Abstract
Ponto–Caspian peracarids (amphipods, isopods, mysids and cumaceans) represent one of the most successful groups of aquatic invaders comprising several high-impact species, such as Chelicorophium curvispinum, Dikerogammarus villosus, or Hemimysis anomala. In the present study we made the first attempt to compare biological traits and the environmental preferences of invasive and non-invasive members of the group based on both literature and field data (Joint Danube Survey 3, 2013) with the goal of identifying factors linked to invasion success and drawing conclusions on future invasion risks. Both datasets indicated substrate preference as an important factor in spontaneous range expansion; all invasive species are lithophilous, whereas the majority of non-invasives are psammo-pelophilous. The remaining seven presently non-invasive lithophilous species deserve special attention when considering potential future invaders; however, due to their rarity and possible negative interactions with earlier colonists we consider the probability of their expansion in the foreseeable future as low. Their potential expansion could most likely be of minor consequence anyway, since no considerable functional novelty can be attributed to them in addition to species already present. In this limited context (regarding habitats dominated by hard substrates and not considering the potential further spread of already invasive species) it might be justified to conclude that ‘the worst is over’. Nevertheless, impending navigation development projects both in the Danube–Main–Rhine and Dnieper–Pripyat–Bug–Vistula systems might favour the future spread of non-lithophilous species, which might imply a new invasion wave of Ponto–Caspian peracarids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predicting future invasions by identifying traits of species determining invasion success is a fundamental endeavor of applied ecology (Williamson and Fitter 1996; Kolar and Lodge 2001; Heger and Trepl 2003). Initial attempts at finding features universally predisposing species to be an invader concluded that there might be inherent limitations to generalization (Williamson 1999). However, it also emerged that not all invasions are idiosyncratic, and carefully designed studies (e.g., distinguishing among stages of the invasion process) might identify informative traits (Kolar and Lodge 2001). How specifically these traits can be defined depends on the scope of the study; a meta-analysis comprising all major groups of organisms ever investigated in this context was only able to demonstrate the universal importance of climate/habitat match, history of invasive success, and the number of arriving/released individuals (Hayes and Barry 2008). Another meta-analysis restricted to plants was able to link invasiveness to more informative but still composite traits related to performance, such as physiology, leaf area allocation, shoot allocation, growth rate, size, and fitness (Van Kleunen et al. 2010b). More accurate predictions can be made if one focuses on a specific taxon in a given region (e.g., fish in the North American Great Lakes; Kolar and Lodge 2002), and it might even be possible to successfully model the potential range and impact of single invader species in yet unaffected areas (e.g., Kulhanek et al. 2011).
Data allowing an in-depth analysis of invasion risks are hard or often impossible to obtain (e.g., propagule pressure in accidental introductions); accordingly, most of the studies deal with a few well-known taxa (i.e., plants, birds, and fishes), and deliberate introductions are strongly overrepresented (Kolar and Lodge 2001; Hayes and Barry 2008). Nevertheless, due to the scale-dependent nature of the issue, specific studies on less tractable but similarly important groups of invaders are indispensable in order to provide predictions as accurate as possible.
Ponto–Caspian peracarids represent one of the most successful groups of aquatic invaders, comprising several high-impact species such as the ‘Caspian mud shrimp’ Chelicorophium curvispinum (G.O. Sars, 1895), the ‘killer shrimp’ Dikerogammarus villosus (Sowinsky, 1894), or the ‘bloody-red mysid’ Hemimysis anomala G.O. Sars, 1907 (Van den Brink et al. 1993; Dick et al. 2002; Ricciardi et al. 2012). Studies dealing with factors of their invasion success so far have concentrated on the comparison with native species, and concluded that life history traits, such as short generation time and high fecundity might be the main factor of their superiority (Devin and Beisel 2007; Grabowski et al. 2007a). Although this approach might reveal important aspects of the explanation of their success, per se it does not allow predictions to be made (Van Kleunen et al. 2010a).
In the present study, we make the first attempt to compare biological traits and the environmental preferences of invasive and non-invasive Ponto–Caspian peracarids based on both literature and field data, with the goal of identifying factors linked to invasion success and making conclusions on future invasion risks.
Materials and methods
Historical context
The expansion of Ponto–Caspian peracarids toward Western and Northern Europe has been promoted mainly by two major inland waterways connecting their native region to other catchments, the so-called southern (Danube–Main–Rhine system) and central corridors (Dnieper–Pripyat–Bug–Vistula–Notec–Oder system connected to German rivers by the Midland Canal). The third, northern corridor (Volga–Neva system) has not played a significant role in this context (Bij de Vaate et al. 2002). After colonizing several interconnected catchments in continental Europe, some of the species were also able to further extend their range to the British Isles and even to North-America (Ricciardi and MacIsaac 2000; Pothoven et al. 2007; Gallardo and Aldridge 2015).
Along the River Danube, Ponto–Caspian peracarids began to expand around the beginning of the twentieth century, parallel to the start of regular mechanized ship traffic; by the middle of the century seven species had established in the middle section of the river (Fig. 1). In the following decades, colonization rate decreased until in 1992 the Danube was connected to the Rhine basin via the Main–Danube canal. Soon after, species which have previously colonized the middle and upper sections of the Danube appeared in the Rhine, and four additional species began to expand in the system (Fig. 1). After this hectic period, however, events apparently slowed down again; presently, large-scale expansions have been detected for more than a decade.
