Abstract
All-male populations of the freshwater prawn Macrobrachium rosenbergii were recently produced by a novel temporal RNA interference (RNAi)-based biotechnology for aquaculture purposes. This biotechnology opens the way to the wide use of all-male prawn populations as sustainable biocontrol agents against invading populations of freshwater snails, for which there is currently no environmentally friendly solution. Among the most damaging of the invasive freshwater snail species are the apple snails (Pomacea spp.), which inflict major damage on natural ecosystems and rice fields. The proposed use of all-male prawn populations as environmentally friendly biocontrol agents against invasive freshwater snails has several advantages: efficient predation by the prawns over a wide range of freshwater snails, the ready availability of the prawns, and the monosex non-reproductive nature of the biocontrol agents. Since the aquatic predators are strongly size selective, we quantified the predation rate as a function of body size of both predator and prey (M. rosenbergii and P. caniculata). Medium-sized and large prawns (~10–30 g) efficiently preyed small and medium-sized snails (up to 15 mm), while small prawns (up to 4 g) immediately and completely eradicated snail hatchlings. Medium-sized prawns (~22 g) exterminated a significant fraction of snail biomass within 24 h (up to 58% of their body mass) after being introduced into a tank of snails. A typical ‘climbing-to-the surface’ anti-predator behavior of the snails was recorded. The potential of all-male prawns as efficient biocontrol agents over hatchling and adult apple snails as part of an integrated pest management program is discussed. Our experiments set the stage for evaluating the ecological and economic implications of this generic solution for a wide variety of habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater snails are distributed worldwide, with both native and non-native invasive species negatively affecting biodiversity, agriculture and human health (Lach and Cowie 1999; Sokolow et al. 2016; EFSA 2014). Nonetheless, until recently there has been little discussion in the scientific literature of their role as agricultural pests, even though invasive species of snails have long been known to cause major losses to a variety of crops around the globe (Barker 2002). Today, however, the agricultural community is coming to the understanding that there is an urgent need to develop efficient management programmes to deal with snail invasions.
Among the most damaging freshwater snails are apple snails (Pomacea spp., Ampullariidae), the largest of the freshwater gastropods (up to 155 mm shell height), which are naturally distributed mostly in humid tropical and subtropical habitats in South and Central America, Africa and South-East Asia (Burky 1974; Pain 1960). Many species are amphibious and are therefore able to spend long periods of time outside the water, a trait that challenges control efforts (Prashad 1925; Burky et al. 1972). Due to their amphibious capabilities, large size and omnivorous nature (Kwong et al. 2009; Naylor 1996), invasive apple snails have the potential to alter wetland ecosystems, a problem that goes hand in hand with their role as agricultural pests (Naylor 1996).
The spread of apple snails throughout the world was triggered by two major market activities—the food industry and the aquarium trade (Perera 1996; Roll et al. 2009; Horgan et al. 2014). Apple snails were introduced into a number of countries outside their South American natural habitat as early as the 1960s, first in Hawaii, and later, during the late 1970s and 1980s, in many Asian countries. Thereafter, snails that escaped or were released from captivity established invasive populations in East Asia. Cases of snail invasions have, however, not been limited to these countries and have been reported in temperate-zone countries throughout the world, including the USA, Chile, China, Egypt, Israel, Japan, Mexico, South Africa, South Korea and Spain. The problem has recently been discussed in a publication of the European Food Safety Authority (EFSA 2014), a “Scientific Opinion on the environmental risk assessment of the apple snail for the EU”, which points to the potential negative impacts of these invasive species on ecosystem traits, ecosystem services and biodiversity.
Here, we propose a sustainable solution for the biocontrol of invasive apple snail populations by harnessing our novel biotechnology for the production of all-male prawn populations as natural and environmentally safe predators of the snails (Ventura et al. 2012; Lezer et al. 2015; Shpak et al. 2017). This technology was originally developed to enhance aquaculture of the freshwater prawn Macrobrachium rosenbergii, and its development is described in brief below.
Many crustacean species display sexual dimorphism, with males being larger than females or vice versa. Sexual differentiation is governed by the androgenic gland (AG), an endocrine organ unique to male crustaceans (Charniaux-Cotton 1954; Sagi and Cohen 1990; Sagi et al. 1997), which controls their masculine traits (Touir 1977; Taketomi et al. 1990; Manor et al. 2004). The biotechnology to produce all-male populations of M. rosenbergii begins with sex-reversal of males into ‘neo-females’ through temporal interference (RNAi) by silencing the expression of the gene encoding the insulin-like androgenic gland hormone (IAG) (Manor et al. 2007; Ventura and Sagi 2012; Ventura et al. 2009, Chung et al. 2011; Mareddy et al. 2011; Savaya-Alkalay et al. 2014; Vázquez-Islas et al. 2014). Crossing the ‘neo-females’ with male animals produces an all-male progeny. This process for the production of all-male populations of M. rosenbergii is completely irreversible (Shpak et al. 2017) and constituted the first commercial use of temporal gene silencing in aquaculture (Ventura et al. 2012). The strength of RNAi in biological applications lies in the fact that the administration of dsRNA does not involve genomic modifications (non GMO) or the use of exogenous hormones (Fire et al. 1998; Lezer et al. 2015). It is thus considered suitable for sustainable aquaculture and environmental applications. In addition to the advantage of producing non-breeding all-male populations, other advantages of the biotechnology include faster growth and larger body sizes of the males and hence increased food (namely, prey) consumption (Sagi et al. 1986), and, importantly, the non-migratory behaviour of the males. Females, in contrast, would migrate downstream in rivers to release their larvae in brackish estuarine water and would thus not remain in the target area (Bauer 2011).
