Abstract
Islands exhibit disproportionally high biodiversity, however high levels of endemism and simplified food webs make their communities susceptible to invasive species. Introduced goats (Capra hircus), a generalist herbivore, are among the most harmful invasive species on islands. Concern about goat impacts on island communities have resulted in eradication programs, which have been generally implemented without comprehensive evaluation and monitoring. Unintended consequences may follow eradication, as grazing can have complex effects on island food webs. Using whole island manipulations, we evaluate the long-term, community-wide effects of goat herbivory, as well as their subsequent removal, in a system of 16 islands in the Aegean Sea (Greece) located within the Mediterranean biodiversity hotspot. Goat grazing on these islands is a major conservation concern, as these support endemic plant communities that have evolved in low herbivory conditions and lack appropriate defenses. We show that goat introductions lead to significant decreases in vegetation height, percent cover, and biomass but not to immediate plant species loss, as native island endemics are replaced with widespread generalist taxa carried in by the livestock. Additionally, goats contribute to the desertification of islands by initiating a long-term soil loss cycle that continues even after goats are removed; however, remaining soil structure and chemistry are not affected. Island arthropod populations do not appear to be significantly impacted by goat introduction or removal, except for a distinct increase in the order Diptera with goat presence. This study also reaffirms the role of seabirds in providing important marine subsidies, rich in nitrogen and phosphorus, to island food webs. Plant species diversity declines following goat removal, and vegetation cover returns only partially, as further recovery is being prevented by the long-term loss of soil. This suggests that following goat removal, island communities may require additional restoration efforts, including seabird reestablishment and reintroduction of extirpated plant populations, to promote island recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the greatest threats to biodiversity is the worldwide spread of invasive species (Hooper et al. 2012). Numbers of both intentional and accidental exotic species introductions have increased exponentially and have reshaped native communities in many parts of the world, particularly islands (Kolar and Lodge 2001). Myers et al. (2000) show that at least 9 of the 25 proposed biodiversity hotspots consist principally or entirely of islands, highlighting their importance for global biodiversity. Islands host over 20% of the world’s biodiversity despite constituting only 5% of the global terrestrial area (Kier et al. 2009). Exotic species introductions have been shown to have the most profound effects on isolated islands, especially those supporting depauperate biotic communities with a high percentage of endemic taxa (Mueller-Dombois 1981). Island ecosystems are highly susceptible to disturbances because they tend to have simplified trophic webs and high rates of endemism (Courchamp et al. 2003). Insular populations are more susceptible to extinction than mainland species (MacArthur and Wilson 1967; Foufopoulos et al. 2011). Reflecting this susceptibility of island taxa, 90% of known reptile and amphibian extinctions, 93% of avian extinctions, and 81% of mammalian extinctions worldwide, have occurred on islands (Honegger 1981; King 1985; Ceballos and Brown 1995). Many of the most detrimental island invasives are human associates, such as rats (Rattus spp.), cats (Felis catus), rabbits (Oryctolagus cuniculus), and escaped livestock (Bowen and Van Vuren 1997; Nogales et al. 2004; Jones et al. 2008). Feral livestock, including pigs (Sus scrofa) and goats (Capra hircus), tend to excessively remove plant biomass, eliminating primary producers from island food webs, harming the native plant communities, causing soil erosion, and damaging island landscapes (Coblentz 1978).
A key factor facilitating the proliferation of introduced mammalian herbivores on islands is increased food availability due to insular vegetation’s lack of defenses. Most plants have evolved some type of anti-herbivore defenses, the extent of which is often proportional to the risk of browsing (Rhoades 1979). Until the arrival of humans, mammalian herbivores were absent from most small island ecosystems (Atkinson 1989). Due to the energetic costs of resistance in plants, many island taxa lack defenses against herbivory such as chemical deterrents, physical armature, or a tolerance to grazing (Coblentz 1978; Vitousek 1988). This lack of resistance can, in turn, lead to pronounced shifts in the diet preferences of herbivores towards less defended endemic flora, giving more heavily defended invasive plants a competitive advantage (Loope and Scowcroft 1985; Van Vuren and Coblentz 1987). In the Mediterranean, plant species vary greatly in their levels of phenols and preferential grazing occurs on species with the lowest phenolic levels (Massei et al. 2000).
Goats are recognized today as the sixth leading threat to species worldwide because of their impact on vegetation (Bellard et al. 2016). In the early twentieth century, Sir Alfred Russell Wallace noted that, “goats are the greatest of all foes to trees” (Wallace 1911). Domesticated in the dry highlands of western Iran 10,000 years ago (Zeder and Hesse 2000), goats are able to utilize many arid habitats unsuitable for other herbivores. This adaptability arises from the combination of a slow metabolism, an efficient digestive system, low water requirements, high reproduction rates, and a generalist diet (Silanikove 2000; Chynoweth et al. 2013). Goats also have the ability to consume tougher, chemically-defended food, making more vegetation available to them (Devendra and McLeroy 1982).
Many experimental studies have been conducted to quantify the effects of goat grazing on island vegetation productivity and species richness. In Hawaii, Mueller-Dombois and Spatz (1972) found that goat exclosures showed a stark increase in vegetation cover. Outside such areas, there were fewer endemic, and more exotic species, and much more exposed soil and rock. Regeneration of the endemic koa tree (Acacia koa), was disrupted in grazed areas, but abundant inside goat exclosures (Spatz and Mueller-Dombois 1973). On the Galápagos, goats have reduced or eliminated 77% of all plant species on the islands (Eckhardt 1972), and compete directly with endemic Galápagos tortoises. Bellingham et al. (2010) found that grazing promoted the growth of dense, unpalatable understories in New Zealand, restricting the succession of native forests. However, it has also been suggested that we should not generalize the negative influences of grazing and focus on individual case studies, as Randriamalala et al. (2016) found no significant grazing impacts on diversity, height, or biomass of vegetation in Madagascar.
Beyond vegetation, invasive goats can have profound impacts on other island taxa including breeding seabird populations (Pafilis et al. 2013). Because islands constitute relatively closed terrestrial systems, breeding seabird populations can provide critically important allochthonous marine nutrient subsidies to the simple local food webs (Sánchez-Piñero and Polis 2000). Seabird-derived guano, food scraps, and carrion can serve as fertilizers for plants, which in turn can boost arthropod and other primary consumer populations (Sánchez-Piñero and Polis 2000). Indeed, nesting seabirds have been found to increase the limiting soil nutrients nitrogen (N) and phosphorus (P) (Wait et al. 2005), which in turn support dense insular primary producer populations (Kolb et al. 2010). Seabirds can also increase CaCO3 content and lower C:N ratios (García et al. 2002). The ratio of C to N is a crucial measurement for organic matter decomposition (Parnas 1975). Thus, one aim of this study is to investigate whether the presence of goats is associated with decreased nesting seabird populations and how goats may affect arthropods as well as soil nutrients. Given the importance of seabirds for nutrient cycling in island systems, factors that reduce their numbers can have cascading impacts (Towns et al. 2009). Overgrazing has also been observed to affect physical soil properties and is associated with soil loss and desertification of many landscapes. In Australia, Bayne et al. (2004) analyzed sediment fluxes in areas with varying grazing intensities and found that there was less vegetation in areas with more goats, therefore promoting soil erosion and subsequently increasing the risk of further plant loss. Important parameters affecting erosion susceptibility are soil organic matter content and grain size distribution (Wischmeier et al. 1971).