Cumulative number of invasive Ponto–Caspian peracarid species in the course of time along the southern corridor, based on first records outside the native range. Points are connected only for the sake of expressiveness. Dashed line illustrates the time having passed until present (2016) since the last new species was detected. References: 1 Unger (1918), 2 Borza (2011), 3 Dudich (1927), 4 Borza et al. (2015), 5 Dudich (1930), 6 Sebestyén (1934), 7 Woynárovich (1954), 8 Nosek and Oertel (1980), 9 Nesemann et al. (1995), 10 Weinzierl et al. (1997), 11 Wittmann et al. (1999), 12 Bernerth et al. (2005), 13 Wittmann (2002)
Along the central corridor, C. curvispinum and Chaetogammarus ischnus (Stebbing, 1899), were first found outside their native range in the early twentieth century, when ship traffic used to be the most active (Grabowski et al. 2007b; Karatayev et al. 2008). After World War II, a dam was built on the Dnieper-Bug canal allowing only occasional ship traffic (Karatayev et al. 2008); still, D. villosus and D. haemobaphes (Eichwald, 1841) were able to reach Poland via this route around the millennium (Grabowski et al. 2007b). Beside them, several other species have expanded their range within the Dnieper basin mainly (but not exclusively) as a result of deliberate introduction (Mastitsky and Makarevich 2007; Semenchenko and Vezhnovetz 2008; Pligin et al. 2014). Ponto–Caspian species were also transported to the Baltic states in the 1960–1970s; four mysid and three amphipod species established in the Baltic region after having been released in Lithuanian or Estonian reservoirs and lakes (Arbaciauskas 2002; Herkül et al. 2009).
Literature data
Pontic and Ponto–Caspian peracarid species occurring in freshwater were considered as potentially invasive (Table 1). Although they might have the potential of range expansion (Grabowski et al. 2012), primarily freshwater species (i.e., Gammarus spp. and Niphargus spp.) were not included in the analysis, since they have markedly different ecological and biogeographical characteristics. In the present paper we use the term ‘invasive’ in a broad, purely biogeographical sense (i.e., species which have considerably widened their distributional range in recent times), without referring to abundance, or ecological/economic impact. We regarded species having spontaneously crossed the borders of their respective native catchment (Danube in the southern corridor and Dnieper in the central corridor) as invasive, but we also discuss deliberate introductions and expansions of smaller magnitude. The species list for the southern corridor is presented after Lyashenko et al. (2012) with slight modifications (Dikerogammarus bispinosus Martynov, 1925, Diamysis pengoi (Czerniavsky, 1882), and Pontogammarus aestuarius (Derzhavin, 1924) added; Chaetogammarus behningi Martynov, 1919 omitted for synonymy with C. ischnus, and Hemimysis serrata Băcescu, 1938 omitted for lack of evidence for occurrence in freshwater). The species list for the central corridor was compiled after Dediu (1980), Komarova (1991), Pligin et al. (2014), and Vasilenko and Jaume (2015). Regarding amphipod taxonomy we conformed to Lowry and Myers (2013) with the modifications of Hou and Sket (2016). We note that the classification of the Ponto–Caspian complex is far from being settled; further substantial rearrangements can be expected from molecular results (Cristescu and Hebert 2005). For this reason, we did not include taxonomic/phylogenetic information in the analysis.
Coherent datasets could be gathered only for a few basic species traits. Body lengths (average size of mature females in the summer generations, if available) were compiled after Băcescu (1951, 1954), Cărăuşu et al. (1955), and after species descriptions for species not included in these. Size data were ordered into four classes (1:[0, 5] mm, 2:[5, 10] mm, 3:[10, 15] mm, 4:[15, ∞] mm) to decrease incoherency. The substrate preference of mysids and amphipods has been classified in the most straightforward way by Dediu (1966, 1980), comprising five categories (litho-, phyto-, psammo-, pelo-, and argyllophilous). We adopted this system and completed the list for the species not dealt with in those publications after descriptions of Cărăuşu et al. (1955), Gruner (1965), and Vasilenko and Jaume (2015). In the case of Katamysis warpachowskyi G.O. Sars, 1893 the classification of Dediu (1966) contradicted to other observations from both the native and non-native range (Băcescu 1954; Wittmann 2002; Borza 2014); therefore, we included both opinions as a compromise. Salinity tolerance was characterized based on field observations in three categories (freshwater, oligohaline, mesohaline) after Băcescu (1954), Cărăuşu et al. (1955), Dediu (1980), Komarova (1991), and Vasilenko and Jaume (2015). Since apparently all species occur in freshwater as well as in oligohaline waters (the sole exception being perhaps D. bispinosus; Cărăuşu et al. 1955), only tolerance to mesohaline conditions (>5‰) was considered in the analysis.
Field data
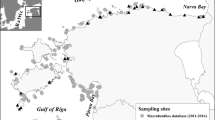
The field samples analyzed in the present study were collected during the 3rd Joint Danube Survey between 13 August and 26 September 2013 at 55 sites of the river ranging from Ulm (river km 2581) to the Delta (river km 18, Kiliya branch). At each site, 4–7 samples consisting of five units covering 25 × 25 cm bottom area were collected in the littoral zone (0.1–1.5 m depth) by hand net (aperture: 25 × 25 cm, mesh size: 500 μm) representing all habitat types available (‘multi-habitat sampling’), as defined in the AQEM protocol (Hering et al. 2004). All samples were preserved in 4% formaldehyde solution in the field, and stored in 70% ethanol after sorting. Sorting was facilitated by fractioning the material on a set of sieves (mesh sizes: 0.5, 2, 5, 10, 20 mm). In several cases, twofold to 64-fold subsampling of the smallest one or two fractions was necessary due to the extremely high number of juvenile animals in the samples. Altogether 41,509 Peracarida specimens were identified to species level whenever possible (usually above 2 mm body length in genera represented by more than one species).
