(), ecospat.niche.equivalency.test(), ecospat.niche.similarity.test(), also from the ecospat R package. Niche overlap between native and non-native ranges was measured by Schoener’s D index, which ranges from no overlap, 0, to complete overlap, 1 (Schoener 1974; Warren et al. 2008).
Observed niche overlap between native and non-native ranges was then compared to random values, using tests of niche equivalence and similarity. The niche equivalence method tests if climatic niches (from native and non-native populations) are indistinguishable from each other (Graham et al. 2004). The rejection of the null hypothesis, therefore, indicates that native and non-native niches are not identical (Broennimann et al. 2012). On the other hand, the niche similarity test asks whether models calibrated on the native range predict invasion occurrences better than expected by chance (Warren et al. 2008). The null hypothesis in niche similarity tests, thus, refers to niche resemblance, so that its rejection indicates that niches are less similar than expected by chance. We caution that the null hypothesis for niche equivalence is much easier to reject than for niche similarity. We ran 1000 iterations for null hypotheses tests and p-values were computed.
Mechanistic links to possible causes of observed differences between native and non-native niches were incorporated by disentangling niche dynamics into the processes of stability, unfilling and expansion (Guisan et al. 2014). Niche stability is observed whenever invasion mostly occurs towards regions that are climatically similar to those from the native range. However, niche unfilling is expected if environmental conditions from native ranges are available, but unoccupied in the non-native environment (Guisan et al. 2014). Finally, observed niche expansion results from occupancy of no-analog climatic conditions.
Thermal and hydric stress tolerance
We characterized the environment within which species are found by assessing the climatic conditions associated with species’ occurrence records. To do so, we extracted the values of mean annual temperature (BIO1) and mean annual precipitation (BIO12) associated with each occurrence record from climate raster files referring to current conditions (Supporting occurrences Ocorr_supp.csv), obtained from WorldClim version 1.4, also used in the previous analysis. The selection of BIO1 and BIO12 to assess species climatic tolerance is due to their known relationship to the natural history of species (Rödder et al. 2009) as a proxy of thermal and hydric stress. We reiterate that such values are not equivalent to physiological tolerance, which can only be rigorously calculated with manipulative experiments but are conditions that species experience and likely tolerate in the wild. As with any presence-only study at the global scale, however, we were not able to define whether each population associated with each occurrence record is in equilibrium with their surrounding environment, so there is the possibility of eventual occurrence in unsuitable or marginal habitats. Therefore, we recommend that the results of this section are interpreted with caution and as an overview. Thermal and hydric stress tolerance differences among groups (native vs non-native) were tested using a generalized linear model. Residuals and assumptions were checked via graphical analysis.
Results
Including occurrences from invaded ranges improved the accuracy of ecological niche models for both studied species (Table 1). Ecological niche models calibrated across pooled ranges (combining occurrence from both native and invaded ranges, Table 1, column Pooled) resulted in models with higher predictive accuracy. Native-based models predicted relatively well the occurrences from native ranges (Table 1, item Native-native), but had poor performance predicting occurrences from invaded ranges (Table 1, item Native-invaded). The inclusion of non-native occurrences in the modelled ecological niche increased the potential distribution of the cane toad by 51% and by 63% for the bullfrog (Table S1, Fig. 2). In other words, occupancy of climates that are currently absent in native ranges increased potential distribution by > 107 Km2 for both species.
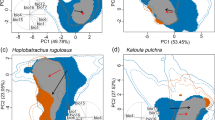
Climate suitability and potentially invadable areas for cane toads, Rhinella marina + R. horribilis, and bullfrogs, Lithobates catesbeianus. In this figure, green areas are considered suitable for each species according to distribution models calibrated with occurrences from native ranges only. Pale-pink colours indicate areas that are only considered available when including occurrences from invaded ranges. Empty yellow polygons indicate species’ native distribution, according to the International Union for the Conservation of Nature (IUCN 2020)
Climatic niches from native and non-native populations were different (the niche equivalence hypothesis) for both species (Table 1). However, we also found evidence that populations from non-native ranges occupy environments that are more similar than expected by chance to conditions from its native range–the niche similarity hypothesis (Table 2), which is corroborated by the high stability (about 94% for both species) observed between native and non-native ranges. Observed values of niche expansion accounted for only 6% and 8% for the cane toad and the bullfrog, respectively. Niche unfilling was relatively low, accounting for less than 1% for the cane toad but up to 8% for the bullfrog.
