Abstract
Human activity can dramatically affect biodiversity, often by introducing non-native species, or by increasing the abundance of a small number of native species. Management strategies aimed at conserving biodiversity need to be informed by the actual impacts of highly abundant species, whether native or introduced. In this study we examined characteristics of two bird species, introduced common mynas and native noisy miners, both of which are highly abundant in urbanised areas along the East coast of Australia. Current managerial practices have a strong focus on eradication of common mynas, while noisy miners are largely ignored. However, in this study noisy miners were found in a broader range of habitats, and in greater abundance, than common mynas; displayed more aggressive behaviour; and were linked to a decline in the diversity and abundance of other species where common mynas were not. We suggest that the adaptability of a species and the variety of habitats it can colonise may be a better predictor of its potential impact, than whether it is native or introduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanised environments are associated with low species diversity (e.g. Clergeau et al. 2001; Jokimäki et al. 1996; McKinney 2002; Sol et al. 2011) and tend to be dominated by large numbers of a few species that are able to take advantage of the unique conditions experienced in an urban landscape (Bezzel 1985; Kark et al. 2007). These dominant species, termed “urban exploiters” (Blair 1996), typically have generalist diets and habits (Evans et al. 2011) and high sociality (Kark et al. 2007). Urban exploiter birds are often introduced (e.g. McKinney 2002; Orchan et al. 2013), but can also be native species existing in greater abundance than historically recorded (Blair 1996; Emlen 1974; Huhtalo and Jarvinen 1977; Jokimäki et al. 1996; Kark et al. 2007). Management bodies have previously focussed heavily on control of introduced species found in such environments, often attempting to reduce numbers or eradicate the species from entire areas (Gurevitch and Padilla 2004; Orchan et al. 2013; Schlaepfer et al. 2011a; Stromberg et al. 2009). The same is clearly not true for native species, which are generally assumed to have positive effects on the environment (Brown and Sax 2004; Davis et al. 2011). Recent scientific opinion (e.g. Carroll 2011; Davis 2011; Davis et al. 2011; Schlaepfer et al. 2011a, b), however, suggests managerial bodies shift from the present focus on whether a species is native or not, and towards objective assessment of each species’ environmental impact in its particular ecosystem. Objective assessments of relative species impacts are often obtained through investigation into several primary predictors, such as range, abundance, and per-capita or per-biomass effect on parameters such as community structure and population dynamics (Parker et al. 1999).
This paper focuses on the potential impact of two common but unrelated bird species, noisy miners Manorina melanocephala (Meliphagidae) and common mynas Sturnus tristis (Sturnidae). Noisy miners are native, communally-breeding honeyeaters found throughout the southern and eastern parts of Australia (Simpson and Day 2004). Common mynas, which belong to the Sturnid family, were introduced to the eastern coast of Australia in large numbers between 1860 and 1972 (Long 1981), and have become common in cities in this range. Common mynas are obligate hollow-nesting birds, and thrive in regions cleared of natural vegetation. They are heavily associated with human activity, taking advantage of food provided in the form of scraps and rubbish (Crisp and Lill 2006), using gutters and rooves of buildings for nesting (Bomford and Sinclair 2002; Counsilman 1974), and even furbishing their nests with human litter (Counsilman 1974; Sengupta 1968, 1982). Similarly, noisy miners, as edge specialists, have benefited greatly from the clearing of vegetation and segregation of remnant patches of woodland within most of their range in Eastern Australia (Catterall et al. 1991; Clarke and Schedvin 1997a; Low 1994; Loyn 1987), as such habitat appears to be optimal for territorial defence (Taylor et al. 2008); however they require natural vegetation for building cup nests (Dow 1978).
Although common mynas are introduced and noisy miners are native, both species have been accused of creating environmental problems and anthropogenic disturbances. Noisy miners are particularly known for their antagonistic, mobbing behaviour and extreme territorial aggression (Dow 1977, 1979; Grey et al. 1997). Presumably due to these behaviours, noisy miner numbers negatively correlate with the diversity of bird species, particularly small insectivorous birds in suburban gardens (et al. Grey 1997, 1998; Parsons et al. 2006) and woodlands (Major et al. 2001), and their aggressive exclusion of other birds has recently been nominated for inclusion in the EPBC Act list of Key Threatening Processes (DSEWPC 2011). They have even been referred to as a ‘reverse keystone’ species, as it appears that low abundance or total absence of the species is necessary for high diversity to be sustainable in some areas (Piper and Catterall 2003). They have recently been found to be highly aggressive in competition over artificially provided food sources (Haythorpe et al. 2012; Sol et al. 2011). In addition, they have increased dramatically in abundance over the last few decades (Barrett et al. 2002; Barrett et al. 2007), creating ever greater potential for negative impact on native wildlife.
Common mynas are also thought to pose a threat to native wildlife, and are typically disliked by members of the public (e.g. Nee et al. 1990; Tidemann 2003). They are now one of only three avian species to be listed by the IUCN as among the ‘World’s 100 worst invasive species’ (Lowe et al. 2000), and a poll by the Australian Broadcasting Company found that Australians considered them to be the “most significant pest/problem” (http://www.abc.net.au/tv/wildwatch/results/award.htm). In their native range in India they are known to compete with other hole-nesting species such as ring doves Streptopelia decaocto (Dhanda and Dhindsa 1993) and rose-ringed parakeets Psittacula krameri (Dhanda and Dhindsa 1996), as well as in parts of their introduced range such as Israel (Orchan et al. 2013). On some islands common mynas are thought to be contributing to decreases in abundance of threatened species, such as magpie robins Copsychus spp. (Huong and Sodhi 1997; Watson et al. 1992) found on the Seychelles Islands and echo parakeets Psittacula eques (Jones 1996) found on Indian Ocean islands near Madagascar. Artificial removal of common mynas on a New Zealand island was associated with an increase in some native bird species, suggesting they may have played a role in their historical decline (Tindall et al. 2007). However, it does not follow that common mynas would pose the same threat in an ecosystem less fragile than that of a smaller island, and until recently, actual quantitative evidence on their ecological impact, particularly in Australia, has been inconclusive. A long-term study on the impact of common mynas on native species at the population level (Grarock et al. 2012) has recently provided strong evidence to suggest that abundance of some cavity-nesting and small bird species may be threatened by common myna establishment. However, this study also found that the numbers of some species previously thought to be threatened by common mynas, such as Eastern rosellas and common starlings (Pell and Tidemann 1997b), may not be. Given the species thought to be at risk from common mynas are still highly abundant (and three are actually introduced and potential pests themselves), the seriousness of this risk is still to be determined. Others have speculated that the overlap of their range with that of some currently abundant species may cause these species to become threatened in the future (Tracey and Saunders 2003). For instance, Pell and Tidemann (1997b) document a possible threat to native crimson rosellas Platycercus elegans and Eastern rosellas P. eximius through competition for suitable nesting hollows. It should be recognised, however, that while common mynas occupy principally urban environments, Eastern rosellas occupy both urban and woodland habitats, and crimson rosellas in fact prefer to colonise dense forests, including rainforest. Thus far there is no indication that the numbers of crimson or Eastern rosellas is declining in response to common mynas, and in fact both rosella species appear to be thriving (Tidemann 2010; Veerman 2002).
