Abstract
Biotic global change agents, such as non-native plants (‘weeds’), non-native earthworms (‘worms’), and overabundant herbivores (white-tailed ‘deer’), can be major stressors in the forest understory. The status and relationships among these global change stressors across large spatial extents and under naturally varying conditions are poorly understood. Here, through an observational study using a network of U.S. National Park Service forest health monitoring plots (n = 350) from eight parks in seven northeastern states, we modeled causal pathways among global change stressors through model selection in a structural equation (SEM) framework. Weeds, worms, and, deer were common across all parks in the study—46% of plots had non-native plants, 42% of plots had evidence of earthworms, and all parks had plots with high deer browse damage. All biotic global change stressors were significantly and positively correlated with one another (all Spearman rank correlations ≥ 0.44). Consequently, 28% of plots had a combination of earthworms absent, low deer browse, and no non-native plants, and 29% of plots included earthworms, non-native plants, and moderate or greater browse damage. Through SEM, we found strong support for pathways among global change stressors, e.g., deer browse positively influenced earthworm presence and both deer and earthworms promoted non-native plants. Warmer air temperatures and higher soil pH also facilitated non-natives. This research highlights the tremendous multipronged management challenge for areas already experiencing the combined effects of weeds, worms, and deer and the future vulnerability of other areas as temperatures warm and conditions become more amenable to biotic global change stressors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global change stressors have far reaching effects across Earth’s ecosystems. In addition to physical changes such as increasing temperatures, pollution, and habitat fragmentation, biotic changes in the form of introduced non-native plants (‘weeds’) and animals (such as earthworms, ‘worms’) and overabundant native herbivores (white-tailed ‘deer’) are major ecosystem drivers (Mack et al. 2000; Craven et al. 2016; Côté et al. 2004). These stressors are present across ecosystem types and protected area designations; management and restoration actions must take into account these multiple, interacting stressors for effective and realistic stewardship (Hobbs et al. 2014). For example, the northeastern U.S. includes national parks established for both significant natural features and to preserve cultural resources such as historic battlefields and military forts. In most cases, these historic parks were not originally designated to protect native biodiversity; yet, these cultural parks, with large areas of native ecosystems, have become important components of the protected area network in the heavily-populated and habitat-fragmented eastern U.S. As these parks look to management under continuously changing climate and related conditions in the near and long term (National Park System Advisory Board (NPSAB) 2012), a major management strategy is to reduce existing stressors such as non-native species and overabundant herbivores (National Fish, Wildlife and Plants Climate Adaptation Partnership (NFWPCAP) 2012). Here, we take advantage of a network of forest monitoring plots in seven northeastern states to assess the status and relationships among non-native plants, non-native earthworms, deer browse pressure, and environmental conditions.
Individually, biotic stressors can have strong impacts on native plant performance, biodiversity, and ecosystem structure and function (Rooney and Waller 2003; Vilà et al. 2011; Craven et al. 2016). For example, chronic herbivory by white-tailed deer (Odocoileus virginianus), a native yet often overabundant species, simplifies understory community structure and can lead to decreases in native biodiversity (Rooney and Waller 2003; Côté et al. 2004). Non-native earthworms are ecosystem engineers, altering the forest floor and soil environment (Frelich et al. 2006; Suárez et al. 2006) and reducing native seedling growth and survival (Dobson and Blossey 2015; Dávalos et al. 2015a). Non-native plant invasions have myriad effects to native flora, including reductions in species diversity, abundance, growth, and fecundity (Mack et al. 2000; Vilà et al. 2011).
Increasingly, studies are finding positive and reinforcing relationships among biotic global change stressors (Nuzzo et al. 2009). Overabundant deer facilitate increases in non-native plant species cover (Waller and Maas 2013) and may be required for successful non-native plant invasion and persistence (Kalisz et al. 2014). Similarly, non-native earthworms promote non-native plant invasion (Bartuszevige et al. 2007; Roth et al. 2015; Craven et al. 2016). In combination, deer and earthworms change the forest floor environment, including leaf litter depth, plant cover and competition, and nutrient availability. These changes together negatively affect native seedlings (Dobson and Blossey 2015) and promote establishment and growth of non-native plants (Dávalos et al. 2015b; Dobson and Blossey 2015). Furthermore, overabundant deer appear to have a facilitative effect on invasive earthworms based on reductions in earthworms after deer exclosures are erected (Rearick et al. 2011; Dávalos et al. 2015c), likely through changes to soil nutrient inputs, litter quality, and microbial communities (Karberg and Lilleskov 2009). Non-native plants may also have some reciprocal effects to promote deer and earthworms (Kourtev et al. 1999; Madritch and Lindroth 2009).