Statistical analysis
Since regression-based methods could not handle the literature dataset due to the low number of cases and zero variance in some of the classes, the importance of the variables was assessed by the more flexible random forest approach (based on conditional inference trees) using the ‘cforest’ function in the ‘party’ package (Hothorn et al. 2006) in R 3.2.5 (R Core Team 2016). When estimating the importance of predictor variables, allowance was made for potential biases arising from different scale types and from the correlation among them (Strobl et al. 2007, 2008). Variable importance scores can be used to rank the predictors, but they are not informative about the strength of the relationship. Therefore, the effect of the variables with scores amounting to >10% of the highest value was further analyzed with Fisher’s exact tests.
We performed redundancy analysis (RDA) to reveal differences in the environmental preferences among Ponto–Caspian peracarids using the ‘rda’ function in the ‘vegan’ package (Oksanen et al. 2016). We restricted the analysis to the lower section of the river (river km <685, comprising 13 sites with 56 samples; Fig. 2) where several of the non-invasive species were present (only P. lacustris occurred upstream of this section), or at least could have been present potentially based on previous records (Borza et al. 2015). We used log(x + 1) and Hellinger-transformed (Legendre and Gallagher 2001) count data (individuals per sample) in the analysis, but we show ind./m2 values in Figs. 3 and 6 for the sake of comparability. Explanatory variables included substrate type (Table 1), depth, current velocity (measured at approx. 5 cm from the bottom), pH, conductivity, dissolved O2, dissolved organic carbon, chlorophyll-a, suspended matter, total nitrogen, and total phosphorus concentration. We performed forward selection (Blanchet et al. 2008a) on the environmental variables with two different adding limits, p = 0.05 and 0.01 (using the ‘ordiR2step’ function in the ‘vegan’ package), and constructed RDA models with each of the two selected variable sets. We tested the variance explained by the models with ANOVA involving 9999 permutations.
Frequency of occurrence versus average abundance (whenever present) ± SE of Ponto–Caspian peracarid species downstream of river km 685 in the Danube during Joint Danube Survey 3. Both scales are log10-transformed. Black triangles: invasive species, white triangles: non-invasive species. Cc, C. curvispinum; Ci, C. ischnus; Cr, Chelicorophium robustum; Cs, Chelicorophium sowinskyi; C_sp, Chelicorophium sp.; Cw, Chaetogammarus warpachowskyi; Dh, Dikerogammarus haemobaphes; Dv, D. villosus; D_sp, Dikerogammarus sp.; Es, Euxinia sarsi; Ha, H. anomala; Js, Jaera sarsi; Kw, Katamysis warpachowskyi; Lb, Limnomysis benedeni; Oc, Obesogammarus crassus; Oo, Obesogammarus obesus; Pb, Paramysis bakuensis; Pi, Paramysis intermedia; Pl, Paramysis lacustris; Pr, Pontogammarus robustoides; P_sp, Paramysis sp.; Pu, Paramysis ullskyi; Ss, Schizorhamphus scabriusculus; Tt, Trichogammarus trichiatus
To provide an insight into the autocorrelation structure of the data, we constructed Mantel correlograms (Borcard and Legendre 2012) using the ‘mantel.correlog’ function in the ‘vegan’ package about the response variables as well as the residuals of the two RDA models. The first distance class in the correlograms represents within-site distances, whereas the subsequent classes were delimited according to the Sturges equation based on river km distances among sites (12 classes with equal widths of 61.4 river km; the last six are not shown). p values of the Mantel correlation coefficients were calculated with Holm-correction. Since the correlograms did not indicate significant residual spatial autocorrelation (Fig. 5), the inclusion of a spatial submodel (e.g., asymmetric eigenvector maps, AEM; Blanchet et al. 2008b) was not necessary.
Results
Literature data
A total of 62 peracarid species could be identified as potentially invasive based on our criteria (Table 2). Overlap was high between the two basins, 59 species being present in the Danube catchment versus 56 in the Dnieper. In the southern corridor, lithophily proved to be the most important variable in explaining invasion success, followed by psammophily (its variable importance score amounting to15% of the score of lithophily), while all remaining variables received scores less than 0.01% of the highest. In numbers, all of the 13 invasive species were lithophilous, whereas 39 out of 46 non-invasive species were not lithophilous, meaning that the two variables are dependent on each other with a high statistical certainty (Fisher’s exact test of independence, p < 0.0001). Psammophily was also strongly associated with invasion success (p < 0.001), but had less explanatory power in terms of numbers (9 out of 13 invasive species not psammophilous, 40 out of 46 non-invasive species psammophilous), and even this arose from the strong negative association with lithophily (p < 0.0001). Substrate preference varied strongly among peracarid orders, but the role of lithophily in relation to invasion success was consistent.
In the central invasion corridor the low number of invasive species did not allow the evaluation of variable importances (all variables were scored zero), but as all four invasive species are lithophilous (the two variables being dependent at p = 0.01), the results are consistent with the southern corridor.
Field data
A total of 22 Ponto–Caspian peracarid species were recorded during the survey of which 21 were present in the section below river km 685 (Fig. 3; D. bispinosus was found only between river km 2258 and 1252). Invasive species tended to occur more frequently than non-invasive ones and were usually more abundant whenever present (Fig. 3).