Although both noisy miners and common mynas are considered pests by the general public, considerable attention is given to controlling common mynas in Australia, which are the target of ongoing eradication efforts. Australian governments and local councils have invested funding into developing specialist traps designed to exterminate large numbers of common mynas. Organisations such as the Hawkesbury Indian Myna Action Group (HIMAG),Footnote 1 the Canberra Indian Myna Action Group (CIMAG) Inc,Footnote 2 the Indian Myna Bird Project (Mid North Coast),Footnote 3 and the Yarra Indian Myna Action Group (YIMAG) Inc,Footnote 4 to name just a few, provide information on trapping and humane euthanasia of common mynas and distribute traps to the public. Noisy miners, on the other hand, are a protected species in each state in which they occur in Australia—for example, in New South Wales under the National Parks and Wildlife Act 1974 (Schedule 11), it is illegal to kill, trap or harm them (Section 98). Despite these highly different management strategies, the actual impact of each of these birds is not clear.
There is evidence that both noisy miners and common mynas may potentially be impacting negatively on biodiversity. It is difficult, however, to make judgements about the relative severity of each species’ impacts when such judgements need to be based on comparisons between studies, which may employ different methodology and measures and be conducted at different sites. With this consideration in mind, the present study directly compares the three primary indicators of impact—range, abundance, and per-capita effect, in this case focussing on effects on population (abundance) and community (diversity; Parker et al. 1999)—between native noisy miners and introduced common mynas across suburb, edge and bush habitats, and across breeding and non-breeding seasons. We predict that the greatest threat to biodiversity would come from a species that (1) was present in a variety of habitat types; (2) was present in great numbers or was the most abundant species in the area; (3) was linked to a decrease in diversity and/or abundance of other species; and (4) would be frequently observed engaging in aggressive behaviours.
Methods
Transect locations and descriptions
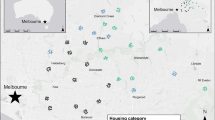
Forty-five transects were mapped within and around the city of Newcastle, Australia, located approximately 160 km north of Sydney. There were 15 ‘suburban’ transects, situated at least 50 m from the nearest bushland; 15 ‘bush’ transects that were at least 50 m into bushland; and 15 ‘edge’ transects placed in regions where bushland of at least 260 hectares met with suburbs (Fig. 1). Bushland was found in urban nature reserves, including Awabakal Nature Reserve, Blackbutt Reserve, Glenrock State Conservation Area, Richley Recreation Reserve, Sygna Close Reserve, and Tingira Heights Nature Reserve, and consisted of dense native remnant vegetation, including tall trees and undergrowth. Edge transects ran along the border of these two habitats, extending into the suburbs on one side and into bushland on the other. Selection of transect locations was random in suburban sites, but limited in edge sites to those that were accessible (e.g. not part of a residential property), and in bush sites limited to reserves that allowed public access.
Each transect was 200 m long and contained four observation stops, at 25, 75, 125, and 175 m along the transect, respectively. The observation area for each stop was a circle, centred on the transect, with a radius of 25 m.
Observation procedure
Surveys of all 45 transects were conducted every 2 months for a year, resulting in three surveys (in April, June and August, respectively) being conducted during the non-breeding season and three surveys (in October, December and February, respectively) during the breeding season of most birds. These are referred to throughout this paper as the observations.
Each survey of a transect totalled 20 min of observation, with a sampling time of 5 min at each of the four stops. Sampling time was based on previous work by Blair (1996, 2004), and initial pilot studies in the study areas determined that practically all observations (>95 %) were recorded within the first 2 min of sampling, a trend that continued throughout the rest of data collection; thus 5 min was considered to be sufficiently long to pick up the majority of relevant behavioural observations. From the centre of the stop, the observer recorded the numbers and species of all birds seen and heard within the observation area, excluding those that were only flying overhead and not spending any other time at the stop. For each survey, the observer also recorded whether or not each bird species initiated an aggressive act on another bird. An aggressive act was defined as a swoop, peck at, physical fight with, or chase of another bird, regardless of whether this resulted in the other bird leaving the area. To avoid bias in number of aggressive interactions due to increased number of any one species, multiple individuals engaging in an aggressive encounter were only counted once, and noisy miners and common mynas were recorded in a yes/no fashion as either engaging in aggressive behaviour or not engaging in aggressive behaviour for each stop, thus multiple attacks in one visit were given the same weight as only one attack.
Overall abundance and species diversity
Species diversity was defined as the total number of species observed and overall abundance was defined as the total number of individuals observed. Both of these measures were calculated separately for each transect (by combining observations over the four stops), during each of the six two-monthly surveys. The scores from the three breeding-season and three non-breeding-season surveys, respectively, were then averaged so that each transect was left with one species diversity score and one overall abundance score for each season.
Transect diversity was defined as the total number of species observed at that transect, tallied over the six surveys of the study. Note that transect diversity is not simply a sum of the six species diversity scores recorded during the six surveys, as a single species observed during four of the surveys, for example, counted as just one species for the purposes of the transect diversity measure, not as four species. Transect abundance was defined as the total number of individuals present at that transect, tallied over all six surveys. This measure is simply a sum of the six overall abundance scores recorded for each transect.
To explore the effect of habitat type on overall abundance and species diversity, we conducted repeated-measures ANOVAs, using each of the 45 transects as the basic replicates of the analyses. Habitat type was entered as a between-transect factor (3 levels: suburb, edge and bush), and season as the within–transect factor (2 levels: breeding and non-breeding).
