Abstract
Ten unusual decapod crustacean species are reported for the coasts of Galicia (NW Spain), eight of them recorded for the first time in this area. Three species: Pilumnopeus africanus, Charybdis hellerii and Pachygrapsus gracilis, are non-indigenous species. The reports of Panopeus africanus and Inachus aguiarii represent the northernmost localities of these African species, whose previous North limits were in the Southwestern European coasts. Remaining five species: Xaiva biguttata, Bathynectes longipes, Parthenopoides massena, Monodaeus couchii and Homola barbata are scarcely known species found within their distribution range. Updated data about these species are given, both from the point of view of their distribution and identification (morphological and molecular methods), as well as the potential pathways for introduction of the non-indigenous ones. According to evidence presented in the present study, biofouling still remains an important vector of species transmission and surely has been undervalued with respect to ballast water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European marine waters comprise the Northeastern Atlantic, Baltic Sea and partially Mediterranean and Black Seas. In this area, there is an unequal repartition of marine decapods, from few species in the Northeastern Atlantic and Black Sea to the more diverse Mediterranean waters (d’Udekem d’Acoz 1999). An important number of these species are non-indigenous and cryptogenic species (Galil et al. 2002; Galil 2009; Zenetos et al. 2010). The most likely pathways of introduction for these species are through the Suez Canal in the Mediterranean Sea (Lessepsian migration), shipping (by fouling and ballast water) and tropicalization processes, in both Mediterranean and Atlantic. In both basins also aquaculture and aquarium trade are responsible for some introductions.
There are about 636–671 marine decapods species in European waters, between 218 and 244 of them are brachyuran crabs (d’Udekem d’Acoz 1999; Türkay 2001). In the Iberian Peninsula 9 non-indigenous brachyuran crab species occur, from a total of 140 listed, most of them reported in the last 10 years (Marco-Herrero et al. 2015). Most of these species present well-established populations, as Hemigrapsus takanoi Asakura and Watanabe, 2005 in the Cantabrian Sea (Dauvin et al. 2009), Rhithropanopeus harrisii (Gould, 1841) and Eriocheir sinensis H. Milne-Edwards, 1853 in the Guadalquivir River (Cuesta et al. 1991; García de Lomas et al. 2010), and Dyspanopeus sayi (Smith, 1869) in the Ebro Delta (Schubart et al. 2012). Other species as Charybdis feriata (Linnaeus, 1758), Callinectes sapidus Rathbun, 1896, and Callinectes exasperatus (Gerstaecker, 1856) have scattered records (Abelló and Hispano 2006; Castejón and Guerao 2013; Cuesta et al. 2015). Two other non-indigenous species, Afropinnotheres monodi Manning, 1993 and Percnon gibbesi (H. Milne-Edwards, 1856) present well-established populations in Iberian waters (Félix-Hackradt et al. 2010; Subida et al. 2011). These two species are native from the East Atlantic and with close populations in Northwestern Africa; therefore they could have arrived by natural range expansion northward, although human mediated introduction cannot be ruled out.
Galicia is a region located in north-western Spain, with over 1200 km of coastline and an important fishery and shell fishing sector. The marine fauna of the coasts of Galicia has been subjected to important changes due to the amount of non-indigenous species established as a consequence of anthropogenic and climatic factors (Bañón 2012). Along these lines, mollusks, fishes and algae are the most intensively studied and best known groups. Among crustaceans, only a few non-indigenous balanid species and the caprellid amphipod Caprella mutica Schurin, 1935 have been reported (Macho et al. 2010; Almón et al. 2014).
The exact origin or pathways for most of the records of new non-indigenous species for European waters are unknown. From Galician waters, aquaculture activities have been identified as the main cause of intentional or unintentional introduction of marine non-indigenous species (Bañón et al. 2008, 2015), but alternative pathways are poorly explored. In the case of decapod crustaceans, the construction of channels, the increase in maritime transport and development of aquaculture are the most reported pathways for expanding their original areas of distribution (Rodríguez and Suárez 2001).
The study of non-indigenous species is of major importance, not only to understand the mechanisms involved, but also for risk assessment and design of appropriate management strategies to preserve marine biodiversity. The aim of this study is to contribute to this main objective, by reporting new records of non-indigenous and rare brachyuran crabs (including new molecular information) from Galician waters, as well as the possible vectors and pathways for the introduction of these species. All this information (DNA barcodes, vectors, pathways) are useful due to lack of studies on brachyuran crabs distribution and for understanding mechanisms involved in the introduction processes. Other data, as sex, size, and distribution are provided.
Materials and methods
Area and period of study
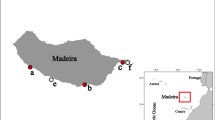
The study area covers most of the Galician coasts, with special emphasis on the Ría de Arousa (Fig. 1), were the GEMM group has their basecamp and develop most of their activities. Sampling period extends from 2001 through 2015. Detailed information of the records can be seen in Table 1.
