Abstract
Range expanding species can have major impacts on marine ecosystems but experimental field based studies are often lacking. The urchin Centrostephanus rodgersii has recently undergone a southerly range expansion to the east coast of Tasmania, Australia. We manipulated densities of C. rodgersii and algal regrowth in urchin barrens habitat to test effects of the urchin on biotic interactions between two native herbivores, black-lip abalone (Haliotis rubra) and another urchin (Heliocidaris erythrogramma), and their benthic habitat. After 13 months, removals of only C. rodgersii resulted in overgrowth of barrens habitat by algae and sessile invertebrates. Densities of abalone increased (+92 %) only in patches from which C. rodgersii was removed and algal regrowth allowed. In contrast, densities of H. erythrogramma increased in all treatments (+45, +28, +25 %) in which C. rodgersii was removed, irrespective of the algal regrowth manipulations. These results suggest that C. rodgersii has a negative influence on the densities of abalone through competition for food and on densities of H. erythrogramma through competition for preferred habitat. Densities of abalone (+65 %) but not H. erythrogramma (+25 %), were lower in the patches from which C. rodgersii and canopy algae regrowth were removed relative to patches from which only C. rodgersii was removed (+92 and +28 %, respectively). These results suggest that C. rodgersii overgrazing of canopy-algae results in loss of structural complexity which could increase abalone susceptibility to predation, cause abalone to seek shelter in cryptic microhabitats and/or prevent their return to patches where canopy algae are absent. The ongoing spread of C. rodgersii and expansion of barrens habitat in eastern Tasmania will continue to negatively affect populations of these two native herbivores and their associated fisheries at a range of spatial scales. This example shows that habitat modifying species which become highly invasive can have disproportionate negative impacts on the structure and dynamics of the recipient community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change is leading to the poleward range expansion of many marine species (Parmesan and Yohe 2003; Hickling et al. 2006; Poloczanska et al. 2008). Range expanding species that create or modify habitat are predicted to have profound effects on ecosystem structure and function (Grosholz 2002; Harley et al. 2006). These ecosystems engineers can alter habitat complexity, environmental chemistry and other physical variables with major effects on the abundances, diversity and context-dependent interactions of native species (Sorte et al. 2010; Walther 2010). Despite their importance, field-based experimental studies on the impacts of many range expanding habitat modifiers on marine ecosystems are lacking (but see Bertness 1984; O’Connor and Crowe 2005; Hollebone and Hay 2008; Ling 2008; Firth et al. 2009).
The southeast coast of Australia has experienced an increase in the abundances of many warmer water species (Stuart-Smith et al. 2009; Johnson et al. 2011) as a result of greater poleward penetration of the East Australia Current (Ridgway 2007), which has been linked in part to Antarctic ozone depletion (Cai et al. 2005; Cai 2006). One of the most conspicuous species that has undergone southerly range expansion from New South Wales to the east coast of Tasmania as a result of greater penetration of the East Australian Current, is the long spined urchin Centrostephanus rodgersii (Johnson et al. 2005, 2011; Ling et al. 2009; Stuart-Smith et al. 2009). This urchin is a habitat modifier, well known for its ability to overgraze filamentous and foliose algae and sessile invertebrates, effecting a catastrophic shift to barrens habitat dominated by the urchin and characterised by bare rock (Johnson et al. 2005; Ling 2008) or, in its native habitat, encrusting red algae (Fletcher 1987).
Centrostephanus rodgersii was first detected off the north east coast of Tasmania in 1978 (Edgar 1997). Since then, the abundances and range of this species have increased, and extensive barrens habitat (>100 m diameter) has formed at some locations on the north east coast of Tasmania as a result of urchin overgrazing seaweeds (Johnson et al. 2005; Ling 2008). While extensive barrens are not yet a widespread feature of the east coast of Tasmania, development of incipient barrens patches (≤10 m diameter) in otherwise intact algal beds are characteristic of much of the coastline (Johnson et al. 2005; Ling 2008). The continued spread and increase in abundances of C. rodgersii poses a major threat to the structure and function of macroalgal beds (Edgar et al. 2004; Ling 2008) and the important commercial fisheries species they support (Johnson et al. 2005, 2011; Strain and Johnson 2009).
