Abstract
The common house crow (Corvus splendens) is one of the best known and most wide spread species of the family Corvidae. It is a successful invasive species able to exploit urban environments, well removed from its natural distribution. It is considered a pest as it attains high population densities, can cause serious economic losses and has many adverse effects on native fauna and flora, including predation, competitive displacement and disease transmission. Little genetic research on the house crow has been undertaken so we have only a limited understanding of its natural genetic population structure and invasion history. In this study, we employ microsatellite and mitochondrial DNA markers to assess genetic diversity, phylogeography and population structure of C. splendens within its native range represented by Sri Lanka and Bangladesh and introduced range represented by Malaysia, Singapore, Kenya and South Africa. We found high levels of genetic diversity in some of the invasive populations for which multiple invasions are proposed. The lowest genetic diversity was found for the intentionally introduced population in Selangor, Malaysia. Sri Lanka is a possible source population for Malaysia Selangor consistent with a documented introduction over 100 years ago, with port cities within the introduced range revealing possible presence of migrants from other unsampled locations. We demonstrate the power of the approach of using multiple molecular markers to untangle patterns of invasion, provide insights into population structure and phylogeographic relationships and illustrate how historical processes may have contributed to making this species such a successful invader.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crows (Family: Corvidae, Genus: Corvus) (Linnaeus 1758), with over 40 described species (Marzluff and Angell 2005), constitute one of the most wide-spread and successful genera of birds. Corvus species are found on all the continents of the world, except South America and Antarctica (Burton and Burton 2002). Crows appear to have evolved in Asia based on an analysis of cytochrome b, myoglobin and β-fibrinogen sequences by Ericson et al. (2005). One of the most successful invasive species of the genus Corvus is the house crow (Corvus splendens), also known as the Indian crow or Colombo crow.
Although the house crow is of oriental origin, breeding populations can now be found in more than 20 countries outside of its native range (Ryall 2013). The native distribution stretches from Pakistan in the west through Myanmar to South Yunnan in the east and from central Nepal in the north to Sri Lanka and Maldives in the south (Ryall 2013). The house crow is strongly associated with human habitation and no population of this species is known to live independently of people (Sibley and Monroe 1990; dos Anjos et al. 2009). Although it generally occurs in lowland habitats, at elevations about 1600 meters or less, it has been reported in Himalayan military bases up to 4240 meters (dos Anjos et al. 2009).
It is hypothesized that intentionally introduced populations may have given rise to some of the current house crow populations in Asia and Africa. There are records of three deliberate, human facilitated introductions of this species into its non-native range, including introduction to Malaysia from Sri Lanka in 1890s (Willey et al. 1903; Wells 2007); introduction to Zanzibar from India in 1890s (Vaughan 1930) and introduction to Yemen (Aden) also from India in 1840s (Barnes 1893). Such introductions were intended to prevent caterpillar plagues and to reduce human refuse (Willey et al. 1903; Vaughan 1930). In addition, the range of the house crow has increased significantly over the last 100 years most likely by hitchhiking on ocean going ships (Ryall 2013). Although it is considered a tropical species, the occurrence of a small breeding population has been reported in Hoek van Holland in the Netherlands as well as in South Africa (Ottens and Ryall 2003). Nyari et al. (2006) highlighted the global invasive capacity of the house crow through ecological niche modelling based on known occurrences and GIS (Geographic Information System) analyses of the native distribution of this species. Areas in Central America, the Caribbean, West Africa and remaining parts of Southeast Asia were identified at high risk for colonisation by house crows.
An invasive species such as the house crow constitutes an excellent model to study the dynamics of the evolution of populations in new environments and identify the roles of selection and genetic drift (Keller and Taylor 2008; Lima et al. 2012). Understanding the patterns of genetic diversity in introduced populations may help to predict establishment success and impacts of invasive species on their new environment (Sakai et al. 2001; Dlugosch and Parker 2008). The establishment of a newly founded population depends on the recurrent colonization, accumulation of new mutations or phenotypic plasticity (Kanuch et al. 2014). By knowing the temporal variation in a species genetic diversity combined with the colonization history, it is possible to determine establishment success (Le Corre and Kremer 1998; Lee 2002; Simmons and Thomas 2004).
Population genetic theory predicts that translocated populations, will usually show reduced levels of genetic variation compared to native populations due to recent colonization (founder effects) and high level of inbreeding (Jensen et al. 2013). Introduced populations derived from low propagule pressure (number of introduced individuals) and/or characterized by slow population growth after introduction usually exhibit significantly reduced genetic diversity (Uller and Leimu 2011). In contrast, those established by a large number of individuals originating from different areas in the native range (intra-specific admixture) usually do not present a reduction in genetic diversity and sometimes even reveal increased genetic variability and high adaptability which may facilitate the invasion process (Allendorf and Lundquist 2003; Wares et al. 2005; Bock et al. 2015). There have been many studies that, indicate admixture could contribute to invasion success (e.g. Kolbe et al. 2004; Wolfe et al. 2007; Keller and Taylor 2010; Verhoeven et al. 2011; Bock et al. 2015). For instance, Keller and Taylor (2010) found that the level of genetic admixture in the invasive plant Silene vulgaris was related to the increased fecundity and thus may contribute to its invasion success.