The forward selection process with p = 0.05 retained six environmental variables, namely substrate type, pH, conductivity, dissolved O2, chlorophyll-a, and total phosphorus concentration which altogether explained 28.8% of the total variation (df = 10, F = 3.23, p < 0.001). With p = 0.01, the only retained variable was substrate type, accounting for 18.8% of the variance (df = 5, F = 3.54, p < 0.001). Comparing the results of the two models revealed that the five physicochemical variables had a minor, individually not interpretable effect on the ordination of the species (Fig. 4, "Appendix"). Nevertheless, their inclusion eliminated spatial autocorrelation in the data, which was still present to some degree when substrate types were considered only (Fig. 5). Consistent differences could be detected between the substrate preferences of invasive and non-invasive species; the former preferred stony substrates while the latter were associated mainly with soft sediments (they were not found on stony substrates at all; Figs. 4, 6). Representatives from both groups occurred on macrophytes and wood (‘phytal’), but invasive species were more abundant on average on these substrates (Fig. 6). Although the separation between invasive and non-invasive species was not perfect on the ordination plane (Fig. 4), the main reason for this was the rarity of certain species in the material (rare species were positioned near the origin). Our dataset does not allow solid conclusions to be made on the environmental preferences of these species.
Triplot of the RDA model including only substrate type. Samples are not shown for the sake of perspicuity. Black triangles invasive species, white triangles non-invasive species, solid line convex hull for invasive species, dashed line convex hull for non-invasive species. Substrate types (explanation in Table 1): ARG argyllal, LIT lithal, PEL pelal, PPE psammopelal, PSA psammal, PHY phytal. Abbreviations of species names as in Fig. 3 (specimens identified to genus level are not included)
Mantel correlograms of the response variables (squares/solid line), the residuals of the RDA model including six explanatory variables (circles/dashed line), and the residuals of the RDA model including only substrate type (triangles/dotted line). The distance class at 0 river km corresponds to within-site distances. Solid symbols indicate significant correlations (*p < 0.05; **p < 0.01; ***p < 0.001). Numbers on the top of the graph indicate the number of pairs involved in the calculation of correlations for each distance class. Symbols are connected only to visualize the trends
Density of invasive (a) and non-invasive (b) Ponto–Caspian peracarid species on different substrate types (explanation in Table 1) downstream of river km 685 in the Danube during Joint Danube Survey 3
Discussion
Factors of invasion success
Both datasets indicated substrate preference, specifically lithophily as the most important factor in determining invasion success among Ponto–Caspian peracarids. The two analyses supplemented each other; literature data showed a comprehensive but somewhat schematic picture about the whole species pool, whereas field data provided a more detailed insight into the environmental preferences of the most frequent species.
The most obvious explanation for the importance of substrate preference is that lithophilous species have a higher chance of establishment and proliferation outside their native range because waters here are dominated by stony substrates (gravel, riprap). This explanation is in accordance with general observations identifying environmental match as the most consistent factor of invasion success across various groups of organisms (Hayes and Barry 2008). On the other hand, it also seems reasonable to assume that substrate preference might also affect the chance of being transported to distant places. Lithophilous species might be more inclined to attach to hard surfaces of ships, the main means of transport (Reinhold and Tittizer 1999). These two alternatives are not mutually exclusive; in all likelihood both explanations have some effect on the chance of passing successive stages of the invasion process.
Since presently all invasive species are lithophilous but not all lithophilous species are invasive, preference for hard substrates can be considered as a necessary but not sufficient prerequisite of invasion success among Ponto–Caspian peracarids. Although the number of factors included in the analysis was rather low, it is not likely that the consideration of more variables would change this conclusion, since the importance of habitat match for invasion success is widely reported and quite evident. Nevertheless, we can presume that some additional factors of invasion success do exist and accounted for presently non-invasive lithophilous species. Similarly, although present invasion patterns do not allow much distinction among non-lithophilous species, invasion potential might vary among them, too. Below we list three factors we consider as potentially relevant in this regard.
-
1.
Invaders already present might impede the establishment of further colonists. Sympatric members of the Ponto–Caspian peracarid assemblage can be assumed to coexist stably through resource partitioning, based on their shared evolutionary history (Gallardo and Aldridge 2015). In contrast, the circumstances allowing their coexistence within their native range might not be provided outside it in all cases. For instance, phytophilous amphipods (e.g., P. robustoides, O. crassus, and C. warpachowskyi) can be assumed to be able to use (and actually prefer) stony substrates (Jermacz et al. 2015b), but the presence of lithophilous species, above all D. villosus, might prevent them from doing so (Jermacz et al. 2015a). This, in the absence of extended macrophyte stands and lentic sandy shoals might impede the establishment of the newcomers, or even result in the decline of their populations already present. This mechanism might explain the extinction of O. crassus from the Middle Danube during the twentieth century concurrent with the appearance of D. villosus, and similarly, the disappearance of Chelicorophium maeoticum (Sowinsky, 1898) in the Serbian section of the Danube and the River Tisza might be linked to the invasion of C. curvispinum (Borza et al. 2015).
-
2.
Propagule pressure, a strong determinant of invasion success (Hayes and Barry 2008; Simberloff 2009), can be expected to be correlated with abundance within the donor region. Accordingly, some of the species might simply be too rare to have a realistic chance of being transported over long distances in numbers high enough to develop a persistent colony. According to Dediu (1980), several species occur generally in very low numbers (1–10 ind./m2), while the density of some others might reach the magnitude of tens of thousands ind./m2. Furthermore, some of the species have only a few known occurrences which often date back to several decades ago (Lyashenko et al. 2012).
-
3.