Urchins and abalone consume predominately understorey filamentous and foliose algae and share similar habitat and predators (Shepherd 1973; Tegner and Levin 1982; Day and Branch 2000; Naylor and Gerring 2001). Surveys along the south east coast of Australia have demonstrated a negative relationship between the abundances of C. rodgersii, and that of H. rubra and H. erythrogramma at a broad range of spatial scales (Johnson et al. 2005; Andrew and Underwood 1992). Previous studies have suggested that C. rodgersii has a negative impact on the abundances of H. rubra and H. erythrogramma through competition for food (Shepherd 1973; Andrew et al. 1998; Strain and Johnson 2009) and/or preferred habitat (Andrew and Underwood 1992) in intact algal beds. Overgrazing of canopy-algae by C. rodgersii could also have a negative effect on the abundances of H. rubra and H. erythrogramma through loss of structural complexity (Andrew 1993; Andrew et al. 1998; Edgar et al. 2004). However, experimental studies designed to separate these hypotheses and test the effects of C. rodgersii on the abundances of both H. rubra and H. erythrogramma in barrens are lacking. In this study we manipulated densities of C. rodgersii and the algal regrowth to determine the impacts of this urchin on the densities of H. rubra and H. erythrogramma through competition for food, preferred habitat and/or loss of canopy-forming algae.
Materials and methods
Study area and experimental manipulations
This study was conducted at two randomly selected sites at the Lanterns (43°8′20′′S, 148°0′21′′S and 43°8′19′′S, 148°0′21′′S) on the east coast of Tasmania, Australia, between August 2005 and September 2006. Both sites have steeply sloping rocky substratum to a depth of >30 m and are moderately exposed.
At each site, flagging tape was used to mark the perimeter of 12 discrete Centrostephanus rodgersii barrens patches (mean width = 5.02 m, ±SE = 0.12 m, mean length = 5.14 m, ±SE = 0.10 m), each supporting 18–22 resident C. rodgersii (mean = 19.833 individuals, ±SE = 1.726 individuals) and of three other patches within the intact algal bed of similar size to the barrens patches but supporting seaweeds and no C. rodgersii. All barrens patches were randomly assigned to the following treatments: T1 = unmanipulated C. rodgersii barrens patches; T2 = removal of C. rodgersii and all regrowth from patches; T3 = removal of C. rodgersii and regrowth of canopy-algae species from patches; and T4 = removal of C. rodgersii only from patches. The intact algal patches without C. rodgersii represented a control treatment (T5). There were n = 3 replicate patches of each treatment. To avoid possible edge effects, the response variables were not monitored within 0.1 m of the tape.
At the beginning of the experiment, divers removed all C. rodgersii from T2, T3 and T4 patches using knives. Throughout the experiment, divers revisited the sites every 2 months to maintain the manipulations. At each visit, all reinvading C. rodgersii were removed (T2, T3 and T4), all algae and sessile invertebrate regrowth removed (T2) by scrubbing the substratum with a copper wire brush, and all regrowth of canopy-algae species (≥300 mm total length) removed by hand (T2 and T3), as appropriate for the treatment. These manipulations were undertaken for 13 months to allow at least one cycle of algal and sessile invertebrate regrowth (Ling 2008).
Benthic community
At both sites, the benthic community was assessed in a two-stage process, using a modification of the methods of Valentine and Johnson (2003). For each patch, the number of stipes and percentage cover of canopy-algae (≥300 mm in height) were assessed by eye in four randomly positioned 0.5 × 0.5 m quadrats. The fronds of these plants were then moved aside and the understorey community was assessed by photography using a digital Canon Powershot camera A95 with 2× Nikonos SB-102 strobes. A grid of 100 equidistant points was overlaid over the photographs and the taxa under each point identified to estimate community structure in terms of percentage cover. Understorey algae and sessile invertebrates that could not be identified to species level were allocated to complexes or higher taxonomic groups (e.g. Zonaria/Lobophora, sponge, ascidian etc.). All visual assessments were conducted by the same diver.
Densities of urchins and abalone
At both sites, divers counted the total number of urchins (C. rodgersii and Heliocidaris erythrogramma) and abalone (Haliotis rubra) see below for sampling details. These counts were converted to total densities (m−2) of C. rodgersii, H. rubra and H. erythrogramma. Patch area was calculated using the formula for an ellipsoid:
Due to time constraints, the schedule of monitoring responses of the benthic community to the manipulations differed between the two sites however manipulations at both sites followed an identical schedule. At the first site, the benthic community was assessed immediately prior to the manipulations, 1 month later and then every 2 months. At the second site, the benthic community was assessed immediately prior to the manipulations and then after 7 and 13 months.
Analyses
The effects of the different treatments on benthic community structure after 13 months described as functional groups (density of stipes and percentage cover of canopy-algae, and percentage cover of bare rock, encrusting red algae, filamentous algae, foliose understorey algae, and sessile invertebrates) were analysed using univariate 3-way nested ANOVAs. In each case the model included the main effects of treatment (fixed, 5 levels = T1–T5) and site (random, 2 levels = A and B), and patches (random, 3 levels = 3 replicates) nested within the treatment and site interaction.
