Abstract
Mutualism between invaders may alter a key characteristic of the recipient community, leading to the entry or in situ release of other exotic species. We considered whether mutualism between invasive yellow crazy ant Anoplolepis gracilipes and exotic honeydew-producing scale insects indirectly facilitated land snails (exotic and native) via the removal of a native omnivore, the red land crab Gecarcoidea natalis. In plateau rainforest on Christmas Island, Indian Ocean, the land snail community was surveyed at 28 sites representing four forest states that differed in the density of red crabs, the abundance of yellow crazy ants and management history. One-way ANOVAs and multivariate analyses were used to determine differences in land snail species abundance and composition between forest states. Sample-based rarefaction was used to determine differences in species richness. The removal of the red land crab by supercolonies of yellow crazy ants was associated with a significant increase in the abundance of both invasive (14 species) and native (four species) land snails. Compositional differences in the land snail community were driven most strongly by the significantly greater abundance of a few common species in forest states devoid of red crabs. In forest where the crab population had recovered following management for ants, the land snail assemblage did not differ from intact, uninvaded forest. The land snail community was dominated by exotic species that can coexist alongside red crabs in rainforest uninvaded by exotic ants and scale insects. However, the ant–scale mutualism significantly increased land snail abundance and altered their composition indirectly though the alteration of the recipient community. We suggest these constitute ‘population-release’ secondary invasion in which the impacts of previously successful invaders facilitate a significant increase in abundance of other exotic species already established at low density within the community. Understanding facilitative interactions between invaders and indirect consequences of impacts will provide invaluable insights for conservation in heavily invaded ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anthropogenic movement of species is so pervasive that virtually all ecosystems now contain introduced species. Whereas early work on the determinants driving the entry, establishment and population increase (hereafter ‘invasion success’) focused on negative interactions (e.g. Elton 1958), positive interactions are increasingly identified as contributing to the invasion success of many exotic species (e.g. White et al. 2006). The invasional meltdown hypothesis (Simberloff and Von Holle 1999) posits that mutualism between invaders generates reciprocal, positive population-level responses in both partners, amplifies invader-specific impacts and facilitates further, secondary invasions. Although widely researched (Ricciardi 2001; Bourgeois et al. 2005; Relva et al. 2010; Green et al. 2011), these interactions are rarely considered in heuristic models describing the determinants of invasion success (i.e. Catford et al. 2009).
Of the three widely recognised determinants of invasion success—propagule pressure, traits of the invader and traits of the recipient community (e.g. Catford et al. 2009; Lockwood et al. 2009)—invader–invader mutualisms seem most likely to facilitate secondary invasions by altering key characteristics of the recipient community. The impacts of invasive species generally on community traits are diverse and varied, from altered soil properties (Vitousek and Walker 1989) and disturbance regimes (Cross 1981) to species loss (Zavaleta et al. 2001) and altered resources (White et al. 2006; Wang et al. 2014). Where exotic species form mutualistic interactions, alterations to community traits may be amplified beyond the impacts of those species independently (Simberloff and Von Holle 1999; Aizen et al. 2008) which may indirectly benefit other species not involved in the mutualism (White et al. 2006; Green et al. 2011; Grinath et al. 2012). For example, Grinath et al. (2012) demonstrated that an exotic ant-scale mutualism indirectly benefited plants via the negative influence of the ant on beetle herbivores. White et al. (2006) suggested these kinds of indirect effects, mediated through altered community traits, might be more common in invasive interaction networks than previously considered. Although examples of mutualisms between invaders are increasingly common (Adams et al. 2003; Bourgeois et al. 2005; Wonham et al. 2005; White et al. 2006; Helms et al. 2011; Flory and Bauer 2014), instances in which these interactions are solely responsible for the invasion success of other exotics species are almost absent (but see Green et al. 2011). The continued study of mutualism between invaders may yield yet many more examples of secondary invasions.
Some of the most frequently studied invader–invader mutualisms are those between ants and sap-sucking Hemiptera (e.g. Styrsky and Eubanks 2007; Helms et al. 2011). The invasion of the rainforest on Christmas Island (Indian Ocean) by the exotic yellow crazy ant (Anoplolepis gracilipes) and a variety of invasive honeydew-secreting scale insects [Tachardina aurantiaca (Kerriidae), Coccus spp. and Saisettia spp. (Coccidae)] has long been considered the strongest evidence for invasional meltdown (Simberloff 2006). The association between the ant and scales leads to positive population-level feedback on both, resulting in the formation of high-density ant supercolonies that accelerate and diversify impacts across rainforest on the Island (O’Dowd et al. 2003; Abbott and Green 2007; Davis et al. 2008, 2010). A key consequence of supercolony formation on Christmas Island is the removal of the dominant omnivore-detritivore, the red land crab Gecarcoidea natalis (hereafter red crab). Occurring at naturally high densities (Green 1997), the red crab plays a key functional role in shaping the forest understory across the island by regulating seedling recruitment and rates of litter decomposition (Green et al. 1997, 1999, 2008). These crabs also provide the recipient community with considerable biotic resistance against invaders in three ways. First, the omnivorous red crabs create high predation pressure on dispersing propagules that is consistent in both time and space. Second, by consuming and redistributing leaf litter, red crabs create spatial and temporal fluctuations in leaf litter cover and biomass (Green et al. 1997, 1999), which could present a challenging environment to the establishment of some invaders. Third, the consumption of seedlings creates a structurally simple rainforest understory (Green et al. 2008), potentially limiting available habitat and resources for some invaders. However, the yellow crazy ant—fuelled by carbohydrate from scale insects—causes local extinctions of red crabs, directly removing a major predator and indirectly increasing resources by deregulating seedling recruitment and leaf-litter breakdown (O’Dowd et al. 2003).
