Abstract
The successful introduction of the common genet (Genetta genetta) into Europe has been traditionally associated to the Muslim invasion of Iberia, although diverse evidence suggested an earlier arrival. In this study, we assessed genetic variation at 11 microsatellite loci in 199 individuals from the Mediterranean Basin and used approximate Bayesian computation (ABC) combining genotypes and published mitochondrial sequences. Our objectives were to (1) test alternative scenarios of introduction of the species in Europe, (2) re-assess the mitochondrial signatures of ‘introduction hotspots’ in Iberia, and (3) evaluate how post-introduction demographic processes have shaped genetic structure in the invaded range. ABC estimates favored a scenario of independent introductions from Maghreb into the Balearic Isl. and Iberia; the latter was dated between the Upper Palaeolithic and the end of Phoenicians’ influence. Patterns of genotypic diversity broadened the Andalusian introduction hotspot to the antique Tartessos Kingdom and suggested multiple introductions and/or long-term genetic drift. The best fit ABC scenario implied a natural spread from Iberia to France, but was in potential conflict with our delimitation of two genetic clusters (France and Iberia) in continental Europe. In fact, southwestern France populations showed a fair proportion of alleles shared with Maghreb and low levels of heterozygosity that may reflect subsequent introduction from Iberia, in line with the high error rates in favor of this alternative scenario. Significant patterns of isolation-by-distance among individuals within both genetic clusters are suggestive of natural dispersal from both Iberian and French introduction sites resulting in a secondary contact zone in northern Iberia. Overall, our study strongly suggests that the common genet was intentionally introduced in southern Iberia at a time antedating the Muslim invasion, possibly via Phoenicians’ commercial routes. Subsequent introduction in France, long-term genetic drift and admixture likely shaped the species genetic variation currently observed in continental Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introductions represent natural experiments to study non-equilibrium processes in population biology, such as colonization and spread under new environmental conditions (Sakai et al. 2001). The genetic architecture of introduced populations has been considered a major component of adaptations to newly invaded ecological niches, possibly more important than ecological tolerance (Lee 2002; Crawford and Whitney 2010). Multiple introductions involving different genetic lineages can play a significant role in counter-balancing the important loss of allelic richness and heterozygosity following introduction bottlenecks (Kolbe et al. 2004; Roman and Darling 2007; Dlugosch and Parker 2008). Secondary contact and gene flow (“admixture”) within the invaded range may occur through the release of selection against admixture in introduced populations (Verhoeven et al. 2011), with the potential consequence of promoting range expansion (Forsman et al. 2008).
Thus, characterizing the introduction pattern of colonizing species is crucial to understand the determinants of successful introductions (Dlugosch and Parker 2008). The accurate traceability of introduction routes has been greatly improved by the use of multi-locus genotyping to detect founder events (Davies et al. 1999). Nevertheless, the detection of multiple introductions may be rendered difficult by subsequent rapid population growth and admixture among invading populations (Khamis et al. 2009). This issue is exacerbated in historically introduced species, where demographic processes occurring over hundreds of generations can blur the genetic signature of early introductions. In this case, organelle genomes such as mitochondrial DNA may prove useful in retaining the genetic imprint of past introductions (Searle 2008; Jones et al. 2013).
The cultural exchanges connecting the borders of the Mediterranean Basin (MB) since prehistoric times represent an outstanding framework to study historical introductions, notably in mammals (Dobson 1998). Human-mediated introductions since the end of the Würmian glaciations (14–12 kyr ago) have deeply impacted current patterns of biodiversity in MB (Blondel and Vigne 1993; Vigne et al. 2009). These led to dramatic levels of endemic extinction, at the same time counter-balanced by the establishment of various allochthonous taxa, which are now paradoxically considered part of the “cultural heritage” of MB (Masseti 2009). The intensity of introductions has significantly increased since the first millennium B.C., following massive human migrations from eastern to western borders of the Mediterranean (e.g. Cucchi et al. 2005) that opened several dozens of potential routes to the human-mediated dispersal of species across MB (Ciolek 2011).
The common genet (Genetta genetta) is an opportunistic meso-predator (Viverridae, Carnivora) naturally distributed across Maghreb (Mauritania, Morocco, Algeria, Tunisia), sub-Saharan Africa and the southern Arabian Peninsula (Delibes and Gaubert 2013). Its establishment in Europe constitutes a unique example of a successful introduction of a wild African carnivore, since the Egyptian mongoose (Herpestes ichneumon)—long thought to be a contemporaneously introduced species—might have dispersed naturally into southwestern Europe (Gaubert et al. 2011). The invaded range of the common genet now spreads from Portugal to continental Spain, Mallorca, Cabrera and Ibiza (Balearic Isl.) and France (west, south-west and south-east). A few records are also known from Italy (Gaubert et al. 2008b). The introduction of the species has traditionally been associated to the Muslim invasion of Iberia (Spain and Portugal) starting 711 A.D. (Morales 1994), although a Greek historical source suggested its presence in Europe as early as the 6th century B.C. (Amigues 1999). Recent investigations based on mitochondrial DNA (mtDNA) reassessed these views, and suggested that the species was introduced from an endemic North African lineage into Andalusia (southern Spain), Catalonia (northwestern Spain), Mallorca and Ibiza (Balearic Isl.), and possibly western France (Gaubert et al. 2009, 2011). The distribution of the haplogroup at the origin of the European populations suggested an early influence of Phoenicians/Carthaginians, possibly later relayed by Muslim conquerors (Gaubert et al. 2011). However, such hypotheses were based on a single locus (mtDNA) and were lacking an explicit test of introduction scenarios, including times of introduction.
Following the postulate that genetic imprints in historically introduced populations are expected to be a good proxy of the history of humans’ dispersal and trading networks, we aimed at refining our mtDNA-based scenario of multiple introductions of the common genet in Europe using multi-locus genotyping (microsatellites). More specifically, we tested different scenarios of introduction from Maghreb and reassessed the delineation of the proposed ‘introduction hotspots’ in Andalusia and Catalonia. In a second step, we questioned how post-introduction events and demographic processes may have shaped the current genetic structure of the common genet in continental Europe.
Methods
Geographic sampling
A total of 199 samples were collected within the Mediterranean rim, covering Maghreb—the geographic source of European populations—and the range invaded by G. genetta, including southwestern continental Europe (Spain, Portugal, France and Italy) and the Balearic Isl. (Fig. 1; Table 1). Samples were gathered through a network of collaborators from the study region, resulting in a variety of sample types that included muscle, blood and ear tissues (146 samples), and guard hairs (53 samples) (see Gaubert et al. 2009, 2011). The samples were preserved in ~90 % ethanol at 4 °C before DNA extraction.