Our expanding understanding of the interrelationships among non-native plants, earthworms, and deer come primarily from designed experiments in field and greenhouse settings, where treatments are applied and controlled (e.g., deer exclosures, removal of invasive plants, and addition of earthworms to microcosms). In this research, we ask whether relationships among biotic global change stressors are detectable in an observational study across a large region using naturally varying conditions, and further, whether our understanding of these relationships among biotic stressors allows us to model the system through structural equations. We hypothesize that deer have a positive effect on earthworm presence and that both deer and earthworms promote non-native plant species richness and cover. Understanding the status and relationships among above- and below-ground global change stressors is critical to land stewardship prioritization and efficacy.
Methods
Study sites
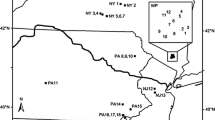
This study included eight national parks in seven states across the Northeast Temperate Network of the National Park Service (NPS) Inventory and Monitoring Program (Fig. 1). Parks range in size from 28 ha (Weir Farm National Historic Site, CT) to 14,577 ha (Acadia National Park, ME). These parks protect a diversity of natural and cultural heritage. For example, Roosevelt-Vanderbilt National Historic Sites (NY) preserve a country palace and the estate and homes of a former First Lady and President. Marsh-Billings-Rockefeller National Historical Park (VT) preserves the evolving history of conservation and forest management in the U.S. Acadia National Park protects the iconic coast, forest, and mountains of coastal Maine. All of the parks experienced intensive land-use prior to preservation. Some parks primarily only experienced timber harvesting (e.g., Marsh-Billings-Rockefeller and Acadia) while others also experienced agriculture and development (e.g., Minute Man National Historical Park, MA).
Locations of national park units included in the study: 1. Acadia National Park, 2. Marsh-Billings-Rockefeller National Historical Park, 3. Saint-Gaudens National Historic Site, 4. Saratoga National Historical Park, 5. Minute Man National Historical Park, 6. Roosevelt-Vanderbilt National Historic Sites, 7. Weir Farm National Historic Site, and 8. Morristown National Historical Park. Study parks are found in three ecological provinces: Laurentian Mixed Forest (light gray), Adirondack-New England Mixed Forest (medium gray), and Eastern Broadleaf Forest (Oceanic) Province (dark gray) (Cleland et al. 2007)
All of these parks now have large areas of forest protected managed by the NPS. These forests are similar to neighboring forests outside park boundaries with the exception that park forests are often older with more complex structure (Miller et al. 2016). The parks are located across three ecological provinces: Laurentian Mixed Forest, Adirondack-New England Mixed Forest, and Eastern Broadleaf Forest (Oceanic) Provinces (Fig. 1). Forest types range from spruce–fir–northern hardwoods in the north (ME, NH, VT) to transition hardwoods-white pine-hemlock (MA, NY) and central hardwoods in the south (CT, NJ).
Study sites have warm, moist, temperate climates. Annual mean temperature ranges across sites from 6.6 to 11.0 °C and summer (June, July, August) mean monthly temperature from 18.7 to 22.2 °C. Annual precipitation is abundant across this landscape, 1087–1447 mm, and similarly summer precipitation varies from 296 to 378 mm. January mean minimum temperatures vary from − 5.78 °C in the south to − 13.3 °C at northern, interiors sites. Climate data are 2001–2010 averages from PRISM Climate Group (2012).
Forest sampling
Forest plot data used in these analyses are from the standard monitoring effort of the NPS Inventory and Monitoring Program. See Tierney et al. (2016) for details on sampling methods. Data are from 350 plots in eight parks (Electronic Supplementary Material Table S1). Each plot was sampled one time during the 4-year period, 2012–2015. Approximately 25% of the plots, distributed across parks, were sampled in each of the 4 years of the study. Forest plots are 225 m2 (15 × 15 m) in Acadia, ME and 400 m2 (20 × 20 m) in the other seven parks. The number of plots per park varies from 10 for the smallest site (Weir Farm) to 176 for the largest site (Acadia). Sampling intensity varies from one plot per 2 hectares of forest (Saint Gaudens, NH and Weir Farm) to one plot per 73 hectares of forest (Acadia). Plot location is based on a generalized random stratified sampling design generated within each park.
Multiple forest health metrics were sampled in each plot, including species and diameter at breast height (DBH) for all trees ≥ 10 cm within the plot. Non-native plant richness and cover data were collected within eight 1-m2 quadrats in each plot. All non-native plant species in each quadrat were identified to species and the percent cover (using a 10 cover-class system) of each species was estimated visually. In addition, we collected multiple stand and site metrics, such as assessing deer browse damage and inspecting soils for evidence of earthworm presence.
Estimating deer population size is difficult. Accurate and standardized measures across large regions, meaningful at plot-level spatial scales, are not available. We did not attempt to calculate deer density, as the more proximate estimate of impact to the understory is deer browse damage to woody stems (Morellet et al. 2007). Thus, each plot was scored on a deer browse index with four impact levels (1-low, 2-moderate, 3-high, 4-very high). Low impact plots had no observed browse damage and palatable woody and herbaceous species present in the understory. Moderate-impact plots had evident but uncommon browse; high-impact plots included common browse evidence and/or rare browse-preferred species and browse-resilient vegetation limited in height growth by deer browsing. Very high impact plots had omnipresent browse evidence, a distinct browse line around 1.5 m above the ground, browse-preferred species were absent, and non-preferred species showed signs of heavy repeated browsing.