The overall effect of the treatments on the benthic community after 13 months was analysed using 3-way PERMANOVA (as per model described above). To depict community structure, we used non-metric multi-dimensional scaling (nMDS) plots. The PERMANOVA and nMDS analyses were based on Bray-Curtis similarity matrices derived from percentage cover data after a square root transformation to reduce the influence of dominant species. All multivariate tests were undertaken using the statistical software Primer 6.0 with the PERMANOVA extension (Clarke and Warwick 2001; Anderson et al. 2008).
The effects of the different treatments on the densities of H. rubra and H. erythrogramma after 13 months were analysed using 2-way ANOVAs. The model included the main effects of treatment (fixed, 5 levels = T1–T5) and site (random, 2 levels = A and B) and their interaction.
The responses of the benthic community H. rubra and H. erythrogramma manipulations are depicted graphically for each assessment. However, the effects of the manipulations on the benthic community and the densities of H. rubra and H. erythrogramma were analysed at an a priori time of interest, i.e. after 13 months, to allow sufficient time for regrowth of both understory and overstorey algae in the barrens patches (see below Table 1).
Prior to all univariate tests, transformations to stabilize variances were determined from the relationship between group standard deviations and means. In all figures raw variables are depicted. Following the main analyses, one or two-tailed t-tests were made as planned comparisons at 13 months to assess the separate effects of competition for food, competition for preferred habitat, and loss of canopy-forming algae to facilitate interpreting overall effects of treatments on densities of H. rubra and H. erythrogramma (Table 1). For all tests α was adjusted using the procedure suggested by Todd and Keough (1994). All univariate tests and all univariate and multivariate graphical representations were undertaken using the statistical software R (www.R-project.org).
Results
A summary of trends through time for each treatment is given in Table 2. Detailed results are outlined below.
Benthic assemblage structure
At the initial assessment the benthic assemblage in all Centrostephanus rodgersii barrens patches was similar and distinctly different to that of the patches within the intact algal bed (Fig. 1). After 13 months, there were significant differences between the treatments and sites (treatment × site: F 4, 119 = 2.040, P = 0.001), particularly in the treatments from which C. rodgersii and all regrowth were removed (T2). The planned comparisons showed clear separation in MDS space between the benthic assemblage structure in the unmanipulated C. rodgersii barrens patches (T1) and the barrens patches from which C. rodgersii and canopy-algae regrowth were removed (T3) (F = 10.518, P = 0.006, α adjusted = 0.0125), and patches from which only C. rodgersii was removed (T4) (F = 5.006, P = 0.010, α adjusted = 0.0125). There was also clear separation between the intact algal patches (T5) and the incipient barrens patches from which C. rodgersii and canopy-algae regrowth (T3) (F = 13.848, P = 0.001, α adjusted = 0.0125) and from which C. rodgersii and all regrowth were removed (T2) (F = 3.557, P = 0.010, α adjusted = 0.0125) (Fig. 1). The barrens patches from which C. rodgersii and all regrowth (T2) was removed and the unmanipulated C. rodgersii barrens patches (T1) were also separated in MDS space, particularly at Site B, however these differences were not significant after adjusting for multiple testing (F = 3.557, P = 0.010, α adjusted = 0.0125) (Fig. 1). In contrast, after 13 months there were no detectable differences in community structure at a functional group level between incipient barrens patches from which only C. rodgersii was removed (T4) and the intact algal patches (T5) (F = 1.000, P > 0.05, α adjusted = 0.0125) (Fig. 1).
Ordinations (nMDS) of benthic community structure, showing the relationship between experimental treatments (n = 3 replicates) at a 0 month prior to manipulations (August 2005), and b 13 months after manipulations (September 2006), at two sites at the Lanterns, Tasmania, Australia. Before manipulations, at both sites the community assemblage in the control patches without C. rodgersii (T5) differed to all other treatments (top panels). After 13 months, clear differences in community structure were evident among several treatments while patches subject only to removal of C. rodgersii (T4) converged with the control plots (T5) (bottom panels). Treatments are, upright triangles unmanipulated C. rodgersii barrens (T1), diamonds removal of C. rodgersii and all regrowth from patches (T2), downward triangles removal of C. rodgersii and canopy-algae regrowth from patches (T3), squares removal of C. rodgersii only (T4), circles no C. rodgersii in intact algal patches (T5). The analysis is based on a Bray-Curtis matrix of square root transformed percentage cover data. Ellipses (95 % confidence interval) are drawn around all treatments for clarity
Similarly, after 13 months of removing only C. rodgersii from barrens patches there were no detectable differences in the cover of foliose understorey algae and sessile invertebrates in the treatment patches (T4) relative to the control patches with no C. rodgersii in the intact algal bed (T5) (Table 3; Figs. 2, 3). There was however still a significantly higher density of stipes of canopy-algae but lower cover of canopy-algae, encrusting red algae and filamentous algae in the T4 patches compared with the intact algal patches (T5), indicating that the development of algae in these patches had not yet achieved the full characteristics of the seaweed community surrounding them (Table 3; Figs. 2, 3). Removals of C. rodgersii and all regrowth to simulate barrens patches (T2) resulted in a higher cover of filamentous algae compared with the unmanipulated C. rodgersii barrens patches (T1) (Table 3; Figs. 2, 3), reflecting that 2 monthly visitations and associated manipulations were insufficient to prevent some development of filamentous algae in this treatment. However, after 13 months there were no detectable differences in the density of stipes and cover of canopy-algae, the cover of encrusting red algae, foliose understorey algae and sessile invertebrates between the T2 patches and unmanipulated incipient barrens patches (T1). Not surprisingly, there was a significantly lower density and cover of canopy-algae and cover of encrusting red algae, filamentous algae, understorey foliose algae and sessile invertebrates in the treatment patches (T2) relative to the intact algal patches (T5) (Table 3; Figs. 2, 3).