A recent study has shown that damming of rivers has, in many cases, resulted in a significant increase in schistosomiasis, a chronic disease caused by parasitic flatworms of the genus Schistosoma, which require two hosts to complete their life cycle, a freshwater snail intermediate host and a vertebrate definitive host (usually a mammal). The rise in this debilitating disease may be attributed to an increase in the snail populations of the dammed rivers due to the disturbance of the prawn-snail predator–prey equilibrium. This sifting of the equilibrium is caused by the blocking of the natural migratory routes of river prawn populations and hence to a decrease in their reproductive activity. It would seem that restoration of native prawn populations could thus play a pivotal role in combatting the disease and reducing the risk of infection for nearly 400 million people (Sokolow et al. 2017). However, the use of local prawns has two inherent disadvantages: they might not exist at particular infected sites (due to damming for example) and, being natural non-domesticated species, they are often not available in sufficiently large numbers for biocontrol use (as the case of the African river prawn, Macrobrachium vollenhovenii). Mass-cultured non-endemic species are equally unsuitable, since they present an invasion risk. Recently, we suggested a novel approach to the problem, which is based on using endemic all-male populations as non-invasive biocontrol agents (Savaya-Alkalay et al. 2014). Our approach is based on previous studies that demonstrated two Macrobrachium species as efficient predators of freshwater snail species of the genera Biomphalaria and Bulinus (Sokolow et al. 2013; Roberts and Kuris 1990; Lee et al. 1982). Thus, we propose that the best solution for biocontrol of a wide range of pest snail species—both endemic and exotic—lies in the use of non-native all-male prawn populations that do not carry the risk of becoming invasive species. All-male prawns seem to be particularly appropriate biocontrol agents in temperate exotic habitats where the winters are cold, resulting in prawn mortality, thus further preventing any unintended establishment of prawn populations.
To the best of our knowledge, only one study has explored the predation potential of crustaceans for Pomacea snails (Yusa et al. 2006). However, the only Macrobrachium species tested in that study (M. formosense) was a small-sized species (2.4 g average weight), whereas the species used here (M. rosenbergii) can reach much higher weights and the specimens used for the present study weighed up to 37 g. Due to its large body mass (Aflalo et al. 2014; Harikrishnan and Kurup 2001) and its worldwide availability as an aquaculture product demanding a premium price, the sustainability of the proposed biocontrol agent is based on the extra income it would generate for local inhabitants, e.g., rice growers, by selling the prawns for local or international markets (Boock et al. 2016).
Numerous predation studies, including those on different species of decapod crustaceans (especially crabs) and mollusks, have revealed that predation preferences vary according to prey body size. Most studies showed that predators prefer smaller prey items, which minimizes handling time. In view of the size selectivity for prey exhibited by aquatic predators, the first step in developing an efficient biocontrol programme for invasive apple snail populations requires quantifying the predation rate by freshwater prawns as a function of the body size of both predator and prey (Torres et al. 2012). Another important aspect of predator–prey interaction is the anti-predator behavior of the snails; for example, in laboratory experiments, Pomacea snails were shown react to chemical cues resulting from the presence of crushed conspecifics in their habitat (Ueshima and Yusa 2015). We therefore also analyzed the behavioural response of snails to cues associated with predation of conspecifics by prawns.
Materials and methods
Animals
All prawns used in this study were M. rosenbergii males grown at Ben-Gurion University of the Negev, Beer-Sheva, Israel and at Colors Ltd., Hatzeva, Israel. Only naive prawns that had never been fed with P. caniculata were used in the experiments. Pomacea caniculata snails were supplied by Kavra Ltd., Israel. Snails were maintained in 100-L plastic tanks with an interior biological and mechanical filter to maintain water quality (Fig. S1A). Snail diameter was measured with Vernier calipers to the nearest 1 mm. During the experiments each tank was fed every other day with shrimp pellets (Raanan Fish Feed Ltd., I.Z Milout, Israel, 40% protein), once a week with frozen food (Artemia and bloodworms, Ocean Nutrition Ltd., CA, USA) and fresh lettuce leaves. Water temperature was maintained at 28 ± 1 °C.
Size-selective predation
A size-selective predation experiment was designed to study the predation preferences of prawns of three different sizes for snails of three different sizes. The experiment was conducted in four experimental tanks of 100 L. Each of these four tanks was stocked with 10 large snails (15–35 mm), 10 medium-sized snails (7–15 mm) and 10 hatchlings (less than 7 mm). A large prawn (27.5 ± 1.5 g) was introduced into the first tank, a medium-sized prawn (10.3 ± 0.9 g) into the second tank, and a small prawn (0.6 ± 0.1 g) into the third tank; the fourth tank served as the no-prawn control. The experiment was replicated nine times. Each round lasted one week, and during that time the numbers and sizes of surviving snails were monitored daily.