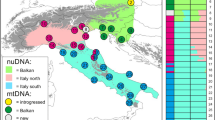
Genetic structure of the common genet in the Mediterranean Basin inferred from microsatellites. a Map of the geographic populations superimposed to the species distribution range in the Mediterranean Basin. Pie charts represent the proportional membership of individuals to clusters inferred from STRUCTURE (K = 4). Circles are proportional to the number of individuals. b Plots of the probabilistic assignments inferred by STRUCTURE per individuals and populations. See Table 1 for population acronyms
Given the opportunistic strategy of sample collection and our large study area, we followed a threshold between geographic proximity and number of individuals to partition our sample set in 20 geographic ‘populations’ including two populations from the native range (Table 1). Six populations with N < 5 were not included in population genetic analyses based on allelic frequencies.
Microsatellite genotyping
Fresh tissue and hair samples were processed in separated lab areas to avoid cross-contamination. Genomic DNA was extracted from muscle and blood using the phenol–chloroform extraction described by Sambrook et al. (1989). A similar protocol was applied to hair samples, and DNA was recovered by ultrafiltration with Microcon® YM-30 (Higuchi et al. 1988). We used a modified salt-chloroform method (Müllenbach et al. 1989) to extract DNA from ear tissues.
Fresh tissues samples were genotyped in singleplex PCR at 12 polymorphic microsatellite loci following protocols detailed in Gaubert et al. (2008a). Hair samples were genotyped through single- or multiplex pre-amplification PCR followed by singleplex PCR. The multiplex pre-amplification step included Mix 1 (loci A5, C101, D4, D111, B103 and B105) and Mix 2 (loci A104, A108, A112, A113, B104, A110). Multiplexing reactions were performed using the QIAGEN Multiplex PCR kit (QIAGEN, Hilden, Germany), including 2X QIAGEN Multiplex PCR Master Mix (1X final concentration), 0.1–0.2 mM primer mix (forward and reverse) and 6 μl DNA template for a final volume of 25 μl. PCR cycling conditions followed the manufacturer’s recommendations (QIAGEN Multiplex protocol), with annealing temperatures of 54 and 53 °C for Mix 1 and Mix 2, respectively. The second PCR run was performed in singleplex for the twelve microsatellite loci following the conditions detailed in Gaubert et al. (2008a), adding 4 μl of pre-amplification product to a 20 μl final volume. For hair samples, we used the modified multiple-tube approach detailed in Ferrando et al. (2008) to circumvent genotyping errors due to null alleles, false alleles and allelic drop-out (Taberlet et al. 1996). We performed a minimum of four PCR replicates per locus and sample. PCR products were analyzed on an ABI 3100 DNA sequencer and allele size was scored with GeneMapper v. 4.0. (Applied Biosystems, Foster City, CA).
Genetic diversity and structure
The 12 loci were examined for null alleles and miss-scoring in each geographic population with MICRO-CHECKER v.2.2.3. (Van Oosterhout et al. 2004). Locus B103 showed a significant level of null alleles in all European populations (P < 0.05), and thus was not considered in subsequent analyses. In fine, our data set consists of individuals with at least seven genotyped loci. The mean number of loci genotyped per individual was 10.2.
Genetic diversity was measured as the average number of alleles (N a ), effective number of alleles (N e ), observed (H o ) and expected (H e ) heterozygosities for each geographic population using GenAlEx v.6.1 (Peakall and Smouse 2006) and FSTAT v. 2.9.3 (Goudet 2001). The proportion of shared alleles, or similarity (ps; Bowcock et al. 1994), between European populations and Maghreb was calculated with Microsatellite analyzer (MSA) 4.05 (Dieringer and Schlötterer 2003). We applied the rarefication method implemented in HP-RARE (Kalinowski 2005) to estimate allelic richness (A R ) and private allelic richness (PA R ). We used GENEPOP v. 4.0 (Rousset 2008) to perform exact tests (Guo and Thompson 1992) for deviations from Hardy–Weinberg equilibrium and linkage disequilibrium among loci for all individuals and for each population within the introduced and native ranges. Significance levels were calculated by the Markov-chain method using 10,000 dememorization steps, 500 batches and 1,000 subsequent iterations per batch to keep the standard error estimates <0.01 (Raymond and Rousset 1995). The inbreeding coefficient (F IS ) (Weir and Cockerham 1984) was calculated for each population using FSTAT with 10,000 permutations. The false discovery rate (FDR) technique was used to eliminate false assignment of significance by chance under α = 0.05 (Verhoeven et al. 2005).
We used STRUCTURE v.2.3.3 (Pritchard et al. 2000) to assess population structure in the Mediterranean rim and admixture events among European populations. We performed 20 independent runs for K = 1–10 using 1 × 105 Markov chain Monte Carlo (MCMC) iterations and a burnin of 104, assuming admixture and uncorrelated allele frequencies, with and without a priori information on geographic populations. The most likely value for K was estimated using the ɅK method (Evanno et al. 2005) as implemented in Structure Harvester (Earl and VonHoldt 2012). Population structure was also explored in a geographic context using Geneland v.3.2.2 (Guillot et al. 2005a, b) as implemented in R (R Development Core Team 2010). We estimated the most likely number of populations (K) according to genotypic and geographic data under the Dirichlet (D) model (Guillot et al. 2005a). The optimal K value was tested from K = 1–10 under default parameters of number of nuclei in the Poisson Voronoi tessellation. D model was run five times for 5 × 105 MCMC iterations, with the first 2 × 104 iterations discarded as burnin. Then, the Falush (F) model (Falush et al. 2003) was run five times under the same conditions with the fixed optimal number of populations (K) as determined above. Maps of posterior probabilities of F model population membership were obtained and the run with the higher probability was selected. In both STRUCTURE and Geneland analyses, most likely K values were estimated including (1) all the geographic populations (Maghreb, Balearic Isl. and continental Europe) and (2) continental Europe only.
Genetic differentiation among geographic populations was also calculated by pairwise estimates of F ST (identical to the extended θ WC ) (Weir and Cockerham 1984) among geographic populations. Significant departure from zero was tested using 10,000 permutations in Arlequin v.3.01 (Excoffier et al. 2005). In addition, we carried out Principal Component Analysis (PCA) (1) among populations, on the basis of F ST estimates using 10,000 permutations in PCAgen v. 1.2 (Goudet 1999), and (2) among individuals, calculating a squared distance matrix (Φ) (Smouse and Peakall 1999) in GenAlEx.