Given the perceived impacts of feral goats on island ecosystems, conservation organizations have attempted to eradicate goats from numerous island sites worldwide—though with variable success. To date, approximately 164 goat eradications have succeeded (DIISE 2015) thanks to advances in technology and improved field techniques including the use of Global Positioning Systems (GPS), aerial hunting, and the use of ‘Judas goats’ (Campbell and Donlan 2005). Results of these eradications have been variable and difficult to predict—island ecosystems may recover on their own, require some restoration or reintroduction, or even become more damaged due to ecological destabilization (Courchamp et al. 2003; Schweizer et al. 2016). Few studies have implemented monitoring programs to evaluate the post-eradication recovery of these systems and predicting the success of eradication attempts has been difficult.
Successful goat eradications can create surprising and unintended conservation problems. Adequate assessments of the consequences of eradication are hindered because ecological relationships between island organisms are often poorly understood prior to eradication (Zavaleta et al. 2001). For example, goat eradications on the Galápagos Islands led to a decline, rather than a recovery, of the endangered Galápagos Hawk (Buteo galapagoensis), because the species had come to depend on goat-altered habitats (Rivera-Parra et al. 2012). Similarly, on the Bonin Islands, unbeknownst to conservation managers, goat grazing kept newly arrived invasive plants under control. Once the goats were eradicated, these exotic plants overran the landscape, impairing the native plant communities (Mack and Lonsdale 2002; Osawa et al. 2016). In the Mediterranean, goat grazing has had profound ecological impacts and whole habitats have been shaped by historical livestock management systems (San Miguel-Ayanz et al. 2010). Therefore, to prevent unintended consequences of goat eradications, it is critical to understand the effects of this important species before any eradication efforts are implemented (Zavaleta et al. 2001).
Goats and their products have always been an integral part of Mediterranean island economies (Hadjigeorgiou et al. 2002); most inhabited islands have been traditionally grazed year-round by roving herds of goats. With the increased availability of boat motors and associated reliability of access, shepherds have expanded their grazing area to previously inaccessible islets. Such relatively small islets are often of great conservation importance, either as refugia for endemic taxa that have been extirpated on larger islands, or as breeding sites for significant seabird populations. Typically, Mediterranean shepherds will release herds of goats onto such islands after the onset of the winter growing season, coming back to collect them after they feed on the spring vegetation flush. Such free-ranging goat herds will typically graze with limited, if any, supplemental feeding (Pafilis et al. 2013). Because islands are generally overstocked, even seasonal presence can have severe impacts on local plant communities. However, in the last 20 years, because of a shift away from traditional livestock husbandry and increased conservation concerns, goat releases have been discontinued on several islands. This sets the stage for an investigation of the potential recovery of local ecosystems after goats have been removed.
In contrast to studies that focus on exclosure plots, we test for effects on an island-wide scale (Greuter 1995). Islands represent spatially discrete entities making them reliable and realistic study systems (Wardle 2002). The present dearth of large-scale experiments has been attributed to a combination of logistic difficulties, moral challenges, and concerns about pseudoreplication (Soulé 1990; Donlan et al. 2002; Oksanen 2001). However, as ecosystem-level approaches become more prevalent in conservation, so will the need for large-scale experimental manipulations (Zavaleta et al. 2001). Here we conduct whole island manipulations and examine the effects of goats and their removal on multiple trophic levels. In particular, we quantify vegetation characteristics, physical and chemical soil traits, arthropod characteristics, and seabird populations on each island. We also provide secondary analyses of the interactions between these variables to further elucidate the ecosystem-level processes mediating effects of goat grazing on island species communities. We hypothesize (1) that goat grazing will have pronounced effects on vegetation characteristics, arthropod community structure, soil, as well as seabird presence and (2) that these changes will become reversed following goat removal.
Materials and methods
Study area
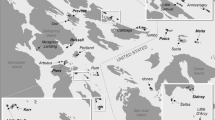
All fieldwork occurred in May–July 2015 on the Cycladic islands (central Aegean Sea, Greece). The climate is typical for the Mediterranean region with warm, dry summers and mild, modestly wet winters (Gikas and Tchobanoglous 2009). The islands are mainly composed of limestone and flysch substrates with shallow to no soil profiles. Located within the Mediterranean biodiversity hotspot, they represent a hyperdiverse landscape covered by summer-deciduous phrygana, semi-arid coastal heaths and sclerophyllous maquis vegetation types (Médail and Quézel 1999). “Islet specialist” plant species—taxa found mostly on small islands—play a major role in the study ecosystems (Bergmeier et al. 2003). All of the study islands have relatively simple food webs in which the top predators are lizards (Podarcis erhardii, Hemidactylus turcicus, and Mediodactylus kotschyi). Sand boas (Eryx jaculus) are present only on the two largest islands in this study (Drionissi and Gramvoussa). All of our study islands are uninhabited and ecologically similar to each other, with no other known introduced herbivores such as rabbits; they were selected to fall into one of three categories: islands currently being grazed by goats (Aspronissi, Fidussa, Agrilou, and Venetiko); islands which have never been grazed by goats (Turlos, Preza, Agia Kali, Drionissi, Grambonissi, North Varvaronissi, and South Varvaronissi); and those with previously extant but now removed goat herds (Mikros Ambelas, Petalidi, Kisiri, Psalida, and Gramvoussa) (Fig. 1). We considered an island to be grazed if goats had been brought onto the island for more than one spring season. From interviews with local shepherds, we determined that all islands considered eradicated have been devoid of goats for at least 10 years. Because the islands are relatively small (0.79–79.25 ha; see Appendix A in ESM), and the habitat open, we were able to census goats visually.
Map of Greece and Cycladic study areas in the Aegean. Study islands: Agia Kali (AK), Agrilou (AG), Aspronissi (AS), Drionissi (DR), Fidussa (FI), Grambonissi (GB), Gramvoussa (GV), Kisiri (KS), Mikros Ambelas (MA), North Varvaronissi (NV), Petalidi (PE), Preza (PR), Psalida (PS), South Varvaronissi (SV), Turlos (TU), and Venetiko (VE), all located near the larger islands of Amorgos (AM), Ios (IO), Irakleia (IR), Naxos (NA), Paros (PA), and Schinoussa (SC)
Quantification of vegetation condition
To assess vegetation characteristics we established four 50 m transects, one in each cardinal direction. On the smallest islands (Kisiri, Mikros Ambelas, and North Varvaronissi), which could not accommodate this design, three transects were used. We measured the presence of vegetation along the entire length of each transect and averaged the values for a measure of percent vegetation cover. We also applied this method to assess percent bedrock and bare soil. To determine vegetation height, we measured the height of plants every 2 m along each transect and averaged the values for each island. We sampled vegetation biomass in five randomly placed 80 cm × 80 cm squares. In these squares, all vegetation was clipped to ground level; all plant matter was collected and dried until no further weight loss was observed, and then weighed. Biomass data were averaged and expressed as a single g/m2 island value. Plant communities were determined from 80 cm × 80 cm quadrats placed every 5 m along each established transect; in each quadrat we recorded the identity of all plants present. Utilizing the program EstimateS (Colwell 2013), we generated a sample-based incidence rarefaction curve (Gotelli and Colwell 2001; Colwell et al. 2004). A bias-corrected form of the Chao2 asymptotic estimator was used to estimate the actual number of plant species on the island (Chao 1987; Colwell and Coddington 1994, Gotelli and Colwell 2011):
where S Chao2 = the estimated number of species, S obs = the observed number of species, m = the total number of samples, q 1 = the number of unique species, and q 2 = the number of duplicate species.