Mean (±SE) (i) density (m−2) and (ii) percentage cover of canopy-algae in treatment patches (n = 3 replicates) through time (months), at two sites at the Lanterns, Tasmania, Australia. Treatments are, upright triangles unmanipulated C. rodgersii barrens (T1), diamonds removal of C. rodgersii and all regrowth from patches (T2), downward triangles removal of C. rodgersii and canopy-algae regrowth from patches (T3), squares removal of C. rodgersii only (T4), circles control no C. rodgersii in intact algal patches (T5). Note the different scale on the y-axes
Mean (±SE) percentage cover of (i) encrusting red algae, (ii) filamentous algae, (iii) foliose algae, (iv) sessile invertebrates in treatment patches (n = 3 replicates) through time (months), at two sites at the Lanterns, Tasmania, Australia. Treatments are, upright triangles unmanipulated C. rodgersii barrens (T1), diamonds removal of C. rodgersii and all regrowth from patches (T2), downward triangles removal of C. rodgersii and canopy-algae regrowth from patches (T3), squares removal of C. rodgersii only (T4), circles control no C. rodgersii in intact algal patches (T5). Note the different scale on the y-axes
Removals of C. rodgersii and canopy-algae regrowth from incipient barrens patches (T3) resulted in significantly higher cover of filamentous algae, understorey foliose algae and sessile invertebrates when compared with the unmanipulated barrens patches (T1) (Table 3; Figs. 2, 3). There were no detectable differences in the density and cover of canopy-algae and cover of encrusting red algae and sessile invertebrates between the treatment patches (T3) and the unmanipulated barrens patches (T1). There was a significantly lower density and cover of canopy-algae and cover of encrusting red algae but no detectable differences in the cover of filamentous algae, foliose understorey algae between the treatment patches (T3) and the intact algal patches (T5). In general, the trends were similar between sites however the cover of overstorey algae, encrusting red algae and filamentous algae, but not sessile invertebrates, in the intact algal patches was higher at site A relative to site B (Table 3; Figs. 2, 3). The cover of foliose algae and sessile invertebrates in the treatment from which C. rodgersii only was removed was also higher at site A than at site B (Table 3; Figs. 2, 3).
Density of urchins and abalone
Throughout the experiment the density of C. rodgersii was higher in the unmanipulated barrens patches (T1) than in the patches from which the urchins were removed (T2, T3, T4) and the intact algal patches (T5) (Appendix 1 in ESM), indicating that manipulations were successful in maintaining removal patches at very low densities of C. rodgersii.
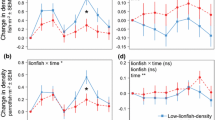
Prior to the manipulations, the densities of Haliotis rubra in the C. rodgersii incipient barrens patches (T1, T2, T3 and T4) were similar, and much lower than the densities of abalone in the intact algal patches (T5) (Fig. 4). After 13 months, there was a significantly higher density of H. rubra in the patches from which C. rodgersii and canopy-algae regrowth was removed (T3) relative to the unmanipulated C. rodgersii barrens patches (T1) (T = 4.583, P = 0.001, α adjusted = 0.025) (Fig. 4). There was a higher density of H. rubra in the patches from which only C. rodgersii was removed (T4) relative to the patches from which C. rodgersii and canopy-algae regrowth were removed (T3) (T = 3.672, P = 0.004, α adjusted = 0.025). There was also a higher density of H. rubra in the patches which C. rodgersii and canopy-algae regrowth (T3) were removed relative to the patches from which C. rodgersii and all regrowth were removed (T2) (T = 7.007, P < 0.001, α adjusted = 0.025) (Fig. 4). In contrast, there were no detectable differences in the densities of H. rubra between the patches from which C. rodgersii and all regrowth (T2) were removed and the unmanipulated C. rodgersii barrens patches (T1) (T = 0.347, P > 0.05, α adjusted = 0.025) (Fig. 4).