We tested for isolation-by-distance (IBD) at individual and population scales within continental Europe by using, respectively, inter-individual genetic distances (a r ) (Rousset 2000) and a linearized Fst index (Fst/1-Fst) versus the logarithm of the geographic distance. The â estimates of the a parameters described in Rousset (2000) were computed using GENEPOP (Rousset 2008). Mantel tests were used to quantify the correlation between genetic and geographic distances (r) after 10,000 permutations using the mantel function in the VEGAN package for R (Oksanen et al. 2013).
Test of alternative introduction scenarios
We estimated the relative likelihood of alternative scenarios of introduction of the common genet in Europe using approximate Bayesian computation (ABC; see Beaumont 2010) as implemented in DIYABC v.2.0.3 (Cornuet et al. 2014), combining our genotypic data to a previously generated mtDNA dataset (cytochrome b and control region; Gaubert et al. 2009, 2011) (Online Resource 1). DIYABC allows the elaboration and comparison of complex scenarios involving bottlenecks, serial or independent introductions and genetic admixture events as they are often suspected in introduced populations (Estoup and Guillemaud 2010). The demographic parameters considered to model the scenarios are the times of split or admixture events (in number of generations), the stable effective population size, the effective number of founders in introduced populations, the duration of the bottleneck during colonization, and the rate of admixture (whenever admixture occurs).
We draw six introduction scenarios following hypotheses posited in the literature and our own results obtained with Bayesian clustering methods (Fig. 2; see below). Since STRUCTURE and Geneland identified four groups (see “Results”) and that an introduction hotspot was suspected in Catalonia (northeastern Spain) by our previous mtDNA analyses (Gaubert et al. 2009, 2011), we delimited a first set of five populations (set1) as follows: France (Fra), Iberia (Ibe; excluding Catalonia), Catalonia (Cat), Balearic Isl. (Bal) and Maghreb (Mag). Since (1) the clustering methods detected highly admixed populations neighboring Catalonia and (2) there was a discordant genetic pattern between Ibiza and Mallorca + Cabrera that may imply different sources of introduction, we ran a second analysis with slightly different groupings (set2). In this case, Cat also included S_NBP and S_NCAST, and Ibizan samples were moved to Mag. Following various lines of evidence (Morales 1994; Amigues 1999; Gaubert et al. 2009, 2011), Maghreb (Pop5) was in each scenario considered as the native population from which European populations were introduced. Scenario 1 considered Fra, Ibe and Bal as originating from three independent introductions, with a secondary introduction of Cat from Bal. Scenario 2 was similar except that Cat was the result of admixture between Fra and Ibe. Scenario 3 fixed a single introduction event for continental Europe in Iberia followed by a natural spread into Cat and Fra, and an independent introduction in the Balearic Isl. Scenario 4 was similar to scenario 1 except that Fra originated from a secondary introduction event from Ibe. Scenario 5 was similar to scenario 4 except that Cat was the result of admixture between Fra and Bal, thus implying an introduction event from Bal (with a bottleneck). Scenario 6 was similar to scenario 4 except that Cat was the result of admixture between Fra and Ibe.
DIYABC graphical representation of the six alternative introduction scenarios of the common genet in Europe used for approximate Bayesian computation simulations. Native population is Maghreb. Time is not to scale. See Materials and methods for details on the scenarios and model parameters. t1–t4 = times of introduction events; ta–tc = times of admixture events; N1–N5 = stable effective population sizes; N1b–N4b = effective numbers of founders in introduced populations; da, dh–dl = times of end of bottleneck since introduction or admixture events; ra–rc = rates of admixture
Prior distributions were uniform and set by default (Online Resource 2), with the exception of (1) the mtDNA mutation model fixed to TrN (Gaubert et al. 2009) and (2) microsatellites and mtDNA mutation rates having their minimum and maximum distributions increased by a factor 10, respectively (so the fit of the observed data with the model simulations were improved). Microsatellite loci followed the generalized stepwise-mutation (GSM) model as implemented by default in DIYABC (Estoup et al. 2002). Priors were also constrained to set up realistic posterior estimates as concerns times of split (prior range, by default: 10–10,000 generation time). The time of split between a given pair of primary introduced and secondary introduced populations was systematically fixed to be younger than the initial split between the related pair of native and introduced populations. Similarly, stable effective population size of the native population (Mag) was constrained to be higher than the effective number of founders in introduced populations.
The ABC method relies on summary statistics calculated from the dataset to represent the maximum amount of information in the simplest possible form (Sunnåker et al. 2013). DIYABC uses a series of standard, one sample and two sample summary statistics traditionally used in population genetics (see Cornuet et al. 2013). Following Cornuet et al. (2010), we used the largest series of summary statistics available in DIYABC, excluding a subset of one and two sample summary statistics that were used to check the goodness-of-fit of our dataset under the posterior predictive distribution of the model for the best scenario. Overall, our models (six scenarios) represented 26 historical parameters and 145 summary statistics applied to microsatellites and mtDNA sequences. The summary statistics used to assess the goodness-of-fit were mean size variance (one sample) and mean size variance and shared allele distance (two sample) for microsatellite data, and variance of pairwise differences and of numbers of the rarest nucleotide at segregating sites (one sample) and mean of pairwise differences (two sample) for mtDNA.
We simulated 6,000,000 datasets per scenario based on the coalescent model to produce robust ABC results, as recommended by the authors of DIYABC (Cornuet et al. 2013). As a first analytical step, we checked whether our dataset fitted the range of our pre-defined models (scenarios and parameter priors) using PCA representation on the summary statistics of the first 10 % simulated datasets. We concluded that our six models were suitable for proceeding to the ABC analyses by evaluating the position of our observed data relative to the distribution of summary statistics (Online Resource 3). The relative posterior probabilities of the different scenarios were calculated through polychotomous logistic regression from the 0.1 % of simulated data sets most closely resembling the observed data using linear discriminant analysis on summary statistics (Estoup et al. 2012). Then, the posterior distributions of parameters were estimated under the most likely scenario by the logit transformation of parameters and linear regression on the 1 % of simulated data sets most closely resembling the observed data. The power of our DIYABC analysis to discriminate between alternative scenarios was evaluated by simulating 500 pseudo-observed data sets per scenario with the same number of loci and individuals as our dataset. The relative posterior probabilities of each competing scenario were used to calculate type I and II errors for the most likely scenario.