Single prawn predation on medium-sized snails
Based on the results of the above experiment, an experiment with medium-sized snails was designed to study the predation capacity of a single prawn for a significant number of snails. This experiment comprised eight experimental 100-L plastic tanks, each containing 60 medium-sized snails with total weight of 9.8 ± 0.2 g (average weight of 0.16 g each) and one prawn weighing between 14 and 37 g (average weight of 21.9 ± 2.7 g), and eight control tanks, each containing 60 snails. Each tank was fitted with an interior biomechanical filter. The tanks were monitored for three days until all the snails had been consumed. One additional encounter was conducted with a non-feeding prawn (possibly due to its stage in the molt cycle). To enable us to monitor the behaviour of both prawns and snails, four of the experimental tanks (including that of the non-feeding prawn) were video recorded with a GOPRO HEROS3 + camera. The camera was placed either above the tank or inside the tank (underwater filming). The ‘above’ camera was positioned such that the whole tank was captured in the frame. The videos were obtained to enable quantification of the number of interactions between the predator and the prey in the filmed time span. In addition, the movement of the snails was followed by recording their dispersal in the tank every 5 min. The tank was regarded as being composed of two different sections: A lower part that included the bottom and the lower part of the tank walls (0–15 cm from the bottom) and a higher part that included the upper part of the tank walls and the water surface (15–30 cm from the bottom). The underwater filming was used to obtain close-ups of the predation process with respect to the different prawn body parts involved.
For the cameras filming from above, a total of 12 h of videos was analyzed. For the underwater filming, a total of 3 h of videos was analyzed. The videos were edited with Windows Movie Maker Version 2012.
Snail hatchling predation
To investigate the biocontrol ability of prawns for snail hatchlings, a third predation experiment was conducted with small prawns and snail hatchlings. A single small prawn (2.3 ± 0.2 g) was placed in a 100-L plastic tank. A clutch of snail eggs that had been laid in one of the plastic holding tanks (Fig. S1A) was placed in mosquito netting (10 × 10 cm) secured above the water level with the aim to imitate natural conditions, namely, that the snail hatchlings fall into the water (Fig. S1A). As soon as the eggs started to hatch, the net was brushed every other day to allow the hatchlings to fall easily into the water. The control tank lacked a prawn. The experiment was repeated nine times with nine different prawns and was terminated a month after all the eggs had hatched and the hatchlings were large enough to enable us to distinguish between the hatchlings and other debris in the tank and to collect and count them. In two different replicates, once the snail eggs had begun to hatch, a camera was placed under water to document prawn predation behaviour. Three hours of such videos were analyzed, and representative short clips are given in the supplemental material (Videos S1 and S2).
Statistical analysis
To minimize mean proportional variance differences in the size-selective predation experiment, an angular transformation [arcsin(\(\sqrt[2]{{P_{i} }}\))] was applied to the portion of surviving snails prior to the analysis. A repeated measure ANOVA was used to analyze the combined effects of prawn and snail sizes on the portion of surviving snails over time. To minimize the number of pairwise comparisons, a set of planned comparisons was used to test separately for the effect of prawn size on the portion of surviving snails in each of the three snail size classes. To quantify the effects of prawn and snail sizes on predation rate of snails by prawns, a Cox proportional hazards model (Hosmer et al. 1999) was used, with treatment (control = 0, predation = 1), initial body masses in grams (of both the prawn and snails) and the respective interaction terms as covariates. Time-to-event data are commonly analyzed using this method, which enables the evaluation of the effects of different predictor variables (i.e., covariates) on the rate at which the event in focus (in our case, predation on snails by a prawn) occurs (Hosmer et al. 1999). Use of this statistical analysis tool allowed us to estimate a coefficient (β) for each one of the covariates and to test for its significance. The exponent coefficient (eβ) estimates the expected change in the event occurrence rate per one unit change in the covariate. For instance, eβ = 0.5 for snail initial body mass means that an increase of 1 g in the initial snail body mass translates into a 50% reduction in snail mortality rate, averaged over the entire duration of the experiment. To avoid pseudo-replication and to account for a possible correlation between individuals within each experimental tank, we used a robust jackknife variance estimator grouped by observations (snails) per tank (Lin and Wei 1989). The results of the snail hatchling predation experiment were analyzed using a t test (independent samples). Statistical analyses were performed using S-PLUS 2000 (MathSoft, Inc., Cambridge, MA, USA).