Core species
For analyses involving all subsequent dependent variables we included only species that were present in at least 5 of the 15 transects for each habitat type. This elimination process resulted in 15 core species for the suburb (including both noisy miners and common mynas), 19 core species for the edge (which included noisy miners but not common mynas), and 20 core species for the bush (also including noisy miners, and not common mynas).
Absolute abundance and relative abundance scores
Absolute abundance (the total number of sightings of a given species) was recorded at every transect for each core species of each habitat type. Absolute abundance scores for each core species during the breeding and non-breeding seasons were calculated for each transect by summing absolute abundance totals observed at that transect during the October, December and February surveys and the April June and August surveys, respectively. An absolute abundance score that combined data from the breeding and non-breeding seasons was also calculated for noisy miners and common mynas only.
Relative abundance scores for each core species at each transect (for the breeding and non-breeding seasons separately, as well as both seasons combined for noisy miners and common mynas as described above) were also calculated using the formula:
where RAx is the relative abundance of species x, AAx is the absolute abundance of species x, AA∑ is the sum of all core species’ absolute abundance scores at that transect, and n is the number of core species observed at that transect. The resulting score was negative if the number of a species was lower than the mean for that transect and positive if the number of a species was higher than the mean for that transect. Species with an absolute abundance score of zero for a given transect (indicating the species was never observed at that transect) were still allocated a relative abundance score for that transect as per the above formula.
Results
Effects of habitat type
Overall abundance did not differ significantly between the habitat types (F(2,42) = 0.576, p = 0.567), or between the breeding and non-breeding seasons (F(1,42) = 1.069, p = 0.307), nor was it affected by an interaction between these variables (F(2,42) = 1.229, p = 0.303; Fig. 2a). Species diversity was significantly different across habitat type (F(2,42) = 3.688, p = 0.033). Post hoc contrasts showed both bush (p = 0.04953) and edge (p = 0.013) habitats had greater diversity than suburban habitats, but bush habitats did not differ from edge habitats (p = 0.579). There was also no main effect of season on species diversity (F(1,42) = 1.409, p = 0.242) and no interaction between season and habitat type (F(2,42) = 0.312, p = 0.734; Fig. 2b).
The overall abundance and species diversity across the different habitat types. Overall abundance was not significantly affected by either habitat or season (a), while species diversity was significantly lower in the suburb than in either the edge or the bush, although this effect was not seasonal (b). Different uppercase letters (A, B) indicate significant differences between variables
Noisy miners were the most abundant species recorded in suburb (333 sightings) and edge (374 sightings) habitats. They were also one of the most abundant species sighted in the bush (157 sightings), outnumbered only by small bush specialist wren species, white-browed scrubwrens Sericornis frontalis (193 sightings) and superb fairy-wrens Malurus cyaneus (233 sightings). Common mynas by contrast were not present in bush habitats, and were present in only three of 15 edge habitat transects, coming to a total of only six sightings. They were, however the second most abundant species (139 sightings) in suburban habitats after noisy miners.
Repeated-measures ANOVAs were used to investigate the effect of habitat type on absolute abundance and relative abundance of noisy miners. Each transect was the basic replicate of the analysis, and habitat type was entered as a between-transect factor (3 levels: suburb, edge and bush) and season (2 levels: breeding and non-breeding) as a within-transect measure.
Noisy miner absolute abundance was not affected by season (F(1,42) = 1.729, p = 0.196) but differed significantly between the habitat types (F(2,42) = 3.229, p = 0.0496). Post hoc simple contrasts showed a significant drop in numbers occurred in bush habitats compared with edge habitats (p = 0.021), but did not occur between suburb and bush (p = 0.059), or between edge and suburb (p = 0.654; Fig. 3a). The relative abundance of noisy miners was not affected by season (F(1,42) = 1.366, p = 0.249) nor by an interaction between season and habitat type (F(1,42) = 0.590, p = 0.559), but the main effect of habitat type did approach significance (F(2,42) = 3.070, p = 0.057). Post hoc simple contrasts confirmed that noisy miner relative abundance was significantly higher in the edge compared to the bush (p = 0.021) habitats and also tended to be higher in the suburb compared to the bush (p = 0.091), but did not differ between the suburb and the edge (p = 0.507; Fig. 3b).
Since 96 % of common myna sightings occurred in suburban transects, formal analyses comparing common myna abundance across the three habitat types were not conducted, however, paired-samples t-tests were used to compare absolute and relative abundance of common mynas between the breeding and non-breeding seasons. Neither relative abundance (t(14) = 1.006, p = 0.331), nor absolute abundance (t(14) = 1.005, p = 0.332) of common mynas differed between the breeding and non-breeding seasons in the suburban habitat.
Transect diversity
Given the nil effect of breeding season in the previous analyses, the species diversity scores from each of the six surveys of the study were combined to produce a transect diversity score, reflecting the total number of species observed at a given transect throughout the study period. Pearson’s bivariate correlations were then used to correlate these transect diversity scores with common myna relative abundance and absolute abundance across the 15 suburban transects. The same analyses were conducted with noisy miner relative abundance and absolute abundance across all transects at each of the three habitat types.
With respect to common mynas, neither absolute abundance (r = 0.036, n = 15, p = 0.898) nor relative abundance (r = 0.007, n = 15, p = 0.979) was significantly correlated with transect diversity in suburban habitat. The same was true for noisy miner absolute abundance (r = −0.034, n = 15, p = 0.904) and relative abundance (r = −0.066, n = 15, p = 0.816) in the suburban habitat and also for absolute abundance (r = −0.087, n = 15, p = 0.757) and relative abundance (r = −0.097, p = 0.731) in the bush habitat. There were, however, significant negative correlations between noisy miner absolute abundance (r = −0.636, n = 15, p = 0.011) and relative abundance (r = −0.680, n = 15, p = 0.005), respectively, and transect diversity in the edge habitat, confirming that as noisy miner numbers increased, and they became a larger proportion of the bird life, the number of other species observed dropped.
Transect abundance
As described above for the species diversity scores, the overall abundance scores were combined across the six surveys resulting in each transect having a transect abundance score (which was the total number of individual birds observed at that transect over the entire study period). For the purposes of correlating the transect abundance scores with noisy miner and common myna abundance scores, as described below, the numbers of noisy miners and common mynas, respectively, were subtracted from the transect abundance scores, such that each correlation examined the association between abundance of a focal species (either noisy miners or common mynas) and abundance of all other core bird species.