Many studies use either microsatellite or mtDNA data to study genetic diversity in invasive species, however the use of a combination of both nuclear and mitochondrial data may provide a more complete understanding of genetic diversity and population relationships (Pavlova et al. 2013; Jackson et al. 2015). Nuclear microsatellite markers and mtDNA sequences have been successfully used separately and jointly to investigate genetic diversity in a number of invasive species (Rollins et al. 2009, 2011; Bock et al. 2015). However there have been only a limited number of genetic studies of the genus Corvus (Haring et al. 2007, 2012; Haas et al. 2009; Jønsson et al. 2012; Kryukov et al. 2012). Most of the previous studies have focused on systematics and phylogeography of the genus Corvus; none of them however have compared genetic diversity in native and introduced populations of C. splendens.
In this study we use both nuclear and mitochondrial DNA markers to investigate genetic diversity in populations of the house crow from its natural distribution and invasive populations. The specific aims of the study are to (1) determine levels of genetic variation within and among populations from native and introduced ranges of the house crow, (2) identify genetic and demographic processes that have shaped population structure and variability (3) determine if there is evidence for multiple introductions of crows to Southeast Asia.
Materials and methods
Sampling
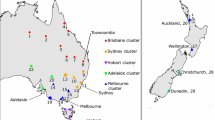
A total of 132 C. splendens samples were collected from four Asian countries representing the species’ native and introduced range. Samples from the native range included Bangladesh (BG) and Sri Lanka (SRI) and the introduced range included Malaysia Selangor (MY), Malaysia Penang (PG) and Singapore (SG). Samples from Malaysia Selangor were collected from two locations: Klang (MY1) and Ampang (MY2). In Bangladesh, two locations, Dhaka (BG1) and Chandpur (BG2) were sampled. For the mitochondrial DNA analysis additional translocated samples from South Africa (SA) and Kenya (KN) were included. Figure 1 and Online Resource 1 summarises the sampling locations and sample sizes respectively.
All specimens were collected between February 2013 and November 2014. Samples were collected at different nesting sites, to avoid sampling close relatives. DNA was extracted from the liver tissue, shed or plucked feathers or from the blood secured on FTA cards.
Laboratory analyses
Genomic DNA was extracted from liver tissue using the DNeasy Blood and Tissue Kit (Qiagen), following the manufacturer’s protocol for purification of total DNA from animal tissues. The same Qiagen kit was used for DNA extraction from feathers, with slight modifications to the protocol as follows: 30 µl of proteinase K and 30 µl of 1 M DTT was added at the tissue lysis stage followed by overnight incubation. DNA from FTA cards was extracted using the Chelex 100 protocol, as described by Jensen et al. (2003). Online Resource 1 provides details of the collected samples type together with the extraction method used. Individuals were genotyped at 12 polymorphic microsatellite loci, selected from universal bird markers (Dawson et al. 2010, 2013; Haas and Hansson 2008) and corvid specific markers (Verdugo et al. 2012). Online Resource 2 includes the details of the microsatellite loci, which were combined into two multiplex panels.
Multiplex polymerase chain reaction (PCR) was performed in 12.5 µl reactions that contained approximately 10 ng of template DNA, 6.25 µl of 2X Qiagen Type-it Multiplex Master Mix and 0.2 µM of each primer. PCR conditions were set as follows: initial activation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, 58 °C for 90 s, 72 °C for 30 s and final extension at 60 °C for 30 min.
Due to the low quantity of DNA (<10 ng) obtained from shed feathers, loci were amplified separately and pooled together in equal volumes, with the number of cycles was increased to 35. Samples from Malaysia Selangor and Singapore were genotyped on an ABI 3130xl genetic analyzer (Applied Biosystems) and samples from Bangladesh, Sri Lanka and Penang were genotyped on an ABI 3730xl genetic analyzer (Applied Biosystems). To ensure consistency in results from the two machines, positive controls were run along with samples on the ABI 3730xl sequencer; 6-FAM, VIC, NED and PET fluorescent dyes together with the size standard 500 LIZ (GeneScan) were used. Resulting data were analysed with GeneMarker v. 2.6.4 (Softgenetics) for fragment size determination and allele scoring. To ensure that the differences in sampling or extraction method did not influence microsatellite genotyping, random samples for which both feather and liver tissue were available, were genotyped and compared.
Microsatellite analyses
The number of alleles (NA), expected (HE) and observed heterozygosity (HO), exact Hardy–Weinberg equilibrium test (HWE) and linkage disequilibrium (LD) between loci were calculated with Arlequin 3.5.1.2 (Excoffier and Lischer 2010). Additional estimators of genetic diversity including allelic richness (Ar) and polymorphic information content (PIC) were calculated using FSTAT (Goudet 2001) and Cervus (Marshall et al. 1998) respectively. A randomization test that uses MonteCarlo randomization as described by Guo and Thompson (1992) and the ‘U’ test statistic described by Raymond and Rousset (1995) were used to predict presence of null alleles as implemented in MICROCHECKER (van Oosterhout et al. 2004).
Subsequent analyses were performed on 2 data sets, one including all 12 loci and the other excluding loci with putative null allele (9 loci).
The program STRUCTURE (Pritchard et al. 2000) was used to identify genetic clusters among tested individuals. Initial analysis was carried out without using any prior information on the sampling location of the individuals. Burn-in of 10,000 and a MCMC length of 50,000 iterations were used and simulated number of populations was set to K = 1–10. To check for the consistency of the runs, five independent simulations were performed for each K. Preliminary results were assessed using Evanno method (Evanno et al. 2005), and most probable K range was determined based on the distribution of delta K calculated by STRUCTURE HARVESTER (Earl and von Holdt 2012). Subsequent runs were performed for the most probable range of K, as indicated by the preliminary results (K = 2–6). An admixture model was run five times for each value of K assumed subpopulations, using 5,000,000 iterations after a burn-in of 500,000 iterations. All analyses were run with allele frequencies as correlated and the admixture model.