Mean densities (±SE) of H. rubra (m−2) in treatment patches (n = 3 replicates) through time (months), at two sites at the Lanterns, Tasmania, Australia. Treatments are, upright triangles unmanipulated C. rodgersii barrens (T1), diamonds removal of C. rodgersii and all regrowth from patches (T2), downward triangles removal of C. rodgersii and canopy-algae regrowth from patches (T3), squares removal of C. rodgersii only (T4), circles control no C. rodgersii in intact algal patches (T5). Note the different scale on the y-axes
At the initial assessment, the density of Heliocidaris erythrogramma in the barrens (T1, T2, T3, T4) and intact algal patches (T5) was similar (Fig. 5). After 13 months, the densities of H. erythrogramma were significantly higher in the patches from which C. rodgersii and all regrowth were removed (T2) (T = 6.306, P < 0.001, α adjusted = 0.025) and the patches from which C. rodgersii and canopy-algae (T3) were removed (T = 7.368, P < 0.001, α adjusted = 0.025) relative to the unmanipulated C. rodgersii barrens (T1) (Fig. 5). In contrast, there was no detectable difference in the density of H. erythrogramma in the patches from which C. rodgersii and all regrowth (T2) were removed and those from which only C. rodgersii was removed (T4) (T = 2.333, P = 0.042, α adjusted = 0.025) (Fig. 5). There was also no detectable difference in the density of H. erythrogramma in the patches from which only C. rodgersii was removed (T4) relative to patches from which both C. rodgersii and canopy-algae regrowth were removed (T3) (T = 2.564, P = 0.038, α adjusted = 0.025) (Fig. 5).
Mean densities (±SE) of H. erythrogramma (m−2) in treatment patches (n = 3 replicates) through time (months), at the Lanterns, Tasmania, Australia. Treatments are, upright triangles unmanipulated C. rodgersii barrens (T1), diamonds removal of C. rodgersii and all regrowth from patches (T2), downward triangles removal of C. rodgersii and canopy-algae regrowth from patches (T3), squares removal of C. rodgersii only (T4), circles control no C. rodgersii in intact algal patches (T5). Note the different scale on the y-axes
Discussion
Effect of habitat modifying species on benthic structure and function
Habitat modifying species can have major impacts on marine ecosystem structure and function (Helmuth et al. 2006; Williams and Grosholz 2008). In many ecosystems, urchins are well known habitat modifiers (Lawrence 1975; Chapman 1981; Chapman and Johnson 1990; Tegner and Dayton 2000). It is already well recognised that Centrostephanus rodgersii grazing has an important influence on the benthic community assemblage in south east Australia (Fletcher 1987; Johnson et al. 2005, 2011; Ling 2008). We demonstrate here that in its new habitat on the east coast of Tasmania, this urchin is responsible for overgrazing the filamentous and foliose algal and sessile invertebrates and maintaining simplistic and homogeneous bare rock benthic habitat which is similar to the barrens described in its endemic range (Fletcher 1987) and broadly typically of urchin barrens habitat throughout the world (Pinnegar et al. 2000).
In our study, experimental removals of C. rodgersii from barrens patches resulted in bare rock being overgrown by filamentous algae (primarily red algae), foliose algae (red, juvenile canopy-forming and understorey foliose brown algae) and sessile invertebrates. However, after 13 months there were still differences in the benthic assemblage between experimental patches where only C. rodgersii was removed and control patches in intact algal beds. Similar to another study on the east coast of Tasmania (Ling 2008), removals of C. rodgersii from barrens patches resulted in a rapid return to an algal dominated state (≥50 % cover), however recovering patches were biased towards smaller and more abundant canopy forming algae and a lower cover of encrusting red algae, relative to the community of the long standing intact algal beds. Complete recovery of the algal community following the removal of C. rodgersii can take 2–3 years (Ling 2008), and is likely to be a function of the size of the cleared area and its proximity to established reproductive algal populations (Fletcher 1987). The recovered patches and intact algal beds provide increased 3 dimensional structural complexity (Graham 2004; Ling 2008), primary and secondary productivity (Chapman 1981; Duggins et al. 1989) and altered nutrient cycling and energy flows (Sauchyn and Scheibling 2009) relative to barrens habitat.