Methods of collection
Various sampling methods were used to collect the different specimens included in this study. During one of the regular samplings carried out by the Grupo de Estudo do medio Mariño (GEMM) in an attempt to catalogue the marine fauna of Ría de Arousa (north-west Spain), the scuba divers found a specimen of Charybdis hellerii that was photographed. Pilumnopeus africanus and Panopeus africanus were collected in both, scuba diving and traps, including underwater inspection of a tuna vessel hull from the Abidjan harbor (Ivory Coast). Specimens of Pachygrapsus gracilis were collected by hand in the lower intertidal area of beaches and breakwaters of harbors and marinas, under stones and between rocks. Other species correspond to contributions from local fishermen who regularly collaborate with GEMM, providing specimens caught in traps for the capture of the velvet swimming crab Necora puber (Linnaeus, 1767) and octopus Octopus vulgaris Cuvier, 1797. Additional records of Monodaeus couchii (Couch, 1851) from Galicia during the period 2010–2014 were obtained of the bottom trawl surveys conducted every autumn (September–October) since 1983 by the Instituto Español de Oceanografía (IEO) to evaluate the status of demersal and megaepibenthic ecosystems on the northern Spanish shelf.
Once collected, specimens were transported to the Laboratory and then preserved and stored in ethanol (80 %). All specimens were initially identified based on morphological characters and identification keys by Monod (1956), Zariquiey Álvarez (1968) and Manning and Holthuis (1981), sexed and measured. Measurements were taken under a stereomicroscope equipped with a calibrated ocular micrometer or using a digital caliper (TesaCalIP65), except for C. hellerii. The following measurements were taken: cephalothorax length (CL), measured from the frontal margin (or tip of the rostrum when present) to the posterior margin of the cephalothorax, and cephalothorax width (CW), measured as the cephalothorax maximum width, commonly between the tips of the last anterolateral teeth. Taxonomic information and valid names were checked on World Register of Marine Species (WORMS) Web service.
The preserved specimens were deposited at the Museo de Historia Natural da Universidade de Santiago de Compostela (MHNUSC, Santiago de Compostela, Spain).
Molecular identification
DNA barcode sequences have been obtained for individuals of P. gracilis, P. africanus, I. aguiarii and P. africanus collected in this study. The molecular identification of the crabs was based on partial sequences of the mitochondrial genes 16S mtDNA and Cytochrome c oxidase subunit I (Cox1). Total genomic DNA was extracted from muscle tissue from the pereiopods following a modified Chelex 10 % protocol by Estoup et al. (1996).
Target mitochondrial DNA from the 16S rRNA and Cox1 genes was amplified with polymerase chain reaction (PCR) using the following cycling conditions: 2 min at 95 °C, 40 cycles of 20 s at 95 °C, 20 s at 45–48 °C, 45 s (16S) or 47 s (Cox1) at 72 °C, and 5 min 72 °C. Primers 1472 (5′-AGA TAG AAA CCA ACC TGG-3′) (Crandall and Fitzpatrick 1996) and 16L2 (5′-TGC CTG TTT ATC AAA AAC AT-3′) (Schubart et al. 2002) were used to amplify 550 bp of 16S, while primers COH6 (5′-TAD ACT TCD GGR TGD CCA AAR AAY CA-3′) and COL6b (5′-ACA AAT CAT AAA GAT ATY GG-3′) (Schubart and Huber 2006) allowed amplification of 670 bp of Cox1. PCR products were sent to CISA-INIA to be purified and then bidirectionally sequenced.
Sequences were edited using the software Chromas version 2.0. The obtained final DNA sequences were compared with those from specimens of Iberian brachyuran crabs obtained in the context of the MEGALOPADN project, and a BLAST search was also executed at the NCBI webpage to get the sequence that matches best. All sequences were deposited in Genbank under accession numbers KU163290-KU163298.
Results
A total of 10 species of brachyuran crabs have been collected in Galician waters in the context of this study. P. africanus, C. hellerii and P. gracilis, are non-indigenous species. The collections of P. africanus and I. aguiarii represent the northernmost reports of these African species. Xaiva biguttata (Risso, 1816), Bathynectes longipes (Risso, 1816), Parthenopoides massena (Roux, 1830), M. couchii and Homola barbata (Fabricius, 1793) are rarely collected in Galician waters found within its distribution range.
-
PILUMNIDAE Samouelle, 1819
-
Pilumnopeus africanus (de Man, 1902)
-
(Fig. 2a)
Material examined
Pobra do Caramiñal, Ría de Arousa, 42.608°N 08.931°W, muddy sand in crustacean traps at 8 m depth, 15 Jan 2013, 1 ♂ 5.04 × 7.28 (DNA voucher, MHNUSC 25041-1). Same locality, inside a fouling colony of the bryozoan Biflustra perambulata Louis and Menon, 2009 growing over tuna vessel hull, 2 m depth, scuba diving, 03 Jul 2013, 1 ♂ 7.65 × 9.60 and 1 ♀ 5.80 × 8.10 (MHNUSC 25041-2 and 25041-3).