The cane toad occupied mostly warm (Mean annual temperature = 26 ± 4 °C) environments, with a vast array of precipitation conditions, from near zero to almost 800 mm3 per year (Fig. 3). We observed no differences in temperature, but the environments invaded by the cane toad were drier than those from native ranges (BIO12|Native mean = 2121 ± 63 mm3; Invaded mean = 1475 ± 62 mm3; F-stat = 50.1, p-value < 0.001). The bullfrog, on the other hand, mostly occupied temperate environments (Mean annual temperature = 14 ± 3 °C), and invaded environments with more extreme climatic conditions (considering precipitation and temperature) than those found within native ranges (Fig. 3). Within its native distribution, the bullfrog occupies environments whose precipitation ranges from zero to < 300 mm3 per year, while non-native occurrences were found in more humid environments (> 400 mm3 per year). Environments invaded by the bullfrog were warmer than those from native ranges (BIO1|Native mean = 12.7 ± 2 °C; Invaded mean = 15.2 ± 3 °C; F-stat = 51.5, p-value < 0.001), although some extreme occurrences were found in environments whose temperatures frequently go above -10 °C. The bullfrog also invaded environments that are more humid than those from native ranges (BIO12|Native mean = 959 ± 16 mm3; Invaded mean = 1222 ± 25 mm3; F-stat = 82, p-value < 0.001).
Amplitude of environmental conditions occupied by bullfrogs (green) and cane toads (brown). Mean and extreme values of precipitation (mm × 1000) and temperature (°C) are shown in invaded and native areas, defined according to the International Union for the Conservation of Nature (IUCN 2020)
Discussion
Anticipating invasion risk is crucial to prevent the harmful effects that exotic species can impose on ecosystems. Here, we assessed the invasion potential of two of the world’s worst invasive species: cane toads, Rhinella marina + R. horribilis, and bullfrogs, Lithobates catesbeianus. These amphibians are large-bodied, prolific breeders, good competitors in all developmental stages and opportunistic feeders (Liu et al. 2015; Heise-Pavlov and Longway 2011; Isaacs and Hoyos 2010), who negatively impact native faunas worldwide (Werner et al. 1995; Wu et al. 2005). Disentangling niche dynamics, we observed evidence for a relatively small niche expansion for both species. However, the inclusion of non-native occurrences on ecological niche models improved predictive accuracy and increased estimates of potential distribution for both species. Overall, our results indicate that climatic information from native ranges is insufficient to explain the current invasive distribution of the studied species and that native-based climate modelling may lead to underestimated invasion potential.
We found that cane toads and bullfrogs invade territories with climatic conditions that are not available within their native distributions. Bullfrogs, for example, were recorded in conditions harsher than those from its native range, effectively increasing by 11 °C the thermal amplitude of environments occupied. The occupancy of harsher climates, which are no-analogs to those found within native ranges, can result from eco-evolutionary changes to fundamental niches (Guisan et al. 2014) or simply from the occupancy of suitable but inaccessible areas (Soberón and Peterson 2005; Soberón 2007; Peterson et al. 1999), due to dispersal limitation or competitive exclusion, for example. Regardless of the mechanism, climatic information on native ranges was not enough to explain patterns of invasion, even though niches were statistically similar. Models trained with occurrence data from the native ranges underestimated the invasion potential of areas that are currently invaded. Native-based models were, therefore, unable to accurately predict invasion potential, which has straightforward implications for invasive risk assessments based on ecological niche models.
We also found that cane toads and bullfrogs can potentially colonize vast areas of the globe. However, co-occurrence between them is unlikely because cane toads occur mostly in warm and humid areas, while bullfrogs preferentially occupy drier and colder regions. Cane toads’ maps of potential distribution suggest high climatic suitability across the African Congo Basin, the Brazilian Atlantic Forest, the east coast of United States, in Florida, in addition to Sundaland, Phillippines and the Wallacea region in southeastern Asia. If intentional or inadvertent introduction by humans allows colonization of those territories, cane toads may outcompete native counterparts and decimate predators who try to consume them (Phillips and Shine 2006a, b). Such interaction with native faunas would likely lead to an overall reduction in richness and diversity, mirroring impacts already reported, for example, in Australia (Crossland et al. 2009; Mayer et al. 2015). Failed eradication protocols and the continued spread of cane toads in the Australian continent (Tingley et al. 2017) are, thus, suggestive of the fate of native biodiversity elsewhere under the introduction of this species.