As preliminary analyses indicted no significant difference between populations MY1 and MY2 these were pooled together (MY) for most subsequent analyses. Similarly, no genetic differentiation was found between the Bangladesh populations (BG1 and BG2) and these were also combined (BG) (Online Resource 7 and Online Resource 9). Analyses were therefore performed on data from five major populations (MY, PG, SG, SRI, BG). In addition Malaysia Selangor (MY) population was compared to Sri Lanka (SRI) to test the hypothetical origin of Selangor population from Sri Lanka. We also compared Malaysia Selangor (MY) to Singapore (SG) and Penang (PG) populations to determine level of gene flow within Malaysia and between Malaysia and Singapore. For the additional analyses simulated number of populations was set to K = 1–3. Principal coordinate analysis (PCoA) was conducted using GenAlEx v. 6.3 (Peakall and Smouse 2006) with all tested populations to graphically represent relationships among populations.
Mitochondrial DNA analysis
A variable fragment of cytochrome b (Cyt b) gene was identified based on the alignment of two full C. splendens mitochondrial genomes (KJ766304, KP019940) (Krzeminska et al. 2014, 2015) and previously studied control region fragment (Haring et al. 2012) were sequenced. The following primers were used: Cyt_b_13814 + AGGCCTATGCCTCATCACAC, Cyt_b_14457-CCTAGGAGGTTTGGGGAAAA, CRCor + ACCCTTCAAGTCCGTAGCAG, Phe-Cor- TTGACATCTTCAGTGTCATGC. Polymerase chain reaction (PCR) was performed in 25 µl reactions that contained approximately 5–10 ng of template DNA, 5 µl of 5× BIOLINE MyTaq Reaction Buffer and 0.4 µM of each primer. PCR conditions were as follow: initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, 58 °C (CR)/56 °C (Cyt b) for 30 s, 72 °C for 45 s and final extension at 72 °C for 4 min. PCR products were purified and sequenced in both directions using Sanger sequencing. Sequences were aligned and manually edited in Geneious 7.1.9 (Biomatters). In total 64 individuals were sequenced, including MY (n = 13), PG (n = 7), SG (n = 10), BG (n = 10), SRI (n = 17), KN (n = 2) and SA (n = 5). A minimum spanning network among haplotypes was generated using Arlequin 3.5.1.3 (Excoffier and Lischer 2010) and visualized with Cytoscape 3.2.1 (Shannon et al. 2003). Molecular diversity indices including haplotype diversity (h), nucleotide diversity (πn) and molecular diversity from number of polymorphic sites (θs) were also calculated in Arlequin 3.5.1.3 (Excoffier and Lischer 2010).
Haplotype richness was calculated using individual based rarefaction as implemented by the Vegan package in the software R 3.2.3 (R CoreTeam 2013). Rarefaction curves are randomized plots of the number of observed haplotypes against the number of observations. Fu’s Fs test of selective neutrality for the haplotype data based on the infinite-site model without recombination was also performed using Arlequin to check for significant departures from 0, which would indicate either population expansion (negative value) or recent population bottleneck (positive value) (Fu 1997).
Genetic differentiation analysis
An analysis of molecular variation (AMOVA) implemented in Arlequin 3.5 was used to test the hierarchic genetic structure of the populations. Significance for AMOVA analysis was ascertained using 10,000 permutations.
Pairwise genetic differentiation (FST) between all pairs of populations was calculated according to Reynolds et al. (1983) and Slatkin (1995) as implemented in Arlequin 3.5. Pairwise FST was tested to determine whether it was significantly different from zero by randomizing the genotypes with 10,000 permutations. Sequential Bonferroni corrections were used for tests involving multiple comparisons. Linear regression between FIS and FST parameters as described by Waples (2014) was used to test for the presence of Wahlund effect using IBM SPSS v.22.
Results
Nuclear genetic diversity
A summary of the genetic diversity parameters for all native and introduced populations across tested loci is given in Table 1. All loci were polymorphic in all populations except for Locus CAM-13, which was monomorphic in the introduced Malaysia Selangor and Singapore populations. None of the 12 loci deviated from HWE after Bonferroni corrections (p < 0.0008) in any of the five populations. Based on the observed heterozygosity and allelic richness there was no overall significant difference between genetic diversity in native and introduced populations as two groups (Table 1). However a noticeably lower genetic diversity was observed for the Malaysia Selangor population that had been intentionally introduced. No LD was found between pairs of loci for the Malaysia Selangor, Singapore and Penang populations after Bonferroni correction (p < 0.0008), however several pairs of loci in Bangladesh and Sri Lanka populations had significant levels of LD after Bonferroni correction. (Online Resource 3).
A significant reduction in heterozygosity (p < 0.00083), which may be due to the presence of one or more null alleles across all 5 populations was observed at loci Cb01, CAM13 and CAM18 (Online Resource 4). For this reason, subsequent analyses were performed on 2 data sets, one with all loci (presented results) and the other excluding null allele loci. No differences in the results were apparent when loci were excluded. For this reason presented results are based on the 12 loci dataset. No indication of Wahlund effect was observed for tested populations (Online Resource 5).