Remarks
This African species is distributed in West Africa, from Guinea to Cameroon, where it inhabits estuaries and lagoons (Manning and Holthuis 1981). This is the first record of this species for European waters, and the first record out of its original distribution.
-
PORTUNIDAE Rafinesque, 1815
-
Charybdis hellerii (A. Milne Edwards, 1867)
-
(Fig. 2b)
Material examined
Santa Uxía de Ribeira. Ría de Arousa, 42.559°N 08.998°W, muddy sand under the hull of a tuna vessel, 10 m depth, 20 Jun 2009, 1 ♀, about 33.4 × 52.5 mm (measures calculated according to the photos). The specimen was not preserved and the identification was made from a series of detailed photographs conducted by one of the authors. The identification was confirmed afterwards by the authors, based on the ample information available of this species. Given that this is a well-known species, misidentification is not likely to occur.
Remarks
This Indo-West Pacific portunid crab has been reported in different places of the Western Atlantic: Colombia, Venezuela, Cuba, Brazil, Florida (Dineen et al. 2001) and Eastern Mediterranean: Egypt, Cyprus, Syria, Lebanon, Turkey (Galil et al. 2002; Özcan et al. 2010). In European waters there are only previous records of this species in the Eastern Mediterranean: Greece and Cyprus (Galil and Kevrekidis 2002; Galil et al. 2002), and there is also a single record of the congeneric C. feriata in Barcelona (Abelló and Hispano 2006). Therefore, this is the first record of this species for European Atlantic waters, and East Atlantic waters in general.
-
GRAPSIDAE MacLeay, 1838
-
Pachygrapsus gracilis (De Saussure, 1858)
-
(Fig. 2c)
Material examined
Santa Uxía de Ribeira, Ría de Arousa, 42.562°N 08.998°W, under stones in lower intertidal of the Marina breakwater, 12 Jan 2013, 2 males 10.41 × 12.91 and 6.51 × 8.02 mm (DNA vouchers, MHNUSC 25042-1 and 25042-2).
Remarks
Amphiatlantic tropical species widely distributed. In western Atlantic: Caribbean, Texas, Florida, French Guiana, Brazil and Argentina, and in eastern Atlantic from Senegal to Angola (Poupin et al. 2005; Manning and Holthuis 1981). In European waters juveniles of this species had been previously cited in Bremerhaven (Germany) where they were found in ship hulls (Gollasch 1999). However, new data concerning the current status of this species in this locality are not available. The specimens here reported are the second record for this species in European waters, and the first for the Iberian Peninsula.
-
PANOPEIDAE Ortmann, 1893
-
Panopeus africanus A. Milne Edwards, 1867
-
(Fig. 2d)
Material examined
Pobra do Caramiñal, Ría de Arousa, 42.608°N 08.931°W, muddy sand in crustacean traps at 8 m depth, 15 Jan 2013, 1 ♂ 14.53 × 20.37 (DNA voucher, MHNUSC 25043-1). Same locality, inside a fouling colony of the bryozoan Biflustra perambulata Louis and Menon, 2009 growing over tuna vessel hull at 2 m of the surface, scuba diving, 03 Jul 2013, 1 ♂ 15.15 × 22.60 (MHNUSC 25043-2).
Remarks
This African species is distributed along the Eastern Atlantic, from Angola to Portugal (Manning and Holthuis 1981), and probably introduced into South Africa (Barnard 1955). In European waters there are only populations in the Gulf of Cadiz (SW Spain) and in some estuaries in the south of Portugal, being the northernmost population that from Mira estuary (Rodríguez and Paula 1993). The presence of P. africanus in Galician waters represents now the northernmost report for this species.
-
INACHIDAE MacLeay, 1838
-
Inachus aguiarii Brito Capello, 1876
-
(Fig. 2e, f)
Material examined
Os Esqueiros, Ría de Arousa, 42.510°N 08.945°W, sand and gravel in octopus traps, 30–40 m depth, between 01 Mar 2012 and 31 Aug 2013, over 40 specimens, 1 ♂ 22.34 × 20.97 mm and 1 ovigerous ♀ 22.20 × 21.87 mm (DNA vouchers, MHNUSC 25044-1 and 25044-2).
Remarks
This is a rare sublittoral species at depths between 20 and 200 m. The distribution comprises the Eastern Atlantic, from Guinea to Portugal, and Mediterranean (Zariquiey Álvarez 1968). In European waters it has been only reported in the Atlantic from several populations in Portugal: Sesimbra, Setubal (topotypical locality), Barra do Sado, Costa da Galé, Malhada, and Baleeira-Quarteira (Neves 1975), and in the Mediterranean from only two records: in the Aegean Sea (Kocatas 1981) and Alboran Sea (Maldonado and Uriz 1992).
Other species
Additionally, five other brachyuran species were collected in these surveys: X. biguttata, B. longipes, P. massena, M. couchii and H. barbata. All of them are within their known range of distribution, but only two, M. couchii and P. massena (as Parthenope massena) have been previously reported in Galician waters.