Likewise, bullfrogs’ suitable areas span across sub-tropical ecosystems in South America, such as the mountainous highlands of the Andes and southern Atlantic Forests, as well as temperate portions of Australia, several scattered zones in Asia, most of the eastern United States and even parts of the Amazon, which could potentially be invaded by bullfrogs. Most of the faunas of these regions have experienced no previous contact with bullfrogs, but elsewhere examples of such novel biotic interactions often led to competitive exclusion, favouring the bullfrog (e.g., Rana boylii and Pseudacris regilla, Kupferberg 1997; R. aurora, Lawler et al. 1999).
The large areas predicted as suitable for cane toad and bullfrog raise another conservation flag: the potential co-introduction of emerging infectious diseases, such as those caused by the chytrid fungus, Batrachochytrium dendrobatidis (O’Hanlon et al. 2018) and ranaviruses (Ruggeri et al. 2019). There is mounting evidence that bullfrogs co-occur with such pathogens (Rödder et al. 2013), and tolerate their presence (Ribeiro et al. 2019), thus likely acting as vectors for their transmission (Rödder et al. 2013; O’Hanlon et al. 2018). Infectious diseases, especially those caused by the chytrid fungus, were responsible for the decline of hundreds of amphibian populations in the last half-century, representing the greatest loss of biodiversity ever related to a single pathogen (Scheele et al. 2019; 2020).
In conclusion, we found that climatic information on native ranges of cane toads and bullfrogs was not enough to explain their distributional patterns of invasion. The occupancy of climates not found across native ranges increased the potential invadable areas of both species. The high invasion potential of such voracious predators, superb competitors, and lethal disease vectors highlights the urgency of strategies to mitigate their impact on native faunas. Further, we caution that invasive risk assessments based on information from species native ranges, alone, are probably underestimated and unrealistic projections of impact. Therefore, we suggest the inclusion of environmental information from non-native occurrences on invasion risk assessments based on climate modelling.
Availability of data, material, and code
The climate data used in this work can be downloaded at www.ipcc-data.org and in http://worldclim.org/version2. All occurrence records and an example of the R codes used in this work are available as Supporting information.
References
Acevedo AA, Lampo M, Cipriani R (2016) The cane or marine toad, Rhinella marina (Anura, Bufonidae): two genetically and morphologically distinct species. Zootaxa 4103:574–586
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Araújo MB, Anderson RP, Barbosa AM, Beale CM, Dormann CF, Early R, Garcia RA, Guisan A, Maiorano L, Naimi B, O’Hara RB, Zimmermann NE, Rahbek C (2019) Standards for distribution models in biodiversity assessments. Sci Adv 5:eaat4858
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338
Barbosa FG, Both C, Araújo MB (2017) Invasive American bullfrogs and African clawed frogs in South America: high suitability of occurrence in biodiversity hotspots. Zool Stud 56:1–10
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, Soberón J, Villalobos F (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell 222:1810–1819
Both C, Grant T (2012) Biological invasions and the acoustic niche: the effect of bullfrog calls on the acoustic signals of white-banded tree frogs. Biol Lett 8:714–716. https://doi.org/10.1098/rsbl.2012.0412
Both C, Santos-Jr LR, A, (2011) Widespread occurrence of the American bullfrog, Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae), in Brazil. South Am J Herpetol 6:127–134
Bradley BA, Early R, Sorte CJ (2015) Space to invade? Comparative range infilling and potential range of invasive and native plants. Global Ecol Biogeogr 24:348–359
Broennimann O, Di Cola V, Guisan A (2016) ecospat: Spatial ecology miscellaneous methods. R package version 2(1):1
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin MJ, Randin C, Zimmermann NE, Graham CH, Guisan A (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21:481–497
Broennimann O, Guisan A (2008) Predicting current and future biological invasions: both native and invaded ranges matter. Biol Lett 4:585–589