All of the species included in the list of potential invaders have been recorded in freshwater; however, low salinity might be suboptimal for some of them, decreasing their chance of ever expanding their ranges in inland waters. Moreover, considering that saltwater can intrude the deltas of rivers, occasionally up to several tens of kilometers in the artificially deepened Sulina arm of the Danube, for example (Bondar 1983), sporadic occurrences in freshwater might not prove independence of saltwater in all of the cases. Thus, detailed studies of their autecology might identify some of the species included in the list as an occasional visitor in freshwaters.
Future prospects
We acknowledge that lithophily is not the only factor affecting invasion success among Ponto–Caspian peracarids; nevertheless, it is worthwhile to consider it with regard to future invasion prospects since it is not trivial among the species. In the southern invasion corridor, the distribution of only seven lithophilous species (six amphipods and one mysid; Table 3) remained restricted to the lower reaches of the river as yet, which deserve special attention when considering potential future invaders. However, due to their rarity (several of them have not been recorded for decades; Table 3) and possibly to other factors (e.g., negative interactions with invasives) we estimate the probability of their large-scale expansion in the foreseeable future as low. Even if some of them became invasive after all, the effect on lithophilous assemblages could most likely be minor, since no considerable functional novelty can be attributed to them in addition to the species already present. Of course, they can be expected to occupy different niches, which might imply changes in resource utilization either by the consumption of previously unused resources or competition for used ones, but this kind of impact is not comparable to the functional novelty represented by the appearance of the first corophiid (Van den Brink et al. 1993), large predatory gammarid (Dick et al. 2002), or zooplanktivore mysid (Ketelaars et al. 1999) in a given ecosystem. Therefore, in this limited context; i.e., regarding habitats dominated by hard substrates and not considering the potential further spread of already invasive species, it might be justified to conclude that ‘the worst is over’.
Apparently, the system has reached a steady state where the pool of lithophilous species has run out (or it is close to it, at least), and non-lithophilous species are not able to expand (over large distances, at least). Nevertheless, most invasions in the history of the corridor occurred in bursts parallel to major developments in navigation (Fig. 1). Is there something that could disrupt the status quo and might induce a new invasion wave? Since considerable economic interests are involved, further development of the conditions of shipping on the Danube is continuously on the agenda, for example in the form of deepening the shipping channel, which would allow larger classes of ships to pass (Anonymous 2016a). This might imply increasing ship traffic, shortening of travel times, and a rearrangement in the importance of traffic hubs both in the donor and recipient regions, which in the end might allow further species to spread. Another issue is the possible construction of dams in the Middle Danube, which might become inevitable one day due to sinking ground water levels in the Great Pannonian Plain. This could result in a more-or-less continuous cascade of reservoirs throughout the upper and middle river sections which might allow the spread of psammo-pelophilous species, as exemplified by Eastern European large rivers, where, besides several deliberate introductions, some of the species began to expand spontaneously (Grigorovich et al. 2002; Filinova et al. 2008; Semenchenko et al. 2015). The secondary spread of several species introduced into the Baltic region as well as the recent appearance of P. lacustris in the Serbian Danube section and in the River Tisza (Borza and Boda 2013) also indicate that spontaneous expansion of non-lithophilous species should be dealt with, at least when the environment is favourable (i.e., it is dominated by soft substrates) and distances are not too large (in the magnitude of several hundred kilometres). So, when planning such projects, further invasions of Ponto–Caspian peracarids should be considered among possible environmental hazards.
In the central invasion corridor, the project aimed at widening the bottleneck represented by the Dnieper–Bug canal is already near the implementation phase (Anonymous 2016b), which might give a boost to the expansion of Ponto–Caspian species in the near future (Karatayev et al. 2008). In this region, much more potential remained in lithophilous species; however, some of them might reach the Baltic basin even sooner from Germany, as in the case of D. villosus and T. trichiatus (Grabowski et al. 2007b; Rachalewski et al. 2013). The lowland character of the rivers constituting this waterway (Semenchenko and Vezhnovetz 2008) combined with a higher vector activity might provide favourable conditions for the spread of non-lithophilous species, as well. Some of them are already present in the Baltic basin, so their potential expansion would be of less consequence, but it could imply the colonization of further areas within the region. On the other hand, several other species are present in the reservoirs of the Dnieper (Pligin et al. 2014), the possible further spread of which also should be dealt with under the altered circumstances.
Conclusions
In our analysis we were able to identify preference for stony substrates as an important factor of invasion success among Ponto–Caspian peracarids, providing a consistent but not full explanation for the presently observable patterns, and allowing general conclusions to be made on future prospects. At the same time, our effort highlighted how insufficient our present knowledge is about the taxonomy, faunistics, autecology, and interactions of this important group. In the light of their already significant impact and still high potential for further expansion, much more effort should be devoted to studying Ponto–Caspian peracarids within their native range, which could allow us to provide a more precise assessment of future invasion risks.
