Abstract
When alien species introduced into a new environment have a strong niche overlap with ecologically similar native species, interspecific competition can cause a decrease in abundance and distribution of native species. Pallas’s squirrel (Callosciurus erythraeus) was introduced in Northern Italy where it currently co-occurs with native Eurasian red squirrels (Sciurus vulgaris). The alien species is known for its invasiveness but so far negative effects of Pallas’s squirrels on native tree squirrels have not been demonstrated. Here, we compare demographic parameters of red squirrel populations between sites without (red-only sites) and with (red-Pallas’s sites) C. erythraeus and present results of trapping and removal of Pallas’s squirrel and its effects on red squirrel population dynamics. The native species was patchily distributed and absent in many trapping sites occupied by the Pallas’s squirrel. Red squirrels occurred at much lower densities and showed reduced adult survival in areas of co-occurrence than in red-only sites, but there were no differences in reproductive rate. Removing invasive squirrels throughout the study period resulted in re-colonisation by the native species only in some trapping sites, and several alternatives to explain the lack of a marked increase in population size are discussed. This study is the first to provide evidence that presence of Pallas’s squirrel reduces viability of local red squirrel populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally accepted that stable, long-term coexistence between two species that compete for the same resources is possible only when some degree of niche differentiation occurs (Emmons 1980; Riege 1991). If there is no (sufficient) niche differentiation and resources are limited, interspecific competition can be severe and affect individual’s fitness (e.g. reduced survival and/or reproductive success), which will be reflected at the population level (distribution, population density, persistence; Holway 1999; Byers 2000; Shuttleworth et al. 2015). When there is no sufficient time for co-evolution to shape niche differentiation, competition can ultimately lead to extinction of one of the two species (Ricciardi et al. 1998; Mooney and Cleland 2001).
This problem of competitive exclusion can occur when an alien species is introduced in a new environment where its niche overlaps strongly with that of an ecologically similar native species (Broennimann et al. 2007). As opposed to naturally co-evolved competitor systems, in the case of human-mediated introductions the two species (alien and native) come into contact abruptly, each one being in some way “trapped” by its evolutionary background. In many cases the outcome, lacking the time for niche differentiation to evolve, is the establishment of mechanisms of competitive exclusion with one species replacing the other (Hardin 1960; Strong and Pemberton 2000; Mooney and Cleland 2001; Bertolino 2008; Paini et al. 2008).
Among mammals, tree squirrels are well-known examples of invasive alien species (IAS) (Jeschke and Strayer 2005; Genovesi et al. 2012). They have been introduced worldwide mainly through the international pet trade (Aprile and Chicco 1999; Long 2003), often followed by subsequent (illegal) translocations, and their capability to establish viable populations from only a few founders, the high vagility and diverse food habits have made them a successful invasive taxon (Palmer et al. 2007; Bertolino 2009; Martinoli et al. 2010). Alien—native squirrel species interactions are good model systems to explore patterns and mechanisms involved in interspecific (resource) competition and disease-mediated competition (Gurnell et al. 2015; Shuttleworth et al. 2015). The replacement of the native Eurasian red squirrel (Sciurus vulgaris Linnaeus 1758) by the alien Eastern grey squirrel (Sciurus carolinensis Gmelin 1788) in the British Isles and Italy is a well-known example of invasion leading to local extinction of the native species. The presence of the invasive grey squirrel negatively affected the food exploitation and, in some forests, habitat use of red squirrels resulting in smaller body size and reduced female reproductive success and juvenile recruitment, ultimately causing population size to decrease until local extinction (Gurnell et al. 2004, 2015). Another case is the competition between the American red squirrel (Tamiasciurus hudsonicus Erxleben 1777) and the Abert’s squirrel (Sciurus aberti Woodhouse 1853), which are naturally sympatric over much of their ranges in the United States (Thorington et al. 2012). However in the Pinaleño Mountains (Arizona, USA) the Abert’s squirrel was recently introduced in an area where the endemic Mt. Graham red squirrel (T. h. grahamensis) has been isolated for approximately 10,000 years from other tree squirrel species (Lomolino et al. 1989; Edelman and Koprowski 2005). Preliminary studies suggested that the non-native species may affect space use of the red squirrel because of extensive home range overlap and use of common food resources, which may force the native species to forage more widely and consume sub-optimal food items (Ferner 1974; Edelman and Koprowski 2005, 2006).
Since 2007, the presence of a tree squirrel native to South-East Asia, the Pallas’s squirrel (Callosciurus erythraeus Pallas 1779), has been documented in Northern Italy, but history of the introduction and numbers of squirrels released are still unknown (Mazzamuto et al. 2016a). So far, Pallas’s squirrel has been introduced in seven countries around the world (Argentina, France, Belgium, Italy, the Netherlands, Japan and China- Hong Kong), and of the 29 known introduction events 21 (including Italy) are documented as successful with a viable population established in the wild (Bertolino and Lurz 2013). The species is known to be invasive and to damage commercial and forest trees, electric wires and parts of buildings (Hori et al. 2006; Guichón and Doncaster 2008; Stuyck et al. 2009). Moreover, in Japan the native squirrel species, Sciurus lis, is locally declining probably because of competition for food and nesting sites (Ministry of the Environment, Japan, 2002; Miyamoto et al. 2004).
In its current range in Italy, Pallas’s squirrel co-occurs with the native Eurasian red squirrel that could suffer from interspecific competition for space and (food) resources, due to ecological niche overlap. In 2011 a removal program of the invasive squirrel started (European Life Project LIFE09 NAT/IT/00095 EC-SQUARE; http://www.rossoscoiattolo.eu/en).