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson A, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Brunner JL, Olson AD, Rice JG, Meiners SE, Le Sage MJ, Cundiff JA, Goldberg CS, Pessier AP (2019) Ranavirus infection dynamics and shedding in American bullfrogs: consequences for spread and detection in trade. Dis Aquat Organ 135:135–150
Chamberlain S (2016) sCrubr: clean biological occurrence records. R Package Version 0.1, 1, 162
Conner JK (2003) Artificial selection: a powerful tool for ecologists. Ecology 84:1650–1660
Crossland MR, Alford RA, Shine R (2009) Impact of the invasive cane toad (Bufo marinus) on an Australian frog (Opisthodon ornatus) depends on minor variation in reproductive timing. Oecol 158:625–632
Crossland MR, Hearnden MN, Pizzatto L, Alford RA, Shine R (2011) Why be a cannibal? The benefits to cane toad, Rhinella marina [=Bufo marinus], tadpoles of consuming conspecific eggs. Anim Behav 82:775–782. https://doi.org/10.1016/j.anbehav.2011.07.009
D’Amore A (2012) Rana (Lithobates) catesbeiana Shaw (American bullfrog). In: Francis R (ed) A handbook of global freshwater invasive species. Earthscan, New York, pp 321–330
Elith JL, JR, (2009) Species distribution models: ecological explanation and prediction across space and time. Ann Rev Ecol Evol Syst 40:677–697
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Env Conserv 24:38–49
Fitzpatrick MC, Gotelli NJ, Ellison AM (2013) MaxEnt versus MaxLike: empirical comparisons with ant species distributions. Ecosphere 4:1–15
Fitzpatrick MC, Hargrove WW (2009) The projection of species distribution models and the problem of no-analog climate. Biodiv Conserv 18:2255–2261
Forti LR, Becker CG, Tacioli L, Pereira VR, Santos ACFA, Oliveira IS, Haddad CFB, Toledo LF (2017) Perspectives on invasive amphibians in Brazil. PLoS ONE 12:e0184703
Frost DR (2021) Amphibian Species of the World: An Online Reference. Version 6.0. http://research.amnh.org/herpetology/amphibia/index.html. Accessed 21 February 2021
Graham CH, Ron SR, Santos JC, Schneider CJ, Moritz C (2004) Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58:1781–1793
Guillera-Arroita G, Lahoz-Monfort JJ, Elith J, Gordon A, Kujala H, Lentini PE, McCarthy MA, Tingley R, Wintle B (2015) Is my species distribution model fit for purpose? Matching data and models to applications. Glob Ecol Biogeogr 24:276–292
Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C (2014) Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29:260–269
Hanselmann R, Rodrıguez A, Lampo M, Fajardo-Ramos L, Aguirre AA, Kilpatrick AM, Rodrıguez JP, Daszak P (2004) Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol Conserv 120:115–119
Heise-Pavlov SR, Longway LJ (2011) Diet and dietary selectivity of Cane Toads (Rhinella marina) in restoration sites: a case study in Far North Queensland, Australia. Ecol Manag Restor 12:230–233
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hijmans RJ, van Etten J (2016) raster: Geographic Data Analysis and Modeling. R package version 2.5–8
Howard RD (1978) The influence of male-defended oviposition sites on early embryo mortality in bullfrogs. Ecology 59:789–798
Howard RD (1981) Sexual size dimorphism in bullfrogs. Ecology 62:303–310
Isaacs P, Hoyos JM (2010) Diet of the cane toad in different vegetation covers in the productive systems of the Colombian coffee region. South Am J Herp 5:45–50
IUCN (2020) The IUCN Red List of Threatened Species. Version 2020–1. https://www.iucnredlist.org. Downloaded on 19 March 2020
Jacowski K, Orchard SA (2013) Stomach contents from invasive American bullfrogs Rana catesbeiana (= Lithobates catesbeianus) on southern Vancouver Island, British Columbia, Canada. NeoBiota 16:17–37
Jiménez-Valverde A, Peterson AT, Soberón J, Overton J, Aragón P, Lobo JM (2011) Use of niche models in invasive species risk assessments. Biol Inv 13:2785–2797
Kupferberg SJ (1997) Bullfrog (Rana catesbeiana) invasion of a California river: the role of larval competition. Ecology 78:1736–1751
Lawler SP, Dritz D, Strange T, Holyoak M (1999) Effects of introduced mosquitofish and bullfrogs on the threatened California red-legged frog. Conserv Biol 13:613–622. https://doi.org/10.1046/j.1523-1739.1999.98075.x
Leivas PT, Leivas FW, Moura MO (2012) Diet and trophic niche of Lithobates catesbeianus (Amphibia: Anura). Zoologia 29:405–412
Leroy B, Delsol R, Hugueny B, Meynard CN, Barhoumi C, Barbet-Massin M, Bellard C (2018) Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J Biogeogr 45:1994–2002
Lever C (2001) The cane toad: the history and ecology of a successful colonist. Westbury Academic & Scientific Pub.