It is necessary to correct for area effects when making inter-island comparisons because the number of species increases with island size (MacArthur and Wilson 1967). This species-area relationship can be defined by the power law S = CAz, where C and z are coefficients, A is the area, and S is the number of species. To estimate species density C (an area-independent metric of species richness given by the equation C = S/Az), we obtained a data-based estimate of the coefficient z by plotting the species-area relationship for our study system and extracting the exponent of the fitted curve (Rosenzweig et al. 2011). Non-metric multidimensional scaling (NMDS) was used to analyze the changing species composition between study sites (Marín et al. 2016). In order to construct a species composition distance matrix, we employed the Bray–Curtis similarity index. Surface fitting was used to interpret the effects of different environmental variables on our species composition.
Quantification of arthropod characteristics
Five pitfall traps, used to sample epigeal arthropods, were installed on each island at randomly chosen locations near each directional transect. The traps were constructed by sinking plastic cups (7 cm in diameter and 11 cm deep), filled 2/3 with ethylene glycol, flush into the ground (Woodcock 2005; Schmidt et al. 2006). Traps were placed under a large, raised flat stone in a manner that protected them from livestock trampling but still allowed free invertebrate access. After approximately 2 weeks (on average 17.19 ± 4.59 days), the samples were collected, rinsed, identified to morphospecies, sorted, counted, dried, and weighed.
Abundance of each species and total number of observed species were recorded for each island. The number of arthropod individuals was counted for each trap and then averaged for each island and divided by the number of collection days. Each sample was dried under a heat lamp until no further weight reductions were observed and then the weight was recorded. Arthropod biomass was averaged for each island and divided by the number of days collected. The program EstimateS (Colwell 2013) was used to construct a sample-based abundance rarefaction curve for each island (Gotelli and Colwell 2001; Colwell et al. 2004). A bias-corrected form of the Chao1 asymptotic estimator was used to estimate the total number of arthropod species on the island (Chao 1987; Colwell and Coddington 1994, Gotelli and Colwell 2011):
where S Chao1 = the number of estimated species, S obs = the observed number of species, f 1 = the number of singleton species, and f 2 = the number of doubleton species.
Since the number of species is intrinsically linked to the size of an island (MacArthur and Wilson 1967), we accounted for area by calculating the coefficient C of the species-area relationship, where C = S/Az (Rosenzweig et al. 2011), and thus obtained comparable metrics. We also used NMDS with surface fitting to analyze arthropod species composition between islands of different grazing statuses and the environmental variables that affect that composition.
Soil classification—geochemistry
Five 1 kg soil samples were collected from each island. Samples were gathered from the four cardinal directions to minimize the effects of aspect on soil characteristics. 80 samples were analyzed for their grain size distribution, total carbon (C), total organic carbon (OC), total nitrogen (N) and total phosphorus (P). Grain size analysis was made using a Malvern Mastersizer 2000. Moment measures were calculated using GRADISTAT V.4 software (Blott and Pye 2001) and based on USDA classification. For C and N, we used a Carlo Erba EA1108 CHNS-O Elemental analyzer. OC content was estimated using the wet oxidation—titration method of Wakley and Black (1934), which has been validated and compared with LECO by Gaudette et al. (1974), and with the combustion-infrared method (Avramidis et al. 2015). We divided the OC by total N to determine a C:N. Traditionally, a conversion factor of 1.72 has been used to convert OC to organic matter based on the assumption that organic matter is 58% C (Nelson and Sommers 1982). We calculated P based on a persulfate digestion method according to APHA 4500-P (2005). CaCO3 was measured using a digital hand-held soil calcimeter (FOGII/Version 2/2014; BD INVENTIONS). More specifically, CaCO3 (%) calculations were based on the measurement of emitted CO2, a method modified from Müller and Gastner (1971). To determine the degree of erosion, soil depth measurements were taken from 30 random locations on each island using a graded metal stake that was sunk into the ground until it encountered bedrock; values were averaged to obtain an island-wide value of soil depth.
Quantification of seabird populations
Relative abundances of nesting seabirds were obtained during extended single island visits coinciding with the peak of the seabird nesting season (Hildén et al. 1995). This reduces any inter-measurement variation resulting from the presence of prospectors on some islands and not others. These estimates are meant to be comparative approximations of resident bird populations and are not true population censuses. We conducted our surveys during similar conditions (sunny with light wind), and during the same time of the day (mid-morning hours). All adult seabirds observed at the island were counted following procedures outlined in Walsh et al. (1995). Breeding seabirds were flushed upon our arrival on the islands and were counted in the air using binoculars by two independent observers for 15 min. Observation sites were chosen so as to encompass the whole island. The focus seabird species was the yellow-legged gull (Larus michahellis). Cliff nesting seabirds were almost completely absent from the islands and were not included in the counts. To our knowledge, none of the study islands harbored any nocturnal seabird colonies (Puffinus yelkouan and Calonectris diomedea). After estimates were calculated, the values were then divided by island area to account for differences in island size which determined our relative seabird abundances (birds/km2).
Statistical analysis
We compared all observed variables across the three island types—no goats, goats, and goats removed. Assumptions of normality were tested using Shapiro-Wilkes tests; variables that failed the test were either log-transformed (arthropod biomass, number of arthropods, C:N), square root-transformed (seabird density), arcsine-square root-transformed (% CaCO3, % Arachnida, % Diptera, % Annuals, and % Perennials), or Box-Cox-transformed using λ = − 0.5 (% N and % P). For all tests, n = 16. We utilized one-way ANOVA tests followed by post hoc Tukey comparisons to test for differences between the groups. If normality criteria could not be met, we used Kruskal–Wallis tests instead. To quantify vegetation and arthropod species diversity, we used a Shannon–Wiener Diversity Index (SWDI) that was developed to take into account both species richness and evenness (Maurer and McGill 2011). Our secondary analyses investigated the relationships between different variables using linear mixed model regressions (LMM), treating grazing status as a random effect. To assess the significance of differences in community compositions following NMDS for vegetation and arthropods, we used Analysis of Similarities (ANOSIM). All analyses were run in RStudio Team (2015).