In this paper we present the results of the control campaign and the effects of Pallas’s squirrel presence on red squirrel population dynamics. In particular we examined red squirrel population density, fecundity and local survival in the experimental area where Pallas’s and red squirrel share the same space and food resources, and in a control area without the invasive squirrel. Based on earlier work on interspecific competition between native red and alien grey squirrels (Wauters et al. 2000; Gurnell et al. 2004, 2015), we tested the hypothesis that the alien Pallas’s squirrel negatively affects the demography of the native red squirrel. Hence, we predict that: (i) local distribution and/or density of red squirrels will be lower in the mixed-species than in the red-only area; (ii) local survival and/or reproductive success of the native species will be lower in the mixed-species than in the red-only area. Finally, we predict that removal of Pallas’s squirrels throughout the study period should result in an increase in the demographic parameters of the red squirrel population over time in the experimental area.
Materials and methods
Study areas
Squirrels were studied in two areas, one red-Pallas area with both red and Pallas’s squirrels present, and one red-only area where only red squirrels occurred. The first study area (326 ha) is part of 9000 ha of continuous mixed deciduous forests with patches of grassland and small villages (North of Varese province, Lombardy region, Italy 45°58′09.2″N, 08°43′57.6″E). The red-only study area (68 ha) is part of an extensive mixed forest of 3500 ha on the northern edge of the upper Po plain in Lombardy (Parco Pineta regional park, 45°46′43.7″N, 8°54′35.1″E). Shortest distance between the two study areas is 23 km and both fall within the Upper Po plain and lower hills of the Insubria region (Tosi and Zilio 2002) (Fig. 1). For comparison, tree species composition in our two study areas is given in Table 1. Forest composition was analysed creating a minimum convex polygon (MCP) from all the locations of the traps to which a further 50 meters buffer zone was added. Within that area, a regular 150 × 150 m grid of points was created using a geographic information system (QGIS Development Team 2015). At each point of the regular grid that fell in the MCP area a 20 × 20 m sampling plot was set up in which we recorded species name of tree/shrub, number of trees/shrubs for every species, percentage cover of tree and shrub layer (see also Cagnin et al. 2000). Although there are slight differences in tree-species composition, both red-only and red-Pallas areas have a diverse forest structure with a variety of seed-bearing species used by both squirrel species for feeding (e.g. Wauters et al. 2001a, b). All study areas have between 30 and 40% of hazel Corylus avellana in the understorey.
Study design
We used an unbalanced study design, with data from different studies on red squirrel ecology (control area) carried out both before and during the period of our monitoring of red and Pallas’s squirrels in several trapping grids in the only area in Italy where they co-occur (experimental area, see also Table 2). We are aware that this implies that the different red-only sites are not true replicates, but we will show that both previously recorded data (years 1996–1998, see Wauters et al. 2001b; Gurnell et al. 2004) and those recorded by us in 2011–2012 in mixed forests of Parco Pineta produce comparable results for population parameters and can be used as replicates of red-only area.
In the red-only area red squirrels were trapped in 2 trapping sites of 31 and 37 ha and monitored from July 1996 to December 1998 and from December 2011 to June 2012 (Fig. 1). As ‘capture session’ we defined a period of consecutive days of trapping while the trap checks during each session are ‘occasions’. During 1996–1998, trapping was carried out for at least 5 days every season (4 trapping sessions/year: January, April, July, October), while during 2011–2012, three 5-day trapping sessions were held over a period of 8 months (December, March, June). In the red-Pallas area squirrels were trapped in 19 different trapping sites and for the entire area, trapping was carried out from May 2011 to February 2015 (Fig. 1; Table 2). Each capture session was generally 5 day long with 8 trap checks, but some changes occurred during the study depending on the weather condition or management needs; consequently the 19 trapping sites were active for different number of sessions and in different years (Table 2). In both control and experimental area, at least one capture session took place during the breeding period (February–October, Wauters and Lens 1995; Wauters et al. 2001b).
Trapping and handling squirrels
Single-capture traps (model 202, Tomahawk Live Trap Co., Tomahawk, WI, USA) and, in the experimental area, both single-capture and multi-capture traps (Long Meadow Publishing, Shaftesbury, Wiltshire, UK; see Mayle et al. 2007) were generally arranged in grids and spaced 70–100 m from each other. In the experimental area, depending on management actions for the eradication of the alien species, some traps were also arranged along transects or irregular grids (Table 2). Traps were fixed on tree trunks at breast height using baling wire. In the red-only area, traps were also set on the ground (Wauters et al. 2001b). Prebaiting started one week before each trapping session: hazelnuts and apple slices were placed inside the blocked traps, and each trap was checked and rebaited every two days. When activated, traps were checked two times per day to reduce the time squirrels were confined in traps. Each trapped squirrel was weighed to the nearest 5 g with a Pesola spring balance, identified to species, sex and reproductive status (Wauters et al. 2007), and the length of the right hind foot (nails excluded) was measured (0.5 mm precision) with a thin ruler (Wauters et al. 2007). Red squirrels were individually marked with numbered metal ear tags (type 1003 S National Band and Tag Co, Newport, Kentucky, USA) and immediately released, while all males and non reproductive females (in order to comply with welfare of newborn animals) of the invasive species were euthanized by CO2 inhalation, following European Commission and American Veterinary Medical Association guidelines (Close et al. 1996, 1997; Leary et al. 2013). For each trapped squirrel, trap ID, date, the capture session number and the occasion number were recorded.
Population parameters and data analyses
Population size estimates of the alien species were based on removal sampling. Maximum likelihood estimation from catch-effort data was used to estimate the maximum number of Pallas’s squirrels in the study area (Gould and Pollock 1997) with the R package fishmethods v. 1.8-0 (Nelson 2015). This model improves standard linear regression methods to estimate the number of individuals present at the start of a series of trapping sessions (Y-variable), based on the number of animals trapped and removed (X-variable) in subsequent sessions, assuming a closed population during the entire trapping period (Leslie and Davis 1939).