Results
No significant linkage disequilibrium was detected between pairs of loci among all the individuals. Departures from Hardy–Weinberg equilibrium (HWE) were detected considering all loci and all individuals, but there was no significant departure for any geographic population (P < 0.001; FDR correction for multiple comparisons, 1 % nominal level). All the loci were polymorphic in all populations, except Cabrera and Ibiza (Balearic Isl.) that showed no allelic variability in eight and five loci, respectively. The mean number of alleles per population (Na) ranged from 1.4 to 4.8, and the allelic richness (A R ) ranged from 1.3 to 4.8 (Cabrera and western Maghreb as minimum and maximum values; Table 1). Mean expected heterozygosity values were moderately high in continental Europe populations (0.43-0.61) and lower in the Balearic Isl. (0.09–0.25), compared to the native range (0.63–0.71). Populations from southwestern Iberia had the highest heterozygosity levels in continental Europe (0.59–0.61). Northeastern Iberia and southwestern France had the highest inbreeding coefficient (F IS ) values in continental Europe (0.11–0.13), whereas the highest values among all the populations were found in the Balearic Isl. (0.18–0.20). Performing Hardy–Weinberg exact test by locus and population and applying FDR correction for multiple comparisons, only four values of F IS were found significant: at loci A108, C101 and D111 in southwestern France, and at locus A108 in western France (data not shown). However, average F IS values across loci for each population were not significant. Private allelic richness (PA R ) was the highest in Maghreb (0.61–1.26), whereas southwestern Spain and southern Portugal had the greatest richness in Europe (0.11–0.24; see Table 1). The highest similarity values (shared alleles) between European populations and Maghreb (ps) were found in Ibiza (0.46) and Catalonia + the eastern, French Pyrenean border (0.43). The rest of the similarity values were slightly inferior (0.33-0.41).
The Bayesian clustering analysis with STRUCTURE identified four clusters (K = 4) within the studied species range (Table 2), including Iberia (cluster 1), France (cluster 2), Cabrera Isl. (cluster 3) and Maghreb + Ibiza Isl. (cluster 4). Mallorca Isl. was admixed between clusters 3 and 4 (posterior probabilities of assignment <0.70). Individuals from Iberia and France were assigned to clusters 1 and 2 (respectively) when restricting our analysis to continental Europe. Admixed populations were found north of Iberia and at the French border (SF_NE, S_NBP, S_NCAST; Fig. 1 and Table 2). The maps of posterior probability obtained with GENELAND supported four similar clusters within the studied species range but only evidenced admixed populations in northwestern Iberia (i.e. lower posterior probability values of assignment to the two clusters in continental Europe; Online Resource 4).
Principal component analysis (PCA) plots showed genetic structure within the species distribution (Fig. 3). Maghreb, Balearic and continental Europe populations separated along PCI (45.05 %, P < 0.05). Along PCII (16,20 %, P < 0.05), Mallorca + Cabrera and Ibiza (Balearic Isl.) separated and European populations stretched from south to north. PCA among individuals yielded a similar pattern, although less clear-cut geographically. The overall measure of genetic differentiation among all populations was high (F ST = 0.276, P = 0.0001), with the majority of pairwise comparisons showing a significant differentiation (Online Resource 5).
Principal components analysis (PCA) among a the geographic populations and b all the individuals representing the common genet. See Table 1 for population acronyms
The two clusters defined by STRUCTURE for continental Europe were used to delineate three groups in the IBD analysis: ‘southern group’ (cluster 1: most of Iberia), ‘northern group’ (cluster 2: most of France) and ‘admixed’ group (populations with global, posterior assignment probabilities <0.70: northern Iberia and French boundary). Whereas Mantel tests showed significant positive relationships between pairwise a r values and geographic distance within each continental group (r values: northern = 0. 1572; southern = 0. 2597; admixed = 0. 1406; in all cases, P < 0.01), a higher correlation was found when grouping all the individuals (r = 0.4052; P < 0.0001) (Fig. 4). At the population level, there was a highly significant correlation between geographic and genetic distances across continental Europe (r = 0.7322, P < 0.001). A similar level of correlation was found when removing the admixed group (northern and southern: r = 0.7377, P < 0.01) and the southern group (northern and admixed: r = 0.7239, P < 0.01). There was a lower correlation when the northern group was removed (southern vs admixed: r = 0.5497, P < 0.001). None of the IBD analyses among populations within the three groups were significant (P > 0.05), probably due to small sample size.
Isolation by distance among a individuals and b populations of the common genet in continental Europe. Linear regressions are given for the northern (dashed line), southern (dotted line) and admixed (long dashed line) groups. Groups were delineated following results from STRUCTURE. The thick solid line shows the linear regression all over continental Europe (i.e. all individuals and groups together)
ABC simulations based on six alternative introduction scenarios gave strong support to scenario 3 (mean posterior probability: 0.92–0.97; 95 % CI = 0.92–0.93 to 0.97–0.97) and similar posterior parameter estimates, independent of the sets (1 and 2) used. Scenario 3 implies two independent introductions from Maghreb into the Balearic Isl. and Iberia, followed by a spread throughout northeastern Iberia and France (see Fig. 2). The five other scenarios showed much lower posterior probabilities (<0.07) (see Online Resource 6). False positive rates (type I errors) for scenario 3 were moderately low (24.2–23.9 %), but false negative rates (type II errors) reached 56.2–55.5 %. Scenario 6 contributed to 41.3–40.7 and 33.8–32.6 % of the scenario 3 false positives and negatives, respectively. Our observed data were nested within the posterior predictive distribution of the model for scenario 3 based on alternative summary statistics, suggesting a good fit of scenario 3 with the observed data (Online Resource 7). Randomly modifying the choice of summary statistics used to assess the goodness-of-fit of our observed data had no influence on the choice of the best scenario, neither had the use of separated datasets (i.e. mtDNA versus microsatellites; data not shown).