Monodaeus couchii was cited from Galician shelf, at 390 m depth (González-Gurriarán and Méndez 1986; García Raso et al. 1987) and recently at 400–500 and 500–650 m depth (Serrano et al. 2011). An update of IEO records of M. couchii from the Galician shelf added 12 new specimens caught during the last 5 years, between 454 and 674 m depth, confirming the regular presence of this species in Galician waters.
For P. massena only one male is reported, collected at 23 m depth in sandy bottom of Punta San Carlos (Ría de Ferrol), 30 Jan 1978 (Urgorri et al. 1990). For the rest of the species, although all were previously reported as probably present in Galicia (González-Gurriarán and Méndez 1986; García Raso et al. 1987) their presence had not been confirmed until now.
The DNA barcode sequences have confirmed the identifications of three species as follows: the 16S sequence (550 bp) of P. gracilis fits 100 % with the sequence of P. gracilis from Ivory Coast (SMF 25978) deposited in Genbank under the accession number FR871304 and obtained in the context of a phylogeny of the family Grapsidae by Schubart (2011), but differs in 2.9 % from the sequence of P. gracilis from the Gulf of Mexico (ULLZ 3789, Genbank code: FR871303) from the same study. The Cox1 sequence of P. africanus presents only one mutation with respect to that from a specimen (ULLZ 4273, Genbank code: KF682774) from Cadiz (SW Spain) obtained by Thoma et al. (2014). I. aguiarii 16S (550 bp) and Cox1 (640 bp) sequences present minimal differences (1.4 and 1.25 %, respectively) with respect to sequences of I. aguiarii from the Alboran Sea, obtained in the context of the MEGALOPADN project (Palero et al. unpublished data). P. africanus sequences could not be used to confirm its identification since there is no DNA data in Genbank or authors database for this species. The DNA barcodes of this species have been deposited in Genbank and may be confirmed when sequences of specimens from native populations will become available.
Discussion
A considerable number of brachyuran crabs species inhabiting European waters originate from distant or contiguous areas, and they arrived through human mediated activities and/or by extension of their distribution ranges (d’Udekem d’Acoz 1999). It is not always easy to distinguish the vector or the pathway for these introductions, and it is difficult to separate natural expansions from accidental human introductions in some cases. For example, in the East Mediterranean Sea the number of non-indigenous species has increased dramatically since the opening of The Suez Canal (Galil 2008, 2009; Zenetos et al. 2010). Suez Canal has facilitated the first entrance of Indo-West Pacific species into Mediterranean European waters (Lessepsian migrants) but also the ship traffic through the Canal has allowed arrival of species from more distant areas, and it is difficult to know which one has more weight in this process (Galil et al. 2002).
Introductions of non-indigenous species may affect marine biology negatively; they may have a strong impact on native species by introduction of new diseases (i.e. parasites, viruses), modification of habitats and trophic webs, among other effects. Once established, aquatic non-indigenous species are really difficult or impossible to eradicate. Therefore prevention and early detection are the only ways to mitigate this problem. For an early detection and to develop preventive measures it is very important to know the pathways and vectors of introduction of non-indigenous species (Puth and Post 2005). The most likely pathways of introduction of brachyuran crabs are construction of channels, shipping (ballast water and fouling), aquaculture, and aquarium trade (Zenetos et al. 2012), but unfortunately in the majority of published new records of non-indigenous species there is no evidence of the vectors, and they are suggested or deduced as most plausible. Several species of crabs, such as Pachygrapsus transversus (Gibbes, 1850), Guinusia chabrus (Linnaeus, 1758) (as Plagusia chabrus), Plagusia depressa (Fabricius, 1775), P. squamosa (Herbst, 1790) (as P. tuberculata), Planes minutus (Linnaeus, 1758), Carcinus maenas (Linnaeus, 1758), Sphaerozius nitidus Stimpson, 1858 (as Menippe convexa Rathbun, 1894), etc., have been observed on ship’s hulls (Wolff 1954). This was a convenient means of dispersal for crabs when the old wooden hulls were in use, but it became less likely with the modern metallic hulls, antifouling paints and reduced stowage time (Rodríguez and Suárez 2001). Despite this, in the present study two different crab species, P. africanus and P. africanus, have been collected inside of the bryozoan B. perambulata attached to the hull of a tuna vessel docked in a fishing harbor (Pobra do Camariñal, Ría de Arousa). Another species, recorded in this study for the first time in the Iberian Peninsula, P. gracilis, was collected inside of a Marina (Santa María de Uxía). This species was also collected in Germany in ship hull fouling by Gollasch (1996, 2002). Taken into account that P. africanus, P. africanus and P. gracilis inhabit the same estuarine and lagoon habitats, and also share part of their distributions, i.e. the Gulf of Guinea (Monod 1956; Manning and Holthuis 1981), and considering that there is a route of tuna vessels between Abidjan harbor (Ivory Coast) and the studied Galician harbors, it is clear that ship hull fouling of tuna vessels was the introduction vector of these three species, and consequently their origin probably is Ivory Coast.