Next, we compared the occurrence of the native and the invasive squirrel in the trapping grids with the Fisher’s exact test and used the Wilcoxon matched-pairs signed-ranks test to compare the catch per unit effort per grid per year (number of squirrels trapped/ number of occasions) between the two species.
Red squirrel population size (N) was estimated using Capture-Mark-Recapture (CMR) models, precisely loglinear models for open population (Baillargeon and Rivest 2007; Rivest and Baillargeon 2014). Population size was first estimated for every capture session and, at a later stage, averaged per year and trapping grid. To allow comparisons with previous papers on squirrel demography, we also used the minimum number of animals known to be alive (MNA, Krebs 1999), from trapping and radio-tracking, in each trapping session as a second estimator of population size. Correlation between the two estimators was calculated.
Next, to express squirrel numbers as density ha−1, we calculated the total trapping area including a buffer around each grid of 160 m for the Pallas’s squirrel, 238 m for the red squirrel in the red-Pallas area and 126 m for red squirrels in the red-only (considering the mean home range size of each species in the two areas, Wauters et al. 2001b; M.V. Mazzamuto unpublished). Since the different size of buffer area could induce a bias in red squirrel density estimates, we also applied the 126 m buffer of the red-only area to the red-Pallas area and recalculated red squirrel density to have also a more conservative estimate. All the areas with the buffer were reshaped eliminating habitats not suitable for squirrels (e.g. large meadows, major village centers, commercial and industrial areas, etc.) using QGIS (QGIS v. 2.8.2 Development Team, 2013). By using this method, differences in size between study areas are taken into account and do not affect our estimates of density.
Local survival was explored using Kaplan-Meier survival curve (Kaplan and Meier 1958). Differences in survival between the sexes and between experimental and control areas were tested with Proportional Hazard Regression (Cox 1972). To allow comparisons with previous papers on squirrel demography, we estimated local survival also with the survival index (number of animals present in year t and still alive in year t + 1/number of animals present in year t) used in earlier studies on squirrel demography (Wauters et al. 2001b; Gurnell et al. 2004; Wauters et al. 2004). The local survival rate was calculated at 6 and 12 months.
Because of low capture-recapture rate in the experimental area, the proportion of adult females that were at least one time in the year in post-oestrus (hence pregnant) or lactating was used as a measure of reproductive rate. Differences of reproductive rate between study areas was tested using Chi squared test and logistic regression. All results are reported as average value and 95% CI in brackets.
Results
Red-Pallas area
A total of 684 Pallas’s squirrels (370 males, 314 females; sex ratio (M/F) 1.18) were removed from our study area in 45 months (117 sessions). During the same period, only 69 different Eurasian red squirrels (34 males, 35 females; sex ratio 0.97) were captured in the same sites (Table 2). Twenty-five (36%) of these were captured only once, and only 32 (46%) individuals were considered residents (more than 4 months or 4 trapping sessions in the trap site). Pallas’s squirrel was trapped in all 19 trapping sites, but red squirrel in only 9 sites (Fisher Exact test p = 0.0004) and the first red squirrel capture was recorded after the removal of on average 25 (95% CI 12–28) Pallas’s squirrels in 36 (95% CI 18–54) occasions (Online Resource 1). The catch per unit effort in all trapping grids was significantly higher for the invasive squirrel than for the native one (n = 36 sites, Wilcoxon matched-pairs signed-ranks test p < 0.001). This was also the case when using only the 15 sites where red squirrel catch per unit effort was >0 (Wilcoxon matched-pairs signed-ranks test p < 0.001) (Table 2).
The estimated mean density of Pallas’s squirrel was 7.84 animals ha−1 while average red squirrel density throughout the study period was 0.05 (95% CI 0.03–0.07) individual ha−1 using the CMR method [MNA: 0.04 ha−1 (95% CI 0.02–0.06); Table 3]. Using the same buffer area as for the red-only area (see methods), the more conservative density estimates remained very low (0.09 and 0.06 red squirrel ha−1 using CMR or MNA respectively). The two methods used to estimate mean density in the area gave highly similar results (Pearson’s correlation coefficient r = 0.99, df = 9, p < 0.001, Online Resource 2). Differences between grids (F 4,5 = 1.017, p = 0.48) and years (F 4,5 = 0.62, p = 0.67) were not significant and density was always lower than 0.2 ha−1 (Online Resource 2).
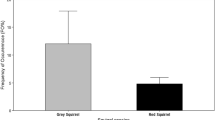
Proportion of female red squirrels breeding ranged from 82 to 100% for the years 2012 (n = 3), 2013 (n = 9) and 2014 (n = 11). Kaplan-Meier survival rate for red squirrels (Fig. 2) was 0.26 at 6 months (95% CI 0.18–0.39) and 0.10 (95% CI 0.05–0.22) at 12 months. The local survival index reported comparable rates of 0.36 (95% CI 0.13–0.59) and 0.20 (95% CI 0–0.50) at 6 and 12 months, respectively (Table 3). Local survival did not differ between sexes (Likelihood ratio test = 0.76, p = 0.38).