Pearson’s bivariate correlations were used to correlate transect abundance scores with noisy miner absolute abundance scores in suburb, edge and bush habitats, and with common myna absolute abundance scores in the suburb only. There was no correlation between noisy miner absolute abundance and transect abundance in suburb (r = 0.174, n = 15, p = 0.534), edge (r = −0.275, n = 15, p = 0.322) or bush (r = −0.068, n = 15, p = 0.809) and also no correlation between common myna absolute abundance and transect abundance in the suburb (r = 0.413, n = 15, p = 0.126). The same analyses were conducted using relative (rather than absolute) abundance scores of noisy miners and common mynas and these also failed to reveal any significant correlation for common mynas in the suburb (r = 0.267, n = 15, p = 0.335) or for noisy miners in the suburb (r = 0.089, n = 15, p = 0.752), edge (r = −0.358, n = 15, p = 0.191) or bush (r = −0.169, n = 15, p = 0.547).
Core species’ relative abundances
To examine the association between the relative abundance of noisy miners and common mynas, respectively, and the relative abundance of other species with which they shared a habitat, we conducted a series of Pearson’s bivariate correlations between each of the core species’ relative abundance scores, and noisy miner relative abundance scores in each of the three habitat types and common myna relative abundance scores in suburban habitats (see Tables 1, 2).
The resulting sets of r-values were converted to z′-values (to achieve normality) using Fisher’s formula:
To determine whether the relative abundance of either noisy miners or common mynas exhibited a stronger association with the relative abundance of the other core species across the 15 suburban habitat transects (and whether the strength of the associations were affected by season), we conducted a repeated-measures ANOVA on the resulting suburban habitat z′ scores with season (2 levels: breeding and non-breeding) and species (2 levels: noisy miner and common myna) both entered as repeated-measures). The z′ value derived from the direct correlation between noisy miner and common myna relative abundance was excluded.
Noisy miner z′ scores were significantly more negative than common myna z′ scores (F(1,12) = 7.845, p = 0.016) and breeding season z’ scores were significantly more negative than non-breeding season z’ scores (F(1,12) = 14.013, p = 0.003). Both these main effects were qualified by a significant species by season interaction (F(1,12) = 17.879, p = 0.001) with post hoc paired comparisons confirming that the noisy miner breeding season z’ average was significantly more negative than the noisy miner non-breeding season z’ average (t(12) = 6.488, p < 0.001) and the common myna breeding (t(12) = 5.075, p < 0.001) and non-breeding (t(12) = 4.690, p = 0.001) averages. No other post hoc comparisons were significant (all p > 0.6). One-sample t-tests also confirmed that only the noisy miner breeding season z’ average was significantly less than zero (t(12) = 8.893, p < 0.001), with no difference found for noisy miner non-breeding (t(12) = 1.714, p = 0.112) common myna breeding (t(12) = 1.856, p = 0.088) or non-breeding (t(12) = 1.032, p = 0.322). Overall, the results demonstrate that, during the breeding season only, higher numbers of noisy miners were significantly associated with lower numbers of other species of birds, with no such association demonstrated for common mynas (Fig. 4a).
The z′-values for noisy miners and common mynas in suburban habitats only (a), and the z′-values for noisy miners only across all habitat types (b). Noisy miner z′-values were significantly lower during the breeding season than during the non-breeding season, and during the breeding season were significantly lower than common myna z′-values in either season (a). Noisy miner z′-values were also significantly lower in both suburb and edge habitats during the breeding season than during the non-breeding season, and lower in both edge and suburban habitats during the breeding season than bush habitats in either season. Different uppercase letters (A, B) indicate significant differences between variables
To further investigate the association between noisy miner abundance and the abundance of other core species, we conducted another repeated-measures ANOVA on the z′ scores calculated for associations between noisy miners and all other core species in the suburb, edge and bush habitats. Season (2 levels: breeding and non-breeding) was entered as a repeated-measure and habitat (3 levels: suburb, edge and bush) was entered as a fixed factor. No significant main effect of habitat was observed (F(2,48)=2.270, p = 0.114) but there was a significant main effect of season (F(1,48) = 36.709, p < 0.001) and a significant season by habitat interaction (F(2,48) = 13.856, p < 0.001). Post hoc paired comparisons confirmed that the negative association between abundance of noisy miners and other core species was significantly stronger in the breeding season for the suburb (t(13) = 4.414, p = 0.001) and edge (t(17) = 5.341, p < 0.001) habitats only, with no difference between seasons in the strength of this association found in the bush (t(18) = 0.821, p = 0.422). One-sample t-tests confirmed that the z′ average was significantly less than zero (signifying a significantly negative average association between the abundance of noisy miners and that of other core species) across all three habitats in the breeding season (suburb: t(13) = 7.067, p < 0.001; edge: t(17) = 6.906, p < 0.001; bush: t(18) = 6.722, p < 0.001) and in the edge (t(17) = 3.536, p = 0.003) and bush (t(18) = 5.042, p < 0.001), though not the suburb (t(13) = 2.056, p = 0.060) in the non-breeding season (Fig. 4b).
Aggression
All sightings of common mynas and noisy miners in suburban habitats and the respective number of aggressive acts conducted by each species in this habitat were tallied. Common mynas were recorded conducting an aggressive act on two occasions out of 139 sightings (1.4 %), while noisy miners conducted aggressive acts on 53 out of 333 (15.9 %) sightings in suburban habitats. A Fisher’s 2 × 2 Exact Test (with α = 0.05) demonstrated a significant association between species and incidence of aggression (p < 0.001) with noisy miners significantly more likely to display aggression than common mynas within the suburban habitats.
As noisy miners occurred across all habitats, all sightings in each of the three habitat types and respective numbers of aggressive acts conducted by noisy miners in these habitats were also tallied. In addition to the 53 aggressive acts (of 333 sightings, 15.9 %) in suburban habitats, 57 aggressive acts out of 374 (15.2 %) sightings in edge habitats, and 14 aggressive acts out of 157 sightings in bush habitats (8.9 %) were recorded. A Pearson’s χ2 test of contingencies (with α = 0.05) was used to determine whether habitat type was associated with the number of aggressive acts conducted by noisy miners. There was no association between frequency of noisy miner aggression and habitat type (\(\chi_{( 2)}^{2} = 4. 7\), p = 0.097).