References
Anonymous (2016a) Danube region strategy projects: waterway inftrastructure. http://www.danube-navigation.eu/projects-ideas. Accessed 28 Nov 2016
Anonymous (2016b) Commission on the development of the E-40 waterway on the Dnieper–Vistula section. http://e40restoration.eu/en. Accessed 28 Nov 2016
Arbaciauskas K (2002) Ponto–Caspian amphipods and mysids in the inland waters of Lithuania: history of introduction, current distribution and relations with native malacostracans. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe. Distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 104–115
Băcescu M (1951) Crustacea: Cumacea. Editura Academiei Republicii Populare Romîne, Bucureşti
Băcescu M (1954) Crustacea: Mysidacea. Editura Academiei Republicii Populare Romîne, Bucureşti
Bernerth H, Tobias W, Stein S (2005) Faunenwandel im Main zwischen 1997 und 2002 am Beispiel des Makrozoobenthos. Faunistisch-ökologische Untersuchungen des Forschungsinstitutes Senckenberg im hessischen Main. Hessisches Landesamt für Umwelt und Geologie, Wiesbaden, pp 15–87
Bij de Vaate A, Jażdżewski K, Ketelaars HAM et al (2002) Geographical patterns in range extension of Ponto–Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174. doi:10.1139/f02-098
Blanchet FG, Legendre P, Borcard D (2008a) Forward selection of explanatory variables. Ecology 89:2623–2632. doi:10.1890/07-0986.1
Blanchet FG, Legendre P, Borcard D (2008b) Modelling directional spatial processes in ecological data. Ecol Model 215:325–336. doi:10.1016/j.ecolmodel.2008.04.001
Bondar C (1983) Zum Eindringen des Wassers des Schwarzen Meeres in die Donau-Arme. Hidrobiol Bucur 17:217
Borcard D, Legendre P (2012) Is the mantel correlogram powerful enough to be useful in ecologicalanalysis? A simulation study. Ecology 93:1473–1481. doi:10.1890/11-1737.1
Borza P (2011) Revision of invasion history, distributional patterns, and new records of Corophiidae (Crustacea: Amphipoda) in Hungary. Acta Zool Acad Sci Hung 57:75–84
Borza P (2014) Life history of invasive Ponto–Caspian mysids (Crustacea: Mysida): a comparative study. Limnologica 44:9–17. doi:10.1016/j.limno.2013.06.001
Borza P, Boda P (2013) Range expansion of Ponto–Caspian mysids (Mysida, Mysidae) in the River Tisza: first record of Paramysis lacustris (Czerniavsky, 1882) for Hungary. Crustaceana 86:1316–1327. doi:10.1163/15685403-00003229
Borza P, Csányi B, Huber T et al (2015) Longitudinal distributional patterns of Peracarida (Crustacea, Malacostraca) in the River Danube. Fundam Appl Limnol 187:113–126. doi:10.1127/fal/2015/0769
Cărăuşu S, Dobreanu E, Manolache C (1955) Amphipoda forme salmastre şi de apă dulce [Freshwater and brackish water Amphipoda]. Editura Academiei Republicii Populare Romîne, Bucureşti
Cristescu ME, Hebert PD (2005) The“Crustacean Seas” an evolutionary perspective on the Ponto Caspian peracarids. Can J Fish Aquat Sci 62:505–517. doi:10.1139/f04-210
Dediu II (1966) Répartition et caractéristique écologique des Mysides des bassins des rivièrs Dniestr et Pruth. Rev Roum Biol Zool 11:233–239
Dediu II (1980) Amphipody presnykh i solonovatykh vod Yugo-Zapada SSSR [Amphipods of fresh and brackish waters of the South-West of USSR]. Shtiintsa, Kishinev
Devin S, Beisel J-N (2007) Biological and ecological characteristics of invasive species: a gammarid study. Biol Invasions 9:13–24. doi:10.1007/s10530-006-9001-0
Dick JT, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084. doi:10.1139/f02-074
Dudich E (1927) Új rákfajok Magyarország faunájában—Neue Krebstiere in der Fauna Ungarns. Arch Balatonicum 1:343–387
Dudich E (1930) A Jaera Nordmanni Rathke, egy új víziászka a magyar faunában [Jaera Nordmanni Rathke, a new aquatic isopod in the Hungarian fauna]. Állattani Közlemények 27:120
Filinova EI, Malinina YA, Shlyakhtin GV (2008) Bioinvasions in macrozoobenthos of the Volgograd Reservoir. Russ J Ecol 39:193–197. doi:10.1134/S1067413608030077
Gallardo B, Aldridge DC (2015) Is Great Britain heading for a Ponto–Caspian invasional meltdown? J Appl Ecol 52:41–49. doi:10.1111/1365-2664.12348
Grabowski M, Bacela K, Konopacka A (2007a) How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia 590:75–84. doi:10.1007/s10750-007-0759-6
Grabowski M, Jażdżewski K, Konopacka A (2007b) Alien Crustacea in polish waters—Amphipoda. Aquat Invasions 2:25–38. doi:10.3391/ai.2007.2.1.3
Grabowski M, Rewicz T, Bacela-Spychalska K et al (2012) Cryptic invasion of Baltic lowlands by freshwater amphipod of Pontic origin. Aquat Invasions 7:337–346. doi:10.3391/ai.2012.7.3.005
Grigorovich IA, MacIsaac HJ, Shadrin NV, Mills EL (2002) Patterns and mechanisms of aquatic invertebrate introductions in the Ponto–Caspian region. Can J Fish Aquat Sci 59:1189–1208. doi:10.1139/f02-088
Gruner HE (1965) Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und ihrer Lebensweise, Teil 51. Krebstiere oder Crustacea, V. Isopoda, 1. Lieferung. Fischer Verlag, Jena
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506. doi:10.1007/s10530-007-9146-5