Fouling still remains an important vector of species transmission and surely has been undervalued against ballast water as it has been showed by Gollasch (2002). This author also found other crab species (juvenile and/or adult forms) in ship hull fouling as Brachynothus sexdentatus (Risso, 1827), H. takanoi (as Hemigrapsus penicillatus) and Pirimela denticulata (Montagu, 1808). de Man (1913, 1914) reported four species of crabs, Macromedaeus voeltzkowi (Lenz, 1905) (as Leptodius voeltzkowi), Latopilumnus truncatospinosus (de Man, 1913) (as Pilumnus truncatospinosus), Latopilumnus malardi (de Man, 1913) (as Pilumnus malardi) and Pilumnus longicornis Hilgendorf, 1879 on a ship hull arriving to Saint-Vaast la Hougue (Normandy, France) from Madagascar; and Godwin (2003) found another three crab species in hull fouling of a vessel docked in the Hawaiian Islands, Plagusia immaculata Lamarck, 1818, Pachygrapsus crassipes Randall, 1840 and Pilumnus oahuensis Edmondson, 1931, these are native species in the Pacific Ocean that could be exported to other areas. Recently Yeo et al. (2010) also found 25 crab species in the fouling of a semisubmersible oil platform dry docked for hull cleaning in Jurong Port (Singapore), 14 of them non-indigenous and two, Glabropilumnus seminudus (Miers, 1884) and Carupa tenuipes Dana, 1852 that currently present established populations out of their original distribution (Central Pacific and Indo-West Pacific, respectively). All these data confirm that biofouling can be an important vector of transport for crabs, even with the improvements of antifouling measures.
Panopeus africanus is present in South Portugal estuaries (Rodríguez and Paula 1993) 600 km southward from Galician rías, but one of the two specimens collected in this study was found inside a bryzoans attached to a tuna vessel hull from Africa, therefore a direct introduction from Africa is more probable than a natural process of northward expansion from Portuguese populations. In contrast, the presence of a well established population of I. aguiarii in Galician waters, including ovigerous females, could be due to a northward expansion of this species that is distributed from Guinea to Portugal, and had until now the northernmost locality at Sesimbra (Portugal).
These processes of northward expansion from tropical areas are known as “tropicalization” and it has been lately favored by the increasing of seawater temperatures in the Northern Hemisphere. Since 1974, there has been a significant increasing trend in sea surface temperature of 0.24 °C by decade in the NW Iberian Peninsula (Gómez-Gesteira et al. 2011). Moreover, a significant decrease in upwelling index (Alvarez-Salgado et al. 2008; Perez et al. 2010), responsible for the presence of colder coastal surface waters, together an increase of the downwelling season (Alvarez-Salgado et al. 2008), which favors the development of the poleward current, were also found. The intensification of the poleward current could explain the new arrival of southern species of tropical origin and the increase in temperature could favor their survival and acclimatization.
The present environmental conditions must be better for I. aguiarii, a deeper species, than the intertidal P. africanus, and maybe for this reason established population of P. africanus are located southernmost (in Portugal), and probably these direct introduction from Africa, recorded in the present study, or other possible secondary introductions from Portuguese estuaries will be not viable.
There are other brachyurans with a similar North limit of distribution: the fiddler crab Uca tangeri (Eydoux, 1835) and the pea crab A. monodi Manning, 1993. Both species are distributed in the West coast of Africa (Manning and Holthuis 1981; Manning 1993) and their present northernmost localities are placed in the Gulf of Cadiz and South of Portugal (Marco-Herrero et al. 2015; Drake et al. 2014). Taken into account that for both species plenty of habitats are available to the north, a different factor must be responsible for their limitation for dispersal to the north, surely the temperature. However this factor is changing during the last years and consequently a natural gradual northward expansion would be expected for I. aguiarii, P. africanus, U. tangeri and A. monodi. A similar process has been recorded for Uca pugnax (Smith, 1870) in the West Atlantic. Johnson (2014) have confirmed that in the last 11 years this species extended its distribution range 80 km North and he related this fact with the increase of 1.3 °C of seawater temperature in the last decade.
Charybdis helleri, the third non-indigenous species found in this study, is native from the Indo-West Pacific, but is well established in the Eastern Mediterranean as well as Western Atlantic coasts from Florida to Brazil. The origin and way of introduction of the single specimen found inside of the Santa Uxía harbor (Ría de Arousa) on the bottom under a docked tuna vessel cannot be stated. In previous records, ballast water has been suggested as vector of introduction (Tavares and Amouroux 2003), although in this case, and taken into account the size of the specimen and the evidences from other species, the possibility of its transport by hull fouling cannot be rejected. However, the origin is probably not Ivory Coast; although the scarcity of updated decapod studies in this area does not allow to rule out this possibility. This African harbor could be a secondary “exporter” of non-indigenous species that it has received without being notice. The lack of accurate studies on the distribution of marine fauna is a handicap for a correct analysis of the process of expansion of species, association with environmental factors and effects on native species. Brachyuran crabs are not an exception, and despite being conspicuous organisms and some of them with economical importance, there is a clear lack of knowledge on species distribution. The Galician carcinofauna is not well known to date, only few studies provide a detailed list of species (González-Gurriarán and Méndez 1986; García Raso et al. 1987; Urgorri et al. 1990). In these works the presence of some species was assumed because Galicia is within their distribution range, as X. biguttata, B. longipes and H. barbata. Now the specimens of these three species collected in the present study confirm their presence, as well as the specimens of P. massena and M. couchii. These two last species have been previously reported in Galician waters based only on a single male of P. massena recorded in 1978 at Ría de Ferrol (Urgorri et al. 1990), and an undetermined number of M. couchii specimens from the Galician shelf at 390 m depth (González-Gurriarán and Méndez 1986) and 400–500 and 500–650 m depth (Serrano et al. 2011).