Discussion
This study examines the potential threat posed by common mynas, an introduced Sturnid, and noisy miners, a native honeyeater, by looking at their range and abundance, and investigating their potential and actual per-capita effect through examination of behaviour and relationship to the diversity and abundance of other species (Parker et al. 1999), across habitat types and seasons. Although mathematically quantifying these parameters as indicators of a species’ total impact is difficult (e.g. Thiele et al. 2010; Thomsen et al. 2011), a broad examination could be expected to reveal important trends. Direct comparison of these two species within the one study area allows us to make judgements about the relative severity of the impacts of each species without the confounds introduced by trying to compare across sites and across studies.
In this study overall abundance did not differ by habitat type, however species diversity was found to be higher in bush and edge habitats than in suburban habitats. These results were not affected by season. Greater levels of diversity in remnant or bushland areas compared to urbanised areas have been found by numerous other studies (e.g. see Blair 1996; Chace and Walsh 2006; McKinney 2008 for reviews).
Noisy miners were found in all three habitat types in high numbers across seasons. They were the most abundant species in both suburb and edge habitats, and third most abundant in bush habitats. These findings are not surprising, as an affinity for edge areas and human modified habitats has been shown by numerous other studies (e.g. Catterall et al. 1991; Grey et al. 1997; Hastings and Beattie 2006; Major et al. 2001) and their ability to penetrate even dense bushland is well known (Clarke and Oldland 2007; Eyre et al. 2009; Howes and Maron 2009). In parts of southern Queensland they are even regularly recorded penetrating more than 20 km into bushland (Maron 2009).
Common mynas by contrast were found primarily in suburban habitats, where they were the second most abundant species after noisy miners, and only rarely in edge habitats, where they probably occupied the more suburban side of the transect. This finding is not surprising, as common mynas are well known as urban habitat specialists that rely heavily on human activity (e.g. Counsilman 1974; Crisp and Lill 2006; Pell and Tidemann 1997a; Sengupta 1968). Contrary to predictions made by Pell and Tidemann (1997b), common mynas in this study did not appear to be capable of penetrating bushland. It should be noted that the bushland surrounding the Newcastle area in which this study was conducted differs dramatically from the sparse, open woodland found around the Canberra region where studies by Pell and Tidemann (1997b) and Grarock et al. (2012) took place, and may explain the difference in these findings.
A negative correlation between noisy miner abundance and species abundance and diversity has been shown by numerous other studies across Australia, including the coastal city of Sydney (Parsons et al. 2006), the inland wheat belt of New South Wales (Major et al. 2001), the foothills of the Great Dividing Range in Victoria (Grey et al. 1997), and a multitude of sites ranging from Victoria to Queensland (Mac Nally et al. 2012). Noisy miner abundance in this study was negatively associated with species diversity in edge habitats. During the breeding season in both the suburb and edge habitats, an increase in noisy miner relative abundance was associated with a decrease in abundance of other species. This effect was not observed during the non-breeding season. Noisy miners are well known for aggressive nest defence behaviour via group mobbing (Arnold 2000; Maron 2009), and it may be that the prominence of this effect during the breeding season is due to differences in territorial behaviour in the presence of nestlings or fledglings. As noisy miners were less abundant in bush habitats than edge habitats, and associated with decreased species diversity in edge habitats, it may be that noisy miners are primarily using edge habitat for breeding, and aggressively excluding other species most prominently in this location. We did not observe a difference in aggression levels across habitats, however, which is inconsistent with this interpretation. We examined aggression only at a fairly coarse level, though, and more detailed recording of the specifics of aggressive acts, such as their nature and efficacy across various habitats, might be informative.
We found no evidence that common myna abundance was associated with diversity or abundance of other species in the suburban habitats where they were found, but no comparable analysis could be conducted in bush or edge habitats as presence of common mynas in these was extremely low. In a study of suburban gardens in Sydney, Parsons et al. (2006) also found common mynas were not associated with a decrease in species diversity. However, this finding is contradicted by more recent findings from Canberra (Grarock et al. 2012). Several possibilities exist here to explain this difference in results. First, it may be that the habitats of Newcastle and Sydney are less conducive to effective competition or exclusion by common mynas than that of Canberra due to different environmental factors. For example, Newcastle and Sydney are both coastal cities that receive high levels of rainfall, which supports substantially more vegetative growth than the inland city of Canberra. It may be that this increase in vegetation gives native birds in Newcastle and Sydney a competitive edge that birds in Canberra do not have. Canberra also frequently experiences lower temperatures during winter than either of the coastal cities, and common mynas may be better able to withstand this than some native birds, again providing a competitive edge. Aside from potential differences in environmental factors, the different methodologies of the studies need to be considered. Grarock et al.’s (2012) findings consider an extensive dataset, spanning 29 years and including over 74,000 surveys, while our study and that of Parsons et al. (2006) are much smaller and span 1 year or less. A finding of impact by common mynas over a lengthy period of time and using a large amount of data, but not during shorter-term studies, indicate that the impact of this species may be gradual and subtle. On the other hand, the impact shown by noisy miners is readily picked up in studies spanning even a few months, suggesting it may be far more acute. These impacts are likely to become even more apparent as the range and abundance of noisy miners continues to increase (Barrett et al. 2007).
The high levels of aggressive behaviour in noisy miners found by this study have been documented elsewhere (e.g. Clarke 1984; Dow 1977, 1979; Loyn 1987). Other closely related miner species, such as bell miners Manorina melanophrys are known to exhibit similar behaviour, and are even capable of completely altering habitat characteristics through aggressive exclusion of all other insectivorous birds, resulting in an increase in abundance of sap-sucking plant parasites such as psyllid insects (Homoptera: Psyllidae) and a corresponding decrease in tree health (Clarke 1984; Clarke and Schedvin 1997b; Dare et al. 2007). Common mynas on the other hand have a long, but mostly anecdotal history of displaying aggression, which was not reflected in the results of this study. Recent studies on common myna feeding behaviour suggests that reports of aggression may have been exaggerated (Haythorpe et al. 2012; Lowe et al. 2011), and it has also been suggested (Haythorpe et al. 2012) that their reputation may be in part due to similarities in the names and appearances of common mynas and noisy miners, leading to identification issues surrounding observations of aggressive interactions by the general public. Previous anecdotal reports of common myna aggression primarily relate to nesting behaviour, including defence of nestlings and competition for nesting resources. While this specific behaviour is clearly of importance in the consideration of impact by common mynas, any bias in this study towards aggressive behaviour due to the proximity of nest sites in relationship to transect locations should actually favour more aggressive behaviour in common mynas than noisy miners, as common mynas are prone to nesting in modified habitats (Counsilman 1974) while noisy miners prefer to nest in natural vegetation, and aggressive behaviour was compared across suburban locations only. However, even with this potential bias noisy miners were significantly more aggressive than common mynas.