Effect of habitat modifying species on abundances of native species
Range expanding habitat modifiers can also alter biotic interactions among native species (Firth et al. 2009; Strain and Johnson 2009; Sorte et al. 2010; Walther 2010). Similar to research in large scale plots (1,000 m−2) in New South Wales (Andrew et al. 1998), we demonstrated that experimental removals of C. rodgersii from barrens patches (mean size 30.654 m−2) resulted in overgrowth of bare rock by filamentous, foliose algae and sessile invertebrates and concomitant increases in the abundances of abalone Haliotis rubra. In other manipulations in Tasmania, the introduction of C. rodgersii into cages (9 m−2) in intact algal beds resulted not only in declines in the percentage cover and standing biomass of foliose algae, but also reduced total weight, dry weight of stomach contents and survivorship of H. rubra relative to controls without C. rodgersii (Strain and Johnson 2009). The combined research strongly suggests that C. rodgersii is a more efficient and effective grazer of attached understorey algae than H. rubra (Andrew et al. 1998; Strain and Johnson 2009). We extend this research by demonstrating that although C. rodgersii is the superior competitor for attached algae in interactions with H. rubra, there is no evidence to suggest that these two herbivores also compete for preferred habitat.
Centrostephanus rodgersii overgrazing of canopy-algae could also potentially increase H. rubra susceptibility to predation (Andrew 1993; Andrew et al. 1998; Edgar et al. 2004) by reducing canopy algae cover. Our observations suggest there were fewer H. rubra, but more rock lobsters and fish predators in patches from which C. rodgersii and canopy-algae regrowth were removed relative to patches where C. rodgersii was removed but regrowth allowed. Since this manipulation does not decrease the availability of food to abalone (because they do not feed on established canopy-forming algae), this result could suggest that predation on abalone is higher in the absence of the canopy, abalone seek cryptic microhabitat in the interstices of the reef, and/or do not return to patches where canopy algae are absent. Our results are consistent with other research in Tasmania, in which removals of canopy-algae from large experimental plots (600 m−2) resulted in decreased abundances of H. rubra and an increase in the density of empty abalone shells (Edgar et al. 2004) relative to controls in intact algal beds. The combined results suggest increased predation on abalone in the absence of large canopy-forming algae. In the reverse manipulation, Andrew (1993) found significant increases in the densities of juvenile abalone (H. rubra and H. coccoradiata) after boulders covered in Ecklonia radiata were transplanted into urchin barrens habitat (10,000 m−2). These results suggest that the continued expansion of C. rodgersii on the east coast of Tasmania will have an ongoing negative impact on the abundances of H. rubra both through loss of food resources (Shepherd 1973; Strain and Johnson 2009) and structural complexity (Andrew 1993; Andrew et al. 1998; Edgar et al. 2004).
In contrast, to the response of abalone, removals of C. rodgersii from barrens patches, irrespective of our manipulations of regrowth, invariably resulted in an increase in the densities of H. erythrogramma relative to unmanipulated C. rodgersii barrens patches. However there were no detectable differences in the densities of the native urchin between the patches from which C. rodgersii and all regrowth were removed and the patches from which C. rodgersii was removed and regrowth allowed. These results strongly suggest that C. rodgersii outcompetes H. erythrogramma through competition for preferred habitat rather than for attached algal food resources. Studies on Diadematidae species have demonstrated that urchins aggressively defend their crevices by biting and pushing conspecifics and congeners (Williams 1977; McClanahan 1988; Shulman 1990) or by dislodging them from areas which are more favourable for catching drift algae, which is an important food resource for urchins inhabiting barrens habitat (Harrold and Reed 1985; Vanderklift and Kendrick 2005; Vanderklift and Wernberg 2008). A similar interaction could be operating in Tasmania although we have never observed it, perhaps because C. rodgersii is highly nocturnal (Flukes et al. 2012). Further manipulations of the availability of crevices and drift algae are required to elucidate the detailed effects of C. rodgersii on H. erythrogramma.
Our experiment also provides insight into the interactions between the two dominant native macroherbivores on the southeast coast of Australia, H. rubra and H. erythrogramma. Interestingly, throughout the 13 months experiment, in the intact algal patches without C. rodgersii, there was a low density of H. erythrogramma but a high density of H. rubra. These results are consistent with broad-scale surveys along the east coast of Tasmania and elsewhere in southeast Australia which have showed that abundances of H. erythrogramma and H. rubra are negatively correlated at a range of spatial scales (Shepherd 1973; Johnson et al. 2005). The nature and effects of interactions between the two native herbivores are poorly understood. However, our results provide some support for the hypothesis that these two native herbivores could also compete for food and/or preferred habitat (Shepherd 1973; Johnson et al. 2005).