Results
Effects of goats
Vegetation
Herbivory by goats has pronounced effects on island plant communities. We identified 119 distinct plant species from the 16 study islands (Appendix B in ESM). Grazing status of an island significantly affects the estimated plant species density (p = 0.00676, F 2,13 = 7.521, ANOVA). Islands with goats removed have significantly fewer species relative to both grazed and ungrazed islands; they have lost 45.4% of their estimated taxa numbers compared to ungrazed islands and 57.9% compared to grazed islands (Fig. 2a). Typical species found on ungrazed islands include Medicago arborea, Matthiola sinuata, and Atriplex halimus. Common plants associated with grazed islands include Plantago coronopus, Tordylium apulum, and Phleum arenarium. The SWDI for plants (p = 0.00412, F 2,13 = 8.628, ANOVA) follows a similar pattern: islands with removed goats have significantly lower SWDI values relative to both grazed and ungrazed islands (Fig. 2b). Percent vegetation cover is significantly lower (p = 0.004, F 2,13 = 8.438, ANOVA) in both grazed (25.96%) and goat-removed (38.56%) islands relative to the ungrazed sample. Plant biomass also decreases significantly (p = 0.00267, F 2,13 = 9.679, ANOVA), with grazed islands experiencing a decline to an average of 66.4% of the baseline (Fig. 2c). Mean plant height (p = 0.013, F 2,13 = 6.174, ANOVA) is on average 63.64% shorter on grazed islands and 64.85% shorter on goat-removed islands relative to ungrazed islands (Fig. 2d). The average percentage of annuals and perennials on the islands changed significantly with grazing regimes (p = 0.0058, F 2,13 = 7.857, ANOVA). Annual plant species increase from 24 to 50.2% of the community with the introduction of goats (subsequently, perennial species decreased from 76 to 49.8% of the community). Plant species composition differed significantly among the islands of different grazing status (p = 0.005, R = 0.357, ANOSIM). The NMDS plot (Fig. 3a) shows a complete separation between the plant communities of islands with goats and islands where goat have been removed. The plot also reveals the relative dissimilarity between the islands without goats and the other two grazing statuses. The surfaces fit onto the NMDS represent environmental variables that were shown to significantly affect community composition similarities: seabird density (p = 0.009), nitrogen content (p = 0.005), phosphorus content (p = 0.004), and soil depth (p = 0.053).
a Comparison of the means of the estimated plant species density. b Comparison of the means of vegetation SWDI. c Comparison of the means of the average aboveground dry vegetation biomass (g/m2). d Grazing effects on average plant height (cm). e Grazing effects on average soil depth (cm). f Grazing effects on seabird population density. Error bars represent standard error
a NMDS plot with surface fitting for plant community composition. b NMDS plot with surface fitting for arthropod community composition. Island abbreviations are the same as in Fig. 1
Arthropods
We collected a total of 7054 individuals from 118 arthropod taxa. Hymenoptera (particularly ants), Isopoda, Coleoptera, and Arachnida were the most abundant taxa, collectively comprising 84.2% of the collected arthropods (Hymenoptera 29.2%; Isopoda 24.3%; Coleoptera 22.7%; Arachnida 8%). Grazing status does not affect significantly estimated arthropod species density (p = 0.489, F 2,13 = 0.757, ANOVA), average arthropod biomass/trap/day (p = 0.611, F 2,13 = 0.511, ANOVA), or average number of arthropods/trap/day (p = 0.561, F 2,13 = 0.604, ANOVA). However, we documented a marginally significant positive effect of goat grazing on the arthropod SWDI (p = 0.064, F 2,13 = 3.417, ANOVA). Within individual arthropod groups, only the % Dipterans is significantly affected by grazing (p = 0.049, F 2,13 = 3.98, ANOVA) where we see a 109.25% increase in areas with goats relative to ungrazed islands. Grazing has a marginally significant effect on % Hymenopterans (p = 0.063, F 2,13 = 3.442, ANOVA) with goat presence resulting in a 53.2% decrease relative to ungrazed islands. The percentages of Arachnids (p = 0.557, F 2,13 = 0.613, ANOVA), Coleopterans (p = 0.146, F 2,13 = 2.235, ANOVA), Isopods (p = 0.281, χ 2 (2, n = 16) = 2.541, Kruskal–Wallis), and Hemipterans (p = 0.92, χ 2 (2, n = 16) = 0.167, Kruskal–Wallis) are not affected by grazing. Arthropod species assemblages appear to not be significantly affected by grazing status (p = 0.159, R = 0.105, ANOSIM). The NMDS plot (Fig. 3b) shows high overlap between the three statuses. The surface fit onto the NMDS represents soil depth (p = 0.01), an environmental variable that was shown to significantly affect arthropod community composition similarities.
Soil
Grazing also is significantly associated with erosion as evidenced by a decrease of 44.41% of average soil depth (p = 0.000983, F 2,13 = 12.36, ANOVA) (Fig. 2e) and an increase of 82.45% in the average amount of exposed bedrock (p = 0.0158, F 2,13 = 5.804, ANOVA) on grazed relative to ungrazed islands. More importantly, this process of soil loss continued even after goats had been removed, resulting in a 146.07% increase in average percent rock and a 68.31% decrease in average soil depth on islands with removed goats as compared to ungrazed islands (see Fig. 4).
While grazing by goats was associated with declining amounts of soil, we found little evidence for effects on soil structure or chemistry. 87.8% of our soil samples were classified as a silt loam while 4.9% were classified as sandy loam, 3.7% silt, and 1.2% sand, loamy sand, and loam, respectively. Grazing status does not significantly affect average % bare ground (p = 0.525, F 2,13 = 0.678, ANOVA), average % organic matter (p = 0.591, F 2,13 = 0.549, ANOVA), average % CaCO3 (p = 0.548, F 2,13 = 0.63, ANOVA), average % N (p = 0.65, F 2,13 = 0.445, ANOVA), average % P (p = 0.845, F 2,13 = 0.171, ANOVA), C:N ratio (p = 0.836, F 2,13 = 0.182, ANOVA), average % sand (p = 0.757, F 2,13 = 0.285, ANOVA), average % silt (p = 0.762, χ 2 (2,n = 16) = 0.545, Kruskal–Wallis), or average % clay (p = 0.464, F 2,13 = 0.814, ANOVA).
Seabirds
On grazed islands, we witnessed a trend towards lower seabird populations relative to ungrazed islands; however this effect does not quite reach statistical significance (p = 0.154, F 2,13 = 2.168, ANOVA) (see Fig. 2f).
Secondary analyses
Impacts of soil erosion
Erosion caused by goats has significant implications for vegetation characteristics. The increased occurrence of bedrock, resultant from goat grazing, is associated with reduced average percent vegetation cover (p < 0.00001, t = − 8.721, LMM), shorter average plant height (p < 0.00001, t = − 4.721, LMM), and marginally diminished plant biomass (p = 0.053, t = − 1.932, LMM). Unexpectedly, we document a significant positive relationship between amounts of exposed bedrock and the SWDI for arthropods (p = 0.012, t = 2.524, LMM), as well as estimated arthropod species density (p = 0.064, t = 1.85, LMM).