The present scenario of global warming will facilitate the establishment of non-indigenous species in two ways, on the one hand favoring the northward expansion of tropical species (tropicalization process) and on the other hand changes in environmental conditions (meridionalization process), which so far prevented that accidentally introduced species were established. The publication of records of single non-indigenous crab specimens has notably increased in recent years (Abelló and Hispano 2006; Cuesta et al. 2015, and present study). These seem simple anecdotal captures, but they are proofs that these species can be transported and released alive far from their original populations. Although at present they do not form established populations because of the propagule pressure, number of release events or environmental conditions, all these factors could be modified in the near future.
Use of DNA barcodes allows accurate identification of species and in some cases markers as Cox1 also can offer information about population origin. The identification of P. gracilis, I. aguiarii and P. africanus have been confirmed in this way. When DNA sequences are not available in general databases (e.g. Genbank), as with P. africanus, the obtained new sequences will be useful helping future researchers to confirm the identification of new specimens of the same species, even larval stages in the plankton.
References
Abelló P, Hispano C (2006) The capture of the Indo-Pacific crab Charybdis feriata (Linnaeus, 1758) (Brachyura: Portunidae) in the Mediterranean Sea. Aquat Invasions 1(1):13–16
Almón B, Pérez J, Bañón R, Trigo J (2014) First record of Caprella mutica from the Iberian Peninsula: expansion southwards in European waters. Mar Biodivers Rec 7(e30):1–4
Alvarez-Salgado XA, Labarta U, Fernández-Reiriz MJ, Figueiras FG, Rosón G, Piedracoba S, Filgueira R, Cabanas JM (2008) Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 7:849–855
Bañón R (2012) Introducción al estudio de las especies exóticas marinas en Galicia. Rev Galega Rec Mariños (Monog.) 3:1–67
Bañón R, Rolán E, García-Tasende M (2008) First record of the purple dye murex Bolinus brandaris (Gastropoda: Muricidae) and a revised list of non native molluscs from Galician waters (Spain, NE Atlantic). Aquat Invasions 3:331–334
Bañón R, Fernández J, Trigo JE, Pérez-Dieste J, Barros-García D, De Carlos A (2015) Range expansion, biometric features and molecular identification of the exotic ark shell Anadara kagoshimensis from Galician waters, NW Spain. J Mar Biol Assoc UK 95:545–550
Barnard KH (1955) Additions to the fauna-list of South African Crustacea and Pycnogonida. Ann S Afr Mus 43:1–107
Castejón D, Guerao G (2013) A new record of the American blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), from the Mediterranean coast of the Iberian Peninsula. Bioinvasions Rec 2:141–143
Crandall KA, Fitzpatrick JFJ (1996) Crayfish molecular systematics: using a combination of procedures to estimate phylogeny. Syst Biol 45:1–26
Cuesta Mariscal JA, García Raso JE, González Gordillo JI (1991) Primera cita de Rhithropanopeus harrisii (Crustacea Decapoda, Brachyura, Xanthidae) en la Península Ibérica. Bol IEO 7:149–153
Cuesta JA, Drake P, Arias AM (2015) First record of the blue crab Callinectes exasperatus (Decapoda, Brachyura, Portunidae) for European waters. Mar Biodivers Rec 8(e36):1–4
d’Udekem d’Acoz C (1999) Inventaire et distribution des crustacés décapodes de l’Atlantique nord-oriental, de la Méditerranée et des eaux continentales adjacentes aunord de 25°N. Collect Patrim Nat 40, Mus natl Hist nat: Paris. ISBN: 2-86515-114-10. X
Dauvin JC, Tous Rius A, Ruellet T (2009) Recent expansion of two invasive crabs species Hemigrapsus sanguineus (de Haan, 1835) and H. takanoi Asakura and Watanabe 2005 along the Opal Coast, France. Aquat Invasions 4:451–465
de Man JG (1913) Sur une nouvelle observation de crabes habitant les coquilles vides des balanes. Bull Mus natl Hist nat Paris 19:9–11
de Man JG (1914) Description de deux espèces nouvelles du genre Pilumnus Leach et d’une jeune femelle du Pil. longicornis Hilgd., découvertes dans des coquilles vides de balanes. Bull Soc Zool France Paris 39:330–343
Dineen JF, Clark PF, Hines AH, Reed SA, Walton HP (2001) Life history, larval description, and natural history of Charybdis hellerii (Decapoda, Brachyura, Portunidae), an invasive crab in the Western Atlantic. J Crustac Biol 21:774–805