Red-only area
A total of 43 red squirrels (24 males, 19 females; sex ratio: 1.26) were trapped in 1996–1998 (10 capture sessions) while 16 red squirrels (7 males, 9 females; sex ratio: 0.78) were trapped in 8 months (3 capture sessions) in Parco Pineta in 2011–2012. The estimated density with CMR was of 0.39 ha−1 and MNA reported a similar density of 0.35 squirrels ha−1 (Table 3). Mean rate of female red squirrels breeding in the years 1996 (n = 3), 1997 (n = 7) and 1998 (n = 11) was 70% (95% CI 34–100) while all adult females in the area reproduced in 2012 (n = 8) (Table 3). No differences in reproductive rate between red-only and red-Pallas area was detected (χ2 = 0.35, df = 1, p = 0.56). Analysing reproductive rate of individual females as a binary variable using logistic regression and exploring the effects of study area (χ2 = 0.02; df = 1; p = 0.89) and year (χ2 = 8.4; df = 6; p = 0.21) gave similar results. Because of the small number of capture sessions in 2011–2012 we could not calculate the Kaplan-Meier survival curve but the survival index at 6 months was 0.78. Regarding data from the late 90-ties, the Kaplan-Meier estimate of local survival in the control area was significantly higher than in the red-Pallas area (Table 3; Likelihood ratio test = 43.06, p < 0.001). Red squirrel densities in the 90-ties were slightly higher than in the 2011–2012 period; in both periods densities were 8 to more than 10 times higher in the red-only than in the red-Pallas area (Table 3).
Discussion
In this study we tested the hypothesis that the alien Pallas’s squirrel negatively affects the demography of the native red squirrel through interspecific competition. Confirming our first prediction, red squirrel densities were significantly higher in areas free of the invasive Pallas’s squirrel than in areas where both species coexist. In addition, red squirrels were distributed in a patches across the red-Pallas area. The second prediction was confirmed in terms of a lower local survival of red squirrels when they shared the forest with the alien species. However, reproductive success of female red squirrels did not seem to be affected by presence of the Pallas’s squirrel. Moreover the removal of the invasive squirrel throughout the study period had only local effects in some trapping sites allowing the return of the native species.
Presence of native and invasive species in the experimental area
We found a low occurrence of Eurasian red squirrels in trapping grids in the area occupied by Pallas’s squirrel. Although forest structure is heterogeneous, the entire study area is covered by mixed deciduous forests dominated by sweet chestnut Castanea sativa mixed with black locust Robinia pseudoacacia and a good understory of hazel Corylus avellana, suitable habitat for red squirrels (Wauters et al. 2001b). However, both occurrence in the trapping grids and catch per unit effort were lower for red than for alien Pallas’s squirrels, demonstrating a higher presence and wider distribution of the invasive species than of the native one. Historically, red squirrels seemed to occur throughout the study area and were regularly observed, suggesting they occurred at relatively high densities (Prigioni et al. 2001). We discard that low catch per unit effort of native red squirrels was affected by lack of traps not occupied by captured Pallas’s squirrels, since high trap-density in the trapping sites assured that during each trapping occasion many traps remained open, thus available for red squirrels to enter (Mazzamuto et al. 2015).
The Pallas’s squirrel density in Italy was 7.8 animals ha−1, higher than reported in fragmented landscape in Belgium (5.6 ha−1, Adriaens et al. 2015). It was also slightly higher than densities of 4.3–6.8 individuals ha−1 reported in Taiwan (tropical monsoon forest in the native range) and Japan (evergreen tropical forest in the introduced range; Tamura et al. 1988, 1989), confirming the adaptability and invasiveness of the species (Parker et al. 2013).
In contrast, density of native red squirrels was much lower than of alien Pallas’s squirrels, with about 150 Pallas’s squirrels for every single red squirrel. Red squirrel population densities in mixed deciduous woodland in other countries varied from 0.2 to 1.6 individuals per ha (Gurnell 1987; Lurz et al. 2000; Wauters et al. 2004; Shuttleworth et al. 2015). Hence, our estimates below 0.1 squirrel ha−1 reported small population size and low densities of red squirrels in the experimental area.
Red squirrel: red-Pallas versus red-only
Comparison of red squirrel density between experimental and control areas confirmed the critically low density, and population size, of red squirrels in the red-Pallas area. Independent of the method used (CMR or MNA), density in the control area (0.39–0.72 individuals ha−1) was about 8–14 times higher than in the experimental area (on average 0.05 individuals ha−1). Within the red-only area, densities had changed little over time, fluctuating between 0.4 and 0.7 squirrels ha−1 with CMR estimates slightly higher than MNA (Table 3). Trapping design in the red-Pallas area was variable in duration with different trapping efforts per trapping site in relation to the management needs and the main aim of the LIFE project (removal of the invasive squirrel). This did not have important implications on our results because, comparing trapping sites constantly monitored with those used for shorter periods, the density estimates were comparable (see Table 2, Online Resource 2). Moreover, although forest composition differed slightly between the experimental and control area and data were gathered, partly, in different years, the observed differences in red squirrel density between the experimental and control area were very large and confirmed a negative effect of interspecific competition by alien Pallas’s squirrel on red squirrel population dynamics.
Interspecific competition was further confirmed by the reduced local survival rate of red squirrels in co-occurrence with Pallas’s squirrels compared to red-only sites. Both methods used, the Kaplan-Meier curve and the survival index, reported similar results. In Parco Pineta in the 90-ties, annual local survival rate was 68%, with female survival significantly higher than that of males (Wauters et al. 2001b). In the red-Pallas area there was no difference in survival rate between sexes, as reported for stable habitats in England and Belgium (Wauters and Dhondt 1990; Kenward et al. 1998). Comparative studies of demographic parameters in red squirrel populations between control and experimental sites, showed that the adult survival of reds was not reduced in co-occurrence with grey squirrels, but recruitment of juveniles of the native species decreased with the increasing density of grey squirrels (Gurnell et al. 2004, 2015). In the red-Pallas area the low survival rate of red squirrels might be related to competition for food resources, suggested by the native species having a poorer body condition than in areas where Pallas’s squirrel is absent (M.V. Mazzamuto unpublished). This could be linked to changes in foraging efficiency of red squirrels when in syntopy or to an indirect competition for cached food which is expressed in a higher mortality. Other potential mechanisms that theoretically could affect the native species’ body condition and survival are parasite spillover and spill-back between alien and native hosts (Tompkins and Poulin 2006; Romeo et al. 2015). However, since Pallas’s squirrels have a poor macro-parasite fauna and only few animals hosted gastro-intestinal helminths, this seems very unlikely. Nevertheless they acquired the flea Ceratophyllus sciurorum from red squirrels and the tick Ixodes ricinus with potential impacts on native red squirrels through processes of spill-back (Mazzamuto et al. 2016b).