Similarly, decreased soil depth is associated with reduced vegetation cover (p = 0.00016, t = 3.767, LMM), diminished average vegetation height (p = 0.0015, t = 3.18, LMM), and marginally fewer arthropods collected per trap (p = 0.077 t = 1.77, LMM). In addition, lower C:N ratios are correlated with higher estimated arthropod species density (p = 0.019, t = − 2.351, LMM). Interestingly, higher N & P values are associated with lower plant SWDI values (p = 0.007, t = − 2.695, LMM & p = 0.0006, t = − 3.423, LMM, respectively). Higher N content is also marginally positively associated with arthropod SWDI values (p = 0.057, t = 1.902, LMM) as higher P values are similarly associated with estimated arthropod species densities (p = 0.054, t = 1.93, LMM). Lastly, we document marginally significant positive relationships between organic matter content and plant height (p = 0.059, t = 1.889, LMM).
Impacts on arthropods
A marginally significant positive relationship was found between both estimated plant species density and average plant height and average arthropod biomass (p = 0.068, t = 1.826, LMM & p = 0.051, t = 1.952, LMM, respectively). A marginally negative relationship was also found between average vegetation biomass and estimated arthropod species density (p = 0.082, t = − 1.74, LMM).
Seabird effects
The vast majority of nesting seabird species found on the study sites were yellow-legged gulls (Larus michahellis) with occasional European shags (Phalacrocorax aristotelis). Relative abundance of these seabirds is associated with significantly increased levels of N (p = 0.00016, t = 3.774, LMM) and P (p < 0.00001, t = 7.941, LMM) in the soil, presumably representing marine subsidies (Fig. 5a, b). A significant negative relationship was found between seabird densities and the vegetation SWDI (p = 0.00006, t = − 3.995, LMM). No significant relationships were detected between seabird densities and estimated plant species densities (p = 0.732, t = − 0.342, LMM), average % vegetation cover (p = 0.616, t = − 0.502, LMM), average vegetation biomass (p = 0.84, t = − 0.202, LMM), average plant height (p = 0.609, t = 0.512, LMM), average soil depth (p = 0.781, t = − 0.279, LMM), average % organic matter (p = 0.197, t = 1.289, LMM), average % CaCO3 (p = 0.136, t = − 1.493, LMM), C:N ratio (p = 0.135, t = − 1.497, LMM), estimated arthropod species density (p = 0.286, t = 1.068, LMM), arthropod SWDI (p = 0.193, t = 1.302, LMM), average arthropod biomass/trap/day (p = 0.67, t = − 0.426, LMM), or average number of arthropods/trap/day (p = 0.524, t = 0.638, LMM).
Discussion
Effects on vegetation
The presence of goats has wide-ranging impacts on vegetation and soil characteristics and has therefore direct implications for island conservation programs. Trampling of soil and removal of vegetation dislodges and triggers soil movement, which sets in motion a positive feedback loop of desertification that persists even after goats have been removed (López-Bermúdez 1990). Independently of direct goat biomass consumption, loss of soil leads to reduced plant cover, less plant biomass, and overall shorter plants, thus further diminishing the ability of vegetation to stabilize soil. Once goats are removed, vegetation cover and plant biomass rebound partially, but remain restricted to the small pockets of soil persisting in rock depressions. While vegetation cover and biomass partially recover, plant growth forms remain prostrate due to prior browsing which diminishes the heterogeneity of the vertical plant structure (Noy-Meir et al. 1989; Dennis et al. 1998). Overgrazing has the ability to even change the hydrology of ecosystems and increase stream flows through reduced infiltration and increased surface runoff (Van Vuren et al. 2001).
Despite these reductions in plant presence, we did not document a significant change in the estimated plant species density or in plant SWDI between islands with, or without, goat grazing. This is in general agreement with other studies done in the Mediterranean (Alrababah et al. 2007). It has been proposed that grazing intensity is not expected to change the diversity of semi-arid grasslands with a long history of grazing (Milchunas et al. 1988; Osem et al. 2002). However, this lack of change in species diversity masks important dissimilarities in plant community composition between islands (Fig. 3a) (Cingolani et al. 2005). While plant assemblages on islands without goats are characterized by relatively rare, native perennial species such as Medicago arborea, Matthiola sinuata, and Atriplex halimus, islands with goats present are characterized by more annual, generalist, low-lying plant species, usually with sticky seeds, such as Plantago coronopus, Tordylium apulum, and Phleum arenarium. Panitsa et al. (2006) suggested that the increase in plant species associated with goat grazing can be traced to the introduction of seeds in the fur or feces of an animal. The most effective animal seed transport mechanisms are usually seen in annual plant species (Shmida and Ellner 1983). A 32-year study by Snogerup and Snogerup (2004) noticed that the main changes in islet flora concerned soil space opened up by grazing animals and erosion, and also classified roughly 100 taxa that were probably transported by grazing animals. Indeed, high grazing pressure can actually increase plant species richness (Hobbs and Huenneke 1992), especially in nutrient-rich ecosystems, such as seabird islands (Proulx and Mazumder 1998). Fernández-Lugo et al. (2009) similarly found that species richness failed to recover after goat grazing but also noticed significant species turnover related to higher soil magnesium content. Our observations indicate that once goats are removed from an island, the island begins to lose some of these introduced species, yet the native species do not return, presumably due to a lack of seed sources, ultimately resulting in an overall decline in alpha diversity. Mulder et al. (2009) found that if there is an expansion of non-native plant species early in the recovery process, islands could have very different successional trajectories than uninvaded islands, leading to two alternative stable states. Depending on the goals of an island conservation project, the actual identities of the plant community may be more important than overall levels of species richness, and re-introduction of focal plant species may be warranted.
Effects on arthropods
We did not observe a consistent effect of goat grazing on arthropod communities (Fig. 3b). In our study, arthropod numbers did not rise with increased vegetation cover or reduced grazing, perhaps because any gains were passed on to lizards which constitute the next trophic level up. However, both goat grazing and declining vegetation cover were associated with increases in insect SWDI, potentially because more microhabitats were available. Other studies have also found that there are groups of arthropods that prefer increased bare ground associated with higher stocking rates of herbivores (García et al. 2009). Specific arthropod guilds have been reported to be differentially influenced by vegetation covers (Gardner et al. 1997; Woodcock et al. 2005). When arthropods were analyzed by order, we only detected a significant increase in Dipterans with grazing, possibly because many Dipteran taxa utilize goat dung to breed (Khan et al. 2012).
General arthropod community characteristics also appear unaffected by direct seabird densities, in general agreement with previous research (Orgeas et al. 2003; Towns et al. 2009), which documented an effect of seabird numbers on arthropod community composition, but no effect on arthropod species richness or biomass. However, we did record a positive association between arthropod species density and elevated soil P levels and between arthropod SWDI and soil N levels, paralleling similar observations in the past (Anderson and Polis 1998; Sayer et al. 2010). Kaspari et al. (2008) observed that P often tends to be the main limiting nutrient for decomposition in most ecosystems and fertilization experiments have shown that P addition stimulates cellulose-decomposing microbes resulting in greater arthropod biomass.
