Abstract
We report results from a 2-year study on the succession pattern of macrofouling assemblages in the Taranto Sea, an important alien species hotspot in the Mediterranean Sea. Four sets of PVC panels were used as macrofouling collectors; each unit was installed at a different time (April 2013, July 2013, October 2013 and January 2014) and then surveyed quarterly for 1 year. The macrofouling community consisted of 93 sessile invertebrate species, of which 16 were NIS and five were cryptogenic. In both years non-indigenous species (NIS) recruitment occurred mainly in the quarter July/October in concert with the settlement of pioneer autochthonous species. This recruitment is independent of immersion time, occurring on both bare substrates and on previously colonized panels. This increase in NIS coverage is influenced by the development stage of the community, suggesting that NIS grow better without potential competitors. Two sets of NIS were distinguished. The first included abundant ascidians, serpulids, and bryozoans that are structuring components of early communities when favorable conditions exist (i.e. a lack of competitive autochthonous species). After settlement, these species are unable to develop in later-stage communities. The second set of NIS was composed of sabellid worms that settle in early and late communities but, unlike the other NIS, are able to persist and become dominant in late macrofouling communities independent of seasonal changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-indigenous species (NIS), coupled with anthropogenic habitat modification (Bulleri and Chapman 2010; Glasby et al. 2007) are considered the most important drivers of biodiversity loss in coastal environments (e.g. MEA 2005; Airoldi and Bulleri 2011). Marine NIS may become invasive, leading to native species displacement, alteration in species interactions, productivity, and energy flow between trophic groups (Sorte et al. 2010; Byers et al. 2010; Wikström and Hillebrand 2012; Lord et al. 2015), shifted community structure (Carlton 1996), and habitat modification, all of which may result in altered ecosystem services and substantial economic losses (Grosholz 2002; Vilà et al. 2009; Katsanevakis et al. 2014). Despite the increasing studies on NIS, much remains to be known relative to how NIS affect indigenous communities (Lasram et al. 2008; Rilov and Galil 2009). Moreover, an expanded understanding of mechanisms used by NIS to invade new localities is clearly needed, including what processes facilitate or hinder their establishment (Bulleri and Airoldi 2005; Wasson et al. 2005). Studies focusing on the variation in temporal recruitment of native versus NIS (Stachowicz et al. 2002), coupled with the potential adaptations of NIS life-cycles to a new environment are still in their infancy.
Marine invasions are often facilitated through the transport of species by ship (such as in ballast water and as hull fouling) and by mariculture (Ruiz et al. 2000; Katsanevakis et al. 2013). Ship hull fouling has enabled the introduction of numerous species including sponges, hydroids, tube worms, barnacles, mussels, bryozoans, sea squirts, and algae. Indeed, every hard artificial substrate in the shallow marine realm (e.g. ship hulls, piers, pontoons, pilings, seawalls and buoys) is subject to biofouling (Wahl 1997; Railkin and Press 2004; Dürr and Thomason 2009).
The majority of marine NIS are reported within lagoons and harbors (Occhipinti-Ambrogi and Savini 2003; Occhipinti-Ambrogi et al. 2011). This could be explained by the low native diversity often characterizing these environments coupled with high vector-mediated propagule pressure and the presence of colonisable artificial substrates (Carlton and Geller 1991; Holle and Simberloff 2005; Clark and Johnston 2009; Minchin et al. 2009; Ruiz et al. 1997; Wonham and Carlton 2005). Pilings, breakwaters, pontoons, seawalls, and jetties serve as novel marine habitats for epibiota (e.g. Bulleri and Chapman 2010; Connell 2000) and often represents the first point of introduction of marine NIS within fouling communities (Ruiz et al. 2000; Wasson et al. 2001; Darbyson et al. 2009). Invasive species are hypothesized to be better adapted for survival on such artificial substrates, possibly out-competing native species for resources (Byers 2002). Therefore, artificial structures should be considered facilitators of NIS invasion (Ruiz et al. 2009) and promoters of their expansion (Connell and Glasby 1999; Glasby 1999).
Biofouling assemblages represent an ideal study system for ecologists due to their amenability for experimentation, their ease of access (without requiring low tides or vessel use), and the organisms’ proclivity to settle on artificial substrates and their subsequent fast growth (Canning-Clode et al. 2010). Studies of ecological succession of these assemblages can be used to address contemporary environmental problems (i.e. changes in biodiversity and ecosystem services) as impacted by invasive species (Prach and Walker 2011).
In the present work, we report results from 2 years of investigation on the succession pattern of NIS foulers and their influence on autochthonous species settlement in the Taranto Seas, one of the most important alien hotspots in the Mediterranean area (Occhipinti et al. 2011). Forty-four NIS are reported in the Taranto Seas (Occhipinti-Ambrogi et al. 2011; Cecere et al. 2015). The Seas host one of the most important commercial ports of the Italian coast, a NATO naval base, and many mussel farms, making the area particularly exposed to the introduction of alien species (Petrocelli et al. 2013). According to Cecere et al. (2015) increases in recent years of mollusc importation and shipping are the most significant visible threats.
A large number of studies have been conducted in the area, permitting characterization of the fouling communities (Parenzan 1969; Tursi et al. 1976; Gherardi 1973; Gherardi 1974; Tursi et al. 1982). More recently, numerous studies have been carried out regarding the discovery of NIS (Brunetti and Mastrototaro 2004; Mastrototaro et al. 2004, Mastrototaro and Brunetti 2006; Longo et al. 2007; Giangrande et al. 2012; Petrocelli et al. 2013; Lezzi et al. 2015). However, to date, most of the work remains descriptive and there are no data on NIS seasonality or community development. We investigated how the coupled effects of starting time and time of immersion can affect settlement trends and growth in native species, NIS, and cryptogenic species during macrofouling community development.