Recently, Green et al. (2011) demonstrated that the elimination of red crabs facilitated the secondary invasion of rainforest by Achatina fulica (Giant African Land Snail) through the creation of enemy-free space. Modelling of A. fulica spread across the entire island showed that invasion was facilitated 253-fold in ant supercolonies but impeded in intact forest where predaceous native red crabs remained (Green et al. 2011). Experimental introductions of tethered A. fulica in intact forest showed that invading propagules would be quickly intercepted and consumed by the abundant red crab (Lake and O’Dowd 1991; Green et al. 2011). However, A. fulica is just one of 22 introduced land snail species present on Christmas Island (Kessner 2006), the remainder of which are considerably smaller (20–1 mm length) and may not be easily detected or handled by red crabs. A further 11 similarly sized species are native to the island (Kessner 2006). Where red crabs have been eliminated by the ant-scale invasion, these native species may also experience ‘release’ from predation or low resource availability imposed on them by these abundant land crabs.
We hypothesized that the invasive ant-scale mutualism on Christmas Island would facilitate the land snail community indirectly through the release of habitat and resources and/or the creation of enemy-free space. We asked: (1) how does land snail abundance, species richness and composition change between different forest states that have arisen as a result of the ant-scale invasion? (2) are exotic species only present where the ant-scale mutualism has altered properties of the recipient community? and (3) do native land snail species also respond positively, in terms of species abundance and richness, to these changes?
Methods
Study system
Christmas Island (105°40′E, 10°30′S) is an isolated oceanic island, 360 km south of Java in the north-eastern Indian Ocean. The island is an Australian external territory that experiences a monsoonal climate where most of the 2000 mm mean annual rainfall occurs between December and May (Falkland 1986). The island rises to a central plateau in a series of cliff and terraces, and these formations largely define the major forest types on the island (Du Puy 1993). Approximately 74 % of the island supports broad-leaved, structurally simple tropical rainforest.
The yellow crazy ant (A. gracilipes), a pantropical invader (Wetterer 2005), forms expansive high-density supercolonies on the Christmas Island when in association with exotic honeydew-secreting scale insects T. aurantiaca (Kerridiae), Coccus celatus, C. hesperidium, and Saisettia coffeae (all Coccidae), among others (O’Dowd et al. 2003; Abbott 2006; Green and O’Dowd 2009). Many tramp ant species form supercolonies, often defined using a combination of criteria including genetic relatedness, intraspecific behavioural interactions, and ant abundance (Giraud et al. 2002; Holway and Suarez 2006; Drescher et al. 2007; Sunamura et al. 2009; Suhr et al. 2011). Although two distinct genotypes of crazy ant occur on Christmas Island, these co-occur at very small spatial scales (Thomas et al. 2010) and behavioural assays pairing individual ants from opposite ends of the island suggest that the population on Christmas Island behaves as a single supercolony (Abbott 2006). Nevertheless, crazy ant supercolonies on Christmas Island have always been defined in terms of very high ant densities. Crazy ant had previously occurred at many locations across the island in very low abundance with no obvious impact on biodiversity, but in 1989, and then again in late 1997, they were discovered in several locations at extremely high densities sufficient to extirpate local populations of the abundant red land crab G. natalis (O’Dowd et al. 2003; Abbott 2006). The local extinction of the red crab results in the deregulation of seedling recruitment and litter decomposition, leading to dramatic changes in forest understory structure and litter dynamics (O’Dowd et al. 2003). The reliance of crazy ants on exotic scale mutualists to form these high-density supercolonies on Christmas Island is well understood (O’Dowd et al. 2003; Abbott 2006); supercolonies have never been observed to form in the absence of outbreak densities of scale insects, and ant densities declined precipitously when excluded from their scale insect mutualists in a large field experiment (Wittman et al. unpublished data; also see Abbott and Green 2007). Since 2001, supercolonies have continued to form and reform resulting in upwards of 5000 ha (~50 % of rainforest on the island) of rainforest being treated with toxic bait containing fipronil (Green and O’Dowd 2009; Boland et al. 2011). This has created a mosaic of patch types in which some areas have a complex history of ant-scale invasion, management, and re-colonisation by either yellow crazy ants, red crabs or not at all.
The most recent survey of the land snail fauna on Christmas Island is Kessner (2006). This survey was stratified by habitat type, and aimed to document all the species occurring on the island, and their habitats. Kessner (2006) recorded 38 species of land snails, of which 11 were presumed natives, 22 were considered introduced species, and five species were of uncertain biogeographic status. The 22 introduced species are classified as such because they are known to be common, anthropogenically introduced ‘tramp’ species with wide tropical distributions (such as Bradybaena similaris). Kessner (2006) determined ‘plateau forest’ (Du Puy 1993), where this study was conducted (see below), to have the highest species richness of land snails, albeit with just four of the 11 native species being recorded in that forest type. No predatory land snail is present and only one species, A. fulica, has been intentionally introduced to the island. The lack of comprehensive surveys prior to Kessner’s makes it impossible to know with any certainty what the native fauna of the island was like at the time of settlement, or what impact the arrival of exotic species had on the native fauna. However, this study was focused on establishing the impact of invasive insects on the contemporary land snail fauna, however much it had been modified from its original state by the time of first supercolony development.