In contrast with survival, breeding success of female red squirrels did not seem to differ between the two areas. Values reported in this study were high both in the red-only area (100%) and in the red-Pallas area (90%). In the control area for the period 1996–1998, annual breeding rate ranged from 61 to 69% (Wauters et al. 2001b). Estimates of red squirrel breeding in broadleaf forests was 51% in England and ranged from 39 to 67 % in Belgium (Wauters and Dhondt 1990; Kenward et al. 1998; Wauters et al. 2004). Thus, so far, our results exclude a negative effect of the invasive squirrel on the reproductive success of the native red squirrel. This is in contrast with the competition mechanism between red and alien grey squirrels in England and Italy. The presence of grey squirrels reduced breeding rate of red squirrels with a significantly smaller percentage of females weaning a summer litter, and consequently, very low number of females raising two litters/year (Wauters et al. 2000; Gurnell et al. 2004, 2015). However, low capture-recapture rate in the red-Pallas site did not allow us to separate spring from summer reproduction as was done in earlier studies. Consequently, our estimates of breeding success are not fully comparable with those of previous studies, but the fact remains that, throughout the study period, most red squirrel females in our experimental area produced at least one litter per year.
A study on fecundity of Pallas’s squirrel in our experimental area using counts of stained uterine scars showed that 58% of females had a spring litter, some of these also produced a summer litter (35%) and a few even a third litter in autumn (10%). Average (±SD) annual litter size at birth of reproducing females was 5.28 ± 1.87 young ranging from 1 to 9 young produced over an entire year by a given female (Santicchia et al. 2015a). Although there are no directly comparable fecundity data for native red squirrels, which can have large litters in some circumstances (Mari et al. 2008), estimates of reproductive rate from percentage females breeding and litter size at weaning in mixed woodlands (Wauters and Dhondt 1995; Wauters and Lens 1995; Wauters et al. 2001b) suggest that the IAS has a higher fecundity than the native species. Hence, in addition to a good adaptation to the new environment, the Pallas’ squirrel has a high capacity to recover after reduction of population size, probably more and faster than the red squirrel (Sakai et al. 2001; Parker et al. 2013).
Pallas’s squirrel management and red squirrel conservation
In some trapping grids the removal of the invasive squirrel allowed red squirrels to gradually recolonize the sites in a relative short period. This confirms the importance of the removal program and indicates that red squirrels are not locally extinct but probably relegated to the borders of the distribution area of Pallas’s squirrel. Red squirrels may avoid good quality patches occupied by many Pallas’s squirrels and shift to poorer-quality habitats or even emigrate and disperse to new areas. Such areas may be of lower quality in terms of tree species diversity, food availability and/or degree of fragmentation (Wauters et al. 1996, 2010; Santicchia et al. 2015b). The permanence of red squirrels in marginal areas, in the long run, could have an adverse effect on population demography, negatively affecting survival and/or reproduction with a consequent decrease in density as suggested for the replacement mechanism of red squirrels by introduced grey squirrels (Gurnell et al. 2015). On the other side, and in contrast with our third prediction, the overall removal program did not result in a marked increase in red squirrel density. After an initial return of red squirrels in several sites in the experimental area, the density in those trapping sites monitored for 3–4 years (e.g. grids 4, 5) remained the same, as demonstrated by the absence of a significant year-effect on red squirrel population size. This could be related to our trapping effort not being sufficiently high to control and drastically reduce the numbers of the invasive species, and/or to red squirrels having a slow recovery rate making our monitoring period too short to see a response. An alternative, but not mutually exclusive, hypothesis is some degree of predation even at low density. Potential squirrel predators occurring in the area, goshawk (Accipiter gentilis), red fox (Vulpes vulpes) and stone marten (Martes foina) are generalists, hence their choice and consumption of prey is affected by prey density (the predator’s functional response, e.g. Smout et al. 2010). Thus, increase of the overall number of squirrels available as prey, because of the high density of Pallas’s squirrels, could increase predation pressure on all squirrels (Smout et al. 2010; Paterson et al. 2015). With the removal of the invasive squirrel, hence with a reduction of total prey availability, even a slight increase of predation on red squirrels may reduce survival and thus the capacity to recover from small population size. So far, however, there is no evidence that predation can have a marked effect on population size (Petty et al. 2003; Bosch and Lurz 2012), and most studies on red squirrel demography show that densities are mainly affected by bottom-up producer (tree seed abundance)—consumer dynamics (e.g. Wauters et al. 2004, 2008; Boutin et al. 2006).
The question is: how can we improve the control of this alien species? Lessons can be learned from studies and projects carried out in the British Isles. Modelling the effects of grey squirrel population control methods on red squirrel population viability in UK, Rushton et al. (2002) indicated that an integrated control strategy, incorporating both trapping and immunocontraception, may be the best option for controlling the alien species. In Scotland, the Saving Scotland’s Red Squirrel project, set up a robust and co-ordinated network of grey squirrel control with three components: Project staff providing intensive but adaptable control effort; landowners funded under the Scottish Rural Development Programme trapping simultaneously over a wide area; and a trap-loan scheme providing assistance in areas beyond the capacity of Project staff control (Tonkin et al. 2011). These could be some options to consider for the next years in the management plan for the invasive Pallas’s squirrel. Worldwide, only one Pallas’s squirrel population was eradicated of the 22 established outside its native range (Bertolino and Lurz 2013; Adriaens et al. 2015). The invasive Pallas’s squirrel is still not eradicated in Italy, despite the four year trapping activity. Hence, in the next management plan a higher trapping effort and a better trapping efficiency (Mazzamuto et al. 2015), supported by alternative control methods, such as planned shooting (Chapuis et al. 2011) should improve the efficiency of the management of this alien species and help the conservation of the native red squirrel.