Alternatively, intact algal beds on moderately exposed coastlines could be unsuitable to support high abundances of H. erythrogramma. Studies on the east coast of Tasmania and elsewhere in southern Australia, have demonstrated that H. erythrogramma are relatively immobile and remain in crevices in intact algal beds in wave exposed habitat (Connolly 1986; Keesing 2006). In these areas, the whiplash like action of canopy-algae is thought to limit the attachment and destructive grazing behaviour of this urchin (Konar 2000; Ling et al. 2010). Certainly, surveys have demonstrated that H. erythrogramma occurs in abundance only in sheltered and, at worst, moderately exposed sites (Johnson et al. 2005). Overall, our results suggest that the initial establishment of C. rodgersii on the east coast of Tasmania and associated overgrazing of canopy-algae might initially benefit populations of H. erythrogramma but, as the densities of the non-native urchin increase, the native urchin will be locally displaced.
Studying invasions
Range expanding species can have complex biotic interactions (competition and/or predation) with native species (Sorte et al. 2010; Walther 2010). A useful extension to this study would be to quantify the rates of predation on H. erythrogramma and H. rubra in C. rodgersii barrens patches, and compare this to predation on these species at comparable sites with intact algal beds. There is already strong evidence that both fishes and rock lobsters have an important influence on the behavior and survival of H. erythrogramma and H. rubra in intact algal beds in marine protected areas in Tasmania, Australia (Pederson and Johnson 2006; Pederson et al. 2008) but the effects of these predators in incipient barrens patches remain unclear. Irrespective, we demonstrate here that C. rodgersii appears to have a stronger effect on the densities of H. rubra and H. erythrogramma through competition for food and preferred habitat rather by overgrazing of canopy-algae per se.
It is predicted that numerous marine species will shift their range polewards in response to global climate change (Parmesan and Yohe 2003). While many of these species will have little or no effect on marine ecosystems (Sorte et al. 2010; Walther 2010), highly invasive range expanding species are often generalist grazers (e.g. sea urchins, gastropods and sea stars) which modify habitat by consuming a wide variety, and large amounts, of prey (Helmuth et al. 2006; Williams and Grosholz 2008; Sorte et al. 2010; Walther 2010). Thus, scientists and managers should focus on developing strategies to control the abundances of newly established generalist grazers to limit their impacts on biodiversity and commercial fisheries. Continued research into the impacts of range expanding species on the biotic interactions between native species and their environment is important for predicting and understanding and managing the effects of invaders on marine community dynamics and ecosystems function and structure (Bertness 1984; O’Connor and Crowe 2005; Ling 2008; Firth et al. 2009).
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMOVA for primer: guide to software and statistical methods, 1st edn. PRIMER-E, Plymouth
Andrew NL (1993) Spatial heterogeneity, urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74:292–302
Andrew NL, Underwood AJ (1992) Associations and density of urchins and abalone on shallow subtidal reefs in southern New South Wales. Mar Freshw Res 43:547–1559
Andrew NL, Worthington DG, Brett PA, Bentley N R, Chick C, Blount C (1998) Interactions between the abalone fishery and urchins in New South Wales. Final Report Series No.12, NSW fisheries, Sydney
Bertness MD (1984) Habitat and community: modification by an introduced herbivorous snail. Ecology 65:370–381
Cai W (2006) Antarctic ozone depletion causes an intensification of the Southern Ocean super-gyre circulation. Geophys Res Lett 33:L03712. doi:10.1029/2005GL024911
Cai W, Shi G, Cowan T, Bi D, Ribbe J (2005) The response of the Southern Annular Mode, the East Australian Current, and the southern mid-latitude ocean circulation to global warming. Geophy Res Lett 32: L23706, 1–4
Chapman ARO (1981) Stability of urchin dominated barren grounds following destructive grazing of kelp in St. Margaret’s Bay, Eastern Canada. Mar Biol 62:307–311
Chapman ARO, Johnson CR (1990) Disturbance and organization of macroalgal assemblages in the northwest Atlantic. Hydrobiologia 192:77–122
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Connolly RM (1986) Behaviour and ecology of the urchin Heliocidaris erythrogramma (Valenciennes). B.Sc. Hons thesis. University of Adelaide, Adelaide
Day E, Branch GM (2000) Evidence for a positive relationship between juvenile abalone Haliotis midae and the urchin Parechinus angulosus in the south western Cape, South Africa. S Afri J Mar Sci 22:145–156
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Edgar GJ (1997) Australian marine life: the plants and animals of temperate waters. Reed New Holland, Sydney