Study sites
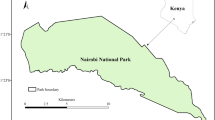
We selected 28 0.25 ha sites representing four forest states across plateau forest (Fig. 1); (1) Intact: forest supporting the naturally high density of red crabs (ca. 0.5 crabs m−2) and where yellow crazy ants were never present (i.e. the reference condition), (2) Supercolonies: forest which was invaded by yellow crazy ants in high densities at the time of study, sufficient to extirpate the local red crab population, (3) Ghosted: forest in which supercolonies had never formed, but in which red crabs were absent or rare (≤0.015 crabs m−2) because the local population had been killed while en route to the ocean during their annual breeding migration (see Davis et al. 2008; Green et al. 2011), and (4) Recovered forest where a yellow crazy ant supercolony had been removed via management action (baiting) and the area re-colonised by red crabs to a density similar to Intact forest. Recovered sites ranged between 6 and 9 years since they were last baited. There were two rounds of sampling, once at the start of a dry season (June–July 2011, n = 5 sites per forest state) and once at the start of a wet season (December 2011–January 2012, (n = 7 sites per forest state; original five sites plus two more). Replication increased for the second survey as additional active Supercolony sites were identified. Sites were surveyed in a random order over the 2-month period of each survey.
Sites were selected from the Island Wide Survey database maintained by Parks Australia. This survey is conducted at ~700 permanent rainforest sites spread in a grid across rainforest on the island, and has been conducted biennially commencing 2001. Data from six surveys (to 2011) was used to select sites according to site history and access. The current state of a site was confirmed by measuring crab density and yellow crazy ant activity (Table 1). Crab density was determined as the number of active crab burrows within a 100 m2 quadrat (50 m × 2 m). Ant abundance was measured as the number of ants crossing a 10 cm × 10 cm white card in 30 s, summed across 11 stations at 5 m intervals along a 50 m transect (Green and O’Dowd 2009; Boland et al. 2011). A total of ≥37 ants signifies a supercolony sufficient to kill red crabs. The presence of abundant scale insects at supercolony sites was confirmed visually on understorey plants, and by observing ants with honeydew-filled, translucent gasters descending tree trunks.
Sampling for land snails
An effort-controlled sampling protocol was used to quantify the land snail community; similar to approaches used by others (Cameron et al. 2003; Cameron and Pokryszko 2005). Each site contained four randomly placed 5 × 5 m quadrats. Sites were surveyed in the morning hours to maximise snail numbers. The land snail community was sampled in two ways. First, all leaf surfaces, branches and tree trunks to 3 m above the ground were searched thoroughly for arboreal snails for 30 min in each 25 m2 quadrat. Second, leaf litter and loose surface soil of two randomly placed 0.25 m × 0.25 m quadrats in each larger 5 × 5 m quadrat (eight samples per site) were collected and taken to the laboratory for immediate processing. There, samples were oven dried at 55 °C for ~20 min to dislodge snails from the wet leaves, prior to being separated into three fractions using a set of graded soil sieves (10, 2 and 0.5 mm). Large shells were picked from the 10 mm fraction, and the leaf litter retained and dried at 55 °C for a further 48 h to obtain an estimate of course-litter-biomass. Smaller snails were picked from the fraction retained by the 2 mm sieve, and that fraction then immersed in water and any further snails that floated also picked. The fraction retained by the 0.5 mm sieve was first immersed in water and any floating snails picked. All floating material was then collected, oven dried, and picked again under a dissecting microscope. Shells that were obviously long-dead (bleached, decalcified and/or broken) at the time of field sampling were omitted from collections, so that the final collection for analysis combined snails that were alive and recently dead at the point of collection (see Tattersfield et al. 2001; Aubry et al. 2005). All snails were collected and preserved in ethanol except for A. fulica that was counted in situ. Collected snails were counted by species. Species identity was confirmed against a complete reference collection provided by Kessner (2006). Voucher specimens were referred to Kessner for final species confirmation. A reference collection from this study is held at La Trobe University, Australia.
Covariate sampling
Habitat variables representing potential covariates were quantified for each study site. Understory habitat complexity was measured using a point-transect method. At 1 m intervals along a 50 m transect (n = 50 points), vegetation structure and ground cover was assessed using a vertical 3 m ranging pole. At each point, the type of substrate was recorded (leaf litter, bare ground, rock, plant or log) in order to calculate percentage ground cover. Vegetation structure was determined by recording the number of vegetation contacts with the vertical pole. The number of contacts was summed to provide a single understorey complexity score for each site. Seedling density was quantified by counting all tree seedlings (>15 cm in height; DBH ≤1 cm) 1 m either side of the 50 m transect (100 m2). Tree density (DBH >1 cm) was assessed by counting all individuals 2 m either side of the 50 m transect (200 m2).
Soil properties and amount of course, loose rocks were assessed once for each site. This was done as land snail abundance has been shown to positively correlate with soil nutrients, particularly exchangeable calcium which is required for shell growth (Labaune and Magnin 2001; Tattersfield et al. 2001). The abundance of loose limestone rocks within a site would also indicate access to calcium. One soil sample per site was collected from combining eight 19.63 cm2 × 5 cm deep circular soil cores (radius = 2.5 cm). Soil properties (pH, conductivity, Ca, NCO3, nitrate, P, K, Mg, CaCO3) were quantified used a Palintest® complete soil test kit. Soil moisture was measured using a probe in which the site value was averaged from 10 random locations. The amount of coarse loose rocks (rockiness) was measured by collecting eight 0.25 × 0.25 m quadrats per site in which loose ground material was collected, passed though a 10 mm sieve, and weighed. This coarse >10 mm measure was used as it provided a rapid assessment without the need to separate many small rocks from quantities of loose soil.