Materials and methods
Field sampling and processing
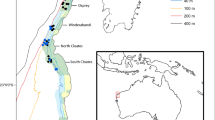
The study was conducted in the south western Mar Grande of Taranto (40°25′56″N 17°14′19″E), in the north western Ionian Sea (eastern-central Mediterranean). The Mar Grande is a semi-enclosed basin with an area of 35.5 km2 and a maximum depth of 42 m, and is bordered by the Chéradi Islands on its western side (Fig. 1a). It shows seasonal temperature variations typical of the coastal Ionian regions with an average annual value of about 18 °C (Fig. 1b); the salinity is about 38‰ and nearly uniform over the entire year.
PVC panels measuring 15 × 15 × 0.5 cm2 were used as macrofouling collectors (e.g. Sutherland and Karlson 1977; Canning-Clode et al. 2009). Prior to deployment each panel was abraded using sandpaper with a grain size of 60 to facilitate larval settlement, and to prevent the detachment of sessile organisms. It was then covered on each side using 1 cm mesh. Panels (as detailed below) made up of three replicates located at different depths (0, 3 and 6 m) were placed in the water, tied to a vertical ballasted nylon rope, which was anchored to an offshore floating raft. The modules were deployed at 2–6 m intervals. The study site (9 m depth) was about 50 m from harbour piers and 30 m from mussel farms, environments characterized by important fouling communities that act as larval supply sources.
In order to study the NIS macrofouling communities over the period of 1 year, four sets of panels, each one consisting of 36 panels (4 collection times × 3 depths × 3 replicates), were installed at four different times: April 2013, July 2013, October 2013 and January 2014, named S1, S2, S3 and S4 respectively, amounting to a total of 144 panels. Each set was surveyed at 3-month intervals to capture four different immersion times: T1 (3 months of immersion), T2 (6 months of immersion), T3, (9 months of immersion), and T4 (12 months of immersion). Three replicates were selected at random during the sampling (Fig. 2).
Only the sessile macrobenthic organisms were considered in the present study. In the laboratory, each panel was in vivo photographed before fixation (Supplementary material, Fig. 1). Ascidians were extracted and anesthetized in menthol before being preserved in 4% formaldehyde-seawater solution. Otherwise, all samples were fixed directly in a solution of 4% formaldehyde-seawater. Sessile organisms were then identified to the lowest possible taxonomic level using stereo and binocular microscopes. Photographs from panels were analysed using the software ImageJ (Abràmoff et al. 2004) which determines the percent coverage of the organisms, thus obtaining coverage matrices of each species detected. Coverage for arborescent organisms was calculated as a projection of each of the branches on the surfaces. The central part of each panel was analysed, excluding an external frame of 1 cm width to avoid sampling of potential edge effects (Cifuentes et al. 2010); the total surface area was 196 cm2. A multi-layer coverage was considered a potential surface coverage of greater than 100%.
Data analysis
Species were categorized into IS (indigenous species), NIS (non-indigenous species), and cryptogenic. References used to assign either “non-indigenous” or “cryptogenic” status are provided in Table 1.
Macro-invertebrates indigenous and non-indigenous species, species richness, and percent coverage were computed as dependent variables using PERMANOVA in an approach similar to parametric ANOVA. Univariate PERMANOVA tests were run on Euclidean distances matrices with 9999 permutations (Anderson 2001). Starting time (S, 4 levels), Immersion time (T, 4 levels) and depth (D, 3 levels) factors were used to detect differences among NIS, IS, total species richness, and coverage in the development of the macrofouling communities. Each NIS and cryptogenic species were individually analyzed using Univariate PERMANOVA tests with the same experimental design.
To further explore the hypotheses that immersion time and/or temperature influenced community development, analysis of covariance (ANCOVA) was applied to the dataset of dependent variables (NIS Richness, Indigenous richness and NIS Coverage), and the continuous (Temperature in the sampling time) and categorical (Age of Immersion) predictors. This procedure first required testing the homogeneity of the slope assumption; if there was no interaction between variables, the interaction’s independent effects of predictors was performed.
Multivariate analyses were used to compare the similarity of macrofouling NIS communities developed on the panels.
Permutational analysis of variance, PERMANOVA (Anderson et al. 2005) was used to test for differences in the NIS community for each starting time (S, 4 levels) using 3-month intervals survey or immersion time (T, 4 levels) and depth (D, 3 levels) as factors. Log transformed data (i.e. to decrease the contribution of the most abundant species to the dissimilarity) on a Bray–Curtis similarity matrix under 9999 permutations was used to perform the analyses (Anderson 2001).
Principal coordinates ordination (PCO) based on the Bray–Curtis dissimilarity matrix of percent cover of NIS was also used to visualize differences in overall community structure through time. To increase the clarity of PCO plots NIS were added as a vector overlay on the plot. This is considered one of the most suitable visual complements to PERMANOVA output (Anderson and Willis 2003).
ANCOVA tests were performed using the STATISTICA software package. The PERMANOVA and PCO, were conducted in PRIMER v6+PERMANOVA software (Anderson et al. 2008).
Results
Species diversity
There were 93 total species of sessile invertebrates found in the macrofouling community of which 72 were IS, 16 NIS and 5 cryptogenic (Table 1). Some species categorized as cryptogenic however, may prove to be NIS once their status is resolved.
The species recognized as NIS are: Paraleucilla magna Klautau, Monteiro and Borojevic, 2004, Polyandrocarpa zorritensis (Van Name, 1931), Branchiomma bairdi (McIntosh, 1885), Branchiomma luctuosum (Grube, 1870), Celleporaria brunnea (Hincks, 1884), Distaplia bermudensis Van Name, 1902, Microcosmus squamiger Michaelsen, 1927, Perophora multiclathrata (Sluiter, 1904), Watersipora subtorquata (d’Orbigny, 1852), Styela plicata (Lesueur, 1823), Styela canopus (Savigny, 1816), Amathia verticillata (delle Chiaje, 1822), Balanus trigonus Darwin, 1854, and Hydroides elegans (Haswell, 1883), Hydroides dirampha Mörch, 1863 and Hydroides dianthus (Verrill, 1873). The species recognized as cryptogenic are Bugula neritina (Linnaeus, 1758), Simplaria pseudomilitaris (Thiriot-Quievreux, 1965), Ecteinascidia turbinata Herdman, 1880, Amphibalanus amphitrite (Darwin, 1854) and Savignyella lafontii (Audouin, 1826) (Table 1).