Field sampling crews assessed direct and indirect evidence of non-native earthworms in three different locations within each plot. This included casts, middens, earthworm burrows, and earthworms detected during soil sampling. This rapid technique is similar to other protocols (Loss et al. 2013; Fisichelli et al. 2013) and captures presence of non-native earthworms but does not capture species composition or abundance.
In addition to biotic stressors, we included several environmental variables assessed at the plot level (Table 1). To quantify soil pH, the upper (A) mineral soil horizon was collected from each plot. In plots where the organic soil horizon was directly above bedrock (no mineral soil horizon present), a sample of the organic horizon was collected instead. Soil pH was measured in distilled water with an electronic pH meter. Organic layer depth was also measured. Climate data for each plot were extracted from PRISM 800 m2 resolution climate surfaces (2001–2010 averages, PRISM Climate Group 2012).
Analyses
All analyses were conducted in R v3.3.2 (R Core Team 2016). All data were averaged to the plot level, e.g., number of non-native species and percent cover from the eight 1-m2 quadrats. We calculated Spearman rank correlations (ρ) and significance tests among biotic global change drivers (deer browse, non-native earthworm presence, non-native plant richness, and non-native plant cover) and among environmental variables. Spearman rank correlations are appropriate for non-normally distributed and ordinal data (Sokal and Rohlf 1995). Due to lack of complete independence between non-native plant richness and cover (cover = 0 whenever richness = 0), this correlation was only calculated for plots with at least one non-native species present (160 of 350 plots).
To more holistically assess relationships among biotic stressors in the understory, we utilized generalized linear mixed-effects models, model comparison with Akaike information criteria (AIC), and a structural equation modeling (SEM) framework. SEM is a useful framework for gaining insights into the direct and indirect relationships among variables in ecological systems (Grace 2006). SEM is a method for inferring and testing causal relationships among variables and is most often used in observational studies (Grace et al. 2012). Recent advances have made this tool more flexible and useful for ecological data (Grace et al. 2012; Lefcheck 2015). Historically, SEM has required assumptions of normally distributed data and independence among observations (Grace 2006). Direct acyclic or piecewise SEM allows for inclusion of response variables with non-normal distributions, random effects, and different correlation structures using local estimation (Lefcheck 2015). In our study, we were able to include response variables with Gaussian, Poisson, and binomial error distributions and the hierarchical structure of the research design (blocks nested in site). Piecewise SEM supports confirmatory path analysis but has some limitations such as inability to evaluate feedback loops or include latent variables. For example, the feedback loop of deer to earthworms and back to deer cannot be assessed here and is likely better examined with longitudinal data. SEM analyses utilized the piecewise SEM package (Lefcheck 2015).
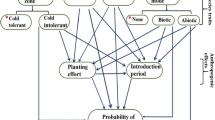
We used pre-existing knowledge about the system to construct a structural equation model and hypothesized causal relationships (pathways) among variables within the model (see Fig. S1 for full hypothesized model, key pathways highlighted below). We are interpreting the relationships uncovered in this observational study as quantitative causal claims based on the assumptions developed from previous experiments and that these causal relationships are likely transferable to other datasets from similar forest types in similar environmental conditions (Pearl 2012). Statistical tests of the pathways in the SEM do not prove causation but rather provide support for the causal claims. In SEM, single-headed arrows represent direct causal relationships, with the variable at the tail of the arrow representing the predictor of the variable at the head of the arrow. The overarching goal was to test the following hypothesized relationships among biotic global change stressors (see “Introduction” for justifications for these relationships):
Earthworm presence ← Deer browse
Non-native plant richness ← Earthworm presence
Non-native plant richness ← Deer browse
Non-native plant cover ← Earthworm presence
Non-native plant cover ← Deer browse
Non-native plant cover ← Non-native plant richness
Two environmental drivers were included in the SEM, soil pH and summer mean monthly air temperature (JJA). Both variables are known to affect earthworms and non-native plants. Higher soil pH is more conducive to non-native plant and earthworm invasion than acidic, low-nutrient soils (Alpert et al. 2000; Fisichelli et al. 2013). Warmer temperatures facilitate non-native species colonization, reproduction, and population persistence (Walther et al. 2009). Furthermore, warmer temperatures are related to non-native plant species occurrence in forest plots in the region (Fisichelli et al. 2014; Oswalt et al. 2015). For earthworms, extremes of hot and cold temperatures can be limiting. Within the north temperate forests in this study, warmer temperatures likely promote earthworm occurrence (Curry 2004). There are many other potential environmental variables that could have been included in analyses. We selected soil pH and temperature due to their known effects to response variables, straightforward measurement techniques, and known correlations with other drivers (Table S2). For example, summer temperature is positively related to summer precipitation (ρ = 0.85, p < 0.001). Soil pH is also positively correlated to summer precipitation (ρ = 0.64, p < 0.001) and negatively correlated with overstory conifer basal area (ρ = − 0.51, p < 0.001), so higher soil pH plots received more summer precipitation and tended to have fewer conifers in the overstory. Thus, these individual environmental drivers most accurately reflect a suite of related understory conditions. Additional explanatory variables such as deer density were not available across all study parks and thus omitted from analyses. Here, we focus on the global change stressors, including deer browse pressure, and their relationships.