Heger T, Trepl L (2003) Predicting Biological Invasions. Biol Invasions 5:313–321. doi:10.1023/B:BINV.0000005568.44154.12
Hering D, Moog O, Sandin L, Verdonschot PF (2004) Overview and application of the AQEM assessmentsystem. Hydrobiologia 516:1–20. doi:10.1023/B:HYDR.0000025255.70009.a5
Herkül K, Kotta J, Püss T, Kotta I (2009) Crustacean invasions in the Estonian coastal sea. Est J Ecol 58:313–323. doi:10.3176/eco.2009.4.06
Hothorn T, Bühlmann P, Dudoit S et al (2006) Survival ensembles. Biostatistics 7:355–373. doi:10.1093/biostatistics/kxj011
Hou Z, Sket B (2016) A review of Gammaridae (Crustacea: Amphipoda): the family extent, its evolutionary history, and taxonomic redefinition of genera. Zool J Linn Soc 176:323–348. doi:10.1111/zoj.12318
Jermacz Ł, Dzierżyńska A, Kakareko T et al (2015a) The art of choice: predation risk changes interspecific competition between freshwater amphipods. Behav Ecol. doi:10.1093/beheco/arv009
Jermacz Ł, Dzierżyńska A, Poznańska M, Kobak J (2015b) Experimental evaluation of preferences of an invasive Ponto–Caspian gammarid Pontogammarus robustoides (Amphipoda, Gammaroidea) for mineral and plant substrata. Hydrobiologia 746:209–221. doi:10.1007/s10750-014-1963-9
Karatayev AY, Mastitsky SE, Burlakova LE, Olenin S (2008) Past, current, and future of the central European corridor for aquatic invasions in Belarus. Biol Invasions 10:215–232. doi:10.1007/s10530-007-9124-y
Ketelaars HA, Lambregts-van de Clundert FE, Carpentier CJ et al (1999) Ecological effects of the mass occurrence of the Ponto–Caspian invader, Hemimysis anomala GO Sars, 1907 (Crustacea: Mysidacea), in a freshwater storage reservoir in the Netherlands, with notes on its autecology and new records. Hydrobiologia 394:233–248. doi:10.1023/A:1003619631920
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. doi:10.1016/S0169-5347(01)02101-2
Kolar CS, Lodge DM (2002) Ecological predictions and risk assessment for alien fishes in North America. Science 298:1233–1236. doi:10.1126/science.1075753
Komarova TI (1991) Fauna Ukrainy. T. 26. Mizidy (Mysidacea). Naukova Dumka, Kiev
Kulhanek SA, Leung B, Ricciardi A (2011) Using ecological niche models to predict the abundance and impact of invasive species: application to the common carp. Ecol Appl 21:203–213. doi:10.1890/09-1639.1
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. doi:10.1007/s004420100716
Lowry JK, Myers AA (2013) A Phylogeny and Classification of the Senticaudata subord. nov. Crustacea: Amphipoda). Zootaxa 3610:1–80. doi:10.11646/zootaxa.3610.1.1
Lyashenko AV, Zorina-Sakharova YY, Makovskiy VV, Sanzhak YO (2012) Modern state of the Ponto–Caspian Complex of the macrofauna of invertebrates in the Lower Reaches of the Danube River within the territory of Ukraine. Hydrobiol J 48:18–37. doi:10.1615/HydrobJ.v48.i4.20
Mastitsky SE, Makarevich OA (2007) Distribution and abundance of Ponto–Caspian amphipods in the Belarusian section of the Dnieper River. Aquat Invasions 2:39–44. doi:10.3391/ai.2007.2.1.4
Nesemann H, Pöckl M, Wittmann KJ (1995) Distribution of epigean Malacostraca in the middle and upper Danube (Hungary, Austria, Germany). Misc Zool Hung 10:49–68
Nosek JN, Oertel N (1980) Zoologische Untersuchungen an Aufwüchsen in der Donau zwischen Rajka und Budapest. Ann Univ Sci Budapestinensis Rolando Eotvos Nomin Sect Biol 22–23:187–204
Oksanen J, Blanchet FG, Kindt R et al (2016) Vegan: community ecology package. R package version 2.3-5. http://CRAN.R-project.org/package=vegan
Pligin YV, Matchinskaya SF, Zheleznyak NI, Linchuk MI (2014) Long-term distribution of alien species of macroinvertebrates in the ecosystems of the Dnieper Reservoirs. Hydrobiol J 50:3–17. doi:10.1615/HydrobJ.v50.i2.10
Popescu-Marinescu V, Năstăsescu M (2005) Amphipods (Gammaridae and Corophiidae) from iron gates I and II Dam lakes–Danube (Romania), concerning especially 2002 situation. Trav Muséum Natl D’Histoire Nat Grigore Antipa 48:501–521
Pothoven SA, Grigorovich IA, Fahnenstiel GL, Balcer MD (2007) Introduction of the Ponto–Caspian bloody-red mysid Hemimysis anomala into the Lake Michigan basin. J Gt Lakes Res 33:285–292. http://dx.doi.org/10.3394/0380-1330(2007)33[285:IOTPBM]2.0.CO;2
Prunescu-Arion E, Elian L (1965) Beitrag zum Studium der Fauna und der Ökologie der Gammariden im rumänischen Abschnitt der Donau. Veröff Arbeitsgemeinschaft Donauforsch 2:65–79
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rachalewski M, Konopacka A, Grabowski M, Bacela-Spychalska K (2013) Echinogammarus trichiatus (Martynov, 1932): a new Ponto–Caspian amphipod invader in Poland with remarks on other alien amphipods from the Oder River. Crustaceana 86:1224–1233. doi:10.1163/15685403-00003228
Reinhold M, Tittizer T (1999) Verschleppung von Makrozoen durch Kühlwasserfilter eines Schiffes. Wasser Boden 51:61–66
Ricciardi A, MacIsaac HJ (2000) Recent mass invasion of the North American Great Lakes by Ponto–Caspian species. Trends Ecol Evol 15:62–65. doi:10.1016/S0169-5347(99)01745-0