The posterior distribution estimates of effective population sizes, duration of bottlenecks and locus-specific evolutionary model parameters were in general little informative (Online Resource 2). Given that the observed genetic diversity of our dataset is expected to be the product of the effective population size of our source population (Mag) and mutation rates (i.e. the majority of mutations/alleles must have existed before the first introduction events), we could not estimate independently effective population sizes and mutation rates. Nevertheless, estimates of time parameters describing introduction and expansion events presented the only informative posterior distributions (sharper distribution and narrower confidence interval than prior ranges) and should thus be usable (see Fountain et al. 2014). Those latter represented the only informative posterior distributions (more pointy aspect and narrower confidence interval than prior ranges). The median value was t4 = 3,130 (set2)–3,320 (set1) generation times (HPD 95 = 853–7,610 to 1,150–8,270) for the introduction event in Iberia, t3 = 1,320 (set1)–1,790 (set2) generation times for the introduction in the Balearic Isl. (HPD 95 = 372–6,060 to 554–6,730) and tb = 717 (set2)–797 (set1) generation times for the expansion from Iberia into Catalonia and France (HPD 95 = 288–1,570 to 338–1,670). Adjusting priors to reflect more realistic effective population sizes (N > 10,000) and mtDNA mutation rate (Gaubert et al. 2009) or to restrict introduction times to more recent periods (e.g. 10–2,000 generation times) systematically resulted in the observed data being off the distribution of the simulated datasets.
Discussion
Scenario of introduction of the common genet from Maghreb to Europe
The descriptive analysis of microsatellite diversity confirmed Maghreb as the source population (Gaubert et al. 2009, 2011) because European populations generally had lower allelic diversity and levels of heterozygosity (Nei et al. 1975; Tsutsui et al. 2000). Our approach using ABC modelling allowed us to explicitly test, for the first time, different scenarios of introduction of the common genet in Europe combining multilocus data (microsatellites and mtDNA). ABC simulations yielded strong support for scenario 3, implying two independent introductions from Maghreb into the Balearic Isl. and Iberia (Fig. 2). Considering a generation time of 2 years in the common genet (Delibes and Gaubert 2013), the posterior distribution estimate of the introduction event in Iberia [6,640 (2,300–16,540) ya for set1 and 6,260 (1,706–15,220) ya for set2] largely antedated the invasion of Iberia by Muslim armies. Although this range is rather large, it covers a realistic time frame from the first trans-migration of humans via the Strait of Gibraltar (Upper Palaeolithic; Derricourt 2005) to the end of the Phoenician influence in the MB (300 BC; Elayi 2013). It is also congruent with the earliest estimate of transportation of a small carnivore (Mustela nivalis) into Mediterranean islands c. 10,000 ya (Lebarbenchon et al. 2010).
Our analyses broadened the Andalusia ‘introduction hotspot’ to southwestern Iberia (i.e. including southwestern Spain and southern Portugal). Indeed, the highest genetic diversity and private allelic richness observed in southern Portugal mirrored the high genetic diversity found in southwestern Spain and added to the peak of unique mtDNA haplotypes found in Andalusia (Gaubert et al. 2009, 2011). The introduction hotspot found in southwestern Iberia superimposes well with the Tartessian Kingdom’s zone of cultural influence from 1,200 to 550 BC (Fig. 1; Chamorro 1987). Previous studies investigating historiographical sources and mtDNA diversity have invoked the possible role of Antique civilizations in the introduction of the common genet into southern Iberia (Amigues 1999; Gaubert et al. 2009, 2011). Indeed, the Greek author Herodotus mentioned the use of the common genet as a bio-control agent against the pullulating of rabbits at the time of the Tartessos Kingdom, 600 BC (Amigues 1999). Tartessos was a rich harbor city located on the western Andalusian coast, which had vibrant trading exchanges with Phoenicians and their nearby commercial harbor in the present-day Cadiz since at least 800 BC (Moscati 1996). It is thus possible that Phoenicians (or Greeks, whom shared almost contemporaneous, similar trading routes) introduced the common genet from their North African colonies through their trading activities with Tartessians. Indeed, Phoenicians are considered the earliest trans-Mediterranean colonizers to southern Iberia having spread ‘exotic’ vertebrate species (Muñiz et al. 1995).
The high genetic diversity and private allelic richness found in southern Portugal and southwestern Spain suggest a bottleneck effect followed by a high rate of population growth (Nei et al. 1975) or long-term genetic drift (Allendorf and Lundquist 2003), in agreement with the ancient time of introduction inferred from ABC. Since multiple introductions may overcome founder effect by producing high genetic diversity in non-native populations (Kolbe et al. 2004) and facilitating rapid adaptation and expansion in the invaded range (Lee 2002), we posit that the common genet in southern Iberia might have repeatedly—and deliberately—been introduced from different sources in Maghreb. This hypothesis is also supported by the co-occurrence of two divergent mtDNA lineages in southwestern Iberia (Gaubert et al. 2009). Although we could not rule on the precise period of introduction of the common genet in Iberia, our results altogether reject the hypothesis of the species being introduced by Muslims from the 8th century, and reinforce the potential role of Phoenicians even though earlier transportations cannot be ruled out.
ABC simulations identified the Balearic Isl. as the second site of introduction of the common genet in Europe, whether Ibiza was grouped with Maghreb or the Balearic Isl. The microsatellite data confirmed that the Balearic Isl. constituted multiple sites of introduction for the common genet (Delibes 1977; Clevenger 1993; Gaubert et al. 2009, 2011). Island populations were genetically close to Maghreb and were mainly assigned to two distinct groups by clustering analyses, including (1) Ibiza, significantly differentiated from but grouped with Maghreb, and (2) Cabrera. Mallorca being admixed between the two genetic clusters and having a small sample size, it remains difficult to establish a definitive scenario of introduction among the Balearic Isl. Nevertheless, our results suggested that severe bottlenecks followed by weak population growth occurred after the introduction of common genets on the islands. The Balearic populations had the lowest allelic richness and levels of heterozygosity in Europe: such a pattern is expected after the introduction of low founder population sizes on relatively small islands followed by genetic drift (Frankham 1998; Broders et al. 1999; Allendorf and Lundquist 2003). Given our limited sample set, we could not clarify whether genets were transported independently to Mallorca and Ibiza, or were translocated from Ibiza to Mallorca in a similar way that they were more recently from Mallorca to Cabrera for regulating rabbit populations (Delibes 1977). Neither we could refine the time frame at which the species was introduced on the islands, given the wide confidence interval that we obtained from ABC estimates either including Ibiza [2,640 (744–12,120) ya, set1] or not [3,580 (1,108–13,460) ya, set2]. The influence of the Phoenicians remains conceivable given that (1) the Antique history of the Balearic Isl. was at its early stage under the influence of Carthaginians (Phoenician colony from Tunisia), as reflected in the Baleares peopling and trade items (Tomàs et al. 2006; Segert 2007), and (2) one of the first records of a small carnivore (Mustela nivalis) in the Balearic Isl. was associated to Carthaginians’ remains (Masseti 1995).