Graphical representation of the STRUCTURE and PCoA analysis using the 12 loci data set, for five tested populations (K = 4) is presented in Figs. 2 and 3. Four most probable genetic clusters among tested populations were estimated following the Evanno et al. (2005) method (Online Resource 6). More detailed analyses between pairs of populations: MY and SRI, MY and PG, MY and SG, are presented in the Online Resource 7.
STRUCTURE analysis of populations in Malaysia Selangor (MY), Malaysia Penang (PG), Singapore (SG), Sri Lanka (SRI) and Bangladesh (BG) (K = 4, as indicated by Evanno et al. (2005) method). Each bar represents an individual, different colours represent the proportion of each genotype comprised of each of four ancestral populations
Mitochondrial DNA analysis
Twenty six haplotypes were identified for 64 sequenced individuals from different locations, with H1 and H2 being the most frequent haplotypes, predominantly present in Malaysia Selangor. Several haplotypes were unique to populations in Bangladesh (H3–H9), Sri Lanka (H18–26) and Singapore (H15–H17) (Fig. 4). The distribution of haplotypes across populations is given in Table 2.
Minimum spanning network for 26 haplotypes (H1–H26). Numbers on branches represent number of mutations separating haplotypes. The size of the node is proportional to the number of individuals. Each location is labelled with a specific colour: yellow—Malaysia Selangor, orange—Malaysia Penang, grey—Singapore, blue—Sri Lanka, green—Bangladesh, pink—South Africa, purple—Kenya
The overall nucleotide diversity (Π n ), haplotype diversity (h) and molecular diversity from number of polymorphic sites (θs) measures were higher in native (Π n = 0.004, h = 0.945, θs = 5.255) than introduced populations (Π n = 0.002, h = 0.713, θs = 2.259). The highest rates of haplotype richness were observed for Sri Lanka and Bangladesh populations, followed by Singapore, Malaysia Penang and Malaysia Selangor (Fig. 5.). For Kenya and South Africa populations (introduced), it was not possible to calculate diversity measures due to small sample size. Haplotype characteristics for each population are presented in Table 3. None of the neutrality tests were significant after Bonferroni corrections. Positive values were obtained for the Fu’s Fs neutrality test in introduced populations with the highest Fs value for Malaysia Selangor (Fs = 5.649), while negative values were obtained for all native populations. Fu’s Fs neutrality test results are also presented in Table 3.
Genetic differentiation among populations
The AMOVA test based on the microsatellite dataset gave an overall FST = 0.0384 (p < 0.001) for native and FST = 0.055 (p < 0.001) for introduced range. The majority of allelic diversity was due to within population variation (96.16 and 94.42 % for native and introduced range respectively). However, less within population variability was observed with the mtDNA dataset for both native (FST = 0.3058, p < 0.001, 69.41 %) and introduced range (FST = 0.4879, p < 0.001, 51.20 %). The results of AMOVA test are presented in Online Resource 8.
Pairwise FST test based on the 12 microsatellite loci, revealed that Malaysia Selangor was significantly different from all tested populations except for Sri Lanka (Online Resource 9). In total seven out of the fifteen tests were found to be significant after sequential Bonferroni correction (p < 0.003); significant differences were also observed between the following populations: Singapore and Bangladesh, Singapore and Penang, Bangladesh and Sri Lanka. The highest level of genetic differentiation was observed between Malaysia Selangor and Bangladesh (FST = 0.0959), and between Malaysia Selangor and Penang populations (FST = 0.0970) (Online Resource 9).
Discussion
In this study we provide insights into invasion processes from an examination of patterns of genetic diversity across the native and introduced range of the house crow and thereby allowing a better understanding of how demographic and genetic processes have shaped the introduced populations and facilitated their success.
The level of genetic diversity measured at 12 microsatellite markers (using measures of heterozygosity and allelic richness) among house crow populations from native and introduced locations did not in general differ significantly. The Malaysian Selangor population showed the least amount of genetic diversity using both nuclear and mitochondrial markers. This can be explained on the basis that this population was founded from a single introduction event over 100 years ago. According to Willey et al. (1903), 56 birds were imported to Klang in Malaysia from Sri Lanka in the 1890s, with the intention to biologically control caterpillars that were damaging crops. Introductions of a limited number of individuals from a single source may result in founder effects, coupled with genetic bottlenecks, resulting in low effective population size. Our results for the Malaysian Selangor population are consistent with the observation of low genetic diversity in other bird introductions and is thought to be due to founder effects (Hawley et al. 2006; Lima et al. 2012).
Based on the nucleotide and haplotype diversity (Table 1; Fig. 4) native populations from Sri Lanka and Bangladesh have the highest level of genetic diversity, which is likely a reflection of a stable demography and a long evolutionary history in those areas. The offshore populations from Penang and Singapore had unexpectedly high levels of gene diversity and allelic richness for introduced populations. As the Penang population was represented by a very small number of individuals, this may have influenced the results and erroneously magnified the level of genetic diversity. However both Singapore and Penang are busy port cities with Singapore being the 2nd and Penang 89th on the world’s busiest ports list based on the total cargo volume and container traffic according to the Institute of Shipping Economics and Logistics (World Port Ranking 2010). International ports may facilitate the introduction of crows from different locations and contribute to intraspecific admixture, which has been proposed to benefit invaders through the introduction of new genotypes into the population (Rius and Darling 2014). Klang (Malaysia Selangor), despite being a port city revealed the lowest levels of genetic diversity. This can be explained by the fact that Klang is a port in the middle zone of the Strait of Malacca; therefore any birds following ships would likely make landfall on the way in due to the narrowness of the Strait. Whereas ships going to Malaysia Penang and Singapore are more likely to come straight from the open oceans.