Results
Size-selective predation
Prawn size had a significant negative effect on the portion of surviving snails (F3,32 = 69.63; P < 0.001, Table S1). As expected, snail survival was highest (89.63%) in the control tank (without prawns), while in the treatment tanks snail survival decreased as prawn size increased (Fig. 1; 81.11, 43.7 and 34.07% for small, medium and large prawns, respectively). Snail size had a significant positive effect on snail survival (F2,64 = 138.18; P < 0.001, Table S1). Large snails exhibited the highest survival (98.6%), followed by medium (54.17%) and small (33.6%) snails. Notably, there was a significant interaction for Prawn size × Snail size × Time (F36,364 = 6.31; P < 0.001, Table S1). Specifically, irrespective of their size, the prawns began by consuming the smaller snails. Once the small snails had been depleted, the prawns switched to the medium-sized snails, but they did not consume the larger snails (Fig. 1). Planned comparisons indicated that the survival of small-sized snails was significantly higher in the control than in the predation treatments (i.e., small, medium and large prawn size classes) in the aggregate (F1,32 = 124.32, P < 0.001, Table 1). Analysis of the different treatments separately revealed significant differences between the small prawns and the control group (F1,32 = 6.62, P = 0.0149, Table 1), and also between the small prawns and the two other prawn size classes in the aggregate (medium and large prawns; F1,32 = 128.1, P = 0.0149, Table 1). Survival of the medium-sized snails was significantly higher in the control than in other predation treatments in the aggregate (F1,32 = 21.67, P < 0.001, Table 2). Further comparisons revealed that the survival of the medium-sized snails was significantly higher in tanks containing small prawns than in those with medium and large prawns (F1,32 = 88.13, P < 0.001, F1,32 = 104.36, P < 0.001, respectively, Table 2), with the difference in the survival rate of medium-sized snails between the medium and large prawns being marginally non-significant (F1,32 = 3.69, P = 0.06, Table 2).
The Cox proportional hazard model (whole model statistics: Wald test = 216, df = 3, P < 0.001), indicated that an increase of 1 g in the initial body mass of the prawn should bring about a ~8% increase (eβ = 1.079) in snail mortality rate (z = 7.458, P < 0.001). In contrast, an increase of 1 g in the average initial body mass of individual snails is expected to cause a ~81% reduction (eβ = 0.203) in their mortality rate (z = −4.562, P < 0.001). The interaction between prawn and snail initial body masses was not significant (z = −0.221, P = 0.83) (Table S2).
Single prawn predation on medium-sized snails
In this experiment, the first 24 h of the encounter between prawns and snails were the most vigorous in terms of extermination of the snails. Table 3 presents all eight cases of such encounters in which the prawns preyed upon dozens of snails, reaching a mass equivalent of 38 ± 4% (mean ± 1SE) of the prawns’ body mass (ranging between 25.8 and 58.5%). The predation pace slowly decreased after the first 24 h; however, all eight prawns eliminated all 60 snails within three days. No snail mortality was observed in the control tanks.
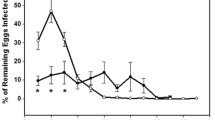
Analysis of the video records (representative frames are shown in Figs. 3, 4) revealed that snail behaviour was strongly affected by the presence of predating prawns (Fig. 2). In the absence of the prawn (Figs. 2a, 3a), most snails stayed at the bottom of the tank, where they had initially been placed. Specifically, during the 100 min of the control experiment, ~80% of the snails remained in the lower part of the tank, while only ~20% climbed up to the higher part of the tank near the water surface. When the non-feeding prawn was introduced to a tank of snails (Fig. 2b), a similar trend was observed. Noteworthy was the observation that the non-feeding prawn did attempt to catch 17 snails during 140 min of video recording, but snail dispersal in the tank did not differ from that observed in the control group, with more than 80% of the snails remaining in the lower part of the tank, where they would be more susceptible to predation (Fig. 3b). In contrast, when a feeding prawn was introduced into a tank of snails (Fig. 2c) and started preying (~23 min after stocking), the snail dispersal pattern began shifting towards the surface. Twenty minutes later, the majority of the remaining snails had moved from the lower part of the tank towards the water surface. After an additional 100 min, ~80% of the surviving snails (27/60) were located near the surface (Fig. 3c). This trend was demonstrated in a representative treatment tank that was documented during the first 140 min of the encounter (Fig. 4). Specifically, when the first snail was eaten (~17 min after stocking), 100% of the snails were still in the lower part of the tank (Fig. 4b, 17″). However, 27 min later (and after the consumption of another eight snails), the majority of the snails had moved near the surface (Fig. 4b, 45″). One day later, an additional 90 min of filming of the representative tank depicted a steady state in which 100% of the surviving snails (31/60) were located close to the water surface (Fig. 4b, 1565″). The videos of prawn behaviour revealed that snail capture took place in the lower part of the tank and that the prawns used their first and second walking legs (small and large chelae) to transfer snails to their maxillipeds (mouth parts).