Post-introduction scenario in continental Europe
ABC estimates selected the sole introduction scenario (scenario 3) that involved the natural spread of the common genet from Iberia to Catalonia (northeastern Spain) and France. Although the posterior probabilities for choosing this scenario were high (0.92–0.97), a fair rate of type I errors (24 %) and more importantly, a high rate of type II errors (56 %) indicate that other scenarios may well fit our dataset. Noticeably, scenario 6 that involved a secondary introduction from Iberia to France and subsequent admixture in Catalonia between French and Iberian populations, was responsible for 41 and 33–34 % of the false positives and negatives, respectively. We thus consider that ABC did not resolve the issue of whether the French populations originated from a geographic spread of introduced Iberian populations (dispersal hypothesis), or were subsequently introduced from Iberia (bridgehead hypothesis).
At first sight, scenario 3 may appear in conflict with our clustering analyses based on microsatellite data in continental Europe, which inferred two genetic groups distributed in western Iberia and France. However, one should keep in mind that the a priori delineation of ‘populations’ in ABC does not correspond to a test of population structure, but to simulations of population history (Cornuet et al. 2010). Moreover, STRUCTURE and other clustering methods have been shown to be sensitive to the IBD effect, marked IBD patterns potentially leading to the biased identification of population clusters along the IBD gradient (Frantz et al. 2009; Meirmans 2012). The strong IBD pattern exhibited within the genotyped populations of Europe is actually in line with scenario 3, which involves northern dispersal from Iberia of the common genet. Indeed, a significant IBD signal may reflect a natural process of diffuse dispersal from introduction sites (e.g. Henry et al. 2009), consistent with the capacity of this small carnivore to colonize new areas in Europe from source populations (Gaubert et al. 2008b). Thus, our results could argue for the biased estimate of two population clusters in continental Europe. Nevertheless, the incidence in southwestern France of (1) a fair proportion of alleles shared with Maghreb and (2) reduced levels of heterozygosity (low Ho, high F IS ) could be the signature of a secondary introduction from southern Europe (bridgehead hypothesis), in line with scenario 6 and as suggested by the distribution of mtDNA haplotypes (Gaubert et al. 2011). In this case, the IBD pattern also found within the French and Iberian population clusters (among individuals) and the lower genetic diversity observed in the other French populations would support a scenario of dispersal from both southwestern Iberia and western France leading to subsequent admixture in a contact zone somewhere between the north of Iberia and the French border (including Catalonia). Although we cannot estimate at which period the species could have been introduced in southwestern France (posterior estimates for scenario 6 were uninformative), the Middle Age fashion for the common genet at French courts (Delort 1978; Gaubert and Mézan-Muxart 2010; Mézan-Muxart 2010) or older practices of offering live small carnivores as political gifts (Morales Muñiz 2000) constitute potential landmarks.
The Catalonia ‘introduction hotspot’ was not recovered as a probable scenario by the ABC analysis. Instead, DIYABC favored a scenario of ancestral coalescence (scenario 3) or secondary admixture (scenario 6) of Catalonia and northern Spanish populations with French and Iberian populations, and consistently disfavored scenarios fixing Catalonia as a population introduced from the Balearic Isl. Nevertheless, we observed (1) lower levels of heterozygosity than in other parts of Iberia (low Ho, high F IS ), (2) a high proportion of alleles shared with Maghreb and (3) the assignment of a small fraction of individuals to cluster 3 (distributed in Mallorca and Cabrera) that could be a signal of introduction of common genets from the Balearic Isl. or Maghreb to Catalonia. It is possible that long-term admixture between Iberian and French populations around the latitude of Catalonia complicated the detection of introduced populations in Catalonia using microsatellite data. All the more since organelle genomes such as mtDNA, which are capable of retaining the genetic imprint of old introductions (Searle et al. 2009), gave a clear signal of independent introduction in Catalonia, either directly from Maghreb or via the Balearic Isl. (Gaubert et al. 2009, 2011).
Finally, clustering analyses revealed a high level of admixture between Iberian and French populations in northern Iberia and at the French boundary, in line with the wide diffusion of the mtDNA haplotype “H1” observed across southwestern Europe (Gaubert et al. 2009, 2011). Whether such admixture may have influenced the local fitness of the species remains unknown.
Conclusion
ABC estimates and descriptive analyses of genetic diversity congruently pointed to two primary introductions of the common genet from Maghreb to Iberia and the Balearic Isl. Nevertheless, given the level of uncertainty in the choice of scenarios 3 and 6, ABC failed to reject the hypothesis of a secondary introduction from Iberia to western France. ABC also failed to detect the Catalonian population as introduced from the Balearic Isl. as a likely scenario. Causes of incongruence between ABC and descriptive analyses of genetic data have been poorly explored and were not the scope of our investigations. We anticipate that they could have been due to low sample size (and thus low statistical power), misspecification of priors, conflict between microsatellites and mtDNA prior requirements (see Templeton 2009), and the non-consideration by the ABC approach of genetic diversity patterns within populations. Eventually, we acknowledge that a wider sampling (e.g. more samples from Maghreb and the Balearic Isl.) and genetic coverage (i.e. more loci) will be necessary to improve the accuracy of the descriptive analyses of genetic diversity in the common genet.
Nevertheless, the descriptive evidence (microsatellite and mitochondrial diversity patterns) used within the permissive range of ABC estimates of scenario choice (scenarios 3 and 6) allowed us to propose that, together with introductions in the Balearic Isl., the common genet may have established thrice in southwestern continental Europe (in southern Iberia, southwestern France and Catalonia). The microsatellite data suggested a scenario of post-introduction gene flow and genetic drift as structuring geographical genetic variation in the invaded range, which is not concordant with the hypothesis of an artificial dispersal of the species by Muslims. Our conclusion raises the question of the specific use of the common genet by humans in historical times, a point that remains almost undocumented in the archaeozoological and historiographical records with the exception of the ‘stories’ of Herodotus. Further investigations covering the fields of archaeozoology and evolutionary genetics will have to be conducted in the MB to better understand the factors having influenced the successful introduction of the common genet in Europe.