The SEM contains three component models, with the response variables earthworms (presence and absence), non-native species richness, and non-native plant cover. We developed generalized linear mixed-effects models with the associated response variable distribution (binomial for earthworm presence and absence, Poisson for non-native species richness, and Gaussian for non-native plant cover). For non-native plant cover, we only included plots with non-native plants present (n = 160) since non-native plant absence was modeled in the non-native plant richness analyses and omission of the plots with 0% cover permitted a Gaussian approach. Each mixed-effects model included a random effect of park subunit (n = 15) nested in park (n = 8) (i.e., block nested in site) and used maximum likelihood estimation with the lme4 package (Bates et al. 2015). The non-native species richness model included an additional individual-level random effect because of overdispersion (Harrison 2014). Based on the hypothesized SEM, we included three explanatory variables for earthworm presence (deer browse, summer temperature, and soil pH) in the full model. For non-native plant richness we included four explanatory variables (deer browse, earthworm presence, summer temperature, and soil pH), and for non-native plant cover we used five explanatory variables (deer browse, earthworm presence, non-native plant richness, summer temperature, and soil pH). Non-native plant cover was natural log transformed to meet statistical assumptions (i.e., linear relationships and homoscedasticity of errors). We used bivariate scatterplots and residual plots to visually assess relationships, model fit, and normality of errors.
We conducted model comparison for each SEM component model using AIC (Burnham and Anderson 2002). We compared models varying in complexity from inclusion of only a single explanatory variable to a model with all additive effects. Earthworm, non-native plant richness, and non-native plant cover models with the lowest AIC values (ΔAIC < 2) and fewest parameters are considered the best fit (Burnham and Anderson 2002). We also calculated and report individual pathway unstandardized coefficient estimates, standard errors, and significance tests using the Kenward–Rogers approximation (Lefcheck 2015). It is not possible to calculate standardized path coefficients for generalized linear models with Poisson or binomially distributed response variables since these response variables cannot be standardized. We calculated variance explained (R2) for each generalized linear mixed-effects model using the methods of Nakagawa and Schielzeth (2013) and report the marginal R2 for the fixed effects included in the SEM.
Results
Biotic global change stressors were abundant across plots (Fig. 2; Table S3). Evidence of non-native earthworm presence was found at 42% of plots (147 of 350 plots). Earthworm presence was lowest at Acadia, ME (9% of plots) and highest at Morristown, NJ (100%) and Weir Farm, CT (100%). Deer browse damage was observed in all study parks (overall index mean = 1.83 on a scale from 1 to 4) and all parks had at least three plots with high browse damage (index = 3). Low browse damage (index = 1) was most common (44%, 153 plots) and very high damage (index = 4) least common (5%, 17 plots). Overall, the lowest browse pressure was found in Acadia [mean (SD) = 1.4 (0.6)], Marsh-Billings-Rockefeller, VT [mean (SD) = 1.6 (0.8)], and Saint-Gaudens, NH [mean (SD) = 1.6 (0.8)]. The highest mean browse pressure was found at Morristown [mean (SD) = 3.2 (0.7)] and Weir Farm [mean (SD) = 2.9 (1.0)]. Non-native plants were present at all parks and in 46% of plots (160 plots) overall. Plots averaged 0.72 non-native species (SD = 1.32) per 1-m2 subplot. Non-native plant cover averaged 6.3% (SD = 16.4), with a maximum plot average of 122%. The most heavily invaded parks include Saratoga National Historical Park, NY [means (SD): 2.9 (2.1) species, 21 (23)% cover], Morristown [means (SD): 2.3 (1.6) species, 34 (35)% cover], and Minute Man, MA [means (SD): 1.8 (0.9) species, 17 (13)% cover]. The least invaded sites include Acadia [means (SD): 0.05 (0.2) species, 0.2 (1.1)% cover] and Marsh-Billings-Rockefeller [means (SD): 0.2 (0.3) species, 0.4 (0.8)% cover].