To determine the impact of any species we must consider in which range or areas they could pose a threat, which species they have the potential to affect, the possible strength or magnitude of this effect, and the method by which it may come about. The inability of common mynas to penetrate dense bushland calls into question their predicted threat to native wildlife and diversity, at least in the Newcastle area. Although the importance of urban regions to biodiversity is clearly higher than historically thought (Goddard et al. 2010), any potential threat common mynas pose appears to be to the species in this region only—typically either other suburban habitat specialists, or habitat generalists.
Noisy miners, in contrast to common mynas, were found to be highly abundant in all habitat types. Threatening behaviour from noisy miners is thus capable of affecting both habitat specialists and habitat generalists. In addition, it is clear that noisy miners do not just exist in these habitats, but thrive in them, as they were the most abundant species recorded in this study. A typical characteristic of species considered to be ‘invasive’—in this case, capable of having a widespread impact on a habitat—is the tendency to be present in comparatively high numbers (Colautti and MacIsaac 2004; Kolar and Lodge 2001; Richardson et al. 2000). In such cases, even relatively benign behaviours or processes may pose serious threats when amplified due to a species’ overabundance. Although common mynas may appear to be highly abundant, they were found in substantially lower numbers than noisy miners. Their localised nature has perhaps led to overestimation of numbers, while high abundance of noisy miners in less frequented bushland areas goes relatively unobserved.
There is substantial evidence to suggest that noisy miner overabundance is correlated with a decrease in the diversity and abundance of other bird species (e.g. Grey et al. 1997, 1998; Mac Nally et al. 2012; Major et al. 2001; Maron et al. 2011; Parsons et al. 2006). In addition the findings of this study suggest that this effect fluctuates under various conditions, such as habitat type and breeding status. While it is possible that this effect could be coincidental, as bird species are affected by the same habitat modifications that favour noisy miners (rather than directly being affected by the noisy miners themselves), a substantial number of experimental studies have addressed this question, and generally conclude that a causal relationship does exist (Debus 2008; Grey et al. 1997, 1998; Kath et al. 2009; Mac Nally et al. 2012; Maron et al. 2011; Piper and Catterall 2003). The most likely method for noisy miners to negatively affect the abundance of small bird species is through aggressive exclusion (Dow 1977, 1979). Aggressive behaviour is likely to cause most impact in locations and during times when breeding is occurring, when noisy miners are aggressively defending nestlings through group mobbing behaviour (Arnold 2000). In support of this, we found noisy miners had an impact on the diversity and abundance of other species in primarily the edge habitat type during the breeding season, although aggressive behaviour was consistent across habitat types, suggesting that actual instances of aggression may not be the only part of a strategy designed to exclude other species.
Common mynas, by contrast, were not correlated with a decrease in diversity or abundance of other species in this study, and other studies (Parsons et al. 2006) have also been unable to find such a correlation. In addition, common mynas in this study were only infrequently observed engaging in aggressive behaviours, and a recent study (Lowe et al. 2011) has similarly found that common mynas rarely initiated interspecific aggressive attacks, and did not interfere with other foraging birds, a finding also supported by our previous research in this area (Haythorpe et al. 2012).
Conclusions
This study provides data on the range, abundance, and behaviours of noisy miners and common mynas and their associations with the abundance and diversity of other bird species in the Newcastle region. Noisy miners in this study were present in all three habitat types, while common mynas were only present in one habitat type (suburbs). While it remains possible that common mynas are negatively affecting some rare species, their restricted distribution suggests that this is unlikely; by contrast a range of rare species may be impacted by noisy miners. Noisy miners were highly abundant in all three habitat types, being the most abundant in suburb and edge habitats, and third most abundant in bush habitats. Common mynas were second most abundant in suburban habitats, the only habitat in which they were found. Noisy miners were associated with a decrease in diversity in edge habitats, and a decrease in abundance in edge and suburban habitats during the breeding season only, while common mynas were not associated with a decrease in diversity or abundance at any time. This effect may have been driven by aggressive exclusion—noisy miners were observed initiating aggressive attacks significantly more often than common mynas, and did so consistently across habitat types. We conclude that noisy miners may have the potential to have a greater impact on wildlife than common mynas in this area.
Perhaps because habitat alteration is so often accompanied by the spread of introduced species (Hobbs and Huenneke 1992; Vitousek et al. 1997), there is a tendency to focus attention on these species when assessing likely impacts on native assemblages. In reality, in today’s increasingly urbanised environment, virtually all species are to some extent introduced, as few exist now in the same environments in which they evolved competitively. For some species this has provided an advantage, allowing them to increase in number dramatically and leading to detrimental impacts on the environment. These are of even greater concern if that species is also capable of subsequently migrating into areas previously unaffected, as this will impact not just the urban environment, which is already highly degraded by other anthropogenic factors, but more diversity-rich remnant bushland areas as well, which are more likely to contain rare or threatened species. Species usually considered to be ‘introduced’ frequently cannot survive outside urban centres, and thus their ability to impact the regions of greatest conservation value is limited.
As the current study suggests, greater risks might be posed by native species with artificially increased numbers than by introduced species with ranges restricted to highly urbanised environments. It may be that in environments that have been dramatically altered by human activity the species with the greatest capacity to adapt to these alterations and colonise a range of different habitats are the most likely to negatively impact on other species. As in the current system, these species need not be introduced.