References
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Amigues S (1999) Les belettes de Tartessos. Anthropozool 29:55–64
Beaumont MA (2010) Approximate Bayesian computation in evolution and ecology. Annu Rev Ecol Evol Syst 41:379–406
Blondel J, Vigne JD (1993) Space, time, and man as determinants of diversity of birds and mammals in the Mediterranean region. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities: historical and geographical perspectives. The University of Chicago Press, Chicago and London, pp 135–146
Bowcock AM, Ruiz-Linares A, Tomfohrde J et al (1994) High resolution of human evolutionary trees with polymorphic microsatellites. Nature 368:455–457
Broders HG, Mahoney SP, Montevecchi WA et al (1999) Population genetic structure and the effect of founder events on the genetic variability of moose, Alces alces, in Canada. Mol Ecol 8:1309–1315
Chamorro JG (1987) Survey of archaeological research on Tartessos. Am J Archaeol 91:197–232
Ciolek TM (2011) 1999–present. Georeferenced historical transport/travel/communication routes and nodes. OWTRAD dromographic digital data archives (ODDDA): old world trade routes (OWTRAD) Project. www.ciolek.com—Asia Pacific Research Online, Canberra, Australia
Clevenger A (1993) Status of martens and genets in the Balearic and Pityusic Islands, Spain. Small Carniv Conserv 9:18–19
Cornuet J-M, Ravigne V, Estoup A (2010) Inference on population history and model checking using DNA sequence and microsatellite data with the software DIYABC (v1.0). BMC Bioinfo 11:401
Cornuet JM, Pudlo P, Veyssier J et al (2013) DIYABC version 2.0: A user-friendly software for inferring population history through approximate Bayesian computations using microsatellite, DNA sequence and SNP data. Institut National de la Recherche Agronomique, Campus International de Baillarguet, Saint-Gély-du-Fesc
Cornuet J-M, Pudlo P, Veyssier J et al (2014) DIYABC v2.0: a software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics 30:1187–1189
Crawford KM, Whitney KD (2010) Population genetic diversity influences colonization success. Mol Ecol 19:1253–1263
Cucchi T, Vigne J-D, Auffray J-C (2005) First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: a zooarchaeological revision of subfossil occurrences. Biol J Linn Soc 84:429–445
Davies N, Villablanca FX, Roderick GK (1999) Determining the source of individuals: multilocus genotyping in nonequilibrium population genetics. TREE 14:17–21
Delibes M (1977) Sobre las ginetas de la isla de Ibiza (Genetta genetta isabelae n. ssp.). Doñana, Acta Vertebrata 4:139–160
Delibes M, Gaubert P (2013) Genetta genetta common genet (small-spotted genet). In: Kingdon JS, Hoffmann M (eds) The mammals of Africa: carnivores, pangolins, equids and rhinoceroses edn, vol 5. Bloomsburry Publishing, London, pp 224–229
Delort R (1978) Le commerce des fourrures en Occident à la fin du Moyen-Âge. Ecole Française de Rome, Palais Farnèse
Derricourt R (2005) Getting “out of Africa”: sea crossings, land crossings and culture in the hominin migrations. JWP 19:119–132
Dieringer D, Schlötterer C (2003) Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Not 3:167–169
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Dobson M (1998) Mammal distributions in the western Mediterranean: the role of human intervention. Mamm Rev 28:77–88
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Elayi J (2013) Histoire de la Phénicie. Perrin, Paris
Estoup A, Guillemaud T (2010) Reconstructing routes of invasion using genetic data: why, how and so what? Mol Ecol 19:4113–4130
Estoup A, Jarne P, Cornuet J-M (2002) Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Mol Ecol 11:1591–1604
Estoup A, Lombaert E, Marin J-M et al (2012) Estimation of demo-genetic model probabilities with approximate Bayesian computation using linear discriminant analysis on summary statistics. Mol Ecol Resour 12:846–855
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol Notes 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Falush D, Stephens M, Pritchard J (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Ferrando A, Lecis R, Domingo-Roura X et al (2008) Genetic diversity and individual identification of reintroduced otters (Lutra lutra) in north-eastern Spain by DNA genotyping of spraints. Conserv Biol 9:129–139
Forsman A, Ahnesjö J, Caesar S et al (2008) A model of ecological and evolutionary consequences of color polymorphism. Ecology 89:34–40
Fountain T, Duvaux L, Horsburgh G et al (2014) Human-facilitated metapopulation dynamics in an emerging pest species, Cimex lectularius. Mol Ecol 23:1071–1084
Frankham R (1998) Inbreeding and extinction: Island populations. Conserv Biol 12:665–675
Frantz AC, Cellina S, Krier A et al (2009) Using spatial Bayesian methods to determine the genetic structure of a continuously distributed population: clusters or isolation by distance? J Appl Ecol 46:493–505
Gaubert P, Mézan-Muxart V (2010) Where have the ‘black genets’ gone? A likely restriction of melanistic cases of the common genet (Genetta genetta) to its introduced range. Mamm Biol 75:353–357
Gaubert P, del Cerro I, Palomares F et al (2008a) Development and characterization of 11 microsatellite loci in a historically introduced carnivoran, the common genet (Genetta genetta). Mol Ecol Resour 8:1375–1377
Gaubert P, Jiguet F, Bayle P et al (2008b) Has the common genet (Genetta genetta) spread into south-eastern France and Italy? Ital J Zool 75:43–57
Gaubert P, Godoy JA, del Cerro I et al (2009) Early phases of a successful invasion: mitochondrial phylogeography of the common genet (Genetta genetta) within the Mediterranean Basin. Biol Invasion 11:523–546
Gaubert P, Machordom A, Morales A et al (2011) Comparative phylogeography of two African carnivorans presumably introduced into Europe: disentangling natural versus human-mediated dispersal across the Strait of Gibraltar. J Biogeogr 38:341–358
Goudet J (1999) PCA-GEN. version 1.2. Lausanne, Switzerland. www.unil.ch/izea/softwares/pcagen.html
Goudet J (2001) FSTAT, version 2.9.3. A program to estimate and test gene diversities and fixation indices. Lausanne University, Lausanne, Switzerland. http://www2.unil.ch/popgen/softwares/fstat.htm
Guillot G, Estoup A, Mortier F et al (2005a) A spatial statistical model for landscape genetics. Genetics 170:1261–1280
Guillot G, Mortier F, Estoup A (2005b) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5:712–715
Guo SW, Thompson EA (1992) Performing the exact tests of Hardy–Weinberg proportion for multiple alleles. Biometrics 48:361–372
Henry P, Le Lay G, Goudet J et al (2009) Reduced genetic diversity, increased isolation and multiple introductions of invasive giant hogweed in the western Swiss Alps. Mol Ecol 18:2819–2831