Bivariate relationships among biotic global change stressors (plot-level averages, n = 350). Spearman rank correlations are shown in the upper left corner of each subpanel (all correlation p values < 0.001). Deer browse damage increases from 1 (low) to 4 (very high). Non-native plant values are plot averages per m2. Boxplot boxes are the interquartile range, thick horizontal lines are the medians, dashed vertical lines are the outer tails (1.5 × interquartile range), and open circles are plot values beyond the outer tails
All biotic global change stressors were significantly correlated with one another (all Spearman rank correlations ≥ 0.44 and p values < 0.001) (Fig. 2). All bivariate plots show positive relationships among biotic stressors, thus earthworm presence increases with deer browse, and non-native plant richness and cover both increase with earthworm presence and deer browse. Consequently, 28% of plots (101 plots) had earthworms absent, low deer browse (index = 1) and no non-native plants. Furthermore, 29% of plots (103 plots) included earthworms, non-native plants, and moderate or more severe browse damage.
The hypothesized SEM fit the data well and supported multiple pathways among global change stressors (Table 2; Fig. 3; Table S4). All supported pathways show positive relationships among variables. Evidence of non-native earthworms was best fit (lowest AIC) with the full hypothesized model including all three explanatory variables (deer browse, soil pH, and summer temperature). The second best fit model omitted deer browse and had a ∆AIC = 7.4. Non-native plant richness was best fit with the model containing soil pH and earthworms (∆AIC = 0.3 and fewest parameters [2] among models with ∆AIC < 2). There was also some support for the hypothesized pathway between summer temperature and non-native richness (path coefficient p value < 0.05 and inclusion in the lowest AIC model). The more complex, hypothesized model including four explanatory variables (soil pH, earthworms, deer, and temperature) performed similarly (∆AIC 1.7) to the simpler two-variable model with only soil pH and earthworms. Similar to richness, non-native plant cover was best explained by a two-parameter model including deer browse and non-native plant richness (∆AIC = 0.5 and fewest parameters [2] among models with ∆AIC < 2). There was marginal support for the pathway between soil pH and non-native plant cover (p value < 0.1 and inclusion in the lowest AIC model). The hypothesized plant cover model with five explanatory variables was not supported (∆AIC = 3.3). Component models explained 33–67% of the variance (R2), based on fixed effects.
Structural equation model (SEM) illustrating supported relationships among biotic global change stressors and environmental variables. Numbers on the arrows are unstandardized path coefficients. Bold black arrows are supported pathways in the best fit, parsimonious model (ΔAIC < 2 and fewest parameters), medium gray arrows are additional pathways in the lowest AIC model (ΔAIC = 0), and fine gray arrows are unsupported pathways (more complicated models with ΔAIC > 0, coefficients not shown). Bold black pathway coefficients have a p value < 0.05 and gray coefficients are marginally significant (p value 0.05–0.10). Three component models within the SEM are generalized linear mixed-effects models with the responses: earthworm presence (binomial), non-native plant richness (Poisson), and (ln)non-native plant cover (Gaussian). Percentages are the variance explained by fixed effects for each response variable (marginal R2)
The SEM supported direct effects of deer browse, soil pH, and air temperature on non-native plant measures as well as indirect positive effects mediated through earthworm presence and non-native plant richness (Fig. 3). For example, soil pH effects on both non-native plant variables occurred through direct pathways (positive effect) and indirectly through soil pH influence on earthworm presence (positive effect). Deer browse and air temperature showed similar direct and indirect relationships to non-native plant variables. Earthworm presence also directly influenced non-native plant richness and indirectly influenced cover through its effect on richness.
Discussion
Deer browse damage, non-native earthworms, and non-native plants are present within forest understories across the northeastern U.S., including within sites receiving the highest levels of protection (i.e., national parks). All these biotic global change stressors are positively correlated with one another; moreover, there was support for four out of six hypothesized relationships among these biotic stressors in the SEM. Environmental conditions, namely higher soil pH, warmer temperatures, and related factors, appear to contribute to the presence and magnitude of these biotic stressors. The variation in and strong relationships among understory stressors across the landscape suggest opportunities to preserve native forest conditions in areas not yet severely impacted as well as the tremendous multipronged management challenge for areas already experiencing the combined effects of weeds, worms, and deer.
Biotic global change agents
As mentioned above, most hypothesized relationships among biotic global change stressors, based on a large body of scientific knowledge (e.g., Côté et al. 2004; Nuzzo et al. 2009; Rearick et al. 2011; Waller and Maas 2013; Craven et al. 2016), were supported in analyses. Thus, across a large number of sites and including plots in all available forest types, deer affect earthworms and deer and earthworms affect non-native plants. These are the types of positive relationships that may lead to invasional meltdowns (Simberloff and Von Holle 1999). Reciprocal relationships among these variables likely also exist in specific circumstances, though we were unable to test for them due to the constraints of the piecewise SEM structure (Lefcheck 2015). For example, palatable non-native plant species with high quality leaf litter may promote both deer and earthworms, especially within local areas with otherwise unpalatable native species producing low-quality litter and acidic soils (Kourtev et al. 1999).