Ricciardi A, Avlijas S, Marty J (2012) Forecasting the ecological impacts of the Hemimysis anomala invasion in North America: lessons from other freshwater mysid introductions. J Gt Lakes Res 38:7–13. doi:10.1016/j.jglr.2011.06.007
Sebestyén O (1934) A vándorkagyló (Dreissensia polymorpha Pall.) és a szövőbolharák (Corophium curvispinum G. O. Sars forma devium Wundsch) megjelenése és rohamos térfoglalása a Balatonban [Appearance and rapid increase of Dreissensia polymorpha Pall. and Corophium curvispinum G. O. Sars forma devium Wundsch in Lake Balaton]. Magy Biológiai Kutint Munkái 7:190–204
Semenchenko V, Vezhnovetz V (2008) Two new invasive Ponto–Caspian amphipods reached the Pripyat River, Belarus. Aquat Invasions 3:445–447. doi:10.3391/ai.2008.3.4.14
Semenchenko VP, Son MO, Novitsky RA et al (2015) Alien macroinvertebrates and fish in the Dnieper River basin. Russ J Biol Invasions 6:51–64. doi:10.1134/S2075111715010063
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102. doi:10.1146/annurev.ecolsys.110308.120304
Strobl C, Boulesteix A-L, Zeileis A, Hothorn T (2007) Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinform 8:25. doi:10.1186/1471-2105-8-25
Strobl C, Boulesteix A-L, Kneib T et al (2008) Conditional variable importance for random forests. BMC Bioinform 9:307. doi:10.1186/1471-2105-9-307
Unger E (1918) A Corophium devium előfordulása a Dunában [Occurrence of Corophium devium in the Danube]. Állattani Közlemények 17:148–149
Van den Brink FWB, Van der Velde G, Bij de Vaate A (1993) Ecological aspects, explosive range extension and impact of a mass invader, Corophium curvispinum Sars, 1895 (Crustacea: Amphipoda), in the Lower Rhine (The Netherlands). Oecologia 93:224–232. doi:10.1007/BF00317675
Van Kleunen M, Dawson W, Schlaepfer D et al (2010a) Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol Lett 13:947–958. doi:10.1111/j.1461-0248.2010.01503.x
Van Kleunen M, Weber E, Fischer M (2010b) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. doi:10.1111/j.1461-0248.2009.01418.x
Vasilenko S, Jaume D (2015) Check-list for Caspian Sea Cumaceans. http://www.zin.ru/projects/caspdiv/caspian_cumaceans.html. Accessed 08 Apr 2015
Weinzierl A, Seitz G, Thannemann R (1997) Echinogammarus trichiatus (Amphipoda) und Atyaephyra desmaresti (Decapoda) in der bayerischen Donau. Lauterbornia 31:31–32
Williamson M (1999) Invasions. Ecography 22:5–12. doi:10.1111/j.1600-0587.1999.tb00449.x
Williamson MH, Fitter A (1996) The characters of successful invaders. Biol Conserv 78:163–170. doi:10.1016/0006-3207(96)00025-0
Wittmann KJ (2002) Weiteres Vordringen pontokaspischer Mysidacea (Crustacea) in die mittlere und obere Donau: Erstnachweise von Katamysis warpachowskyi für Ungarn, die Slowakei und Österreich mit Notizen zur Biologie und zum ökologischen Gefährdungspotential. Lauterbornia 44:49–63
Wittmann KJ, Theiss J, Banning M (1999) Die Drift von Mysidacea und Decapoda und ihre Bedeutung für die Ausbreitung von Neozoen im Main-Donau-System. Lauterbornia 35:53–66
Woynárovich E (1954) Vorkommen der Limnomysis benedeni Czern. im ungarischen Donauabschnitt. Acta Zool Acad Sci Hung 1:177–185
Acknowledgements
Joint Danube Survey 3 was organized by the International Commission for the Protection of the Danube River (ICPDR). We would like to thank everyone involved in the organization, field work, and evaluation of the survey for their effort. This work was supported by the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded by the European Union under the 7th Framework Programme, grant Agreement No: 603378. Péter Borza was supported by the Scholarship of the Scholarship Foundation of the Republic of Austria for Post-docs from October 2013 until March 2014 (funding organization: OeAD-GmbH on behalf of and financed by the Scholarship Foundation of the Republic of Austria). We thank Karl J. Wittmann for data on D. pengoi distribution, Michał Grabowski for remarks on amphipod taxonomy, and Ferenc Jordán, Dénes Schmera and two anonymous referees for useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Fig. 7.
Triplot of the RDA model including six explanatory variables. Samples are not shown for the sake of perspicuity. Black triangles invasive species, white triangles non-invasive species, solid line convex hull for invasive species, dashed line convex hull for non-invasive species. Substrate types (explanation in Table 1): ARG argyllal, LIT lithal, PEL pelal, PPE psammopelal, PSA psammal, PHY phytal. Abbreviations of continuous variables: con conductivity, dis dissolved O2, chl chlorophyll-a, toP total phosphorus. Abbreviations of species names as in Fig. 3 (specimens identified to genus level are not included)
Rights and permissions
About this article
Cite this article
Borza, P., Huber, T., Leitner, P. et al. Success factors and future prospects of Ponto–Caspian peracarid (Crustacea: Malacostraca) invasions: Is ‘the worst over’?. Biol Invasions 19, 1517–1532 (2017). https://doi.org/10.1007/s10530-017-1375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1375-7