Community development: NIS and IS richness and coverage
During community development, total species richness was affected by the starting time (S) and immersion time (T) and their interaction. The same was observed when indigenous species (IS) and non-indigenous species (NIS) richness were analyzed separately, with significant interaction occurring in conjunction with the depth (D) observed for IS and NIS richness (univariate PERMANOVA Table 2); this was confirmed by a pairwise test (Table 2). Starting time (S) and immersion time (T) revealed how species richness changed for the panels at different immersion periods (Fig. 3). Total species richness for the set immersed in April (S1), showed a significant peak after 6 months of immersion (T2-July/October), followed by a subsequent decrease at 9 and 12 months (T3 and T4). The set immersed in July (S2) showed the highest species richness starting from the first immersion time, while sets immersed in October (S3) and in January (S4) were characterized by a similar increasing in richness during immersion time (Fig. 3a). The trend observed in the indigenous species is similar to that described for the whole community and with an increasing trend in S2, S3, and S4; in S1 the species richness showed no significant change (Fig. 3b). Both years of the study were characterized by a peak of NIS species in the quarter July/October (i.e. 6 months of immersion in S1, T2; 3 months of immersion in S2, T1; 12 months of immersion in S3, T4; 9 months of immersion in, S4T3) (Fig. 3c). Afterwards in S1, S2 and S4 NIS richness decreases; in S3 the peak corresponds to the last sampling time.
The NIS trend was confirmed by univariate analysis, although no significant differences in the panels collected during October of 2014 and January of 2015 belonging to the set immersed in January of 2014 was observed.
Depth is responsible for differences in the panels immersed in July and October 2013 (S2 and S3) as shown in the pairwise tests (Table 2). Depth causes a significant difference in the 12 month panels from July (S2T4), where 6 m panels have the highest values of NIS richness.
Total Coverage, NIS, and IS coverage were affected by the interaction of starting time (S) and immersion time (T) according to the univariate PERMANOVA tests found in Table 3. Interaction with the depth could be observed concerning both total coverage and IS coverage (S × T × D), and NIS coverage (T × D). The trends are shown in Fig. 4, in which the factors starting time (S) and immersion time (T) are plotted. Total macrofaunal coverage of the set immersed in April 2013 (S1) reached the maximum value after 6 months of immersion (T2) and remained successively constant (Fig. 4a). The set immersed in July 2013 (S2) showed a maximum of coverage after the first immersion quarter July/October (T1) with a subsequent decrease in the quarter of October 2013/January 2014 (T2), and then increasing again in the panels collected during April 2014 and July 2014 (T3 and T4). The set immersed in October 2013 (S3) followed a constant significant increase of macrofaunal coverage, while the set immersed in January 2014 (S4) after an initial high coverage, due to the unique abundance of the colonial ascidian Diplosoma listerianum, strongly decreased after 6 month of immersion (T2) and subsequently increased after 9 and 12 months of immersion (T3 and T4). Excluding S2, the described trend pattern was the same for the coverage of native species (Fig. 4b). By contrast, in each panel set, the NIS coverage trend always showed a maximum at the first time during the quarter July/October of both years (Fig. 4c). These data are overlapped with the maximum NIS richness as showed above. Pairwise test (Table 3) showed a general trend characterized by a major coverage of indigenous species in the shallowest panels, and greater NIS coverage in deeper panels (Pairwise Test, Table 3).
The ANCOVA analysis showed that NIS coverage, and NIS richness were significantly associated with the age of immersion and the temperature at the time of sampling (Table 4). The data, analyzed using a series of linear regressions between NIS coverage and temperature, shown as different age of immersions, are significantly related to different slopes, with the lowest age of immersion showing the higher slope (Fig. 5a). At high water temperatures, NIS coverage shows high values in the initial successional stages and lower values with the increase of the age of immersion of the substrate. At low temperatures, NIS coverage is low in both early and late successional stages. The number of NIS (Fig. 5b) increases with increasing temperature. At high temperatures they recruit independently from age of immersion, while at low temperatures the NIS richness increases with the age of the immersion, reaching values lower than that observed at highest ones. By contrast, autochthonous species richness increased with increasing temperature, and age of the substratum in which they recruit (Fig. 5c), as confirmed by the ANCOVA test with a two-way interaction among age of immersion and sampling time temperature (Table 4).
Non indigenous species in the community development
Univariate PERMANOVA tests (Table 5) showed that most of the NIS (i.e. P. zorritensis, P. magna, B. bairdi, C. brunnea, D. bermudensis, H. elegans, H. dirampha, S. plicata, S. canopus and A. verticillata) were influenced by the starting time, immersion time, and their interaction. The depth and/or its interaction with immersion time and starting time may also be significant for P. zorritensis, B. bairdi, B. luctuosum, and C. brunnea. In particular, P. zorritensis was more abundant at 3 m depth, and B. bairdi was more common in the deepest panels (Table 5). Other NIS such as W. subtorquata, B. trigonus, H. dianthus, and P. multiclathrata did not appear to be influenced by these factors, and so their distribution appeared stochastic, independent to the age of the substratum and the period in which they recruit.
Figure 6 shows the trend of coverage regarding single NIS. The colonial ascidian P. zorritensis, which was the most abundant NIS, recruits in the July/October quarter, with great difference in presence depending on the depth and the immersion time of panels. Its abundance in the first year of the study (in panels immersed in April and July 2013) reached a maximum coverage of 40%, showing a strong decrease in the subsequent quarter. In the second year of study (in panel immersed in October 2013 and January 2014) its presence was reduced to less than the 2% with few zooids present in panels immersed for 9 and 12 months. Thus, the abundance of P. zorritensis in the quarter July/October is strongly related to the immersion time of each set (Fig. 6a). Similarly, other species such as A. verticillata, P. magna, C. brunnea, H. elegans and S. plicata showed high abundance in the early stages of the succession and mainly after the warmest quarters (Fig. 6l, c, j, m, d), evidence of the pioneering traits of these species. The sabellid B. bairdi had the same recruitment pattern as most of the NIS (July/October) related to the highest temperatures of the year, but its population persisted in the subsequent quarter (Fig. 6f). A similar pattern is observed in B. luctuosum, which is found to recruit on the panels deployed between July and October, and subsequently remaining on the panels. Its large coverage values in the following quarter are due to the growth of individuals, and not to new recruits (Fig. 6g). Lastly, H. dirampha recruits in all of the panels sets, but mainly in the quarter July/October of both years of the study (Fig. 6n).