The presence and strengths of biotic global change stressors across large landscapes are better understood for non-native plants than earthworms or deer browse pressure. Patterns of non-native plants across parks in the study generally match nearby parks in the National Park System (Fisichelli et al. 2014) and USFS Forest Inventory and Analysis (FIA) data that includes plots under all ownership types in the study region (Oswalt et al. 2015). This research captures overall non-native plant richness and cover, which both relate strongly to other biotic stressors and environmental conditions. Although we did not look at individual non-native plant species, the most abundant species in these parks include Berberis thunbergii, Frangula alnus, and Lonicera spp., all species known to be associated with deer and earthworm impacts (Kourtev et al. 1999; Frelich et al. 2006).
This study also provides evidence for ubiquitous earthworm presence across multiple states in the northeast U.S., similar to findings for mid-Atlantic states (Dávalos et al. 2015c) and the western Great Lakes region (Loss et al. 2013; Fisichelli et al. 2013). We did not collect data on earthworm density, biomass, or species composition, although all have been shown to influence understory plants and soil conditions (Suárez et al. 2006; Craven et al. 2016). Non-native earthworm community characteristics have been documented in research at smaller spatial scales in the region, though abundances and species distributions remain uncertain at larger spatial scales (Hendrix and Bohlen 2002).
Similar to earthworms, we found pervasive evidence of browse damage within plots at all study sites. Deer densities across eastern forests are considered chronically high (McShea 2012), though we did not attempt to compare browse pressure with an historical range of conditions to quantitatively assess whether the influence of deer can be considered extreme and a global change stressor. Browse damage is a more proximal measure of deer influence on vegetation than deer population estimates but may not fully capture variation in browse preference and vegetation availability within the browse layer (0.2–2 m above ground level). Regardless, selective herbivory is a major driver of understory plant performance (Fisichelli et al. 2012) and the high browse levels found in many plots and the browse relationships to weeds and worms confirm the importance of this stressor.
This research included a single census (completed over 4 years) and thus is a snapshot in time and does not reflect biotic stressor temporal dynamics within the system. The rate of responses (e.g., increase in percent cover of non-native plants) and interannual variability are not known. However, deer browse damage to woody plants is detectable for many years and non-native plants in the study include perennial herbaceous and woody species likely detectable each year. Similarly, evidence of earthworms, including middens and removal of the organic layer are relatively persistent signs. Thus, although some variables may show some interannual variability (e.g., percent cover and number of non-native species), the relationships quantified here likely persist over time. The plots included in this study are permanent, long-term monitoring plots sampled on a 4-year cycle and thus temporal variability can be assessed in the future.
Environmental conditions
We selected a small number of environmental variables and recognize that these may reflect other important and unmeasured factors, such as are present in any observational study of a complex system. Soil pH was a predictor of all biotic stressors in the SEM. Soil pH directly influences the soil microbial community (Fierer and Jackson 2006) and the cycling and availability of nutrients and metals (e.g., aluminum) in the soil (Schoenholtz et al. 2000), with cascading effects on earthworms and non-native plants (Kourtev et al. 1999; Frelich et al. 2006). Similarly, more basic and nutrient rich soils also harbor larger numbers and cover of non-native species (Dávalos et al. 2015c). Soil pH reflects the current overstory (e.g., the conifer component and acidic needleleaf litter inputs), past vegetation composition, and historical land use (e.g., soil tilling) (Schoenholtz et al. 2000). Soil pH was also correlated with summer temperature; cooler northern sites were more acidic (and had a greater conifer overstory component). Thus, soil pH likely influenced the system through multiple effects and multiple related factors.
Similar to soil pH, temperature relates to multiple factors. Warmer sites in the study are to the south and are in generally more fragmented landscapes with greater residential and commercial development, agriculture, and transportation corridors (Theobald 2013). These areas likely have had greater opportunity for human-mediated introduction of non-native plants and earthworms onto the landscape (Hendrix and Bohlen 2002; Oswalt et al. 2015). Furthermore, fragmented landscapes with abundant edge habitat and adjacent agricultural fields can support larger numbers of deer than intact forests (Côté et al. 2004). Thus, landscape conditions and history likely promote biotic stressors at a broader spatial scale while plot level conditions (e.g., soil pH) determine local-level presence and severity of biotic stressors.
Restoration and adaptation
The chronic nature of browse damage in forest understories (McShea 2012) and linkages to earthworms, non-native plants, and other global change stressors such as temperature (Fisichelli et al. 2012) indicate browse pressure as a key stressor and potential intervention point for successful management. Focusing effort solely on non-native plant eradication may not have desired outcomes if deer browse pressure and earthworms are not simultaneously addressed (Nuzzo et al. 2009; Waller and Maas 2013). Although often challenging to implement due to social and political hurdles, reductions in deer populations are possible and can have desired effects (McShea 2012). Unfortunately for non-native earthworm management, we are unaware of any feasible control measures to eradicate established populations.