References
Adriaens T, Baert K, Breyne P et al (2015) Successful eradication of a suburban Pallas’s squirrel Callosciurus erythraeus (Pallas 1779) (Rodentia, Sciuridae) population in Flanders (northern Belgium). Biol Invasions 17:2517–2526. doi:10.1007/s10530-015-0898-z
Aprile G, Chicco D (1999) Nueva especie exotica de mamifero en la Argentina: la ardilla de vientre rojo. Mastozool Neotrop 6:7–14
Baillargeon S, Rivest L-P (2007) Rcapture: loglinear models for capture-recapture in R. J Stat Softw 19:1–31
Bertolino S (2008) Introduction of the American grey squirrel (Sciurus carolinensis) in Europe: a case study in biological invasion. Curr Sci 95:903–906
Bertolino S (2009) Animal trade and non-indigenous species introduction: the world-wide spread of squirrels. Divers Distrib 15:701–708. doi:10.1111/j.1472-4642.2009.00574.x
Bertolino S, Lurz PWW (2013) Callosciurus squirrels: worldwide introductions, ecological impacts and recommendations to prevent the establishment of new invasive populations. Mammal Rev 43:22–33. doi:10.1111/j.1365-2907.2011.00204.x
Bosch S, Lurz PW (2012) The Eurasian red squirrel: Sciurus Vulgaris. Westarp Wissenschaften-Verlagsgesellschaft, Hohenwarsleben
Boutin S, Wauters LA, McAdam AG, Humphries MM, Tosi G, Dhondt AA (2006) Anticipatory reproduction and population growth in seed predators. Science 314:1928–1930. doi:10.1126/science.1135520
Broennimann O, Treier UA, Müller-Schärer H et al (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709. doi:10.1111/j.1461-0248.2007.01060.x
Byers JE (2000) Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81:1225–1239. doi:10.2307/177203
Cagnin M, Aloise G, Fiore F et al (2000) Habitat use and population density of the red squirrel, Sciurus vulgaris meridionalis, in the Sila Grande mountain range (Calabria, South Italy). Ital J Zool 67:81–87. doi:10.1080/11250000009356299
Chapuis J-L, Dozieres A, Pisanu B, et al (2011) Plan national de lutte relatif à l’écureuil à ventre rouge (Callosciurus erythraeus) dans les Alpes-Maritimes. Muséum National d’Histoire Naturelle, Paris, Muséum d’Histoire Naturelle de Nice, DREAL Provence-Alpes-Côte d’Azur
Close B, Banister K, Baumans V et al (1996) Recommendations for euthanasia of experimental animals: part 1. Lab Anim 30:293–316
Close B, Banister K, Baumans V et al (1997) Recommendations for euthanasia of experimental animals: part 2. Lab Anim 31:1–32
Cox DR (1972) Regression models and life-tables. J R Stat Soc Ser B Methodol 34:187–220
Edelman AJ, Koprowski JL (2005) Introduced Abert’s squirrels in the Pinaleño Mountains: a review of their natural history and potential impacts on the red squirrel. In: Sanderson HR, Koprowski JL (eds) Proceedings of the endangered Mount Graham red squirrel symposium. University of Arizona Press, Tucson
Edelman AJ, Koprowski JL (2006) Seasonal changes in home ranges of Abert’s squirrels: impact of mating season. Can J Zool 84:404–411. doi:10.1139/z06-009
Emmons LH (1980) Ecology and resource partitioning among nine species of African rain forest squirrels. Ecol Monogr 50:31–54. doi:10.2307/2937245
Ferner JW (1974) Habitat Relationships of Tamiasciurus hudsonicus and Sciurus aberti in the Rocky Mountains. Southwest Nat 18:470–473. doi:10.2307/3670306
Genovesi P, Carnevali L, Alonzi A, Scalera R (2012) Alien mammals in Europe: updated numbers and trends, and assessment of the effects on biodiversity. Integr Zool 7:247–253. doi:10.1111/j.1749-4877.2012.00309.x
Gould WR, Pollock KH (1997) Catch-effort maximum likelihood estimation of important population parameters. Can J Fish Aquat Sci 54:890–897. doi:10.1139/f96-327
Guichón ML, Doncaster PC (2008) Invasion dynamics of an introduced squirrel in Argentina. Ecography 31:211–220. doi:10.1111/j.2007.0906-7590.05308.x
Gurnell J (1987) Natural History of Squirrels. Christopher Helm Publishers Ltd, London
Gurnell J, Wauters LA, Lurz PWW, Tosi G (2004) Alien species and interspecific competition: effects of introduced eastern grey squirrels on red squirrel population dynamics. J Anim Ecol 73:26–35
Gurnell J, Lurz PWW, Wauters AL (2015) Years of interactions and conflict in Europe: competition between Eurasian red squirrels and North American grey squirrel. In: Shuttleworth CM, Lurz PWW, Hayward MW (eds) Red squirrels: ecology, conservation & management in Europe. England, pp 19–37
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297
Holway DA (1999) Competitive mechanisms underlying the displacement of native ants by the invasive argentine ant. Ecology 80:238–251. doi:10.2307/176993
Hori M, Yamada M, Tsunoda N (2006) Line census and gnawing damage of introduced Formosan squirrels (Callosciurus erythraeus taiwanensis) in urban forests of Kamakura, Kanagawa, Japan. Assessment and control of biological invasion risks. Shoukadoh Book Sellers, IUCN, Kyoto and Gland, pp 204–209
Jeschke JM, Strayer DL (2005) Invasion success of vertebrates in Europe and North America. Proc Natl Acad Sci USA 102:7198–7202. doi:10.1073/pnas.0501271102