Soil effects
In our study, soil chemical characteristics do not seem to be influenced by grazing. Instead, it is primarily the amount, and not the chemical properties of the soil, that are affected in this system. We find that pronounced losses of what are already shallow, erosion-prone soils, are the main symptom of goat grazing, as well as the driver for further degradation, and result in sparse, structurally simplified habitats. Our observations suggest that erosion associated with goat presence is not entirely caused by exposure due to the removal of plant cover; additionally the breaking of the fragile soil crust by goat hooves allows for the notoriously strong local winds to carry soil particles out to the sea. Other studies in Mediterranean ecosystems have seen varying and contrasting influences of grazing on soil nutrients (Yates et al. 2000; Sánchez-Marañón et al. 2002; Peco et al. 2006; Fernández-Lugo et al. 2009). Increasing N & P, primarily driven by seabird density, were found to lower plant SWDI, which is suggested to be caused by a few competitive plant species increasing in abundance while the majority decline (Tilman 1987; Farrer et al. 2013).
Effects of seabirds
Seabirds appear to shape the study island communities through bottom-up trophic interactions. Seabirds are thought to prefer nesting on smaller islands (Vidal et al. 2001), an observation that was confirmed in this study. Previous studies in the region (Pafilis et al. 2013) have detected a significant inverse relationship between seabird density and grazing. Our results, although failing to meet statistical significance, are consistent with a similar negative trend between goats and resident seabird populations reported nearby (Pafilis et al. 2013). The reduction in seabird abundance and the subsequent nutrient transport has been shown to have cascading effects on below-ground organisms and the ecosystem processes they drive (Fukami et al. 2006). We also detected a strongly significant increase in soil N and P levels with higher seabird densities. Allochthonous nutrient inputs from marine subsidies are critically important for these isolated terrestrial islands (Sánchez-Piñero and Polis 2000). These nutrients may support higher plant biomass and more nitrophilous plant species (Polis et al. 2002). However, these nutrients were only found to stimulate primary productivity during wet periods (Polis et al. 2002), which may explain why—given the generally arid conditions in the study region—we do not see an effect of soil nutrients on vegetation characteristics in our study.
Seabirds can reduce plant vegetation by trampling and burrowing activities or by creating locally toxic conditions through extremely high nutrient levels (Hata et al. 2014). These negative effects may cancel out some of the positive effects in our study. Seabirds can affect island arthropods in at least 2 ways—first, by providing carcasses and food scraps for scavengers such as Tenebrionidae or Dermestidae beetles (Polis and Hurd 1996), and second, by increasing herbivorous or detritivorous and eventually even predatory arthropods, via increased plant productivity (Anderson and Polis 1999; Polis et al. 2002). Lastly, gulls can also act as important seed dispersers between islands; we regularly found large Pistacia lentiscus seeds among the investigated guano. Given this prominent role that seabirds play in Mediterranean island ecosystems, it is important to protect, and even restore seabird colonies on islands from which goats were eradicated.
Conclusion
This study demonstrates that goat herbivory has severe and long-term effects on many, but not all aspects of island ecosystems. Goats set in motion a positive feedback loop of desertification, which persists even after their removal. The grazing and associated soil erosion contribute to changing plant communities, lower plant biomass, less vegetation cover, and shorter plants. There is also some support for an impact on seabirds, an important source of N and P in our system, although this relationship fails to meet statistical significance. Subsequent studies may benefit from including grazing intensity in the analyses. This study suggests that goat removal programs should be accompanied by restoration of the native vegetation ideally before there is extensive loss of soils. Full restoration of small island ecosystems will be slow given the very long periods needed for soil regeneration.
References
Alrababah MA, Alhamad MA, Suwaileh A, Al-Gharaibeh M (2007) Biodiversity of semi-arid Mediterranean grasslands: Impacts of grazing and afforestation. Appl Veg Sci 10:257–264
Anderson WB, Polis GA (1998) Marine subsidies of island communities in the Gulf of California: evidence from stable carbon and nitrogen isotopes. Oikos 81(1):75–80
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118(3):324–332
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington
Atkinson IAE (1989) Introduced animals and extinctions. In: Western D, Pearl MC (eds) Conservation for the twenty-first century. Oxford University Press, New York, pp 54–69
Avramidis P, Nikolaou K, Bekiari V (2015) Total organic carbon and total nitrogen in sediments and soils: a comparison of the wet oxidation—titration method with the combustion-infrared method. Agric Agric Sci Proc 4:425–430
Bayne P, Harden R, Davies I (2004) Feral goats (Capra hircus L.) in the Macleay River gorge system, north-eastern new South Wales, Australia. I. Impacts on soil erosion. Wildl Res 31(5):519–525
Bellard C, Genovesi P, Jeschke JM (2016) Global patterns in threats to vertebrates by biological invasions. Proc R Soc B 283:1–9
Bellingham PJ, Wiser SK, Wright AE, Cameron EK, Forester LJ (2010) Disperser communities and legacies of goat grazing determine forest succession on the remote Three Kings Islands, New Zealand. Biol Conserv 143(4):926–938
Bergmeier E, Dimopoulos F, Dimopoulos P (2003) The vegetation of islets in the Aegean and the relation between the occurrence of islet specialists, island size, and grazing. Phytocoenologia 33(2–3):447–474
Blott SJ, Pye K (2001) GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf Proc Land 26(11):1237–1248
Bowen L, Van Vuren D (1997) Insular endemic plants lack defenses against herbivores. Conserv Biol 11(5):1249–1254
Campbell K, Donlan CJ (2005) Feral goat eradications on islands. Conserv Biol 19(5):1362–1374
Ceballos G, Brown JH (1995) Global patterns of mammalian diversity, endemism, and endangerment. Conserv Biol 9(3):559–568
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43(4):783–791
Chynoweth MW, Litton CM, Lepczyk CA, Hess SC, Cordell S (2013) Biology and impacts of Pacific island invasive species. 9. Capra hircus, the feral goat (Mammalia: Bovidae). Pac Sci 67(2):141–156
Cingolani AM, Noy-Meir I, Díaz S (2005) Grazing effects on rangeland diversity: a synthesis of contemporary models. Ecol Appl 15(2):757–773
Coblentz BE (1978) The effects of feral goats (Capra hircus) on island ecosystems. Biol Conserv 13(4):279–286
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9. User’s guide and application published at: http://purl.oclc.org/estimates
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc (Ser B) 345:101–118
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85(10):2717–2727
Courchamp F, Chapuis J, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78(3):347–383