Data analyses
Univariate and multivariate analyses were performed using SPSS Statistics v. 21 (SPSS Inc.,) and PRIMER v. 6.1.13 (PRIMER-E Ltd.) respectively. Many analyses were undertaken in order to provide a comprehensive account of the land snail diversity patterns we observed. One-way ANOVAs (p < 0.05) with Bonferroni post hoc tests corrected for multiple testing (p < 0.008) were used to compare snail abundance, species density and site characteristics, such as ground cover abundance and understory complexity, between states. Data were log10-transformed if assumptions of normality and homogeneity of variances were not met, and non-parametric Kruskal–Wallis H (test statistic = χ2) or Mann–Whitney U (test statistic = Z) tests were used when these transformations were not sufficient. Means for total snail abundance (arboreal plus ground-dwelling components) are presented as area-based densities (m−2), although they come from a sample volume of 75 m3.
Randomized species accumulation curves (sample-based rarefaction curves) were calculated using EstimateS v. 9.1.0 (Colwell 2013) for each forest state using only the ground-dwelling component of the community. Each 0.25 m × 0.25 m quadrat was used as a replicate and pooled across sites within forest states. Only the small litter quadrats were used for these analyses because there were insufficient large quadrats (that sampled the arboreal snails), and because there were no arboreal species that did not occur in the litter quadrats. Repeated, averaged sample-based rarefaction, allows standardization of sampling by producing smooth curves for comparison. Species richness was compared by the Chao1 estimates (±95 % CI) of asymptotic species richness (Gotelli and Colwell 2001) for curves produced for each forest state. The Chao1 estimator was used because it takes into account species abundance, not simply incidence.
Land snail species composition was visualised by non-metric multidimensional scaling (NMDS) ordination, using the Bray–Curtis dissimilarity coefficient. Ordinations were performed in two dimensions using both abundance and presence/absence data as stress values were below 0.2 (except for one case in which no significant difference was observed). Differences between states were compared using one-way analysis of similarities (ANOSIM) (critical p < 0.05) and pairwise tests corrected for multiple sampling (corrected critical p = 0.05/6 comparisons = 0.008). One-way analysis of similarity percentages (SIMPER) was used to identify species contributing to any dissimilarity between states.
Results
A total of 23,329 snails in 19 species and nine families were sampled during this study (Table S1). Of these, 14 species were exotic, four were native and one species was of uncertain biogeographic status. The cryptogenic Georissa sp. (Hydrocendae) was the most common species, occurring at all but one site and accounting for 45 % (10,604) of observed individuals. The majority (72 %) of the remaining 12,725 snails represented exotic taxa. The exotic Liardetia scandens (Helicarionidae) and Georissa williamsi (Hydrocendae) were widespread, occurring at 100 and 93 % of sites respectively. All species were recorded in the ground-dwelling sampling, however only 12 species were found on understory vegetation. Ten species were recorded exclusively from the <2 mm litter fraction. A. fulica (Achatinidae) and Kaliella cruda (Helicarionidae) were the only exotic species not recorded in Intact forest.
Species abundance and species density
Land snail abundance did not differ between forest states during the dry season for either total abundance (χ2 = 6.74, df = 3, p = 0.08; Fig. 2a), the abundance of just the arboreal component (F3,16 = 2.07, p = 0.35; Fig. 2b) or just the ground-dwelling component (F3,16 = 2.72, p = 0.08; Fig. 2c). However, highly significant differences in abundance between forest states were evident in the wet season (F3,24 = 5.68, p = 0.004; Fig. 2a). The total abundance of land snails was low (ca. 150–300 snails per site) where red crabs were abundant in Intact and Recovered sites, but fourfold or fivefold higher (ca. 1000 snails per site) were red crabs were completely absent in supercolonies, and of intermediate abundance (ca. 700 snails per site) where red crabs were rare in Ghosted sites. This pattern held when considering the arboreal (χ2 = 11.39, df = 3, p = 0.01; Fig. 2b) and ground-dwelling (F3,24 = 4.654, p = 0.011; Fig. 2c) components independently.
Mean (±1 SE) land snail abundance and number of species between forest states for total sample (a, d), arboreal component (b, e), and ground-dwelling component (c, f). Light grey bars dry season data, dark grey bars wet season data. Letters above bars indicate where significant differences were identified between forest states as calculated by Bonferroni post hoc tests corrected for multiple testing (p < 0.008)
This increase in individuals did not correspond with an increase in species in forests devoid of crabs, as no difference in species density was observed between forest states for both dry (χ2 = 1.328, df = 3, p = 0.722) and wet season (F3,24 = 0.487, p = 0.694) periods (Fig. 2d). Location within a site (arboreal vs ground-dwelling) did not alter this pattern (Fig. 2e, f). Generally, arboreal species were also located on the ground so little difference was observed between total and ground-dwelling species density.
Species richness
Chao1 species richness estimates (±95 % CI) completely overlapped between all forest states for both dry (Fig. 3a) and wet (Fig. 3b) season sampling periods. The estimated asymptotic species richness was 15-16 species for each forest state, with large upper confidence intervals in the dry season (Fig. 3a) and greater confidence in the wet season (Fig. 3b).
Species composition
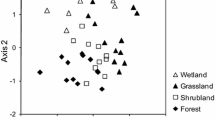
Significant differences between forest states and land snail species composition depended on both season and how the data were treated (Fig. 4). During the dry season, composition did not differ between states for abundance data (R = 0.117, p = 0.076; Fig. 4a), but differed significantly for incidence data (R = 0.222, p = 0.009; Fig. 4b). Intact forest contained a significantly different species assemblage to other forest states (Table 2). The greater occurrence of the exotics Elasmias manilensis (Achatinellidae) and Pupisoma orcula (Pupillidae), and absence of A. fulica and Subulina octona (Subulinidae) in Intact forest contributed highly to the observed differences (Table 3). The native species Lamprocystis mildredae (Helicarionidae) and Japonia wallacei (Cyclophoridae) also contributed strongly to the dissimilarity as they were less common in Intact forest.