Higuchi R, von Beroldingen CH, Sensabaugh GF et al (1988) DNA typing form single hairs. Nature 332:543–546
Jones EP, Eager HM, Gabriel SI et al (2013) Genetic tracking of mice and other bioproxies to infer human history. Trends Genet 29:298–308
Kalinowski ST (2005) HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189
Khamis FM, Karam N, Ekesi S et al (2009) Uncovering the tracks of a recent and rapid invasion: the case of the fruit fly pest Bactrocera invadens (Diptera: Tephritidae) in Africa. Mol Ecol 18:4798–4810
Kolbe JJ, Glor RE, Rodriguez Schettino L et al (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Lebarbenchon C, Poitevin F, Arnal V et al (2010) Phylogeography of the weasel (Mustela nivalis) in the western-Palaearctic region: combined effects of glacial events and human movements. Heredity 105:449–462
Lee CE (2002) Evolutionary genetics of invasive species. TREE 17:386–391
Masseti M (1995) Quaternary biogeography of the Mustelidae family on the Mediterranean islands. Hystrix 7:17–34
Masseti M (2009) Mammals of the Mediterranean Islands: homogenisation and the loss of biodiversity. Mammalia 73:169–202
Meirmans PG (2012) The trouble with isolation by distance. Mol Ecol 21:2839–2846
Mézan-Muxart V (2010) Genette et janette: devises de Jeanne de France au XVe siècle. Reinardus 22:104–125
Morales A (1994) Earliest genets in Europe. Nature 370:512–513
Morales Muñiz DC (2000) La fauna exótica en la Península Ibérica: apuntes para el estudio del coleccionismo animal en el Medievo hispánico. Espacio, Tiempo y Forma, Serie III, Ha Medieval 13:233–270
Moscati S (1996) La grande Cadice dei Fenici. Accademia nazionale dei Lincei, Roma
Müllenbach R, Lagoda PJ, Welter C (1989) An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet 5:391
Muñiz AM, Pecharroman MAC, Carrasquilla FH et al (1995) Of mice and sparrows: commensal faunas from the Iberian Iron Age in the Duero valley (Central Spain). Int J Osteoarchaeol 5:127–138
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Oksanen JF, Blanchet G, Kindt R et al (2013) vegan: Community Ecology Package. R package version 2.0-8. http://CRAN.R-project.org/package=vegan
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. TREE 22:454–464
Rousset F (2000) Genetic differentiation between individuals. J Evol Biol 13:58–62
Rousset F (2008) GenePop’007: a complete re-implementation of the GenePop software for Windows and Linux. Mol Ecol Resour 8:103–106
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Sambrook E, Fritsch F, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Press, New York
Searle JB (2008) The genetics of mammalian invasions: a review. Wild Res 35:185–192
Searle JB, Jamieson PM, Gündüz İ et al (2009) The diverse origins of New Zealand house mice. Proc R Soc B Biol Sci 276:209–217
Segert S (2007) Phoenician and Punic morphology. In: Kaye AS (ed) Morphology of Asia and Africa, vol 1. Eisenbrauns, Winona Lake, pp 75–84
Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573
Sunnåker M, Busetto AG, Numminen E et al (2013) Approximate Bayesian computation. PLoS Comput Biol 9:e1002803
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucl Acids Res 24:3189–3194
Templeton AR (2009) Statistical hypothesis testing in intraspecific phylogeography: nested clade phylogeographical analysis vs. approximate Bayesian computation. Mol Ecol 18:319–331
Tomàs C, Jiménez G, Picornell A et al (2006) Differential maternal and paternal contributions to the genetic pool of Ibiza Island, Balearic Archipelago. Am J Phys Anthropol 129:268–278
Tsutsui ND, Suarez AV, Holway DA et al (2000) Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA 97:5948–5953
Van Oosterhout C, Hutchinson WF, Wills DPM et al (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Verhoeven KJF, Macel M, Wolfe LM et al (2011) Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc R Soc B Biol Sci 278:2–8
Vigne J-D, Zazzo A, Saliège J-F et al (2009) Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. Proc Natl Acad Sci USA 106:16135–16138
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Acknowledgments
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10530_2015_846_MOESM1_ESM.xls
Detailed list of the samples used in this study, together with their genotypes and mitochondrial sequences. Numbering of mitochondrial sequences (‘Hapx’) refer to haplotype combinations in Gaubert et al. (2011) (XLS 100 kb)
10530_2015_846_MOESM2_ESM.docx
Prior and posterior distributions of model parameters estimated for scenario 3 and two different population groupings (sets 1 and 2) from simulations using DIYABC. Historical parameters have been constrained as follows: t4>tb; N2>N2b; N4>N4b; t4>dk; t3>dj.(DOCX 16 kb)
10530_2015_846_MOESM3_ESM.docx
Principal component analysis of summary statistics of 600,000 simulated data sets generated with six demographic scenarios and two different population groupings (sets 1 and 2). Observed dataset (large yellow dot) is projected on the plane formed by the first two principal components. Above: set1; below: set2. (PDF 139 kb)
10530_2015_846_MOESM4_ESM.pdf
Geneland clustering analysis among populations of the common genet in the Mediterranean Basin (K=4). Clusters are the same as delineated by STRUCTURE. Circle in clusters 1 and 2 graphical representations show admixed populations detected in northwestern Iberia. (PDF 140 kb)
10530_2015_846_MOESM5_ESM.docx
Genetic differentiation (F ST ) among the geographic populations of the common genet. See Table 1 for population acronyms. (DOCX 16 kb)
10530_2015_846_MOESM6_ESM.docx
Graphical representation of the relative posterior probabilities of the six scenarios of introduction of the common genet in Europe calculated through a polychotomous logistic regression from the 0.1% of simulated data sets most closely resembling the observed data (direct method). Left: set1; right: set2. (DOCX 44 kb)
10530_2015_846_MOESM7_ESM.docx
Principal component analysis of summary statistics from 500 pseudo-observed datasets generated from the posterior predictive distribution of scenario 3 (large blue dots), plotted along with summary statistics of simulations generated from priors of scenario 3 (small blue dots). The observed dataset (large yellow dot) is projected on the plane formed by the first two principal components. Above: set1; below: set2. (DOCX 130 kb)
Rights and permissions
About this article
Cite this article
Gaubert, P., Del Cerro, I., Centeno-Cuadros, A. et al. Tracing historical introductions in the Mediterranean Basin: the success story of the common genet (Genetta genetta) in Europe. Biol Invasions 17, 1897–1913 (2015). https://doi.org/10.1007/s10530-015-0846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0846-y