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. doi:10.2307/2281868
Kenward RE, Hodder KH, Rose RJ et al (1998) Comparative demography of red squirrels (Sciurus vulgaris) and grey squirrels (Sciurus carolinensis) in deciduous and conifer woodland. J Zool 244:7–21. doi:10.1111/j.1469-7998.1998.tb00002.x
Krebs CJ (1999) Ecological methodology. Benjamin/Cummings, Menlo Park
Leary S, Underwood W, Anthony R, et al (2013) AVMA guidelines for the euthanasia of animals: 2013 edition
Leslie PH, Davis DHS (1939) An attempt to determine the absolute number of rats on a given area. J Anim Ecol 8:94–113. doi:10.2307/1255
Lomolino MV, Brown JH, Davis R (1989) Island biogeograhy of montane forest mammals in the American southwest. Ecology 70:180–194. doi:10.2307/1938425
Long JL (2003) Introduced mammals of the world. Their History, Distribution and Influence. CSIRO Publishing. Wallingford, United Kingdom
Lurz PWW, Garson PJ, Wauters LA (2000) Effects of temporal and spatial variations in food supply on the space and habitat use of red squirrels (Sciurus vulgaris L.). J Zool 251:167–178. doi:10.1111/j.1469-7998.2000.tb00601.x
Mari V, Martini S, Romeo C et al (2008) Record litter size in the Eurasian Red Squirrel (Sciurus vulgaris). Hystrix Ital J Mammal 19:61–65
Martinoli A, Bertolino S, Preatoni DG et al (2010) Headcount 2010: the multiplication of the grey squirrel populations introduced to Italy. Hystrix Ital J Mammal 21:127–136
Mayle B, Ferryman M, Harry P (2007) Controlling Grey Squirrel Damage to Woodlands. Forestry Authority, UK
Mazzamuto MV, Panzeri M, Wauters L et al (2015) Knowledge, management and optimization: the use of live traps in control of non-native squirrels. Mammalia 80:305–311. doi:10.1515/mammalia-2015-0006
Mazzamuto MV, Galimberti A, Cremonesi G et al (2016a) Preventing species invasion: a role for integrative taxonomy? Integr Zool 11:214–228. doi:10.1111/1749-4877.12185
Mazzamuto MV, Pisanu B, Romeo C et al (2016b) Poor parasite community of an invasive alien species: macroparasites of Pallas’s squirrel in Italy. Ann Zool Fenn 53:103–112. doi:10.5735/086.053.0209
Miyamoto A, Tamura N, Sugimura K, Yamada F (2004) Predicting habitat distribution of the alien Formosan squirrel using logistic regression model. Glob Environ Res 8:13–22
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci 98:5446–5451. doi:10.1073/pnas.091093398
Nelson GA (2015) Fishmethods: fishery science methods and models in R
Paini DR, Funderburk JE, Reitz SR (2008) Competitive exclusion of a worldwide invasive pest by a native. Quantifying competition between two phytophagous insects on two host plant species. J Anim Ecol 77:184–190. doi:10.1111/j.1365-2656.2007.01324.x
Palmer GH, Koprowski J, Pernas T (2007) Tree squirrels as invasive species: conservation and management implications. In: Witmer GW, Pitt WC, Fagerstone KA (eds) Managing vertebrate invasive species: proceedings of an international symposium. USDA/APHIS Wildlife Services, National Wildlife Research Center, Fort Collins, Colorado, USA
Parker JD, Torchin ME, Hufbauer RA et al (2013) Do invasive species perform better in their new ranges? Ecology 94:985–994
Paterson RA, Dick JTA, Pritchard DW et al (2015) Predicting invasive species impacts: a community module functional response approach reveals context dependencies. J Anim Ecol 84:453–463. doi:10.1111/1365-2656.12292
Petty SJ, Lurz PW, Rushton SP (2003) Predation of red squirrels by northern goshawks in a conifer forest in northern England: can this limit squirrel numbers and create a conservation dilemma? Biol Cons 111:105–114. doi:10.1016/S0006-3207(02)00254-9
Prigioni C, Cantini M, Zilio A (2001) Atlante dei Mammiferi della Lombardia, 2001st edn. Regione Lombardia e Università degli Studi di Pavia
Ricciardi A, Neves RJ, Rasmussen JB (1998) Impending extinctions of North American freshwater Mussels (Unionoida) following the Zebra Mussel (Dreissena polymorpha) invasion. J Anim Ecol 67:613–619
Riege DA (1991) Habitat specialization and social factors in distribution of red and gray squirrels. J Mammal 72:152–162. doi:10.2307/1381990
Rivest L-P, Baillargeon S (2014) Capture-recapture methods for estimating the size of a population: dealing with variable capture probabilities. In: Statistics in action: a Canadian outlook. CRC Press, pp 289–304
Romeo C, Ferrari N, Lanfranchi P et al (2015) Biodiversity threats from outside to inside: effects of alien grey squirrel (Sciurus carolinensis) on helminth community of native red squirrel (Sciurus vulgaris). Parasitol Res 114:2621–2628. doi:10.1007/s00436-015-4466-3
Rushton SP, Gurnell J, Lurz PWW, Fuller RM (2002) Modeling impacts and costs of gray squirrel control regimes on the viability of red squirrel populations. J Wildl Manag 66:683–697. doi:10.2307/3803135
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Santicchia F, Romeo C, Grilli G et al (2015a) The use of uterine scars to explore fecundity levels in invasive alien tree squirrels. Hystrix Ital J Mammal 26:95–101