Drake P, Marco-Herrero E, Subida MD, Arias AM, Cuesta JA (2014) Host use pattern of the pea crab Afropinnotheres monodi: potential effects on its reproductive success and geographical expansion. Mar Ecol Prog Ser 498:203–215
Estoup A, Largiadèr CR, Perrot E, Chourrout D (1996) Rapid one tube DNA extraction for reliable PCR detection of fish polymorphic marker and transgenes. Mol Mar Biol Biotech 5:295–298
Félix-Hackradt F, Hackradt C, Treviño-Otón J, García-Chartón JA (2010) Continued expansion of Percnon gibbesi (Crustacea: Decapoda: Plagusiidae) into western Mediterranean waters. Mar Biodivers Rec 3(e22):1–3
Galil BS (2008) Alien species in the Mediterranean Sea—which, when, where, why? Hydrobiologia 606:105–116
Galil BS (2009) Taking stock: inventory of alien species in the Mediterranean Sea. Biol Invasions 11:359–372
Galil BS, Kevrekidis K (2002) Exotic decapods and stomatopods off Rhodes Island (Greece) and the eastern Mediterranean Transient. Crustaceana 75:925–930
Galil B, Froglia C, Noël P (2002) Crustaceans: decapods and stomatopods. In: Briand F (ed) CIESM atlas of exotic species in the mediterranean, vol 2. CIESM Publishers, Monaco
García-de-Lomas J, Dana ED, López-Santiago J, González R, Ceballos G, Ortega F (2010) Management of the Chinese mitten crab, Eriocheir sinensis (H. Milne Edwards, 1853) in the Guadalquivir Estuary (Southern Spain). Aquat Invasions 5:323–330
García-Raso JE, González-Gurriarán E, Sarda F (1987) Estudio comparativo de la fauna de Crustáceos Decápodos Braquiuros de tres áreas de la Península Ibérica (Galicia, Málaga y Cataluña). Investig Pesq 51:43–55
Godwin LS (2003) Hull fouling of maritime vessels as a pathway for marine species invasions to the Hawaiian Islands. Biofouling 19:123–131
Gollasch S (1996) Untersuchungen des Arteintrages durch den internationalen Schiffsverkehr unter besonderer Berücksichtigung nichtheimischer Arten. Dissertation, Universität Hamburg
Gollasch S (1999) The Asian decapod Hemigrapsus penicillatus (de Haan, 1835) (Grapsidae, Decapoda) introduced in European waters: status quo and future perspective. Helgol Meeresunters 52:359–366
Gollasch S (2002) The importance of ship hull fouling as a vector of species introductions into the North Sea. Biofouling 18:105–121
Gómez-Gesteira M, Gimeno L, De Castro M et al (2011) The state of climate in NW Iberia. Climate Res 48:109–144
González-Gurriarán E, Méndez M (1986) Crustáceos Decápodos das Costas de Galicia. I. Brachyura. Cuadernos da Área de Ciencias Biolóxicas, Seminario de Estudos Galegos, vol 2, 2ª edn. O Castro-Sada, A Coruña, Ed. Do Castro
Johnson DS (2014) Fiddler on the roof: a northern range extension for the marsh fiddler crab Uca pugnax. J Crustac Biol 34:671–673
Kocatas K (1981) Liste preliminaire et repartition des Crustacés Décapodes des eaux turques. Rapp Comm Int Mer Médit 27:161–162
Macho G, Vázquez E, Giráldez R, Molares J (2010) Spatial and temporal distribution of barnacle larvae in the partially mixed estuary of the Ría de Arousa (Spain). J Exp Mar Biol Ecol 392:129–139
Maldonado M, Uriz MJ (1992) Relationships between sponges and crabs: patterns of epibiosis on Inachus aguiarii (Decapoda, Majidae). Mar Biol 113:282–286
Manning RB (1993) West African pinnotherid crabs, subfamily Pinnotherinae (Crustacea, Decapoda, Brachyura). Bull Mus natl Hist nat Paris, sér. 4. Sect A 15:125–177
Manning RB, Holthuis LB (1981) West African brachyuran crabs (Crustacea: Decapoda). Smithson Contrib Zool 306:1–379
Marco-Herrero M, Abelló P, Drake P, García Raso JE, González-Gordillo JI, Guerao G, Palero F, Cuesta JA (2015) Annotated checklist of brachyuran crabs (Crustacea, Decapoda) of the Iberian Peninsula (SW Europe). Sci Mar 79:243–256
Monod TH (1956) Hippidea et Brachyura ouest africains. Mem Inst Franç Afrique Noire 45:1–674
Neves AM (1975) Sobre uma colecção de Crustáceos Decápodes da Baía de Setúbal (Portugal). Est Fauna Port 5:1–48
Özcan T, Katağan T, Irmak E (2010) An exotic crab, Charybdis hellerii (A. Milne-Edwards, 1867) along the Turkish coasts. Bihar Biol 4:1–5
Perez FF, Padin XA, Pazos Y, Gilcoto M, Cabanas M, Pardo PC, Doval MD, Farina-Bustos L (2010) Plankton response to weakening of the Iberian coastal upwelling. Glob Change Biol 16:1258–1267