Liu C, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28:385–393
Liu X, Luo Y, Chen J, Guo Y, Bai C, Li Y (2015) Diet and prey selection of the Invasive American bullfrog (Lithobates catesbeianus) in southwestern China. Asian Herpetol Res 6:34–44
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database, vol 12. Invasive Species Specialist Group, Auckland Zealand
Mayer M, Brown GP, Zimmermann B, Greenlees MJ, Shine R (2015) Habitat use of the introduced cane toad (Rhinella marina) and native frog species in tropical Australia. J Trop Ecol 31:531–540
Merow C, Silander JA Jr (2014) A comparison of Maxlike and Maxent for modelling species distributions. Methods Ecol Evol 5:215–225
Meshaka WE Jr, Powell R (2000) Benefits of eating conspecifics: effects of background diet on survival and metamorphosis in the Cuban treefrog (Osteopilus septentrionalis). Copeia 2000:469–474
Naimi B, Hamm NA, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37:191–203
Nori J, Urbina-Cardona JN, Loyola RD, Lescano JN, Leynaud GC (2011) Climate change and American bullfrog invasion: what could we expect in South America? PLoS ONE 6:e25718
O’Hanlon SJ, Rieux A, Farrer RA, Rosa GM, Waldman B, Bataille A, Kosh TA, Murray KA, Brankovics B, Fumagalli M, Martin MD, Wales N, Alvarado-Rybak M, Bates KA, Berger L, Böll S, Brookes L, Clare F, Courtois EA, Cunningham AA, Doherty-Bone TM, Gosh P, Gower DJ, Laurila A, Loyau A, Martel A, Meurling S, Miaud C, Minting P, Pasmans F, Shmeller DS, Schmidt BR, Shelton JMG, Skerrat LF, Smith F, Soto-Azat C, Spagnoletti M, Tessa G, Toledo LF, Valenzuela-Sánchez A, Wombwell E, Zamudio KR, Aanensen DM, James TY, Gilbet MTP, Weldon C, Bosh J, Balloux F, Garner TWJ, Fisher MC (2018) Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360:621–627
Pamintuan PE, Starr CK (2016) Diet of the giant toad, Bufo marinus (Amphibia: Salientia), in a coastal habitat of the Philippines. Trop Agric 93:323–327
Peterson AT, Soberón J, Sánchez-Cordero V (1999) Conservatism of ecological niches in evolutionary time. Science 285:1265–1267
Peterson AT (2011) Ecological niche conservatism: a time-structured review of evidence. J Biogeogr 38:817–827
Phillips BL, Shine R (2006) Allometry and selection in a novel predator-prey system: Australian snakes and the invading cane toad. Oikos 112:122–130. https://doi.org/10.1111/j.0030-1299.2006.13795.x
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc R Soc B 273:1545–1550
Pikacha P, Lavery T, Leung LKP (2015) What factors affect the density of cane toads (Rhinella marina) in the Solomon Islands? Pac Conserv Biol 21:200–207
Pyron RA, Costa GC, Patten MA, Burbink FT (2015) Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biol Rev 90:1248–1262. https://doi.org/10.1111/brv.12154
Ribeiro LP, Carvalho T, Becker CG, Jenkinson TS, Leite DS, James TY, Greenspan SE, Toledo LF (2019) Bullfrog farms release virulent zoospores of the frog-killing fungus into the natural environment. Sci Rep 9:1–10
Rödder D, Schmidtlein S, Veith M, Lötters S (2009) Alien invasive slider turtle in unpredicted habitat: a matter of niche shift or of predictors studied? PLoS ONE 4:e7843
Rödder D, Schulte U, Toledo LF (2013) High environmental niche overlap between the fungus Batrachochytrium dendrobatidis and invasive bullfrogs (Lithobates catesbeianus) enhance the potential of disease transmission in the Americas. North-West J Zool 9:178–184
Rodrigues ASL, Pilgrim JD, Lamoreux JF, Hoffman M, Brooks TM (2006) The value of the IUCN Red List for conservation. Trends Ecol Evol 21:71–76. https://doi.org/10.1016/j.tree.2005.10.010
Royle JA, Chandler RB, Yackulic C, Nickols JD (2012) Likelihood analysis of species occurrence probability from presence-only data for modelling species distributions. Methods Ecol Evol 3:545–554. https://doi.org/10.1111/j.2041-210X.2011.00182.x
Ruggeri J, Ribeiro LP, Pontes MR, Toffolo C, Candido M, Carriero MM, Zanella N, Sousa RLM, Toledo LF (2019) Discovery of wild amphibians infected with Ranavirus in Brazil. J Wildl Dis 55:897–902
Sales LP, Ribeiro BR, Hayward MW, Paglia A, Passamani M, Loyola R (2017) Niche conservatism and the invasive potential of the wild boar. J Anim Ecol 86:1214–1223. https://doi.org/10.1111/1365-2656.12721
Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De La Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosh TA, La Marca H, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J III, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel MO, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–463. https://doi.org/10.1126/science.aav0379
Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De La Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosh TA, La Marca H, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson III J, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel MO, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S (2020). Response to Comment on “Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity”. Science 367:eaay2905
Schloegel LM, Picco AM, Kilpatrick AM, Davies AJ, Hyatt AD, Daszak P (2009) Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol Conserv 142:1420–1426
Schoener TW (1974) Some methods for calculating competition coefficients from resource-utilization spectra. Am Nat 108:332–340
Scott A, Ram K, Hart T, Chamberlain S (2016) Package ‘spocc’: Interface to Species Occurrence Data Sources.
Simon MP, Toft CA (1991) Diet specialization in small vertebrates: mite-eating in frogs. Oikos 61:263–278
Snow NP, Witmer G (2010) American bullfrogs as invasive species: A review of the introduction, subsequent problems, management options, and future directions. Proc Vert Pest Conf 24:86–89
Soberón J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10:1115–1123
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inform 2:1–10
Strubbe D, Beauchard O, Matthysen E (2015) Niche conservatism among non-native vertebrates in Europe and North America. Ecography 38:321–329. https://doi.org/10.1111/ecog.00632
Stuart JN, Painter CW (1993) Rana catesbeiana (bullfrog) cannibalism. Herpetol Rev 24:103
Tingley R, Vallinoto M, Sequeira F, Kearney MR (2014) Realized niche shift during a global biological invasion. Proc Natl Acad Sci USA 111:10233–10238
Tingley R, Ward-Fear G, Schwarzkopf L, Greenlees MJ, Phillips BL, Brown G, Clulow S, Webb J, Capon R, Shine R (2017) New weapons in the Toad Toolkit: a review of methods to control and mitigate the biodiversity impacts of invasive cane toads (Rhinella marina). Q Rev Biol 92:123–149
Toledo LF, Silva RR, Haddad CFB (2007) Anurans as prey: an exploratory analysis and size relationships between predators and their prey. J Zool 271:170–177
Urban MC (2020) Climate-tracking species are not invasive. Nat Clim Change 10:382–384
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Werner EE, Wellborn GA, McPeek MA (1995) Diet composition in postmetamorphic bullfrogs and green frogs: implications for interspecific predation and competition. J Herpetol 29:600–607
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36:519–539. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431
Williams JW, Jackson ST, Kutzbach JE (2007) Projected distributions of novel and disappearing climates by 2011 AD. Proc Natl Acad Sci USA 104:5738–5742
Wu Z, Li Y, Wang Y, Adams MJ (2005) Diet of introduced bullfrogs (Rana catesbeiana): predation on and diet overlap with native frogs on Daishan Island, China. J Herpetol 39:668–674
Yanar M, Erdoğan E, Kumlu M (2019) Thermal tolerance of thirteen popular ornamental fish species. Aquaculture 501:382–386
Zug GR, Zug PB (1979) The marine toad, Bufo marinus: a natural history resume of native populations. Smithsonian Contributions to Zoology.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Finance Code 001). Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2016/25358–3; #2019/18335–5). Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #300896/2016–6; 140874/2019–4).
Author information
Authors and Affiliations
Contributions
LPS: Conceptualization, formal analysis, methodology, writing, discussion, review, and editing. RR: Conceptualization, writing, discussion, review, and editing. LFT: Conceptualization, supervision, discussion, review, and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sales, L.P., Rebouças, R. & Toledo, L.F. Native range climate is insufficient to predict anuran invasive potential. Biol Invasions 23, 2635–2647 (2021). https://doi.org/10.1007/s10530-021-02528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02528-1