Variability of a Polyandrocarpa zorritensis, b Paraleucilla magna, c Styela plicata, d Bugula neritina, e Branchiomma bairdi, f Branchiomma luctuosum, g Styela canopus, h Ecteinascidia turbinata, i Celleporaria brunnea, j Distaplia bermudensis, k Amathia verticillata, l Hydroides elegans, m Hydroides dirampha in each panel set (S) and immersion time (T) (bars represent standard error)
The NIS W. subtorquata, M. squamiger P. multiclathrata, B. trigonus and H. dianthus were not abundant in any series or immersion time and their presence is considered stochastic in the succession.
The cryptogenic species identified in the community shows different recruitment and abundance patterns. Among them, B. neritina and E. turbinata were influenced by the starting time, immersion time, and their interaction (Table 5). Bugula neritina shows maximum abundance and recruitment pressure in the coldest period (Fig. 6e), while E. turbinata (Fig. 6i) shows its maximum abundance in the early stages of the succession and after the warmest quarters, similar to other non-native species such as A. verticillata, P. zorritensis and S. plicata.
Multivariate analysis
Multivariate analysis conducted on alien species showed that starting time, immersion time and depth affect the community of NIS in the panels (PERMANOVA Table 6). Pairwise comparison showed significant differences on NIS assemblage at each immersion time of sets immersed in April 2013 (S1). No significant differences are observed in the set during July 2013 (S2) after 6 months of immersion (T2). In the set immersed in October 2013 (S3), NIS assemblage showed significant differences after 6 months of immersion (T2), and lastly in the set immersed in January of 2014 (S4) the NIS assemblage did not change significantly after 9 months (T3). The assemblages were well distinguished in the PCO plot (Fig. 7) where NIS are grouped to the left of the PCO1 plot. In the right of the plot were successional stages of sets immersed during October 2013 and January 2014 (S3 and S4), where NIS recruitment did still not occur. Differences along the PCO2 are due to NIS patterns characterizing each panel set at different immersion times and depths. In particular, the highest points corresponded to the shallowest panels collected after 3 and 6 months in October 2013 (S1T2 and S2T1) and characterized by the dominating presence of P. zorritensis, A. verticillata, P. magna and D. bermudensis. The intermediate points along the PCO2 corresponded to panels collected at 3 and 6 m depths after 3 and 6 months in October 2013 and characterized by an increasing importance of the sabellids B. bairdi and B. luctuosum. Lastly, the lower points along the PCO2 corresponded to panels from the set collected after the immersion period of July/October 2013. They are characterized by B. bairdi and B. luctuosum, because of the disappearance and/or the reduction in abundance of the NIS (i.e. P. zorritensis, A. verticillata, S plicata, P. magna, H. dirampha, D. bermudensis), and S3 and S4 mainly are characterized by B. bairdi and B. luctuosum.
PCO plot based on Bray–Curtis distance of NIS community development in each panel set (S) and immersion time (T) and depths. Solid lines represent the principal clusters similarity. Numbers used as abbreviations for the taxa variables: 1. Hydroides elegans; 2. Hydroides dirampha; 3. Paraleucilla magna; 4. Polyandrocarpa zorritensis; 5. Branchiomma bairdi; 6. Branchiomma luctuosum; 7. Celleporaria brunnea; 8. Distaplia bermudensis; 9. Microcosmus squamiger; 10. Perophora multiclathrata; 11. Watersipora subtorquata; 12. Styela plicata; 13. Styela canopus; 14. Amathia verticillata; 15. Hydroides dianthus; 16. Balanus trigonus
Discussion
The Taranto Seas (including Mar Grande and Mar Piccolo) are characterized by distinctive fouling communities, mainly dominated by filter feeder species of sponges, ascidians, tube worms and bivalves. Four introduced species, C. brunnea, B. bairdi, P. multiclathrata, and W. subtorquata were newly recorded for the region. Microcosmus squamiger was previously erroneously reported in the Taranto basin as M. exasperatus (Cecere et al. 2015).
Previous studies on NIS conducted in the area (e.g. Brunetti and Mastrototaro 2004; Longo et al. 2007; Cecere et al. 2015) were descriptive. The present study documented NIS spatial and temporal settlement patterns, demonstrating differences based on the different starting time that reflects different propagule pressure, and the stage of community development (early community stage vs. late community stage).
Species richness, both of NIS and IS, is influenced by the starting point of the community development and by the period in which the community was monitored, as influenced by seasonal larval supply (Underwood and Anderson 1994; Stachowicz and Byrnes 2006) and the ecological mechanisms (such as facilitation and inhibition) that may shape the community (Connell and Slatyer 1977; Berlow 1997).
We have shown that propagule pressure of most NIS has a seasonality pattern that is higher in the warmest period (summer months) when the peak of abundances of larval production and settlement generally occurs (Osman 1977; Lu and Wu 2007). The seasonality pattern of NIS propagule pressure occurs at the same time of maximum indigenous propagule pressure, and the NIS recruitment on bare substrate co-occur with that of IS pioneer species.