References
Arnold KE (2000) Group mobbing behaviour and nest defence in a cooperatively breeding Australian bird. Ethol 106:385–393
Barrett GW, Silcocks A, Cunningham R (2002) Australian bird atlas (1998–2001). Supplementary report no. 1—comparison of Atlas 1 (1977–1981) and Atlas 2 (1998–2001). Environment Australia Natural Heritage Trust Fund, Hawthorn East, Victoria
Barrett GW, Silcocks AF, Cunningham R, Oliver DL, Weston MA, Baker J (2007) Comparison of atlas data to determine the conservation status of bird species in New South Wales, with an emphasis on woodland-dependent species. Aust Zool 34:37–77
Bezzel E (1985) Birdlife in intensively used rural and urban environments. Ornis Fennica 62:90–95
Blair RB (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–519
Blair RB (2004) The effects of urban sprawl on birds at multiple levels of biological organization. Ecol Soc 9:2. Available at http://www.ecologyandsociety.org/vol9/iss5/. Accessed 19 Nov 2013
Bomford M, Sinclair R (2002) Australian research on bird pests: impact, management and future directions. Emu 102:29–45
Brown JH, Sax DF (2004) An essay on some topics concerning invasive species. Aust Ecol 29:530–536
Carroll SP (2011) Conciliation biology: the eco-evolutionary management of permanently invaded biotic systems. Evol Appl 4:184–199
Catterall CP, Green RJ, Jones DN (1991) Habitat use by birds across a forest-suburb interface in Brisbane: implications for corridors. In: Saunders DA, Hobbs RJ (eds) Nature conservation 2: the role of corridors. Surrey Beatty & Sons, Sydney, pp 247–258
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69
Clarke MF (1984) Interspecific aggression within the genus Manorina. Emu 84:113–115
Clarke M, Oldland J (2007) Penetration of remnant edges by noisy miners (Manorina melanocephala) and implications for habitat restoration. Wildl Res 34:253–261
Clarke MF, Schedvin N (1997a) An experimental study of the translocation of noisy miners Manorina melanocephala and difficulties associated with dispersal. Biol Conserv 80:161–167
Clarke MF, Schedvin N (1997b) Removal of bell miners Manorina melanophrys from Eucalyptus radiata forest and its effect on avian diversity, psyllids and tree health. Biol Conserv 88:111–120
Clergeau P, Jokimäki J, Savard JP (2001) Are urban bird communities influenced by the bird diversity of adjacent landscapes? J Appl Ecol 38:1122–1134
Colautti RI, MacIsaac HJ (2004) A neutral terminology to define ‘invasive’ species. Divers Distrib 10:135–141
Counsilman JJ (1974) Breeding biology of the Indian myna in city and aviary. Notornis 21:318–333
Crisp H, Lill A (2006) City slickers: habitat use and foraging in urban common mynas Acridotheres tristis. Corella 30:9–15
Dare AJ, McDonald PG, Clarke MF (2007) The ecological context and consequences of colonisation of a site by bell miners (Manorina melanophrys). Wildl Res 34:616–623
Davis MA (2011) Do native birds care whether their berries are native or exotic? No. Bioscience 61:501–502
Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, Brown JH, Rosenzweig ML, Gardener MR, Carroll SP, Thompson K, Pickett STA, Stromberg JC, Del Tredici P, Suding KN, Ehrenfeld JG, Grime JP, Mascaro J, Briggs JC (2011) Don’t judge species on their origins. Nature 474:153–154
Debus SJS (2008) The effect of noisy miners on small bush birds: an unofficial cull and its outcome. Pac Conserv Biol 14:185–189
Dhanda SK, Dhindsa MS (1993) Eviction of ring dove, Streptopelia decaocto, from a nest box by common myna, Acridotheres tristis. Pavo 31:35–38
Dhanda SK, Dhindsa MS (1996) Breeding performance of Indian myna Acridotheres tristis in nestboxes and natural sites. Ibis 138:788–791
Dow DD (1977) Indiscriminate interspecific aggression leading to almost sole occupancy of space by a single species of bird. Emu 77:115–121
Dow DD (1978) Breeding biology and development of the young of Manorina melanocephala, a communally breeding honeyeater. Emu 78:207–222
Dow DD (1979) Agonistic and spacing behaviour of the noisy miner Manorina melanocephala, a communally breeding honeyeater. Ibis 121:423–436
DSEWPC (2011) Finalised priority assessment list for the assessment period commencing 1 October 2011. DSEWPC, Canberra. Available at http://www.environment.gov.au/biodiversity/threatened/pubs/priority-assessment-list-2011.pdf. Accessed 31 Aug 2013
Emlen JT (1974) An urban bird community in Tuscon, AZ: derivation structure, regulation. Condor 76:184–197
Evans KL, Chamberlain DE, Hatchwell BJ, Gregory RD, Gaston KJ (2011) What makes an urban bird? Glob Chang Biol 17:32–44
Eyre TJ, Maron M, Mathieson MT, Haseler M (2009) Impacts of grazing, selective logging and hyper-aggressors on diurnal bird fauna in intact forest landscapes of the Brigalow Belt, Queensland. Aust Ecol 34:705–716
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98
Grarock K, Tidemann CRWJ, Lindenmayer D (2012) Is it benign or is it a pariah? Empirical evidence for the impact of the common myna (Acridotheres tristis) on Australian birds. PLoS ONE 7:e40622
Grey M, Clarke MF, Loyn RH (1997) Initial changes in the avian communities of remnant eucalyptus woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala. Wildl Res 24:631–648
Grey MJ, Clarke MF, Loyn RH (1998) Influence of the noisy miner Manorina melanocephala on avian diversity and abundance in remnant Grey Box woodland. Pac Conserv Biol 4:55–69
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474
Hastings RA, Beattie AJ (2006) Stop the bullying in the corridors: can including shrubs make your revegetation more noisy miner free? Ecol Manag Restor 7:105–112
Haythorpe KM, Sulikowski D, Burke D (2012) Relative levels of food aggression displayed by common mynas when foraging with other bird species in suburbia. Emu 112:129–136
Hobbs RF, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Howes AL, Maron M (2009) Interspecific competition and conservation management of continuous subtropical woodlands. Wildl Res 36:617–626
Huhtalo H, Jarvinen O (1977) Quantitative composition of the urban bird community in Tornio, Northern Finland. Bird Study 42:179–185
Huong SL, Sodhi NS (1997) The status of the oriental magpie robin, Copsychus saularis, in Singapore. Malay Nat J 50:347–354
Jokimäki J, Suhonen J, Inki K, Jokinen S (1996) Biogeographical comparison of winter bird assemblages in urban environments in Finland. J Biogeogr 23:379–386