Due to the range of sample sizes used, rarefaction curves were used to estimate haplotype richness. In this method the rate at which new haplotypes are added to the collections, provides important information about the haplotype richness of the population as a whole (Lindblom 2009). In addition it allows an assessment of the extent to which the haplotypes potentially present in the population were sampled and how many are potentially missing from the dataset. The rarefaction curves indicate that with the current sample sizes not all possible haplotypes were detected in some populations. There were 2 groups of populations clearly identified: one consisting of the native Bangladesh and Sri Lankan populations and the invasive Singaporean crows that show a strong positive correlation between mean haplotype richness and subsamples indicating haplotype richness is likely to be underestimated, especially for the 2 native populations; and the second group consisting of the invasive Selangor and Penang populations which show a strong plateauing effect (especially for the former) indicating the sample sizes for these populations are sufficient for estimating haplotype diversity.
Three main mitochondrial haplotypes (H1, H2, H12) were identified among individuals from Malaysia Selangor, Singapore and Penang, of which two (H2, H12) are also found in the Sri Lankan sample. This is therefore consistent with crow populations from Sri Lanka or other closely related populations being a source for Malaysian population and one of the sources for Singaporean population. The presence of haplotype H1, at high frequency within invasive populations, suggests there may be other native crow populations, not sampled in this study, that have contributed as a source population. Nevertheless as this haplotype (H1) is only 1 and 2 mutational steps from H14 and H24 found in Sri Lankan birds, this country or the nearby Indian mainland may be the source. Similarly, it should be noted that some of the haplotypes found in Singapore and nowhere else (H15, H16, H17) are genetically similar to Bangladeshi haplotypes, suggesting the possibility of invasion from Bangladesh or neighbouring northern populations into Singapore. Even though Bangladesh does not share any haplotype with other populations, because of the small sample size, we cannot exclude the case that haplotypes in common were simply not sampled, as suggested by the haplotype richness rarefaction curves (Fig. 5). The Penang population has a unique haplotype that is not present within other introduced populations, again suggesting the possibility of invasion from multiple source populations. Based on the phylogenetic network (Fig. 4) Sri Lankan haplotypes can be split into two haplogroups, separated by a haplogroup from Bangladesh. This suggest population fragmentation within the native range or the origin of some of the crows in Sri Lanka from other locations within the species native range.
According to Vaughan (1930), crows in Africa were first introduced to Zanzibar from India, to reduce human refuse. This small population has acted as a reservoir for introductions to the mainland of Tanzania and Kenya and has subsequently spread to other neighbouring countries. In this study South Africa crows were found to have Sri Lankan haplotypes, but these are different to those from Kenya. This is consistent with the historical records that house crow population in South Africa most probably resulted from a separate introduction in 1970s, facilitated by the increased marine traffic down the east African coast during the closure of the Suez Canal between 1967 and 1980 (Berutti 1997). Interestingly Kenya and Singapore share a haplotype that has a unique 17 bp deletion within the control region not found in any other population. Unique mutations within the CR have been previously observed in invasive populations of other bird species and are claimed to be related to adaptation during invasion (Rollins et al. 2016). The African samples were included in this study to give a larger geographic perspective but the results should be interpreted with caution due to the small sample sizes. Nevertheless the results do suggest that future studies would benefit from more comprehensive geographical sampling and the global pattern of crow invasions maybe varied and complex.
The majority of variations in microsatellite allele frequency was explained by the within population differences (96.16 and 94.42 % for native and introduced range respectively). Lower within-population variability, was observed for mtDNA dataset of both native (69.41 %) and introduced range (51.20 %). Nevertheless, there is still more variation observed within each population than between populations. These results suggest that the invasion process within the introduced range, except for the Malaysia Selangor population probably occurred with a large number of introduced individuals and high population growth.
STRUCTURE results are congruent with the haplotype analysis. In a STRUCTURE analysis, four genetic clusters (K = 4) were identified for the five major populations (MY, PG, SG, SRI, BG).
Based on the presence of four genetic groups we conclude that all three invasive populations have different demographic and invasive histories. There are also genetic structural differences between the two native populations tested. Some overlap was noted in the genotype clustering between the Bangladeshi and Penang populations consistent with the presence of a unique haplotype from Penang population and the possibility of multi-origins for crows in this location.
Both pairwise FST comparisons as well as population structure results revealed that introduced populations from Singapore, Selangor and Penang, despite their geographical proximity were genetically different (Online Resource 9; Fig. 2). These findings indicate that crows are actually a relatively low dispersing species with a limited gene flow over land.
No LD was observed between tested loci in introduced populations whilst numerous LD between loci was noted in both native populations (Bangladesh and Sri Lanka). This may be explained by the random genetic drift acting upon native populations (Ohta and Kimura 1969), assortative mating or epistatic natural selection (Lewontin 1964), or simply by hybridization of two closely related species within a population, which otherwise remain quite distinct (Rolando 1993; Harisson and Bogdanowicz 1997). Further studies would need to be conducted in order to identify the possible cause of LD in native populations of C. splendens.