NMDS ordinations of land snail species composition between forest states for a dry season abundance data, b dry season presence/absence data, c wet season abundance data, and d wet season presence/absence data. Circle Intact, filled diamond Supercolony, filled square Ghosted, triangle Recovered. Open symbols indicate where red crabs are abundant compared to closed symbols where they are absent or uncommon. ANOSIM results presented show test statistic (R) and significance probability (p) for each ordination. Bold p values show significant differences (p < 0.05)
Different patterns emerged during the wet season, with an overall difference in composition observed for abundance data (R = 0.117, p = 0.003; Fig. 4c) but not for incidence data (R = −0.006, p = 0.517; Fig. 4d). Intact forest again differed significantly from Ghosted and Supercolony sites, but not from Recovered sites (Table 2). Differences were driven in every case by common species (Georissa spp., J. wallacei and S. octona) that occurred in greater abundance where red crabs were absent or rare compared to where they were common (Table 3).
Covariates
In both seasons, litter biomass was higher where red crabs were absent or rare (Supercolony and Ghosted) than where they were common (Intact and Recovered) (Table 4). Similarly, Intact and Recovered forest contained significantly less leaf litter and more bare ground cover compared to Supercolony and Ghosted (dry season F3,16 = 21.43, p < 0.001; wet season F3,24 = 7.51, p = 0.001; Table 4). Understorey complexity, seedling density and tree density were significantly higher in impacted states compared to Intact forest (Table 4). Although the amount of loose limestone (rockiness) and levels of soil nutrients were highly variable across sites, there were no differences between these variables and forest state (Table 4).
Discussion
Compared to the uninvaded, Intact state, we found evidence that the invasive mutualism between yellow crazy ants and several species of honeydew-producing scale insects was associated with an increase in abundance and shifts in community composition of the land snail fauna in some forest states in one or both seasons. Most of the non-native land snail species occurred in Intact forest at low densities meaning they could be considered secondary invaders in the sense that their established populations were released due to the impacts of primary invaders. A similar interpretation could be made of other studies, where successful invaders indirectly facilitated exotic species already present in the community (e.g. Grosholz 2005; Johnson et al. 2009; Montgomery et al. 2011). On Christmas Island, the invasive mutualism between yellow crazy ants and scale insects has directly or indirectly affected about two-thirds of island rainforest (Green et al. 2011), so the changes to the recipient community associated with this invasive mutualism, and the patterns we report here for the land snail community, should be very widespread.
Two lines of evidence indicate that these impacts are not due to any direct effect of invasive ants on the snails, and are almost certainly driven by indirect effects. First, there were no differences in snail abundance, species density, asymptotic species richness, or species composition between Ghosted and Supercolony forest states, in either season of observation. These states were similar in all respects for forest structure, ground conditions and soil variables (Table 4), but were starkly different in terms of the abundance of yellow crazy ants (Table 1). Despite the high densities of ants on the forest floor in Supercolonies, there was no measurable impact of yellow crazy ants on the land snail community in those sites, suggesting that the yellow crazy ant is not a predator of land snails. This has been confirmed experimentally for A. fulica (Green et al. 2011), and there are no reports in the literature of crazy ants predating land snails. The invasive myrmecine ant Solenopsis geminata uses its mandibles and stinger to predate live snails (Forys et al. 2001) and snail eggs (Yusa 2001), but like other formicine ants, the yellow crazy ant relies on formic acid to subdue prey. Clearly, this is ineffective against the protections afforded to snails by their mucus secretions and their ability to withdraw into their shells. Even if yellow crazy ants can kill small land snails, that must happen comparatively rarely; the highest land snail densities, and all but one of the 19 species in our all our samples, occurred in Supercolonies.
Second, some metrics of the land snail community differed between Intact and Ghosted forest. Crazy ant supercolonies had never formed in either type of forest, but they differed significantly in measures of red crab density (Table 1), understory complexity, litter biomass and ground cover (Table 4). Given the empirically demonstrated impact of red land crabs on seedling recruitment and litter dynamics (Green et al. 1997, 1999, 2008), the altered composition of land snails in Ghosted forest was almost certainly an indirect effect caused by the absence of red crabs, and the associated increases in litter biomass and understory complexity.
We observed significantly greater numbers of land snails in areas devoid of red crabs only in our wet season sampling. In both seasons, land snail abundance was highly variable between sites of all states. Habitat heterogeneity plays an important role in determining patterns of land snail abundance and diversity (Aubry et al. 2005; Hylander et al. 2005) and previous work has demonstrated significantly dissimilar community composition between sites within a relatively small patches of rainforest (de Winter and Gittenberger 1998). Regardless of this inherent variability, our approach identified greater land snail abundance where red crabs had been directly (Supercolony sites) or indirectly removed (Ghosted sites) by yellow crazy ant supercolonies.
We did not detect a corresponding increase in species density with increased abundance; a land snail community pattern generally observed (Tattersfield et al. 2001; Aubry et al. 2005; Liew et al. 2010). Barker and Mayhill (1999) attribute high species density to micro-niche partitioning and minimal competition between land snail species. However, this pattern is just as likely an artefact of sampling significantly more individuals, which can be alleviated by using asymptotic species richness estimators and rarefaction curves (Gotelli and Colwell 2001). When we corrected for sampling significantly more individuals in those impacted forest states, we found no difference in mean asymptotic species richness in either the dry or wet season (Fig. 2). We can conclude from this that essentially all land snail species present in plateau rainforest on Christmas Island can be found in every forest state, and the impacts of the ant-scale invasion are not facilitating new species to establish. Also, the small confidence intervals around the wet season estimates suggest our sampling regime was sufficient to accurately predict true species richness.