Another global change agent, warming temperatures, is likely to affect forest understory dynamics and promote the biotic stressors (based on the SEM results). Cold, acidic, conifer dominated forests currently have relatively low stressor levels. All parks in the study have already experienced significant warming (Monahan and Fisichelli 2014) and it is unclear how or if forest composition will change in the future as temperatures continue to warm. Northern conifer species present at parks in the study are projected to undergo substantial decreases in potential suitable habitat as temperatures warm, while at the same time broadleaf species gain suitable habitat (Fisichelli et al. 2014). Because deer, earthworms, and non-native plants are already present at all parks, decline of the current overstory and shift in tree composition to broadleaf species could allow these biotic stressors to establish, assuming soil pH and other conditions are suitable. Early detection and rapid response to control these stressors may be effective in these areas where they are not yet widely established (Abella 2014). It is important to note that responses by native biota are species-specific and some may perform well in the presence of non-native plants (Dávalos et al. 2015a), benefit from browse pressure on competing palatable plants (Rooney and Waller 2003), or respond positively to leaf litter loss, such as from earthworm consumption (Warren et al. 2012). Understanding the status and relationships among understory global change stressors and associated environmental variables is critical to identifying vulnerable sites and developing successful management strategies tailored to individual site conditions and priority native species.
References
Abella S (2014) Effectiveness of exotic plant treatments on National Park Service lands in the United States. Invasive Plant Sci Manag 7:147–163
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66
Bartuszevige AM, Hrenko RL, Gorchov DL (2007) Effects of leaf litter on establishment, growth and survival of invasive plant seedlings in a deciduous forest. Am Midl Nat 158:472–477
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cleland DT, Freeouf JA, Keys JE, Nowacki GJ, Carpenter CA, McNab WH (2007) Ecological subregions: sections and subsections for the conterminous United States. Gen Tech Report WO-76D. U.S. Department of Agriculture, Forest Service, Washington, DC
Côté SD, Rooney TP, Tremblay JP, Dussault C, Waller DM (2004) Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst 35:113–147
Craven D, Thakur MP, Cameron EK, Frelich LE, Beauséjour R, Blair RB, Blossey B, Burtis J, Choi A, Dávalos A, Fahey TJ, Fisichelli NA, Gibson K, Handa IT, Hopfensperger K, Loss SR, Nuzzo VA, Maerz JC, Sackett T, Scharenbroch BC, Smith SM, Vellend M, Umek L, Eisenhauer N (2016) The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob Change Biol 23:1065–1074
Curry JP (2004) 6 factors affecting the abundance of earthworms in soils. In: Edwards CA (ed) Earthworm ecology, 2nd edn. CRC Press, Boca Raton, pp 91–113
Dávalos A, Nuzzo V, Blossey B (2015a) Single and interactive effects of deer and earthworms on non-native plants. For Ecol Manag 351:28–35
Dávalos A, Nuzzo V, Blossey B (2015b) Interactive effects of deer, earthworms and non-native plants on rare forest plant recruitment. Biol Conserv 187:173–181
Dávalos A, Simpson E, Nuzzo V, Blossey B (2015c) Non-consumptive effects of native deer on introduced earthworm abundance. Ecosystems 18:1029–1042
Dobson A, Blossey B (2015) Earthworm invasion, white-tailed deer and seedling establishment in deciduous forests of north-eastern North America. J Ecol 103:153–164
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Fisichelli NA, Frelich LE, Reich PB (2012) Sapling growth responses to warmer temperatures ‘cooled’ by browse pressure. Glob Change Biol 18:3455–3463
Fisichelli NA, Frelich LE, Reich PB, Eisenhauer N (2013) Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biol Invasions 15:1057–1066
Fisichelli NA, Abella SR, Peters MP, Krist FJ Jr (2014) Climate, trees, pests, and weeds: change, uncertainty, and biotic stressors in eastern U.S. National Park forests. For Ecol Manag 327:31–39
Frelich LE, Hale CM, Scheu S, Holdsworth AR, Heneghan L, Bohlen PJ, Reich PB (2006) Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol Invasions 8:1235–1245
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Grace JB, Schoolmaster DR, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW (2012) Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3:1–44
Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2:e616
Hendrix PF, Bohlen PJ (2002) Exotic earthworm invasions in North America: ecological and policy implications. Bioscience 52:801–811
Hobbs RJ, Higgs E, Hall CM, Bridgewater P, Chapin FS, Ellis EC, Ewel JJ, Hallett LM, Harris J, Hulvey KB (2014) Managing the whole landscape: historical, hybrid, and novel ecosystems. Front Ecol Environ 12:557–564
Kalisz S, Spigler RB, Horvitz CC (2014) In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proc Natl Acad Sci USA 111:4501–4506