In our study a possible presence of homozygous excess was indicated for some microsatellite loci (Cb01, CAM-13, CAM-18). It has been previously raised that removing such loci from the analysis may in fact result in losing very important information and characteristic features of the population (Sunnucks and Hansen 2013). In fact it is more important to recognize why the loci show homozygous excess. According to Sunnucks and Hansen (2013), there are three possible reasons: a presence of multiple demes (i.e. Wahlund effect), sex-linkage or null alleles. Only in the last case one would remove loci from the dataset; the remaining reasons of the homozygote excess are important population features. There was no evidence of sex linkage based on the previously described results in the selected loci (Dawson et al. 2010; Haas and Hansson 2008; Verdugo et al. 2012). In this study no positive correlation between FIS and FST was observed in tested populations (Online Resource 5) excluding Wahlund effect as a possible reason for the homozygote excess. This may suggest that the homozygous excess is due to the presence of null alleles, however no difference in the results was observed regardless of the loci being included or excluded from the analysis.
When comparing mito-nuclear diversity, it is important to remember that mitochondrial data is haploid, maternally inherited and represents only quarter of the effective population size when compared to nuclear data. Therefore it is much more vulnerable to the effects of small population size and demographic processes such as founder effect, genetic bottlenecks or genetic drift (Galtier et al. 2009; Teacher et al. 2012). In addition, mitochondrial DNA encodes proteins involved in the vital processes such as energy production via oxidative phosphorylation pathway (OXPHOS), therefore it is more likely to be subject to selection pressure and mutational change during invasion (Boratynski et al. 2014; Stager et al. 2014; Morales et al. 2015). In a meta-analysis, Bazin et al. (2006) demonstrated that genetic diversity of mtDNA was generally not related to genetic diversity of nuclear DNA across studies of many distantly related taxa, and they concluded that this was because of adaptive evolution on the mitochondrial genome.
Conclusions
We found significant diversity among populations of house crow, including surprisingly high levels of variation within some but not all invasive populations. We suggest that the invasion of new areas by the house crow can be associated with multiple introduction events and most probably high population growth which is reflected by high genetic diversity within some of the introduced populations. These results are supported by both the nuclear and mitochondrial data sets. The nuclear data is consistent with division of the combined studied population into a minimum of four genetically distinct population clusters with Malaysia Selangor being the least diverse population in this study. We suggest the origin of the Malaysian Selangor population from Sri Lanka or nearby locations. This conclusion is also consistent with the mitochondrial data. For the remaining introduced populations we propose multiple introductions, which are associated with hitchhiking of birds on shipping from various locations and increased intra-specific admixture levels. We reject the alternative model that a single founding event in Peninsular Malaysia followed by local dispersal gave origin to the neighbouring populations in Penang or Singapore. Genetic structure within the native range indicates the possibility of multiple source populations, therefore more comprehensive studies of the house crow over its natural range are crucial. Genetic differences between native populations as well as between geographically close introduced populations show that the house crow is a relatively low dispersal species, when dispersal or colonization is not facilitated by human activity. Birds hitchiking on ships from diverse source populations may have increased genetic diversity in the introduced range, potentially increasing invasion success.
References
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Barnes HE (1893) On the birds of Aden. Ibis 6:57–83
Bazin E, Glemin S, Galtier N (2006) Population size does not influence mitochondrial genetic diversity in animals. Science 312:570–572
Berutti A (1997) House crow. In: Harrison JA, Allan DG, Underhill LG et al (eds) The atlas of Southern African birds, 108. Bird Life South Africa, Johannesburg
Bock DG, Caseys C, Cousens RD, Hahn MA, Heredia SM, Hubner S, Turner KG, Kenneth DW, Rieseberg LH (2015) What we still don’t know about invasion genetics. Mol Ecol 24:2277–2297
Boratyński Z, Alve PC, Berto S, Koskela E, Mappes T, Melo-Ferreira J (2014) Introgression of mitochondrial DNA among Myodes voles: consequences for energetics? BMC Evol Biol 11:355
Burton M, Burton R (2002) Crow. The international wildlife encyclopedia, vol 10. Marshal Cavendish, New York
Dawson DA, Horsburgh GJ, Kupper C, Stewart IRK, Ball AD, Durrant KL, Hansson B, Bacon I, Bird S, Klein A, Krupa AP, Lee JW, Galvez DM, Simeoni M, Smith G, Spurgin LG, Burke T (2010) New methods to identify conserved microsatellite loci and develop primer sets of high cross-species utility-as demonstrated for birds. Mol Ecol Resour 10:475–494
Dawson DA, Ball AD, Spurgin LG, Galvez DM, Stewart IR, Horsburgh GJ, Potter J, Morales MM, Bicknell AW, Preston SA, Ekblom RA, Slate J, Burke T (2013) High-utility conserved avian microsatellite markers enable parentage and population studies across a wide range of species. BMC Genom 14:176. doi:10.1186/1471-2164-14-176