As a whole, most of the recorded species, both IS and NIS, recruit on the bare substrate in the warmer period; this is in accordance with what is observed in previous studies on the succession and variability of fouling communities in other Mediterranean areas (e.g. Relini 1964; Gherardi 1974). Although some authors (Fargione et al. 2003; Stachowicz and Tilman 2005) suggested that successful recruitment of NIS is often due to a non-overlapping recruitment period with native species, we observed that the high recruitment of NIS occurs at the same time of high recruitment of native species. Thus, NIS of the macrofouling community in the Gulf of Taranto, do not appear to fill any temporal gap with minimum recruitment of natives; rather, they become established despite high competition in settlement rate during their recruitment. Moreover, NIS recruitment occurs during the warmer periods (July–October) in early communities as well as in late communities, independent of the age of immersion of the panels. Lower recruitment rates and/or a disappearance NIS were observed in the subsequent quarters (October–January), suggesting that the NIS persistence in the community is affected by seasonality.
Assuming that NIS reproductive cycles depend primarily on abiotic factors, the raising in water temperatures that usually occurs in the study site during June may promote the recruitment and the extensive colonization of NIS species on fouling panels in the warmer period (July–October). These patterns may reflect the species exotic origins and warm-water affinities (Arias et al. 2013; Brunetti and Mastrototaro 2004; Zenetos et al. 2012; Lezzi et al. 2015).
Macrofouling coverage, affected by starting time and immersion time, shows variations depending on the life history of the species, and on the successional events regulated by inhibition/facilitation of species assemblages. For instance, high coverage can be dependent on a single species that initially covers all of the surface (i.e. Diplosoma listerianum in S4 T1), or can be due to a high species pool. As a general trend, the successional study shows an increase of coverage with increasing immersion time, as recently observed in most successional studies in the Mediterranean (Antoniadou et al. 2010, 2011; Pierri et al. 2010). These results support the hypothesis that in temperate areas a rapid increase in species cover, reaching 75–100%, can be observed in less than 1 year, whereas at higher latitudes the process is much slower (Bowden et al. 2006).
Non-indigenous species coverage showed a peak in the quarter July–October in both years of study. However contrary to the NIS richness, NIS coverage is influenced by the developmental stage of the community. Indeed, NIS coverage is higher at early successional stages and at high water temperature, and assumes low values in late successional stages and/or at low water temperatures. Therefore, the low temperature appears to be a limiting factor for NIS coverage both in early and late successional stages, while at high temperatures the limiting factor is the developmental stage of the community.
Our results support the hypothesis by Davis et al. (2000) and Stachowicz and Byrnes (2006) that community invasibility may be influenced by resource availability, here represented as the space available for colonization. Well-established assemblages are less likely to become dominated by NIS than early community assemblages or bare substrates that characterize disturbed or newly created habitats (Clark and Johnston 2011). Thus, late stages of fouling community may protect against NIS invasion even though recruitment of the latter can still occur.
Lastly, differences in the macrofouling assemblages were observed according to the different depth at which panels were immersed. In particular, NIS could be more abundant in panels at 3 or 6 m depth compared to the shallower ones. However, it was observed that some NIS, such as Polyandrocarpa zorritensis, were more abundant in shallow panels and others such as Branchiomma spp. were more abundant in the deepest ones.
An increase in taxonomic richness was observed compared to previous studies, although new NIS appear to be added to the community without any complete displacements of previous species. However, a robust comparison between present and past data is not possible because past studies refer to the more confined location of the basin, the Mar Piccolo (Gherardi 1974; Pierri et al. 2010), historically less rich than the Mar Grande. In these previous studies, during the warmer season the coverage was dominated by bryozoans (B. neritina, S. errata), serpulids, especially H. elegans, and ascidians (e.g. A. aspersa, B. schlosseri, S. plicata, C. lepadiformis). All these taxa were recorded in the present work, but with a low coverage rate. This may be due to the recent addition of NIS, such as P. zorritensis and B. bairdi, that occupy most of the available space, and seem to play a major role in the community development.
Polyandrocarpa zorritensis is the component of the community with the highest coverage values during the early stage of the community in the quarter July/October, while its presence is strongly reduced to only a few zooids in the same quarter when it recruits in the late stages of the community. Polyandrocarpa zorritensis dynamics reveal a strong decrease in abundance in the subsequent quarter (October–January). This species is able to produce a bud stage that persists during unfavourable environmental conditions (i.e. coldest period after the summer months) and makes it possible for the rebuilding of the colony in favourable conditions (Brunetti and Mastrototaro 2004). This life history trait is observed in the S2, where a decrease in abundance during winter months is followed by a colony rebuilding in the early summer. Even though the rebuilding process did not totally recover the colony, the rebuilder zooids should be sufficient to undergo sexual reproduction, supplying new larval propagules that are able to recruit on new substrates. Besides P. zorritensis, the species C. brunnea, P. magna, H. elegans, H. dirampha, S. plicata, A. verticillata are more abundant on bare substrates in the warmest period acting as seasonal pioneer species, highlighting a similar strategy.
By contrast, other species, such as B. bairdi and B. luctuosum, although recruiting in the summer quarter July–October, as most of the other NIS, remain in the macrofouling communities and seem to not be influenced by the development stage of the community, being able to recruit with the same magnitude in early and late community stages.
Other NIS that are poorly represented (i.e. P. multiclathrata, W. subtorquata and H. dianthus, M. squamiger) did not show any clear settlement or recruitment patterns. Among cryptogenic species, E. turbinata appears to be the only one that shares a life strategy similar to those of the other NIS, being present in the early stages of succession and in the warmer period. This species is increasing its distribution in the Mediterranean (Maciver et al. 2016), and the present data show the same thermophilic and pioneering traits that characterize the other NIS recorded in the Taranto Seas.
It is thus possible to distinguish two sets of NIS. The first set includes P. zorritensis, P. magna, H. elegans, H. dirampha, S. plicata, A. verticillata and D. bermudensis that behave as “r-selected” pioneer species structuring components of the early communities only in favourable conditions, but which through competition with IS, after settlement, are not able to develop in late communities. The second set include the sabellid worms B. bairdi and B. luctuosum, that persist and become dominant in late macrofouling communities independent of seasonal changes.