Karberg NJ, Lilleskov EA (2009) White-tailed deer (Odocoileus virginianus) fecal pellet decomposition is accelerated by the invasive earthworm Lumbricus terrestris. Biol Invasions 11:761–767
Kourtev P, Huang W, Ehrenfeld J (1999) Differences in earthworm densities and nitrogen dynamics in soils under exotic and native plant species. Biol Invasions 1:237–245
Lefcheck JS (2015) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7(5):573–579
Loss SR, Hueffmeier RM, Hale CM, Host GE, Sjerven G, Frelich LE (2013) Earthworm invasions in northern hardwood forests: a rapid assessment method. Nat Areas J 33:21–30
Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Madritch MD, Lindroth RL (2009) Removal of invasive shrubs reduces exotic earthworm populations. Biol Invasions 11:663–671
McShea WJ (2012) Ecology and management of white-tailed deer in a changing world. Ann NY Acad Sci 1249:45–56
Miller KM, Dieffenbach FW, Campbell JP, Cass WB, Comiskey JA, Matthews ER, McGill BJ, Mitchell BR, Perles SJ, Sanders S (2016) National parks in the eastern United States harbor important older forest structure compared with matrix forests. Ecosphere. https://doi.org/10.1002/ecs2.1404
Monahan WB, Fisichelli NA (2014) Climate exposure of US national parks in a new era of change. PLoS ONE 9(7):e101302. https://doi.org/10.1371/journal.pone.0101302
Morellet N, Gaillard J, Hewison A, Ballon P, Boscardin Y, Duncan P, Klein F, Maillard D (2007) Indicators of ecological change: new tools for managing populations of large herbivores. J Appl Ecol 44:634–643
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
National Fish, Wildlife and Plants Climate Adaptation Partnership (NFWPCAP) (2012) National fish, wildlife and plants climate adaptation strategy. Association of Fish and Wildlife Agencies, Council on Environmental Quality, Great Lakes Indian Fish and Wildlife Commission, National Oceanic and Atmospheric Administration, and U.S. Fish and Wildlife Service, Washington
National Park System Advisory Board (NPSAB) (2012) Revisiting leopold: resource stewardship in the national parks. National Park System Advisory Board, Washington. http://www.nps.gov/calltoaction/PDF/LeopoldReport_2012.pdf
Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conserv Biol 23:966–974
Oswalt CM, Fei S, Guo Q, Iannone BV III, Oswalt SN, Pijanowski BC, Potter KM (2015) A subcontinental view of forest plant invasions. NeoBiota 24:49
Pearl J (2012) The causal foundations of structural equation modeling. In: Hoyle RH (ed) Handbook of structural equation modeling. Guilford Press, New York, pp 68–91
PRISM Climate Group (2012) Oregon State University. http://prism.oregonstate.edu
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://CRAN.R-project.org/
Rearick D, Kintz L, Burke KL, Ransom TS (2011) Effects of white-tailed deer on the native earthworm, Eisenoides carolinensis, in the southern Appalachian Mountains, USA. Pedobiologia 54:S173–S180
Rooney TP, Waller DM (2003) Direct and indirect effects of white-tailed deer in forest ecosystems. For Ecol Manag 181:165–176
Roth AM, Whitfeld TJ, Lodge AG, Eisenhauer N, Frelich LE, Reich PB (2015) Invasive earthworms interact with abiotic conditions to influence the invasion of common buckthorn (Rhamnus cathartica). Oecologia 178:219–230
Schoenholtz SH, Van Miegroet H, Burger J (2000) A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. For Ecol Manag 138:335–356
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. WH Freeman, New York
Suárez ER, Fahey TJ, Yavitt JB, Groffman PM, Bohlen PJ (2006) Patterns of litter disappearance in a northern hardwood forest invaded by exotic earthworms. Ecol Appl 16:154–165
Theobald DM (2013) A general model to quantify ecological integrity for landscape assessments and US application. Landsc Ecol 28:1859–1874
Tierney GL, Mitchell BR, Miller KM, Comiskey JA, Kozlowski AJ, Faber-Langendoen D (2016) Northeast Temperate Network long-term forest monitoring protocol: 2016 revision Natural Resource Report NPS/NETN/NRR—2016/1184
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Waller DM, Maas LI (2013) Do white-tailed deer and the exotic plant garlic mustard interact to affect the growth and persistence of native forest plants? For Ecol Manag 304:296–302
Walther G, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Warren RJ, Bahn V, Bradford MA (2012) The interaction between propagule pressure, habitat suitability and density-dependent reproduction in species invasion. Oikos 121:874–881
Acknowledgements
This research was supported by the Maine Timberlands Charitable Trust. We thank the National Park Service Northeast Temperate Network staff for their tremendous work which makes such studies possible.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fisichelli, N.A., Miller, K.M. Weeds, worms, and deer: positive relationships among common forest understory stressors. Biol Invasions 20, 1337–1348 (2018). https://doi.org/10.1007/s10530-017-1630-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1630-y