Representative snail distributions from the ‘single prawn predation upon medium-sized snails experiment’. a Control tank 65 min after the introduction of snails. b Snail distribution 115 min after the introduction of a non-feeding prawn. c Snail distribution 135 min after the introduction of a natural (feeding) prawn. The red dashed line represents half the water column depth. Hence, snails found within the internal part of this line represent snails residing at the lower part of the water column, while snails found in the external part are located in the higher part of the water column. Red circles indicate live snails in the higher part. White arrows show live snails in the lower part, and the remainder of the ‘dots’ are dead snails or debris. Bar represents 5 cm
Snail dispersal during 140 min in a representative tank at steady state over 26 h in the presence of a natural (feeding) prawn. a Percentage of snails in the lower part of the tank (open circles) and in the higher part (black triangles). Black circles represent the percentage of live snails. b Representative snail distributions depicted from Fig. 4a: 17, 45 and 1565 min after the beginning of the experiment. Lines, arrows and circles as in Fig. 3. Bar represents 5 cm
Predation on snail hatchlings
Egg masses that had been placed above the water level started hatching 10–14 days after oviposition. One month after hatching, the control tanks contained 89, 144, 116, 302, 159, 255, 92, 374 and 382 (average of 212 ± 39) snails, while in the nine treatment tanks, containing small prawns, no snails were found (t-test, t = 5.4; df = 8, P < 0.001). To demonstrate the process of hatchling predation, 3 h of underwater videos were documented in two representative replicates (see supplemental videos); these videos revealed voracious prawn predation upon the snail hatchlings. During 12 min of video on one of the tanks (5:45 min, Supplemental Video 1), the small prawn encountered 20 snails on the tank bottom and lower tank walls and successfully consumed 16 of them. In an additional video (Supplemental Video 2), the prawn is seen climbing towards the water surface capturing eggs and snails as they fell into the tank from the egg mass suspended above the tank. Once the prawn had caught a snail/snail eggs, it returned to the bottom of the tank to consume the prey. The climb-to-the-surface behavior to capture the hatchlings at the water surface before they sank into the bottom was repeated several times. The close-up videos also revealed that these prawns used both pairs of chelae to catch the snail hatchlings and to transfer them into their mouth parts. Once the snail had been delivered to the maxillipeds, the chelae were free to catch another snail.
Discussion
Although the control of apple snail invasions presents a pressing global need, most efforts to do so have largely failed (Yusa and Wada 1999), even with use of pesticides (Halwart 1994). The failure of chemical pest control methods together with their negative environmental effects indicate that an alternative more efficient and environmentally suitable approach is desirable. The use of biocontrol agents is generally considered the preferable approach for pest control, but such agents may themselves pose different threats to ecosystems when used without an evaluation of their non-target impacts. Ideally, biocontrol agents should be specific for the target species, while causing minimal non-target impacts. Therefore, an environmental risk assessment is required as a pre-condition for any field study, in order to raise awareness of the problem and to prevent potential negative impacts. In addition to the impact on non-target species, there are potential risks in the use of biocontrol agents in terms of direct or indirect competition with native species and unexpected effects (Simberloff and Stiling 1996). The predation of snails by decapod crustaceans has previously been suggested for the regulation of invasive populations of snails (Yamanishi et al. 2012). We offer substantial risk reduction compared to the previous use of native or exotic decapods because our approach is based on an all-male prawn model. Such prawns will have a minimal ecological footprint. The use of readily available all-male prawn populations (Shpak et al. 2017) that, being non-reproductive, will decrease in numbers over time, is both sustainable and ecologically safe. Furthermore, in temperate regions, where the water temperature falls below 13 °C, the safety of the use of such prawns as biocontrol agents would be ensured, since the prawns cannot survive the winter season (Herrera et al. 1998). We confirmed this notion in cold-resistance experiments with our all-male juvenile and adult prawns (see Fig. S2).
The current study presents promising results regarding the effectiveness of the proposed biocontrol agent over the target species. Prawns are voracious predators of Pomacea snails of various sizes, especially smaller snails (up to 15 mm shell size). These findings support those of Roberts and Kuris (Roberts and Kuris 1990) and Sokolow et al. (Sokolow et al. 2013), who tested Macrobrachium species as biocontrol agents of schistosomiasis-intermediate host snails (Biomphalaria and Bulinus spp.). Those studies demonstrated efficient consumption of snails, with a daily biomass removal of snails ranging from 12 to 39% of the prawn body mass. Here, M. rosenbergii tested with different snails seemed to be even more efficient, exhibiting a daily biomass removal of snails ranging from 25.8 to 58.5% of prawn body mass. Snail hosts of schistosomes are much smaller than the large Pomacea snails used here. Therefore, it was particularly necessary to study the predation abilities of the prawns on larger snails (15–35 mm). Importantly, large snails were never consumed by the prawns of the tested body sizes, namely, up to of 37 g. It is, however, possible that larger prawns would be able to consume the large snails, as confirmed by personal observation (Savaya-Alkalay, unpublished data). At present, removal of larger snails from invaded areas is often carried out manually, especially in rice paddies in Asia, where they have been causing serious damage for almost three decades (Wada 1997; Naylor 1996; Halwart 1994; Litsinger and Estano 1993; Yoshie and Yusa 2011; Carlsson et al. 2004c; Aizaki and Yusa 2009).
To the best of our knowledge, this is the first study to examine predation on snail egg masses, thereby imitating the natural situation in which Pomacea snails lay their eggs on objects, such as plant stems, a few centimeters above the water surface (EFSA 2014; Adalla et al. 2006; Carlsson et al. 2004b). The experiment demonstrated the potential for local eradication of snail hatchlings by a single small prawn. Moreover, the underwater videos showed climb-to-the-surface prawn behaviour, resulting in immediate snail eradication. Indeed, these results demonstrate the ability of a single small prawn to significantly alter the establishment of newborn snails, which is crucial for the control of snail populations.