As a general trend, alien species tend to rapidly colonize and grow on fouled panels in the summer months, taking advantage of suitable environmental conditions. The trend of recruitment and abundance of NIS related to the period of higher temperature observed in the present paper was already noted by Vaz-Pinto et al. (2014) for the Azores. The temporal distribution and abundance of NIS when viewed in the framework of global warming (Occhipinti-Ambrogi 2007; Sorte et al. 2010; Walther et al. 2009; Bianchi et al. 2017) is thus of interest relative to the potential expansion of their reproductive periods due to increasing temperatures. Our observations suggest the potential for establishing certain management approaches that could reduce or prevent the spreading of NIS. For example, the availability of artificial substrates (such as net cages, ropes, platforms, and buoys) that would serve as settlement platforms during warmer periods could conceivably be controlled through the use of anti-fouling technologies or by altering mooring times. Indeed, the Taranto Seas, acting as a NIS hotspot, could play a significant role as the origin of further introductions to bordering areas through natural spreading or fouling on boat hulls, in particularly during the summer, when an increase of local and foreign maritime touristic traffic is usually observed (Apostolopoulos et al. 2014). The observed autecology traits of the NIS (i.e. summer recruitment, fast growth) together with their structural dominance in early stage assemblages can be factors that could increase the spread of NIS foulers in the Mediterranean. Popular yachting destinations are at highest risk of harbouring or becoming inoculated with NIS and/or their propagules (Hewitt et al. 1999; Ruiz et al. 2000).
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Airoldi L, Bulleri F (2011) Anthropogenic disturbance can determine the magnitude of opportunistic species responses on marine urban infrastructures. PLoS ONE 6:e22985
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anderson M, Gorley RN, Clarke RK (2005) PERMANOVA. Permutational multivariate analysis of variance, a computer program Department of Statistics, University of Auckland 24
Anderson M, Gorley RN, Clarke RK (2008) Permanova + for Primer: guide to software and statistical methods. PRIMER-E, Plymouth
Antoniadou C, Voultsiadou E, Chintiroglou C (2010) Benthic colonization and succession on temperate sublittoral rocky cliffs. J Exp Mar Biol Ecol 382:145–153. https://doi.org/10.1016/j.jembe.2009.11.004
Antoniadou C, Voultsiadou E, Chintiroglou C (2011) Seasonal patterns of colonization and early succession on sublittoral rocky cliffs. J Exp Mar Biol Ecol 403:21–30. https://doi.org/10.1016/j.jembe.2011.04.001
Apostolopoulos Y, Leontidou L, Loukissas P (2014) Mediterranean tourism: facets of socioeconomic development and cultural change. Routledge, Abingdon
Arias A, Giangrande A, Gambi MC, Anadon N (2013) Biology and new records of the invasive species Branchiomma bairdi (Annelida: Sabellidae) in the Mediterranean Sea. Med Mar Sci 14(1):162–171
Berlow EL (1997) From canalization to contingency: historical effects in a successional rocky intertidal community. Ecol Monogr 67:435–460
Bianchi CN, Caroli F, Guidetti P, Morri C (2017). Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J Mar Biol Assoc UK 1–12. doi:10.1017/S0025315417000819
Bowden DA, Clarke A, Peck LS, Barnes DKA (2006) Antarctic sessile marine benthos: colonisation and growth on artificial substrata over three years. Mar Ecol Prog Ser 316:1–16
Brunetti R, Mastrototaro F (2004) The non-indigenous stolidobranch ascidian Polyandrocarpa zorritensis in the Mediterranean: description, larval morphology and pattern of vascular budding. Zootaxa 528(1):1–8. https://doi.org/10.11646/zootaxa.528.1.1
Bulleri F, Airoldi L (2005) Artificial marine structures facilitate the spread of a non-indigenous green alga, Codium fragile ssp. tomentosoides, in the north Adriatic Sea. J Appl Ecol 42:1063–1072
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35
Byers JE (2002) Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97:449–458
Byers JE, Wright JT, Gribben PE (2010) Variable direct and indirect effects of a habitat-modifying invasive species on mortality of native fauna. Ecology 91:1787–1798
Canning-Clode J, Bellou N, Kaufmann MJ, Wahl M (2009) Local–regional richness relationship in fouling assemblages–effects of succession. Basic Appl Ecol 10:745–753
Canning-Clode J, Maloney KO, McMahon SM, Wahl M (2010) Expanded view of the local–regional richness relationship by incorporating functional richness and time: a large-scale perspective. Global Ecol Biogeogr 19:875–885
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77:1653–1655
Carlton JT, Geller JB (1991) Ecological roulette: the global transport of nonindigenous marine organisms. Chem Phys Lett 179:53
Cecere E, Petrocelli A, Belmonte M, Portacci G, Rubino F (2015) Activities and vectors responsible for the biological pollution in the Taranto Seas (Mediterranean Sea, southern Italy): a review. Environ Sci Pollut Res 23(13):12797–12810
Cifuentes M, Krueger I, Dumont CP, Lenz M, Thiel M (2010) Does primary colonization or community structure determine the succession of fouling communities? J Exp Mar Biol Ecol 395:10–20
Çinar ME (2006) Serpulid species (Polychaeta: Serpulidae) from the Levantine coast of Turkey (eastern Mediterranean), with special emphasis on alien species. Aquat Invasions 1(4):223–240
Çinar ME (2013) Alien polychaete species worldwide: current status and their impacts. J Mar Biol Assoc UK 93(05):1257–1278
Clark GF, Johnston EL (2009) Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 118:1679–1686
Clark GF, Johnston EL (2011) Temporal change in the diversity–invasibility relationship in the presence of a disturbance regime. Ecol Lett 14:52–57