Jones C (1996) Bird introductions to Mauritius: status and relationships with native birds. In: Holmes JS, Simons JR (eds) The introduction and naturalisation of birds. Her Majesty’s Stationery Office, London, pp 113–123
Kark S, Iwaniuk AN, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an ‘urban exploiter’? Biogeography 34:638–651
Kath J, Maron M, Dunn PK (2009) Interspecific competition and small bird diversity in an urbanizing landscape. Landsc Urban Plan 92:72–79
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Long J (1981) Introduced birds of the world. Reed, Sydney
Low T (1994) Invasion of the savage honeyeaters. Aust Nat Hist 24:26–33
Lowe S, Browne M, Boudjelas S, de Poorter M (2000) 100 of the world’s worst invasive alien species. A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG), a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), Auckland
Lowe KA, Taylor CE, Major RE (2011) Do common mynas significantly compete with native birds in urban environments? J Ornithol 152:909–921
Loyn RH (1987) Effects of patch area and habitat on bird abundances, species numbers and tree health in fragmented Victorian forests. In: Saunders DA, Arnold GW, Burbidge AA, Hopkins AJM (eds) Nature conservation: the role of remnants of native vegetation. Surrey Beatty and Sons, Chipping Norton, pp 65–77
Mac Nally R, Bowen M, Howes A, McAlpine C, Maron M (2012) Despotic, high-impact species and the subcontinental scale control of avian assemblage structure. Ecology 93:668–678
Major RE, Christie FJ, Gowing G (2001) Influence of remnant and landscape attributes on Australian woodland bird communities. Biol Conserv 102:47–66
Maron M (2009) Nesting, foraging and aggression of Noisy Miners relative to road edges in an extensive Queensland forest. Emu 109:75–81
Maron M, Main A, Bowen M, Howes A, Kath J, Pillette C, McAlpine C (2011) Relative influence of habitat modification and interspecific competition on woodland bird assemblages in eastern Australia. Emu 111:40–51
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McKinney ML (2008) Effect of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Nee K, Sigurdsson JB, Hails CJ, Counsilman JJ (1990) Some implications of resource removal in the control of Mynas (Acridotheres spp.) in Singapore. Malay Nat J 44:103–108
Orchan Y, Chiron F, Shwartz A, Kark S (2013) The complex interaction network among multiple invasive bird species in a cavity-nesting community. Biol Invasions 15:429–445
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Parsons H, Major RE, French K (2006) Species interactions and habitat associations of birds inhabiting urban areas of Sydney, Australia. Aust Ecol 31:217–227
Pell AS, Tidemann CR (1997a) The ecology of the common myna in urban nature reserves in the Australian Capital Territory. Emu 97:141–149
Pell AS, Tidemann CR (1997b) The impact of two exotic hollow-nesting birds on two native parrots in savannah and woodland in eastern Australia. Biol Conserv 79:145–153
Piper SD, Catterall CP (2003) A particular case and a general pattern: hyperaggressive behaviour by one species may mediate avifaunal decreases in fragmented Australian forests. Oikos 101:602–614
Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107
Schlaepfer MA, Pascal M, Davis MA (2011a) How might science misdirect policy? Insights into the threats and consequences of invasive species. J Consum Prot Food Saf 6(Suppl 1):S27–S31
Schlaepfer MA, Sax DF, Olden JD (2011b) The potential conservation value of non-native species. Conserv Biol 25:428–437
Sengupta S (1968) Studies on the life of the common myna. Proc Zool Soc Calcutta 21:1–27
Sengupta S (1982) The common myna. S. Chand and Company, New Delhi
Simpson K, Day N (2004) Field guide to the birds of Australia. Viking, Victoria
Sol D, Bartomeus I, Griffin AS (2011) The paradox of invasion in birds: competitive superiority or ecological opportunism? Oecologia 169:553–564
Stromberg JC, Chew MK, Nagler PL, Glenn EP (2009) Changing perceptions of change: the role of scientists in Tamarix and river management. Restor Ecol 17:177–186
Taylor RS, Oldland JM, Clarke MF (2008) Edge geometry influences landscape-scale habitat preferences of an edge specialist in south-eastern Australia. Landsc Ecol 23:377–389
Thiele J, Kollmann J, Markussen B, Otte A (2010) Impact assessment revisited: improving the theoretical basis for management of invasive alien species. Biol Invasions 12:2025–2035
Thomsen MS, Olden JD, Wernberg T, Griffin JN, Silliman BR (2011) A broad framework to organize and compare ecological invasion impacts. Environ Res 111:899–908
Tidemann CR (2003) Mitigation of the impact of mynas on biodiversity and public amenity. The Australian National University, Canberra
Tidemann CR (2010) Investigation into the potential for broad-scale control of mynas by trapping at communal roosts. Final report. Report to the Hermon Slade Foundation, the Australian Rainforest Foundation and the NSW Department of Environment, Climate Change and Water. Australian National University, Canberra
Tindall SD, Ralph CJ, Clout MN (2007) Changes in bird abundance following common myna control on a New Zealand island. Pac Conserv Biol 13:202–212
Tracey J, Saunders G (2003) Bird damage to the wine grape industry. Vertebrate Pest Research Unit, NSW Agriculture, Orange
Veerman PA (2002) Canberra birds: a report on the first 18 years of the Garden Bird Survey. Philip A. Veerman, Canberra
Vitousek PM, D’Antonio CM, Loope LL, Rejmanek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. NZ J Ecol 21:1–16
Watson J, Warman C, Todd D, Laboudallon V (1992) The Seychelles magpie robin (Copsychus sechellarum): ecology and conservation of an endangered species. Biol Conserv 61:93–106
Acknowledgments
All work was conducted under the Newcastle University Animal Ethics Committee ethics protocols A-2008-173 and A-2011-103. This work was supported by a grant from the Lake Macquarie City Council to KMH. Thanks to numerous volunteers for significant help with field work, in particular Grace Bourke and Terry Bignell.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haythorpe, K.M., Burke, D. & Sulikowski, D. The native versus alien dichotomy: relative impact of native noisy miners and introduced common mynas. Biol Invasions 16, 1659–1674 (2014). https://doi.org/10.1007/s10530-013-0598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0598-5