We also demonstrated size-selective predation preferences that have implications for the applicability of the proposed method. The experimental outcomes and the video recordings for the small prawns and snail hatchlings imply that stocking of rice paddies and irrigation canals with post-larval (small) prawns could be an efficient biocontrol strategy for eliminating/controlling snail hatchlings.
An additional important aspect of the proposed biocontrol method is the anti-predator behavioural response of snails to prawns found in the present study. A recent study using fish (common carp) and turtles as biocontrol agents found that Pomacea snails react to chemical cues present in the water by exhibiting predator-specific avoidance, namely, burrowing into the substrate in the presence of turtles (which feed from the water surface) or escaping out of the water in the presence of carp (bottom feeders) (Ueshima and Yusa 2015). That study and the study of Aizaki and Yusa also highlighted the importance of chemical cues to elicit the anti-predator response by exposing the snails to crushed conspecifics (Aizaki and Yusa 2009; Carlsson et al. 2004a; Covich et al. 1994; Ueshima and Yusa 2015). In the current study, the snails (lacking a soft substrate for burrowing) responded to the bottom-feeding prawns by moving upwards, as they did in response to carp (although other methods for avoiding prawns in a more realistic system, could not be ruled out). Furthermore, the anti-predator response of the snails was not a response to the presence of prawns; it was a response to predation of conspecifics. In light of previous studies of the anti-predator response of the snails (Aizaki and Yusa 2009; Carlsson et al. 2004a; Covich et al. 1994; Ueshima and Yusa 2015) and the findings of the current study, it appears that this behaviour should be taken into account in future integrated control management schemes.
Our results indicate that the readily available novel all-male prawn biocontrol method offers an effective control approach under a variety of conditions, ranging from rice paddies to other enclosed bodies of water. Our laboratory results now require field validation and environmental risk assessment with respect to rice management and non-target effects. We note here that the fact that testing these non-reproducing monosex populations in the field could be terminated at any time simply by refraining from further stocking of prawns contributes significantly to the environmental safety of this biocontrol method. In a real-world scenario, this environmental safety comes at the cost of the need for repeated restocking, as is done in inundative biocontrol programmes (Van Lenteren et al. 2003). However, such a cost is dwarfed by the losses attributable to snail pests; for example, the annual global loss for rice production due to apple snails has been estimated at US $55–248 billion (Joshi and Sebastian 2006). In the Philippines alone, the annual loss during the 1980s was estimated at US $1.2 billion (Naylor 1996) due to a yield loss varying between 5 and 100%, depending on the level of infestation. Studies have shown that 1 snail/m2 can reduce a rice crop by up to 20%, while 8 snails/m2 can reduce the yield by over than 90% (Halwart 1994). In additional advantage of the proposed biocontrol method is the increased profitability offered by rice-prawn integrated production, with the gross revenue in rice-prawn system being almost 2.5 times greater than that from rice monoculture (Boock et al. 2016). It has been suggested that a stocking density of 2 post-larvae prawns/m2 without any additional feed would be the most profitable scenario. This premise supports our findings in the predation experiments that a single post-larval prawn can exterminate a batch of ~200 snail hatchlings. The cost of post-larval prawns thus seems to be negligible with respect to rice yield improvement and additional revenue from prawn production. In a broader perspective, it would be worthwhile to examine the predation ability of these all-male prawns as a generic solution for other damaging species of freshwater snails. In conclusion, it seems that in heavily invaded ecosystems and in rice fields, a temporal intervention with all-male prawns (even where they are an exotic species) embodies more potential benefit than cost and should therefore be evaluated in field experiments.