Connell SD (2000) Floating pontoons create novel habitats for subtidal epibiota. J Exp Mar Biol Ecol 247(2):183–194
Connell S, Glasby T (1999) Do urban structures influence local abundance and diversity of subtidal epibiota? A case study from Sydney Harbour, Australia. Mar Environ Res 47:373–387
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Darbyson E, Locke A, Hanson JM, Willison JM (2009) Marine boating habits and the potential for spread of invasive species in the Gulf of St. Lawrence. Aquat Invasions 4:87–94
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Dürr S, Thomason JC (2009) Biofouling. Wiley, Chichester
Fargione J, Brown CS, Tilman D (2003) Community assembly and invasion: an experimental test of neutral versus niche processes. Proc Natl Acad Sci 100:8916–8920
Fehlauer-Ale KH, Mackie JA, Lim-Fong GE, Ale E, Pie MR, Waeschenbach A (2014) Cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zool Scripta 43(2):193–205
Gherardi M (1973) Ricerche sulle comunità fouling del Mar Piccolo di Taranto. In: Atti III Simposio Nazionale sulla Conservazione della Natura, Università di Bari, 2–6 May 1973, pp 55–73
Gherardi M (1974) Lepore E Insediamenti stagionali delle popolazioni fouling del mar Piccolo di Taranto. Atti IV Simposio Nazionale sulla Conservazione della Natura, Università di Bari 23–28:235–258
Giangrande A, Cosentino A, Presti CL, Licciano M (2012) Sabellidae (Annelida) from the Faro coastal lake (Messina, Ionian Sea), with the first record of the invasive species Branchiomma bairdi along the Italian coast. Mediterr Mar Sci 13:283–293
Glasby T (1999) Differences between subtidal epibiota on pier pilings and rocky reefs at marinas in Sydney, Australia. Estuar Coast Shelf Sci 48:281–290
Glasby TM, Connell SD, Holloway MG, Hewitt CL (2007) Nonindigenous biota on artificial structures: could habitat creation facilitate biological invasions? Mar Biol 151:887–895
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17:22–27
Harmelin JG (2014) Alien bryozoans in the eastern Mediterranean Sea-new records from the coast of Lebanon. Zootaxa 3893(3):301–338
Hewitt CL, Campbell M, Thresher R, Martin R (1999) Marine biological invasions of Port Phillip Bay. CSIRO Marine Research Hobart, Tasmania, Victoria
Holle BV, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218
Izquierdo Muñoz A, Díaz Valdés M, Ramos-Esplá AA (2009) Recent non-indigenous ascidians in the Mediterranean Sea. Aquat Invasions 4(1):59–64
Katsanevakis S, Zenetos A, Belchior C, Cardoso AC (2013) Invading European Seas: assessing pathways of introduction of marine aliens. Ocean Coast Manag 76:64–74
Katsanevakis S, Wallentinus I, Zenetos A, Leppäkoski E, Çinar ME, Oztürk B (2014) Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat Invasions 9:391–423
Kott P (1985) The Australian Ascidiacea part 1, Phlebobranchia and Stolidobranchia. Mem Qd Mus 23:1–440
Lasram FBR, Tomasini JA, Guilhaumon F, Romdhane MS, Do Chi T, Mouillot D (2008) Ecological correlates of dispersal success of Lessepsian fishes. Mar Ecol Prog Ser 363:273–286
Lezzi M, Pierri C, Cardone F (2015) Presence of Celleporaria brunnea (Bryozoa: Lepraliellidae) in the Central Mediterranean: first occurrence in the Gulf of Taranto. Mar Biodivers Rec 8:e137
Longo C, Mastrototaro F, Corriero G (2007) Occurrence of Paraleucilla magna (Porifera: Calcarea) in the Mediterranean sea. J Mar Biol Assoc UK 87(06):1749–1755
Lord JP, Calini JM, Whitlatch RB (2015) Influence of seawater temperature and shipping on the spread and establishment of marine fouling species. Mar Biol 162:2481–2492. doi:10.1007/s00227-015-2737-2
Lu L, Wu RSS (2007) Seasonal effects on recolonization of macrobenthos in defaunated sediment: a series of field experiments. J Exp Mar Biol Ecol 351:199–210. https://doi.org/10.1016/j.jembe.2007.06.008
Maciver SK, Evans J, Borg JA, Ramos-Esplá AA, Schembri PJ (2016) Status of the ‘Mangrove tunicate’ Ecteinascidia turbinata (Ascidiacea: Perophoridae) in the Mediterranean Sea. J Mar Biol Assoc UK. https://doi.org/10.1017/s0025315416000473
Marchini A, Ferrario J, Minchin D (2015) Marinas may act as hubs for the spread of the pseudo-indigenous bryozoan Amathia verticillata (Delle Chiaje, 1822) and its associates. Sci Mar 79(3):355–365
Mastrototaro F, Brunetti R (2006) The non-indigenous ascidian Distaplia bermudensis in the Mediterranean: comparison with the native species Distaplia magnilarva and Distaplia lucillae sp. nov. J Mar Biol Assoc UK 86(01):181–185
Mastrototaro F, Petrocelli A, Cecere E, Matarrese A (2004) Non indigenous species settle down in the Taranto Seas. Biogeographia 25:47–54
ME Assessment (2005) Ecosystems and human well-being: desertification synthesis. World Resources Institute, Washington
Minchin D, Gollasch S, Cohen AN, Hewitt CL, Olenin S (2009) Characterizing vectors of marine invasion. In: Biological invasions in marine ecosystems. Springer, Berlin, pp 109–116
Occhipinti-Ambrogi A (2007) Global change and marine communities: alien species and climate change. Mar Pollut Bull 55:342–352
Occhipinti-Ambrogi A, Marchini A, Cantone G, Castelli A, Chimenz C, Cormaci M, Froglia C, Furnari G, Gambi MC, Giaccone G, Giangrande A, Gravili C, Mastrototaro F, Mazziotti C, Orsi-Relini L, Piraino S (2011) Alien species along the Italian coasts: an overview. Biol Invasions 13(1):215–237
Occhipinti-Ambrogi A, Savini D (2003) Biological invasions as a component of global change in stressed marine ecosystems. Mar Pollut Bull 46:542–551
Osburn RC (1952) Bryozoa of the Pacific coast of America Part 2, Cheilostomata—Ascophora. Allan Hancock Pac Exped 14:271–611
Osman RW (1977) The establishment and development of a marine epifaunal community. Ecol Monogr 47(1):37–63
Parenzan P (1969) Il Mar Piccolo e il Mar Grande di Taranto. Thalassia Salent 3:19–36