Multivariate analyses indicated that snail communities in Intact forest differed significantly from snail communities in Supercolony and Ghosted sites. However, the results were season specific, with dry season analysis only identifying differences using incidence data and wet season states only separating based on abundance data. The dry season result implies species turnover between sites, evoking a classic view of secondary invasion that posits successful invaders facilitate the entry of other exotics (Simberloff 2006; Green et al. 2011). However, it was only A. fulica and K. cruda that were completely absent from Intact forest but present in many Supercolony and Ghosted areas. Conversely, the wet season result of altered community composition based on significant abundance increases suggests a ‘population-release’ view of secondary invasion (Grosholz 2005; Johnson et al. 2009; Montgomery et al. 2011) and reflects the pattern of invasion success already observed in our other results. As the wet season sampling time was more robust in terms of capturing the true land snail composition (Clergeau et al. 2011), we are inclined to conclude that significant changes in species abundances were driving the observed dissimilarity between forest states.
A limited number of highly abundant species contributed most to the compositional dissimilarity between forest states. In any pairwise comparison, the difference in abundance between the same four species contributed at least 85 % to the observed dissimilarity. These were the ground-dwelling exotics S. octona and G. williamsi, the mainly arboreal native J. wallacei, and the highly abundant but of unknown taxonomic status Georissa sp. It is not uncommon for only a few land snail species to numerically dominate even species-rich assemblages in mainland rainforest (de Winter and Gittenberger 1998). It has been suggested that little competition exists between land snail species (Cameron 1992; Barker and Mayhill 1999), and increased habitat and food resources in the form of leaf litter are almost always associated with increased land snail abundance (Aubry et al. 2005; Liew et al. 2010; de Chavez and de Lara 2011). On Christmas Island, some land snail species appear to respond to these changes more strongly than others. Although we cannot confidently infer the mechanism behind these species-specific responses, we have demonstrated a significant increase in land snail abundance associated with increased leaf litter at the whole community level.
Only two common exotic species (A. fulica and K. cruda) were never observed in Intact forest. Similarly, Green et al. (2011) found A. fulica was never present in Intact forest, as invading propagules were quickly intercepted and consumed by highly abundant red crabs. As such, A. fulica is considered a secondary invader of rainforest on the island due to the creation of enemy-free space following supercolony formation. We also found that the increase in land snail community abundance was strongly associated with the absence of the red crab; which was further supported by the observation that land snail assemblages in areas were red crabs had recolonised former crazy ant supercolonies (i.e. Recovered), were not dissimilar to those of Intact forest. However, because almost all exotic species were present in Intact forest, we suggest that red crabs do not providing the same level of biotic resistance to these much smaller species. We suggest that red crabs are limiting the invasion success of the smaller exotic snail species not directly in the form of predation, but indirectly through their consumption of seedlings and leaf litter which limits available habitat and resources for these species.
This facilitated success was not limited to exotic land snails; we found that the few native species also responded positively to the ant-scale invasion. Only recently have facilitative interactions between invasive and native species been identified as important associations in re-structuring novel ecosystems (Rodriguez 2006). In our study, the native snail J. wallacei was a significant contributor to the dissimilarity between Intact and impacted forest, being more abundant where crabs were absent. This is a mostly arboreal species, and is likely limited in Intact forest by the minimal understory complexity created by red crabs through their consumption of seedling germinants. These findings are analogous with secondary invasion occurring in Bodega Harbor, USA (Grosholz 2005) in which the removal of the previously dominant clam species by an invasive crab also resulted in greater abundances of several native benthic invertebrates (Grosholz et al. 2000). Interestingly, no native land species displayed the opposite pattern of being more abundant in Intact forest and inhibited in someway by the impacts of the ant-scale invasion. The removal of the red crab via the ant-scale invasion has facilitated all land snails, whether exotic or native, by indirectly releasing a limiting resource and increasing habitat complexity.
Conclusions
The formation of yellow crazy ant supercolonies on Christmas Island, supported by honeydew from sap-sucking hemiterans has long been recognised as creating conditions favourable to secondary invasions (O’Dowd et al. 2003; Simberloff 2006), and recently Green et al. (2011) provided solid evidence in support of this for one species of exotic land snail. Our results show that the broader community of land snails was also facilitated by the ant-scale invasion, due to the direct removal of the red crab and associated indirect increases in available habitat and resources. Whereas the large A. fulica was unable to enter forest where crabs were abundant (Lake and O’Dowd 1991; Green et al. 2011), the smaller exotic species that make up the majority of the community were able to establish relatively low density populations within Intact forest that were then indirectly facilitated by the invasive mutualism. These phenomena suggest contrasting models of secondary invasions—the ‘true entry’ model in which an exotic species that was previously barred from an intact recipient community was permitted entry as a result of the impact of other invaders, and the ‘population release’ model in which exotic species invade and persist in intact communities, significantly increase in abundance when other invaders enter the system and change it in their favour.
Although the rainforest of Christmas Island currently contains abundant populations of invasive land snails, there may be little reason for management intervention, for at least two reasons. First, land snails can play an important role in leaf litter decomposition and promotion of microbial growth in tropical systems (Meyer et al. 2013). Their higher abundance in impacted forest may provide partial redundancy in this role against the loss of the dominant detritivore (the red land crab) from areas invaded by yellow crazy ants. Second, our results indicate that once red crabs re-establish their formerly high abundances in areas from which management activity has eliminated yellow crazy ant supercolonies, the land snail community is not different to that found in Intact forest as leaf litter again becomes a limiting resource. While disentangling the net effects of invaders within a community context continues to be a challenge for ecologists (Preston et al. 2012), empirical evidence of facilitative interactions between invaders and indirect consequences of impacts will provide invaluable insights to understanding the outcomes of novel ecosystems.