References
Adalla CB, Magsino EA, Joshi R, Sebastian L (2006) Understanding the golden apple snail (Pomacea canaliculata): biology and early initiatives to control the pest in the Philippines. Glob Adv Ecol Manag Gold Apple Snails: 199–213
Aflalo ED, Dandu RVSN, Verghese JT, Rao N, Samraj TYC, Ovadia O, Sagi A (2014) Neo-females production and all-male progeny of a cross between two Indian strains of prawn (Macrobrachium rosenbergii): population structure and growth performance under different harvest strategies. Aquaculture 428:7–15
Aizaki K, Yusa Y (2009) Field observations of the alarm response to crushed conspecifics in the freshwater snail Pomacea canaliculata: effects of habitat, vegetation, and body size. J Ethol 27:175–180
Barker GM (2002) Molluscs as crop pests. CABI publishing, New York
Bauer RT (2011) Amphidromy and migrations of freshwater shrimps. ii. Delivery of hatching larvae to the sea, return juvenile upstream migration, and human impacts. In: Asakura A (ed) New Frontiers in crustacean biology. Koninklijke Brill NV, Leiden
Boock MV, de Almeida Marques HL, Mallasen M, Barros HP, Moraes-Valenti P, Valenti WC (2016) Effects of prawn stocking density and feeding management on rice–prawn culture. Aquaculture 451:480–487
Burky AJ (1974) Growth and biomass production of an amphibious snail, Pomacea urceus (Müller), from the Venezuelan savannah. J Molluscan Stud 41:127–143
Burky AJ, Pacheco J, Pereyra E (1972) Temperature, water, and respiratory regimes of an amphibious snail, Pomacea urceus (Müller), from the Venezuelan savannah. Biol Bull 143:304–316
Carlsson N, Kestrup Å, Mårtensson M, Nyström P (2004a) Lethal and non-lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshw Biol 49:1269–1279
Carlsson NO, Brönmark C, Hansson L-A (2004b) Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580
Carlsson NOL, Bronmark C, Hansson LA (2004c) Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580
Charniaux-Cotton H (1954) Discovery in, an amphipod crustacean (Orchestia gammarella) of an endocrine gland responsible for the differentiation of primary and secondary male sex characteristics. Comptes rendus de l’Acad des Sci 239:780–782
Chung JS, Manor R, Sagi A (2011) Cloning of an insulin-like androgenic gland factor (IAG) from the blue crab, Callinectes sapidus: implications for eyestalk regulation of IAG expression. Gen Comp Endocrinol 173:4–10
Covich AP, Crowl TA, Alexander JE Jr, Vaughn CC (1994) Predator-avoidance responses in freshwater decapod-gastropod interactions mediated by chemical stimuli. J N Am Benthol Soc 13:283–290
EFSA (2014) EFSA panel on plant health (PLH), scientific opinion on the environmental risk assessment of the apple snail for the EU. EFSA J 12(4):3641, 97 pp
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Halwart M (1994) The golden apple snail Pomacea canaliculata in Asian rice farming systems: present impact and future threat. Int J Pest Manag 40:199–206
Harikrishnan M, Kurup BM (2001) Fishery of Macrobrachium rosenbergii (de Man) in the Vembanad lake and confluent rivers. Indian J Fish 48:189–198
Herrera FDA, Uribe ES, Ramirez LFB, Mora AG (1998) Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: Palaemonidae). J Therm Biol 23:381–385
Horgan FG, Stuart AM, Kudavidanage EP (2014) Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica Int J Ecol 54:90–100
Hosmer D, Lemeshow S, May S (1999) Applied survival analysis: regression modelling of time to event data. John Wiley & Sons, New York
Joshi RC, Sebastian LS (2006) Global advances in ecology and management of golden apple snails. Philippine Rice Research Institute (PhilRice), Philippines
Kwong K-L, Chan RK, Qiu J-W (2009) The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia 51:343–356
Lach L, Cowie R (1999) The spread of the introduced freshwater apple snail Pomacea canaliculata(Lamarck)(Gastropoda: Ampullariidae) on Oahu, Hawaii. Bish Mus Occas Pap 58:66–71
Lee PG, Rodrick GE, Sodeman WA Jr, Blake NJ (1982) The giant Malaysian prawn, Macrobrachium rosenbergii, a potental predator for controlling the spread of schistosome vector snails in fish ponds. Aquaculture 28:293–301
Lezer Y, Aflalo ED, Manor R, Sharabi O, Abilevich LK, Sagi A (2015) On the safety of RNAi usage in aquaculture: the case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone. Aquaculture 435:157–166
Lin DY, Wei L-J (1989) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84:1074–1078
Litsinger J, Estano DB (1993) Management of the golden apple snail Pomacea canaliculata (Lamarck) in rice. Crop Prot 12:363–370
Manor R, Aflalo ED, Segall C, Weil S, Azulay D, Ventura T, Sagi A (2004) Androgenic gland implantation promotes growth and inhibits vitellogenesis in Cherax quadricarinatus females held in individual compartments. Invertebr Reprod Dev 45:151–159
Manor R, Weil S, Oren S, Glazer L, Aflalo ED, Ventura T, Chalifa-Caspi V, Lapidot M, Sagi A (2007) Insulin and gender: an insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen Comp Endocrinol 150:326–336
Mareddy VR, Rosen O, Thaggard HB, Manor R, Kuballa AV, Aflalo ED, Sagi A, Paterson B, Elizur A (2011) Isolation and characterization of the complete cDNA sequence encoding a putative insulin-like peptide from the androgenic gland of Penaeus monodon. Aquaculture 318:364–370
Naylor R (1996) Invasions in agriculture: assessing the cost of the golden apple snail in Asia. Ambio 25:443–448