Poupin J, Davie PJF, Cexus JC (2005) A revision of the genus Pachygrapsus Randall, 1840 (Crustacea: Decapoda: Brachyura, Grapsidae), with special reference to the Southwest Pacific species. Zootaxa 1015:1–66
Puth LM, Post DM (2005) Studying invasion: have we missed the boat? Ecol Lett 8:715–721
Rodríguez A, Paula J (1993) Larval and postlarval development of the mud crab Panopeus africanus Milne Edwards (Decapoda, Xanthidae) reared in the laboratory. J Crustac Biol 13:296–308
Rodríguez G, Suárez H (2001) Anthropogenic dispersal of decapod crustaceans in aquatic environments. Interciencia 26:282–288
Schubart CD (2011) Reconstruction of phylogenetic relationships within Grapsidae (Crustacea: Brachyura) and comparison of trans-isthmian and anphi-atlantic differentiation based on mtDNA. Zool Anz 250:472–478
Schubart CD, Huber MGJ (2006) Genetic comparisons of German populations of the stone crayfish, Austropotamobius torrentium (Crustacea: Astacidae). Bull Fr Pêche Piscic 380–381:1019–1028
Schubart CD, Cuesta JA, Felder DL (2002) Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea. J Crustac Biol 22:28–44
Schubart CD, Guerao G, Abelló P (2012) First record and evidence of an established population of the North American mud crab Dyspanopeus sayi (Brachyura: Heterotremata: Panopeidae) in the western Mediterranean. Sci Mar 76:79–85
Serrano A, Sánchez F, Punzón A, Velasco F, Olaso I (2011) Deep sea megafaunal assemblages off the northern Iberian slope related to environmental factors. Sci Mar 75:425–437
Subida MD, Arias AM, Drake P, García-Raso JE, Rodríguez A, Cuesta JA (2011) On the occurrence of Afropinnotheres monodi Manning, 1993 (Decapoda: Pinnotheridae) in European waters. J Crustac Biol 31:367–369
Tavares M, Amouroux JM (2003) First record of the non-indigenous crab, Charybdis hellerii (A. Milne-Edwards, 1867) from French Guyana (Decapoda, Brachyura, Portunidae). Crustaceana 76:625–630
Thoma BP, Guinot D, Felder DL (2014) Evolutionary relationships among American mud crabs (Crustacea: Decapoda: Brachyura: Xanthoidea) inferred from nuclear and mitochondrial markers, with comments on adult morphology. Zool J Linn Soc 170:86–109
Türkay M (2001) Decapoda. 284–292. In: Costello MJ, Emblow C, White R (eds) European register of marine species. A check-list of the marine species in Europe and a bibliography of guides to their identification. Collect Patrim Nat 50. Mus natl Hist nat, Paris. ISBN: 2-85653-538-0
Urgorri V, Solorzano MR, Besteiro C, Ramil F (1990) Crustáceos Decápodos Braquiuros de Galicia existentes en las colecciones del Museo de Historia natural `Luis Iglesias’ (Galicia). Bol R Soc Esp Hist Nat (Secc Biol) 85:5–15
Wolff T (1954) Occurrence of two East American species of crabs in European waters. Nature 174:188–189
Yeo DCJ, Ahyong ST, Lodge DM, Ng PKL, Naruse T, Lane DJW (2010) Semisubmersible oil platforms: understudied and potentially major vectors of biofouling-mediated invasions. Biofouling 26:179–186
Zariquiey Álvarez R (1968) Crustáceos decápodos ibéricos. Invest Pesq 32:1–510
Zenetos A, Gofas S, Verlaque V et al (2010) Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr Mar Sci 11:381–493
Zenetos A, Gofas S, Morri C et al (2012) Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr Mar Sci 13:328–352
Acknowledgments
We wish to thank the fisherman Alejandro Argibay (vessel “Xa me Vedes”) for providing the specimens and data of various crab species caught in Galician waters. We gratefully acknowledge the assistance of the members of Grupo do Estudo do Medio Mariño (GEMM) in conducting sampling dives. Thanks are due to Carlos Sánchez Nieto for his help on molecular laboratory work. Special thanks to Alberto Serrano and Marian Blanco (Instituto Español de Oceanografía of Santander) for providing additional data of M. couchii. Four anonymous reviewers provided useful comments and corrections that clearly improved the manuscript. Funds for DNA analysis were provide by MEGALOPADN project (CGL2009-11225) funded by the “Ministerio de Economía y Competividad” Spanish Plan R + D + I and FEDER.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuesta, J.A., Almón, B., Pérez-Dieste, J. et al. Role of ships’ hull fouling and tropicalization process on European carcinofauna: new records in Galician waters (NW Spain). Biol Invasions 18, 619–630 (2016). https://doi.org/10.1007/s10530-015-1034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-1034-9