Dennis P, Young MR, Gordon IJ (1998) Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecol Entomol 23(3):253–264
Devendra C, McLeroy GB (1982) Goat and sheep production in the tropics. Longman, London
DIISE (2015) The database of island invasive species eradications, developed by Island Conservation, Coastal Conservation Action Laboratory UCSC, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research New Zealand. http://diise.islandconservation.org
Donlan CJ, Tershy BR, Croll DA (2002) Islands and introduced herbivores: conservation action as ecosystem experimentation. J Appl Ecol 39(2):235–246
Eckhardt RC (1972) Plants and animals in the Galápagos Islands. Bioscience 22(10):585–590
Farrer EC, Herman DJ, Franzova E, Pham T, Suding KN (2013) Nitrogen deposition, plant carbon allocation, and soil microbes: changing interactions due to enrichment. Am J Bot 100(7):1458–1470
Fernández-Lugo S, de Nascimento L, Mellado M, Bermejo LA, Arévalo JR (2009) Vegetation change and chemical soil composition after 4 years of goat grazing exclusion in a Canary Islands pasture. Agric Ecosyst Environ 132(3):276–282
Foufopoulos J, Kilpatrick AM, Ives AR (2011) Climate change and elevated extinction rates of reptiles from Mediterranean islands. Am Nat 177(1):119–129
Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, Yeates GW, Bonner KI, Durrett MS, Grant-Hoffman MN, Williamson WM (2006) Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol Lett 9:1299–1307
García LV, Marañón T, Ojeda F, Clemente L, Redondo R (2002) Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi-arid Mediterranean islands. Oikos 98:75–86
García RR, Jáuregui BM, García U, Osoro K, Celaya R (2009) Effects of livestock breed and grazing pressure on ground-dwelling arthropods in Cantabrian heathlands. Ecol Entomol 34(4):466–475
Gardner SM, Hartley SE, Davies A, Palmer SCF (1997) Carabid communities on heather moorlands in northeast Scotland: the consequences of grazing pressure for community diversity. Biol Conserv 81(3):275–286
Gaudette HE, Flight WR, Toner L, Folger DW (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Petrol 44(1):249–253
Gikas P, Tchobanoglous G (2009) Sustainable use of water in the Aegean Islands. J Environ Manag 90(8):2601–2611
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4(4):379–391
Gotelli NJ, Colwell RK (2011) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, New York, pp 39–54
Greuter W (1995) Origin and peculiarities of Mediterranean island floras. Ecol Mediter 21(1–2):1–10
Hadjigeorgiou I, Vallerand F, Tsimpoukas K, Zervas G (2002) The socio-economics of sheep and goat farming in Greece and the implications for future rural development. Options Méditer Ser B 39:83–93
Hata K, Kohri M, Morita S, Hiradate S, Kachi N (2014) Complex interrelationships among aboveground biomass, soil chemical properties, and events caused by feral goats and their eradication in a grassland ecosystem of an island. Ecosystems 17(6):1082–1094
Hildén O, Ulfvens J, Pahtamaa T, Hästbacka H (1995) Changes in the archipelago bird populations of the Finnish Quark, Gulf of Bothnia, from 1957–1960 to 1990–1991. Ornis Fennica 72:115–126
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: Implications for conservation. Conserv Biol 6(3):324–337
Honegger RE (1981) List of amphibians and reptiles either known or thought to have become extinct since 1600. Biol Conserv 19:141–158
Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486(7401):105–108
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22(1):16–26
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11(1):35–43
Khan HAA, Shad SA, Akram W (2012) Effect of livestock manures on the fitness of house fly, Musca domestica L. (Diptera: Muscidae). Parasitol Res 111:1165–1171
Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W (2009) A global assessment of endemism and species richness across island and mainland regions. PNAS 106(23):9322–9327
King WB (1985) Island birds: will the future repeat the past? In: M. P. J. ICBP (ed) Conservation of island birds, vol 3. Technical Publication, Salt Lake, pp 3–15
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204
Kolb GS, Ekholm J, Hambäck PA (2010) Effects of seabird nesting colonies on algae and aquatic invertebrates in coastal waters. Mar Ecol Prog Ser 417:287–300
Loope LL, Scowcroft PG (1985) Vegetation response within exclosures in Hawai’i: a review. In: Stone CP, Scott JM (eds) Hawaii’s terrestrial ecosystems: preservation and management. Cooperative National Park Resources Studies Unit, University of Hawaii, Honolulu, pp 377–402
López-Bermúdez F (1990) Soil erosion by water on the desertification of a semi-arid Mediterranean fluvial basin: the Segura basin, Spain. Agric Ecosyst Environ 33(2):129–145
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Mack RN, Lonsdale WM (2002) Eradicating invasive plants: hard-won lessons for islands. In: Veitch CR, Clout MN (eds) Turning the tide: the eradication of invasive species. IUCN, pp 164–172
Marín L, Philpott SM, De la Mora A, Núñez GI, Tryban S, Perfecto I (2016) Response of ground spiders to local and landscape factors in a Mexican coffee landscape. Agric Ecosyst Environ 222:80–92
Massei G, Hartley SE, Bacon PJ (2000) Chemical and morphological variation of Mediterranean woody evergreen species: do plants respond to ungulate browsing? J Veg Sci 11:1–8
Maurer BA, McGill BJ (2011) Measurement of species diversity. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, New York, pp 55–65
Médail F, Quézel P (1999) Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conserv Biol 13(6):1510–1513
Milchunas DG, Sala OE, Lauenroth WK (1988) A generalized model of the effects of grazing by large herbivores on grassland community structure. Am Nat 132(1):87–106
Mueller-Dombois D (1981) Island ecosystems: what is unique about their ecology? In: Mueller-Dombois D, Bridges KW, Carson HL (eds) Island ecosystems: biological organization in selected Hawaiian communities. Hutchinson Ross, Stroudsburg, pp 485–501
Mueller-Dombois D, Spatz G (1972) The influence of feral goats on the lowland vegetation in Hawaii Volcanoes National Park. Island Systems IRP. Technical Report No. 13
Mulder CPH, Grant-Hoffman MN, Towns DR, Bellingham PJ, Wardle DA, Durrett MS, Fukami T, Bonner KI (2009) Direct and indirect effects of rats: does rat eradication restore ecosystem functioning of New Zealand seabird islands? Biol Invasions 11:1671–1688
Müller G, Gastner M (1971) The “Karbonat-Bombe”, a simple device for the determination of the carbonate content in sediments, soils, and other materials. Neues Jahrbuch für Mineralogie-Monatshefte 10:466–469
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, Agronomy Monograph 9.2, American Society of Agronomy, Soil Science Society of America, Madison, pp 539–579
Nogales M, Martín A, Tershy BR, Donlan CJ, Veitch D, Puerta N, Wood B, Alonso J (2004) A review of feral cat eradication on islands. Conserv Biol 18(2):310–319
Noy-Meir I, Gutman M, Kaplan Y (1989) Responses of Mediterranean grassland plants to grazing and protection. J Ecol 77:290–310
Oksanen L (2001) Logic of experiments in ecology: is pseudoreplication a pseudoissue? Oikos 94(1):27–38
Orgeas J, Vidal E, Ponel P (2003) Colonial seabirds change beetle assemblages on a Mediterranean island. Ecoscience 10(1):38–44