Pain T (1960) Pomacea (Ampullariidae) of the Amazon river system. J Conchol 24:421–432
Perera G (1996) Apple snails in the aquarium: Ampullariids-their identification, care, and breeding. TFH Publications, New Jersey
Prashad B (1925) Anatomy of the common Indian apple-snail, Pila globosa. Zoological Survey of India, India
Roberts JK, Kuris AM (1990) Predation and control of laboratory populations of the snail Biomphalaria glabrata by the freshwater prawn Macrobrachium rosenbergii. Ann Trop Med Parasitol 84:401–412
Roll U, Dayan T, Simberloff D, Mienis HK (2009) Non-indigenous land and freshwater gastropods in Israel. Biol Invasions 11:1963–1972
Sagi A, Cohen D (1990) Growth, maturation and progeny of sex-reversed Macrobrachium rosenbergii males. World Aquac 21:87–90
Sagi A, Ra’Anan Z, Cohen D, Wax Y (1986) Production of Macrobrachium rosenbergii in monosex populations: yield characteristics under intensive monoculture conditions in cages. Aquaculture 51:265–275
Sagi A, Snir E, Khalaila I (1997) Sexual differentiation in decapod crustaceans: role of the androgenic gland. Invertebr Reprod Dev 31:55–61
Savaya-Alkalay A, Rosen O, Sokolow SH, Faye YP, Faye DS, Aflalo ED, Jouanard N, Zilberg D, Huttinger E, Sagi A (2014) The prawn Macrobrachium vollenhovenii in the Senegal river basin: towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis 8(8):e3060
Shpak N, Manor R, Aflalo ED, Sagi A (2017) Three generations of cultured prawn without W chromosome. Aquaculture 467(C):41–48
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Sokolow SH, Lafferty KD, Kuris AM (2013) Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop 132C:64–67
Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, Lafferty KD, Kuris AM, Rickards C, De Leo GA (2016) Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis 10(7):e0004794
Sokolow SH, Jones IJ, Jocque M, La D, Cords O, Knight A, Lund A, Wood CL, Lafferty KD, Hoover CM (2017) Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Phil Trans R Soc B 372:20160127
Taketomi Y, Murata M, Miyawaki M (1990) Androgenic gland and secondary sexual characters in the crayfish Procambarus clarkii. J Crustac Biol 10:492–497
Torres MV, Giri F, Williner V (2012) Size selective predation on an invasive bivalve, Limnoperna fortunei (Mytilidae), by a freshwater crab, Zilchiopsis collastinensis (Trichodactylidae). J Crustac Biol 32:698–710
Touir A (1977) New data concerning sexual endocrinology of hermaphroditic and gonochoristic crustacea decapoda natantia. 2. Maintenance of gonia and evolution of gametogenesis invivo and invitro. Comptes Rendus Hebd Des Seances De L Acad Des Sci Serie D 284:2515–2518
Ueshima E, Yusa Y (2015) Antipredator behaviour in response to single or combined predator cues in the apple snail Pomacea canaliculata. J Molluscan Stud 81(1):51–57
van Lenteren J, Babendreier D, Bigler F, Burgio G, Hokkanen H, Kuske S, Loomans A, Menzler-Hokkanen I, van Rijn P, Thomas M (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. Biocontrol 48:3–38
Vázquez-Islas G, Garza-Torres R, Guerrero-Tortolero DA, Campos-Ramos R (2014) Histology of the androgenic gland and expression of the insulin-like androgenic gland hormone precursor gene in the genital organ of Pacific white shrimp Litopenaeus vannamei. J Crustac Biol 34:293–299
Ventura T, Sagi A (2012) The insulin-like androgenic gland hormone in crustaceans: from a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol Adv 30:1543–1550
Ventura T, Manor R, Aflalo ED, Weil S, Raviv S, Glazer L, Sagi A (2009) Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150:1278–1286
Ventura T, Manor R, Aflalo ED, Weil S, Rosen O, Sagi A (2012) Timing sexual differentiation: full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn, Macrobrachium rosenbergii. Biol Reprod 86:6
Wada T (1997) Introduction of the apple snail Pomacea canaliculata and its impact on rice agriculture. In: Proceedings of the international workshop on biological invasions of ecosystems by pests and beneficial organisms, pp 170–180
Yamanishi Y, Yoshida K, Fujimori N, Yusa Y (2012) Predator-driven biotic resistance and propagule pressure regulate the invasive apple snail Pomacea canaliculata in Japan. Biol Invasions 14:1343–1352
Yoshie H, Yusa Y (2011) Indirect interactions in a rice ecosystem: density dependence and the interplay between consumptive and non-consumptive effects of predators. Freshw Biol 56:302–310
Yusa Y, Wada T (1999) Impact of the introduction of apple snails and their control in Japan. Naga ICLARM Q 22:9–13
Yusa Y, Sugiura N, Wada T (2006) Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: ampullariidae), in southern Japan. Biol Invasions 8:137–147
Acknowledgements
We would like to thank Mr Yossi Savaia, Mr Anton Fennec, Mr Dan Davidi, Kavra Ltd. and courtesy of Tiran Shipping group Ltd. through their subcontractor Mr. Ran Epshtein, Colors Ltd. for animal supply. In addition we thank Mr Shalev Goldferb, Mrs Nurit Levi, Mr Ran Marziano, Mr Shahar Sagie and Mr Yishai Shuchalter for animal maintenance at BGU.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 4025 kb)
Supplementary material 3 (MP4 7490 kb)
Rights and permissions
About this article
Cite this article
Savaya-Alkalay, A., Ovadia, O., Barki, A. et al. Size-selective predation by all-male prawns: implications for sustainable biocontrol of snail invasions. Biol Invasions 20, 137–149 (2018). https://doi.org/10.1007/s10530-017-1522-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1522-1