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
dos Anjos L, Debus SJS, Madge SC, Marzluff JM (2009) Family corvidae (crows). In: del Hoyo J, Brugarolus RM, Pascual C, Ruiz-Olalla P, Sargatal J (eds) Handbook birds of the world, vol 14. Lynx Edicions, Barcelona, pp 494–640
Earl DA, von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Ericson GP, Jansen AL, Johansson US, Ekman J (2005) Inter-generic relationships of the crows, jays, magpies and allied groups (Aves: Corvidae) based on nucleotide sequence data. J Avian Biol 36:222–234
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Fu Y-X (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Galtier N, Nabholz B, Glemin S, Hurst GD (2009) Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol Ecol 18:4541–4550
Goudet J (2001) FSTAT: a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm
Guo S, Thompson E (1992) Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 48:361–372
Haas F, Hansson B (2008) Identification of 20 polymorphic microsatellite loci in European crow (Corvus corone) from existing passerine loci. Mol Ecol Resour 8:846–850
Haas F, Pointer MA, Saino N, Brodin A, Mundy NI, Hansson B (2009) An analysis of population genetic differentiation and genotype-phenotype association across the hybrid zone of carrion and hooded crows using microsatellites and MC1R. Mol Ecol 18:294–305
Haring E, Gamauf A, Kryukow A (2007) Phylogeographic patterns in widespread corvid birds. Mol Phylogenet Evol 45:840–862
Haring E, Daubl B, Pinsker W, Kryukov A, Gamauf A (2012) Genetic divergences and intraspecific variation in corvids of the genus Corvus (Aves: Passeriformes: Corvidae)—a first survey based on museum specimens. J Zool Syst Evol Res 50(3):230–246
Harisson RG, Bogdanowicz SM (1997) Patterns of variation and linkage disequilibrium in a field cricket hybrid zone. Evolution 51(2):493–505
Hawley D, Hanley D, Dhondt A, Lovette IJ (2006) Molecular evidence for a founder effect in invasive house finch (Carpodacus mexicanus)populations experiencing an emergent disease epidemic. Mol Ecol 15:263–275
Jackson H, Strubbe D, Tollington S, Prys-Jones R, Matthysen E, Groombridge JJ (2015) Ancestral origins and invasion pathways in a globally invasive bird correlate with climate and influences from bird trade. Mol Ecol 24:4269–4285
Jensen T, Pernasetti FM, Durrant B (2003) Conditions for rapid sex determination in 47 avian Species by PCR of genomic DNA from blood, shell-membrane, blood vessels and feathers. Zoo Biol 22:561–571
Jensen H, Moe R, Hagen IJ, Holand AM, Kekkonen J, Tufto J, Saether BE (2013) Genetic variation and structure of house sparrow populations: is there an island effect? Mol Ecol 22:1792–1805
Jønsson KA, Fabre PH, Irestedt M (2012) Brains, tools, innovation and biogeography in crows and ravens. BMC Evol Biol 12:72
Kanuch P, Berggren A, Cassel-Lundhagen A (2014) Genetic diversity of successful colonizer: isolated populations of Metrioptera roeselii regain variation at an unusually rapid rate. Ecol Evol 4:1117–1126
Keller SR, Taylor DR (2008) History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol Lett 11:852–866
Keller SR, Taylor DR (2010) Genomic admixture increases fit- ness during a biological invasion. J Evol Biol 23:1720–1731
Kolbe JJ, Glor RE, Schettino LRG et al (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Kryukov A, Spiridonova L, Nakamura S, Haring E, Suzuki H (2012) Comparative phylogeography of two crow species: jungle crow Corvus macrorhynchos and carrion crow Corvus corone. Zool Sci 29(8):484–492
Krzeminska U, Wilson R, Rahman S, Song BK, Gan HM, Tan MH, Austin CM (2014) The complete mitochondrial genome of the invasive house crow Corvus splendens (Passeriformes: Corvidae). Mitochondrial DNA. [Epub ahead of print]
Krzeminska U, Wilson R, Rahman S, Song BK, Seneviratne S, Gan HM, Austin CM (2015) Mitochondrial genomes of the jungle crow Corvus macrorhynchos (Passeriformes: Corvidae) from shed feathers and a phylogenetic analysis of genus Corvus using mitochondrial protein-coding genes. Mitochondrial DNA 15:1–3
Le Corre V, Kremer A (1998) Cumulative effects of founding events during colonisation on genetic diversity and differentiation in an island and stepping-stone model. J Evol Biol 11:495–512
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lewontin R (1964) The interaction of selection and linkage. I. General considerations; heterotic models. Genetics 49:49–67
Lima MR, Macedo RHF, Martins TLF, Schrey AW, Martin LB, Bensch S (2012) Genetic and morphometric divergence of an invasive bird: the introduced house sparrow (Passer domesticus) in Brazil. PLoS One 7(12):e53332
Lindblom L (2009) Sample size and haplotype richness in population samples of the lichen-forming ascomycete Xanthoria perietina. Lichenol 41(5):529–535
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Marzluff JM, Angell T (2005) In the company of crows and ravens. Yale University Press, New Haven
Morales HE, Pavlova A, Joseph L, Sunnucks P (2015) Positive and purifying selection in mitochondrial genomes of a bird with mitonuclear discordance. Mol Ecol 24:2820–2837
Nyari A, Ryall C, Peterson AT (2006) Global invasive potential of the house crow Corvus splendens based on ecological niche modeling. J Avian Biol 37(4):306–311
Ohta T, Kimura M (1969) Linkage disequilibrium at steady state determined by random genetic drift and recurrent mutation. Genetics 69:229–238
Online document Institute of Shipping Economics and Logistics. World Port Ranking 2010. http://aapa.files.cms-plus.com/statistics/world%20port%20rankings%202010.pdf. Accessed 06 Sept 2015
Ottens G, Ryall C (2003) House crows in the Netherlands and Europe. Dutch Bird 23:312–319