Petrocelli A, Cecere E, Verlaque M (2013) Alien marine macrophytes in transitional water systems: new entries and reappearances in a Mediterranean coastal basin. BioInvasions Records 2:177–184
Pierri C, Longo C, Giangrande A (2010) Variability of fouling communities in the Mar Piccolo of Taranto (Northern Ionian Sea, Mediterranean Sea). J Mar Biol Assoc UK 90:159–167
Prach K, Walker LR (2011) Four opportunities for studies of ecological succession. Trends Ecol Evol 26:119–123
Railkin AI, Press C (2004) Marine biofouling: colonization processes and defenses. Taylor & Francis, Routledge
Relini G (1964) Andamento stagionale degli organismi sessili del Porto di Genova. Archo Oceanogr Limnol 13:281–296
Rilov G, Galil B (2009) Marine bioinvasions in the Mediterranean Sea-history, distribution and ecology. In: Biological invasions in marine ecosystems. Springer, Heidelberg, pp 549–575
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Ruiz GM, Freestone AL, Fofonoff PW, Simkanin C (2009) Habitat distribution and heterogeneity in marine invasion dynamics: the importance of hard substrate and artificial structure. In: Marine hard bottom communities. Springer, Berlin, Heidelberg, pp 321–332
Sordino P, Gambi MC (1992) Prime osservazioni sulla biologia riproduttiva e sul ciclo vitale di Branchiomma luctuosum (Grube, 1869) (Polychaeta, Sabellidae). Oebalia 17(2):425–427
Sorte CJ, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecol Biogeogr 19:303–316
Stachowicz JJ, Byrnes JE (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar Ecol Prog Ser 311:251–262
Stachowicz JJ, Tilman D (2005) Species invasions and the relationships between species diversity, community saturation, and ecosystem functioning. Species invasions: insights into ecology, evolution, and biogeography:41-64
Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW (2002) Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc Natl Acad Sci 99:15497–15500
Sutherland JP, Karlson RH (1977) Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monogr:425-446
Torres P, Costa AC, Dionísio MA (2011) New alien barnacles in the Azores and some remarks on the invasive potential of Balanidae. Helgol Mar Res 66(4):513–522
Turon X, Nishikawa T, Rius M (2007) Spread of Microcosmus squamiger (Ascidiacea: Pyuridae) in the Mediterranean Sea and adjacent waters. J Exp Mar Biol Ecol 342(1):185–188
Tursi A, Gherardi M, Lepore E, Chieppa M (1976) Settlement and growth of Ascidians on experimental panels in two harbours of Southern Italy. In: Proceedings of the IVth international congress on marine corrosion and fouling, Antibes, Juan-les-Pins, pp 535–543
Tursi A, Matarrese A, Sciscioli M, Vaccarella R, Chieppa G (1982) Biomasse benthoniche nel Mar Piccolo di Taranto e loro rapporto con ibanchi naturali di mitili. Nat Sicil 2:263–268
Underwood A, Anderson M (1994) Seasonal and temporal aspects of recruitment and succession in an intertidal estuarine fouling assemblage. J Mar Biol Assoc UK 74:563–584
Vaz-Pinto F, Torrontegi O, Prestes A, Álvaro NV, Neto AI, Martins GM (2014) Invasion success and development of benthic assemblages: effect of timing, duration of submersion and substrate type. Mar Environ Res 94:72–79
Vilà M et al (2009) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8:135–144
Wahl M (1997) Living attached: aufwuchs, fouling, epibiosis. In Fouling Organisms of the Indian Ocean: Biology and Control technology 31-83
Walther GR et al (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Wasson K, Zabin CJ, Bedinger L, Diaz MC, Pearse JS (2001) Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biol Conserv 102:143–153
Wasson K, Fenn K, Pearse JS (2005) Habitat differences in marine invasions of central California. Biol Invasions 7:935–948
Wikström SA, Hillebrand H (2012) Invasion by mobile aquatic consumers enhances secondary production and increases top-down control of lower trophic levels. Oecologia 168:175–186
Winston JE (1982) Marine bryozoans (Ectoprocta) of the Indian River area, Florida. Bull Am Mus Nat Hist 173:99–176
Wonham MJ, Carlton JT (2005) Trends in marine biological invasions at local and regional scales: the Northeast Pacific Ocean as a model system. Biol Invasions 7:369–392
Wyatt ASI, Hewitt CL, Walker DJ, Ward TJ (2005) Marine introductions in the Shark Bay World Heritage Property, Western Australia: a preliminary assessment. Diver Distrib 11:33–44
Zenetos A, Ballesteros E, Verlaque M (2012) Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr Mar Sci 13:328–352
Zullo VA (1992) Balanus trigonus Darwin (Cirripedia, Balaninae) in the Atlantic basin: an introduced species? Bull Mar Sci 50:66–74
Acknowledgements
The authors thank Justin Hillard and Chaise Lawson from Texas A&M University at Galveston for their help in the revision of the English of the manuscript. The authors thank Dr. F. Mastrototaro for suggestions in Tunicata identification. The authors thank the two anonymous referees and the editor, Dr. James Carlton, whose remarks and comments contributed to a great improvement of the readability of this article.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ML, AG, and DP; Taxa Identification: Polychaeta: ML, AG, MDP; Crustacea: ML, MDP; Tunicata: ML; Porifera: ML, DP; Bryozoa: ML; Analysed the data: ML; Wrote the paper: ML, AG, MDP and DP.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Fig. 1
Panels collected at 0-meter depth in October 2013 after 63 months of immersion. (JPEG 8234 kb)
Rights and permissions
About this article
Cite this article
Lezzi, M., Del Pasqua, M., Pierri, C. et al. Seasonal non-indigenous species succession in a marine macrofouling invertebrate community. Biol Invasions 20, 937–961 (2018). https://doi.org/10.1007/s10530-017-1601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1601-3