Osawa T, Hata K, Kachi N (2016) Eradication of feral goats enhances expansion of the invasive shrub Leucaena leucocephala on Nakoudo-jima, an oceanic island. Weed Res 56(2):168–178
Osem Y, Perevolotsky A, Kigel J (2002) Grazing effect on diversity of annual plant communities in a semi-arid rangeland: interactions with small-scale spatial and temporal variation in primary productivity. J Ecol 90:936–946
Pafilis P, Anastasiou I, Sagonas K, Valakos ED (2013) Grazing by goats on islands affects the populations of an endemic Mediterranean lizard. J Zool 290(4):255–264
Panitsa M, Tzanoudakis D, Triantis KA, Sfenthourakis S (2006) Patterns of species richness on very small islands: the plants of the Aegean archipelago. J Biogeogr 33:1223–1234
Parnas H (1975) Model for decomposition of organic material by microorganisms. Soil Biol Biochem 7(2):161–169
Peco B, Sánchez AM, Azcárate FM (2006) Abandonment in grazing systems: consequences for vegetation and soil. Agric Ecosyst Environ 113(1):284–294
Polis GA, Hurd SD (1996) Allochthonous input across habitats, subsidized consumers, and apparent trophic cascades: examples from the ocean-land interface. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns & dynamics. Springer, Berlin, pp 275–285
Polis GA, Rose MD, Sánchez-Piñero F, Stapp PT, Anderson WB (2002) Island food webs. In: Case TJ, Cody ML, Ezcurra E (eds) A new island biogeography of the sea of Cortés. Oxford University Press, New York, pp 362–380
Proulx M, Mazumder A (1998) Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology 79(8):2581–2592
Randriamalala JR, Radosy HO, Razanaka S, Randriambanona H, Hervé D (2016) Effects of goat grazing and woody charcoal production on xerophytic thickets of southwestern Madagascar. J Arid Environ 128:65–72
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal G, Janzen D (eds) Herbivores: their interaction with secondary plant metabolites. Academic Press, New York, pp 3–53
Rivera-Parra JL, Levenstein KM, Bednarz JC, Vargas FH, Carrion V, Parker PG (2012) Implications of goat eradication on the survivorship of the Galapagos hawk. J Wildl Manag 76(6):1197–1204
Rosenzweig ML, Donoghue J, Li YM, Yuan C (2011) Estimating species density. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, New York, pp 276–288
RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/
Sánchez-Marañón M, Soriano M, Delgado G, Delgado R (2002) Soil quality in Mediterranean mountain environments: Effects of land use change. Soil Sci Soc Am J 66(3):948–958
Sánchez-Piñero F, Polis GA (2000) Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81(11):3117–3132
San Miguel-Ayanz A, Perea García-Calvo R, Fernández-Olalla M (2010) Wild ungulates vs. extensive livestock. Looking back to face the future. Options Méditer Ser A 92:27–34
Sayer EJ, Sutcliffe LME, Ross RIC, Tanner EVJ (2010) Arthropod abundance and diversity in a lowland tropical forest floor in Panama: the role of habitat space vs. nutrient concentrations. Biotropica 42(2):194–200
Schmidt MH, Clough Y, Schulz W, Westphalen A, Tscharntke T (2006) Capture efficiency and preservation attributes of different fluids in pitfall traps. J Arachnol 34(1):159–162
Schweizer D, Jones HP, Holmes ND (2016) Literature review and meta-analysis of vegetation responses to goat and European rabbit eradications on islands. Pac Sci 70(1):55–71
Shmida A, Ellner S (1983) Seed dispersal on pastoral grazers in open Mediterranean chaparral, Israel. Isr J Bot 32:147–159
Silanikove N (2000) The physiological adaptation in goats to harsh environments. Small Rumin Res 35:181–193
Snogerup S, Snogerup B (2004) Changes in the flora of some Aegean islets 1968–2000. Plant Syst Evol 245(3):169–213
Soulé ME (1990) The onslaught of alien species, and other challenges in the coming decades. Conserv Biol 4(3):233–240
Spatz G, Mueller-Dombois D (1973) The influence of feral goats on koa tree reproduction in Hawaii Volcanoes National Park. Ecology 54(4):870–876
Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr 57(3):189–214
Towns DR, Wardle DA, Mulder CPH, Yeates GW, Fitzgerald BM, Parrish GR, Bellingham PJ, Bonner KI (2009) Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118(3):420–430
Van Vuren D, Coblentz BE (1987) Some ecological effects of feral sheep on Santa Cruz Island, California, USA. Biol Conserv 41(4):253–268
Van Vuren D, Johnson ML, Bowen L (2001) Impacts of feral livestock on island watersheds. Pac Sci 55(3):285–289
Vidal E, Roche P, Bonnet V, Tatoni T (2001) Nest-density distribution patterns in a yellow-legged gull archipelago colony. Acta Oecol 22(5–6):245–251
Vitousek PM (1988) Diversity and biological invasions of oceanic islands. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington, pp 181–189
Wait DA, Aubrey DP, Anderson WB (2005) Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. J Arid Environ 60(4):681–695
Wakley A, Black IA (1934) An examination of the Degthareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science 27:29–38
Wallace AR (1911) Island life, 3rd edn. Macmillan, London, p 295
Walsh PM, Halley DJ, Harris MP, del Nevo A, Sim IMW, Tasker ML (1995) Seabird monitoring handbook for Britain and Ireland. Published by JNCC/RSPB/ITE/Seabird Group, Peterbourough, p 150
Wardle DA (2002) Islands as model systems for understanding how species affect ecosystem properties. J Biogeogr 29(5–6):583–591
Wischmeier WH, Johnson CB, Cross BV (1971) Soil erodibility nomograph for farmland and construction sites. J Soil Water Conserv 26(5):189–193
Woodcock BA (2005) Pitfall trapping in ecological studies. In: Leather SR (ed) Insect sampling in forest ecosystems. Blackwell Science Ltd., Hoboken, pp 37–57
Woodcock BA, Pywell RF, Roy DB, Rose RJ, Bell D (2005) Grazing management of calcareous grasslands and its implications for the conservation of beetle communities. Biol Conserv 125:193–202
Yates CJ, Norton DA, Hobbs RJ (2000) Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: implications for restoration. Austral Ecol 25(1):36–47
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16(8):454–459
Zeder MA, Hesse B (2000) The initial domestication of goats (Capra hircus) in the Zagros Mountains 10,000 years ago. Science 287(5461):2254–2257
Acknowledgements
This study would not have been possible without support from the University of Michigan Rackham Graduate School and the Greek State (Permit Nr.: ΑΔΑ: ΩΛΔΠ465ΦΘΗ-Υ5Ε). We especially acknowledge the efforts of Joanna Thelen, Rebecca Brewster, and Sydney Watkins who assisted with every aspect of field work, as well as Prof. Dimitris G. Hatzinikolaou, in the Department of Biology at the University of Athens for sorting and preparing the soil samples for chemical analysis. We would like to thank multiple University of Michigan faculty and especially Bobbi Low and Ivette Perfecto for advice.
Author information
Authors and Affiliations
Contributions
The study primary sponsor, UM Rackham Graduate School, played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit paper for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gizicki, Z.S., Tamez, V., Galanopoulou, A.P. et al. Long-term effects of feral goats (Capra hircus) on Mediterranean island communities: results from whole island manipulations. Biol Invasions 20, 1537–1552 (2018). https://doi.org/10.1007/s10530-017-1645-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1645-4