Pavlova A, Amos JN, Joseph L et al (2013) Perched at the mito-nuclear crossroads: divergent mitochondrial lineages correlate with environment in the face of ongoing nuclear gene flow in an Australian bird. Evolution 67:3412–3428
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283
Reynolds J, Weir BS, Cockerham CC (1983) Estimation of the coancestry coefficient: basis for a short-term genetic distance. Genetics 105:767–779
Rius M, Darling JA (2014) How important is intraspecific genetic admixture to the success of colonising populations? Trends Ecol Evol 29:233–242
Rolando A (1993) A study on the hybridization between Carrion and Hooded Crow in northwestern Italy. Ornis Scand 24:80–83
Rollins LA, Woolnough AP, Wilton AN (2009) Invasive species can’t cover their tracks: using microsatellites to assist management of starling (Sturnus vulgaris) populations in Western Australia. Mol Ecol 18:1560–1573
Rollins LA, Woolnough AP, Sinclar R, Mooney NJ, Sherwin WB (2011) Mitochondrial DNA offers unique insights into invasion history of the common starling. Mol Ecol 20:2307–2317
Rollins LA, Woolnough AP, Fanson BG, Cummins ML, Crowley TM, Wilton AN, Sinclar R, Butler A, Sherwin WB (2016) Selection on mitochondrial variants occurs between and within individuals in an expanding invasion. Mol Biol Evol. doi:10.1093/molbev/msv343
Ryall C (2013) House crow monitor Corvus splendens. http://www.housecrow.com/. Accessed 11 June 2015
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Sibley CG, Monroe BL (1990) Distribution and taxonomy of birds of the world. Yale University Press, New Haven
Simmons AD, Thomas CD (2004) Changes in dispersal during species’ range expansions. Am Nat 164:378–395
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Stager M, Cerasale DJ, Dor R, Winkler DW, Cheviron ZA (2014) Signatures of natural selection in the mitochondrial genomes of (Tachycineta) swallows and their implications for latitudinal patterns of the ‘pace of life’. Gene 546:104–111
Sunnucks P, Hansen BD (2013) Guest Box 5: Null alleles and Bonferroni ‘abuse’: treasure your exceptions (and so get it right for Leadbeater’s possum). In: Allendorf FW, Luikart GH, Aitken SN 2013. Conservation and the Genetics of Populations, 2nd ed, p. 93. Wiley, Oxford
Teacher AGF, Andre C, Merila J, Wheat C (2012) Whole mitochondrial genome scan for population structure and selection in the Atlantic herring. BMC Evol Biol 12:248
Uller T, Leimu R (2011) Founder events predict changes in genetic diversity during human-mediated range expansion. Glob Change Biol 17:3478–3485
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vaughan JH (1930) The birds of Zanzibar and Pemba. Ibis 5:577–608
Verdugo C, Clark AM, Prakoso D, Kramer LD, Long MT (2012) Multiplexed microsatellite loci in American crow (Corvus brachyrhynchos): a serverely affected natural host of West Nile Virus. Infect Genet Evol 12:1968–1974
Verhoeven KJF, Macel M, Wolfe LM, Biere A (2011) Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc R Soc Biol Sci 278:2–8
Waples RS (2014) Testing for Hardy-Weinberg proportions: have we lost the plot? J Hered 106(1):1–19
Wares JP, Hughes AR, Grosberg RK (2005) Mechanisms that drive evolutionary change: insights from species introductions and invasions. In: Sax DF, Stachowicz JJ, Gaines MS (eds) Species invasions: insights into ecology, evolution and biogeography. Sinauer Associates, Inc., Sunderland, pp 229–257
Wells DR (2007) The birds of the Thai-Malay Peninsula: passerines, vol 2. Christopher Helm, London
Willey A, Treacher WH, Carey EV, Cochrane CWH, Neubronner AD, Marks O (1903) Acclimatization of Ceylon crow Corvus splendens in the Malay Peninsula. Spolia Zeylandica 1:23–35
Wolfe LM, Blair AC, Penna BM (2007) Does intraspecific hybridization contribute to the evolution of invasiveness: an experimental test. Biol Invasions 9:515–521
Acknowledgments
We would like to acknowledge Agri-Food and Veterinary Authority Singapore, Subang Jaya Council, University of Colombo, University of Dhaka, Mr Mfundo Tafeni from the Environmental Resource Management Department in Cape Town, Mr Duncan Mitchell from Kenya and the Assistant Veterinary Officer, Meor Amri Md. Noor from the Penang Council for providing some of the samples used in this project. We also thank Centre for Research in Biotechnology for Agriculture at the University of Malaya for providing us access to their laboratory equipment, and the Monash University Malaysia Genomics Facility for generating part of data used in this project. We are also extremely grateful to Dr Colin Ryall, The Persistence and Adaptation Research Team (PART), Dr Alexandra Pavlova and Prof. Paul Sunnucks for their help in parts of this study. Funding for this study was provided by the Monash University Malaysia School of Science and Monash University Malaysia Tropical Medicine and Biology Multidisciplinary Platform.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Funding for this study was provided by the Monash University Malaysia School of Science and Monash University Malaysia Tropical Medicine and Biology Multidisciplinary Platform. Samples in Sri Lanka were collected under the research permit granted by the Department of Wildlife Conservation, Sri Lanka (Permit no. WL/3/2/41/14). The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krzemińska, U., Wilson, R., Song, B.K. et al. Genetic diversity of native and introduced populations of the invasive house crow (Corvus splendens) in Asia and Africa. Biol Invasions 18, 1867–1881 (2016). https://doi.org/10.1007/s10530-016-1130-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1130-5