References
Abbott KL (2006) Spatial dynamics of supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes, on Christmas Island, Indian Ocean. Divers Distrib 12:101–110
Abbott KL, Green PT (2007) Collapse of an ant-scale mutualism in a rainforest on Christmas Island. Oikos 116:1238–1246
Adams MJ, Pearl CA, Bury RB (2003) Indirect facilitation of an anuran invasion by non-native fishes. Ecol Lett 6:343–351
Aizen MA, Morales CL, Morales JM (2008) Invasive mutualists erode native pollination webs. PLoS Biol 6:e31
Aubry S, Magnin F, Bonnet V, Preece RC (2005) Multi-scale altitudinal patterns in species richness of land snail communities in south-eastern France. J Biogeogr 32:985–998
Barker GM, Mayhill PC (1999) Patterns of diversity and habitat relationships in terrestrial mollusc communities of the Pukeamaru Ecological District, northeastern New Zealand. J Biogeogr 26:215–238
Boland CRJ, Smith MJ, Retallick K et al (2011) Heli-baiting using low concentration fipronil to control invasive yellow crazy ant supercolonies on Christmas Island, Indian Ocean. In: Veitch CR, Clout MN, Towns DR (eds) Isl. invasives erad. manag. IUCN, Gland, pp 252–256
Bourgeois K, Suehs CM, Vidal E, Medail F (2005) Invasional meltdown potential: facilitation between introduced plants and mammals on French Mediterranean islands. Ecoscience 12:248–256
Cameron RAD (1992) Land snail faunas of the Napier and Oscar Ranges, Western Australia; diversity, distribution and speciation. Biol J Linn Soc 45:271–286
Cameron RAD, Pokryszko BM (2005) Estimating the species richness and composition of land mollusc communities: problems, consequences and practical advice. J Conchol 38:529–547
Cameron RAD, Mylonas M, Triantis K et al (2003) Land-snail diversity in a square kilometre of Cretan maquis: modest species richness, high density and local homogeneity. J Molluscan Stud 69:93–99
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40
Clergeau P, Tapko N, Fontaine B (2011) A simplified method for conducting ecological studies of land snail communities in urban landscapes. Ecol Res 26:515–521
Colwell R (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9 and earlier. User’s guide and application. http://purloclc.org/estimates
Cross JR (1981) The establishment of Rhododenron ponticum in the Killarney Oakwoods, SW Ireland. J Ecol 69:807–824
Davis NE, O’Dowd DJ, Green PT, Mac Nally R (2008) Effects of an alien ant invasion on abundance, behavior, and reproductive success of endemic island birds. Conserv Biol 22:1165–1176
Davis NE, O’Dowd DJ, Mac Nally R, Green PT (2010) Invasive ants disrupt frugivory by endemic island birds. Biol Lett 6:85–88
de Chavez ERC, de Lara AV (2011) Diversity and spatial distribution patterns of macro land snails in Mount Makiling Forest Reserve, Philippines. Asia Life Sci 20:185–201
de Winter AJ, Gittenberger E (1998) The land snail fauna of a square kilometer patch of rainforest in southwestern Cameroon, high species richness, low abundance and seasonal fluctuations. Malacologia 40:231–250
Drescher J, Blüthgen N, Feldhaar H (2007) Population structure and intraspecific aggression in the invasive ant species Anoplolepis gracilipes in Malaysian Borneo. Mol Ecol 16:1453–1465
Du Puy DJ (1993) Christmas Island. In: George AS, Orchard AE, Hewson HJ (eds) Flora of Australia, vol 50. Oceanic islands 2. Australian Government, Canberra, pp 1–30
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Falkland AC (1986) Christmas Island (Indian Ocean) water resources development, study in relation to proposed waterfall. Unpublished report prepared by the Hydrology and Water Resources Unit, Transport Territories, and Works Division for the Department of Territories
Flory SL, Bauer JT (2014) Experimental evidence for indirect facilitation among invasive plants. J Ecol 102:12–18
Forys EA, Allen CR, Wojcik DP (2001) The likely cause of extinction of the tree snail Orthanlicus reses (Say). J Molluscan Stud 67:369–376
Giraud T, Pedersen J, Keller L (2002) Evolution of supercolonies: the Argentine ants of southern Europe. Proc Natl Acad Sci USA 99:6075–6079
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Green PT (1997) Red crabs in rain forest on Christmas Island, Indian Ocean: activity patterns, density and biomass. J Trop Ecol 13:17–38
Green PT, O’Dowd DJ (2009) Management of invasive invertebrates: lessons from the management of an invasive alien ant. In: Clout MN, Williams PA (eds) Invasive species manag. a handb. princ. tech. Oxford University Press, Oxford, pp 153–172
Green PT, Odowd DJ, Lake PS (1997) Control of seedling recruitment by land crabs in rain forest on a remote oceanic island. Ecology 78:2474–2486
Green PT, Lake PS, O’Dowd DJ (1999) Monopolization of litter processing by a dominant land crab on a tropical oceanic island. Oecologia 119:435–444
Green PT, O’Dowd DJ, Lake PS (2008) Recruitment dynamics in a rainforest seedling community: context-independent impact of a keystone consumer. Oecologia 156:373–385
Green PT, O’Dowd DJ, Abbott KL et al (2011) Invasional meltdown: invader–invader mutualism facilitates a secondary invasion. Ecology 92:1758–1768
Grinath JB, Inouye BD, Underwood N, Billick I (2012) The indirect consequences of a mutualism: comparing positive and negative components of the net interaction between honeydew-tending ants and host plants. J Anim Ecol 81:494–502