Santicchia F, Romeo C, Martinoli A et al (2015b) Effects of habitat quality on parasite abundance: do forest fragmentation and food availability affect helminth infection in the Eurasian red squirrel? J Zool 296:38–44. doi:10.1111/jzo.12215
Shuttleworth CM, Lurz PWW, Hayward MW (2015) Red Squirrels: ecology, conservation and management in Europe. European Squirrel Initiative, Woodbridge
Smout S, Asseburg C, Matthiopoulos J et al (2010) The functional response of a generalist predator. PLoS ONE 5:e10761. doi:10.1371/journal.pone.0010761
Strong DR, Pemberton RW (2000) Biological control of invading species-risk and reform. Science 288:1969–1970. doi:10.1126/science.288.5473.1969
Stuyck J, Baert K, Breyne P, Adriaens T (2009) Invasion history and control of a Pallas squirrel Callosciurus erythraeus population in Dadizele, Belgium. In: Proceedings of the science facing Aliens conference brussels. Belgian Biodiversity Platform, Brussels, Belgium
Tamura N, Hayashi F, Miyashita K (1988) Dominance hierarchy and mating behavior of the formosan squirrel, Callosciurus erythraeus thaiwanensis. J Mammal 69:320. doi:10.2307/1381382
Tamura N, Hayashi F, Miyashita K (1989) Spacing and kinship in the Formosan squirrel living in different habitats. Oecologia 79:344–352
QGIS Development Team (2015) Quantum GIS. Open Source Geospatial Foundation Project
Thorington RWJ, Koprowski JL, Steele MA, Whatton JF (2012) Squirrels of the World. Johns Hopkins University Press, Baltimore
Tompkins DM, Poulin R (2006) Parasites and biological invasions. Biological invasions in New Zealand. Springer, Berlin, pp 67–84
Tonkin M, Mackenzie I, House H (2011) The evaluation of grey squirrel control in the Saving Scotland’s Red Squirrel partnership project. Report available at: http://www.scottishsquirrels.org.uk/docs/008__042__general__Saving_Scotlands_Red_Squirrels_Evaluation_Report_Final__1347452857.pdf
Tosi G, Zilio A (2002) Conoscenza delle risorse ambientali della provincia di Varese. Settore politiche per l’agricoltura e gestione faunistica, Provincia di Varese
Wauters LA, Dhondt AA (1990) Red squirrel population dynamics in different habitats. Z Säugetierkd 55:161–175
Wauters LA, Dhondt AA (1995) Lifetime reproductive success and its correlates in female Eurasian red squirrels. Oikos 72:402–410. doi:10.2307/3546126
Wauters LA, Lens L (1995) Effects of Food Availability and Density on Red Squirrel (Sciurus vulgaris) Reproduction. Ecology 76:2460–2469. doi:10.2307/2265820
Wauters LA, Dhondt AA, Knothe H, Parkin DT (1996) Fluctuating asymmetry and body size as indicators of stress in red squirrel populations in woodland fragments. J Appl Ecol 33:735–740. doi:10.2307/2404944
Wauters LA, Lurz PWW, Gurnell J (2000) Interspecific effects of grey squirrels (Sciurus carolinensis) on the space use and population demography of red squirrels (Sciurus vulgaris) in conifer plantations. Ecol Res 15:271–284. doi:10.1046/j.1440-1703.2000.00354.x
Wauters LA, Gurnell J, Martinoli A, Tosi G (2001a) Does interspecific competition with introduced grey squirrels affect foraging and food choice of Eurasian red squirrels? Anim Behav 61:1079–1091. doi:10.1006/anbe.2001.1703
Wauters LA, Gurnell J, Preatoni D, Tosi G (2001b) Effects of spatial variation in food availability on spacing behaviour and demography of Eurasian red squirrels. Ecography 24:525–538. doi:10.1111/j.1600-0587.2001.tb00487.x
Wauters LA, Matthysen E, Adriaensen F, Tosi G (2004) Within-sex density dependence and population dynamics of red squirrels Sciurus vulgaris. J Anim Ecol 73:11–25
Wauters AL, Vermeulen M, Van Dongen S et al (2007) Effects of spatio-temporal variation in food supply on red squirrel Sciurus vulgaris body size and body mass and its consequences for some fitness components. Ecography 30:51–65. doi:10.1111/j.2006.0906-7590.04646.x
Wauters AL, Githiru M, Bertolino S et al (2008) Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31:104–114. doi:10.1111/j.2007.0906-7590.05251.x
Wauters LA, Verbeylen G, Preatoni D et al (2010) Dispersal and habitat cuing of Eurasian red squirrels in fragmented habitats. Popul Ecol 52:527–536. doi:10.1007/s10144-010-0203-z
Acknowledgements
Authors thank Regione Lombardia, Provincia di Varese, Parco Pineta and all the students involved in the survey. A special thanks to A. Molinari, M. Morandini, G. Zardoni and F. Santicchia. We would like to thank both reviewers for their insightful comments on the paper, as these comments led us to an improvement of the work. This work was supported by the EU and realized under the LIFE09 NAT/IT/000095 EC-SQUARE Project. This is paper n. 12 of the ECSQUARE project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazzamuto, M.V., Bisi, F., Wauters, L.A. et al. Interspecific competition between alien Pallas’s squirrels and Eurasian red squirrels reduces density of the native species. Biol Invasions 19, 723–735 (2017). https://doi.org/10.1007/s10530-016-1310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1310-3