Grosholz ED (2005) Recent biological invasion may hasten invasional meltdown by accelerating historical introductions. Proc Natl Acad Sci USA 102:1088–1091
Grosholz ED, Ruiz GM, Dean CA et al (2000) The impacts of a nonindigenous marine predator in a California bay. Ecology 81:1206–1224
Helms KR, Hayden CP, Vinson SB (2011) Plant-based food resources, trophic interactions among alien species, and the abundance of an invasive ant. Biol Invasions 13:67–79
Holway DA, Suarez AV (2006) Homogenization of ant communities in mediterranean California: the effects of urbanization and invasion. Biol Conserv 127:319–326
Hylander K, Nilsson C, Gunnar Jonsson B, Göthner T (2005) Differences in habitat quality explain nestedness in a land snail meta-community. Oikos 108:351–361
Johnson PTJ, Olden JD, Solomon CT, Vander Zanden MJ (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159:161–170
Kessner V (2006) Report on the March 2006 survey of land snails (Mollusc: Pulmonata) of Christmas Island, Indian Ocean. Unpublished report to Parks Australia
Labaune C, Magnin F (2001) Land snail communities in Mediterranean upland grasslands: the relative importance of four sets of environmental and spatial variables. J Molluscan Stud 67:463–474
Lake PS, O’Dowd DJ (1991) Red crabs in rain forest, Christmas Island: biotic resistance to invasion by an exotic snail. Oikos 62:25–29
Liew TS, Schilthuizen M, bin Lakim M (2010) The determinants of land snail diversity along a tropical elevational gradient: insularity, geometry and niches. J Biogeogr 37:1071–1078
Lockwood JL, Cassey P, Blackburn TM (2009) The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib 15:904–910
Meyer WM, Ostertag R, Cowie RH (2013) Influence of terrestrial molluscs on litter decomposition and nutrient release in a Hawaiian Rain Forest. Biotropica 45:719–727
Montgomery WI, Lundy MG, Reid N (2011) “Invasional meltdown”: evidence for unexpected consequences and cumulative impacts of multispecies invasions. Biol Invasions 14:1111–1125
O’Dowd DJ, Green PT, Lake PS (2003) Invasional “meltdown” on an oceanic island. Ecol Lett 6:812–817
Preston DL, Henderson JS, Johnson PTJ (2012) Community ecology of invasions: direct and indirect effects of multiple invasive species on aquatic communities. Ecology 93:1254–1261
Relva MA, Nunez MA, Simberloff D (2010) Introduced deer reduce native plant cover and facilitate invasion of non-native tree species: evidence for invasional meltdown. Biol Invasions 12:303–311
Ricciardi A (2001) Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? Can J Fish Aquat Sci 58:2513–2525
Rodriguez LF (2006) Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions 8:927–939
Simberloff D (2006) Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecol Lett 9:912–919
Simberloff D, Von Holle B (1999) Positive interactions on nonidigenous species: invasional meltdown? Biol Invasions 1:21–32
Styrsky JD, Eubanks MD (2007) Ecological consequences of interactions between ants and honeydew-producing insects. Proc Biol Sci 274:151–164
Suhr E, O’Dowd D, McKechnie S, Mackay D (2011) Genetic structure, behaviour and invasion history of the Argentine ant supercolony in Australia. Evol Appl 4:471–484
Sunamura E, Espadaler X, Sakamoto H et al (2009) Intercontinental union of Argentine ants: behavioral relationships among introduced populations in Europe, North America, and Asia. Insectes Soc 56:143–147
Tattersfield P, Warui CM, Seddon MB, Kiringe JW (2001) Land-snail faunas of afromontane forests of Mount Kenya, Kenya: ecology, diversity and distribution patterns. J Biogeogr 28:843–861
Thomas ML, Becker K, Abbott K, Feldhaar H (2010) Supercolony mosaics: two different invasions by the yellow crazy ant, Anoplolepis gracilipes, on Christmas Island, Indian Ocean. Biol Invasions 12:677–687
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen-fixation, eccosystem effects. Ecol Monogr 59:247–265
Wang B, Geng X-Z, Ma L-B et al (2014) A trophic cascade induced by predatory ants in a fig–fig wasp mutualism. J Anim Ecol 83:1149–1157
Wetterer JK (2005) Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology 45:77–97
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455
Wonham MJ, O’Connor M, Harley CDG (2005) Positive effects of a dominant invader on introduced and native mudflat species. Mar Ecol Ser 289:109–116
Yusa Y (2001) Predation of eggs of the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae) by fire ant Solenopsis geminata. J Molluscan Stud 67:275–279
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgments
This project was undertaken with funding from the Hermon Slade Foundation and the Holsworth Wildlife Endowment. Fieldwork was conducted within the Christmas Island National Park under Permit AU-COM2011107. Thanks to all at Christmas Island National Parks for logistical support. Dion Maple and Dethklok provided invaluable insights into the Christmas Island ecosystem. Thea Shell and Max Cameron assisted with data collection. Vince Kessner provided his expertise in land snail identification. This manuscript benefitted from the helpful comments of three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Loughlin, L.S., Green, P.T. Invader–invader mutualism influences land snail community composition and alters invasion success of alien species in tropical rainforest. Biol Invasions 17, 2659–2674 (2015). https://doi.org/10.1007/s10530-015-0903-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0903-6