Edgar GJ, Barrett SN, Morton A, Samson RC (2004) Effects of algal canopy clearance on plant, fish and macroinvertebrate communities on eastern Tasmanian reefs. J Exp Mar Biol Ecol 312:67–87
Firth LB, Crowe TP, Moore P, Thompson RC, Hawkins SJ (2009) Predicting impacts of climate-induced range expansion: an experimental framework and a test involving key grazers on temperate rocky shores. Glob Chang Biol 15:1413–1421
Fletcher WJ (1987) Interactions among subtidal Australian urchins, gastropods and algae: effects of experimental removal. Ecol Monogr 57:89–109
Flukes EB, Johnson CR, Ling SD (2012) Forming sea urchin barrens from the inside out: an alternative pattern of overgrazing. Mar Ecol Prog Ser 464:179–194
Graham MH (2004) Effects of local deforestation of the diversity and structure of southern California giant kelp forests food webs. Ecosystems 7:341–357
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17(1):22–27
Harley CDG, Hughs RA, Hulgren KM, Miner BF, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impact of climate change in coast marine systems. Ecol Lett 9:228–241
Harrold C, Reed DC (1985) Food availability, urchin grazing, and kelp forest community structure. Ecology 66:1160–1169
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Ann Rev Ecol Evol Syst 37:373–404
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Chang Biol 12:450–455
Hollebone AL, Hay ME (2008) An invasive crab alters interaction webs in a marine community. Biol Invasions 10:347–358
Johnson C, Ling S, Ross J, Scoresby S, Miller K (2005) Establishment of the long-spined urchin (Centrostephanus rodgersii) in Tasmania: first assessment of the potential threats to fisheries. Final report, Fisheries Research Development Corporation, Hobart, Tasmania
Johnson CR, Banks S, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M et al (2011) Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol 400(1–2):17–32
Keesing J (2006) Ecology of Heliocidaris erythrogramma. In: Lawrence JM (ed) Edible urchins: biology and ecology, 2nd edn. Elsevier, New York, pp 329–341
Konar B (2000) Seasonal inhibitory effects of marine plants on urchins: structuring communities the algal way. Oecologia 125(2):208–217
Lawrence JM (1975) On the relationship between marine plants and urchins. Ocean Mar Biol Ann Rev 13:213–286
Ling SD (2008) Range expansion of a habitat modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156(4):883–894
Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M (2009) Climate driven range extension of a urchin: inferring future trend by analysis of recent population dynamics. Glob Chang Biol 15:719–731
Ling SD, Ibbott S, Sanderson JC (2010) Recovery of canopy-forming macroalgae following removal of the enigmatic grazing urchin Heliocidaris erythrogramma. J Exp Mar Biol Ecol 395:135–146
McClanahan TR (1988) Coexistence in a urchin guild and its implications to coral reef diversity and degradation. Oecologia 77:210–218
Naylor R, Gerring P (2001) Interaction between pauna and kina. Water Atmos 9:16–17
O’Connor NE, Crowe TP (2005) Biodiversity loss and ecosystem functioning: distinguishing between number and identity of species. Ecology 86:1783–1796
Parmesan C, Yohe G (2003) A globally coherent fingerprint of change. Nature 421:37–42
Pederson HG, Johnson CR (2006) Predation of the urchin Heliocidaris erythrogramma by rock lobsters (Jasus edwardsii) in no-take marine reserves. J Exp Mar Biol Ecol 336:120–134
Pederson HG, Barrett ND, Frusher SD, Buxton CD (2008) Effect of predator-prey and competitive interactions on size at emergence in the black-lip abalone Haliotis rubra in a Tasmania MPA. Mar Ecol Prog Ser 366:91–98
Pinnegar JK, Polunin NVC, Francour P, Badalamenti F, Chemello R, Harmelin-Vivien ML, Hereu B, Milazzo M et al (2000) Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ Conserv 27:179–200
Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT (2008) Modelling the response of populations of competing species to climate change. Ecology 89:3138–3149
Ridgway K (2007) Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett 34:L13613
R Development Core Team (2010) R: a language and environment for statistical computing. R project for statistical computing, Vienna, Austria. http://www.R-project.org
Sauchyn LK, Scheibling RE (2009) Degradation of urchin feces in a rocky subtidal ecosystem: implications for nutrient cycling and energy flow. Aquat Biol 6:99–108
Shepherd SA (1973) Competition between urchins and abalone. Aust Fish 4:4–7
Shulman MJ (1990) Aggression among urchins on Caribbean coral reefs. J Exp Mar Biol Ecol 140:197–207
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biol 19:303–316
Strain EMA, Johnson CR (2009) Competition between an invasive urchin and commercially fished abalone: effect on body condition, reproduction and survivorship. Mar Ecol Prog Ser 377:169–182
Stuart-Smith RD, Barrett NS, Stevenson DG, Edgar GJ (2009) Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming. Glob Chang Biol 16(1):122–134
Tegner MJ, Dayton PK (2000) Ecosystem effects of fishing in kelp forest communities. ICES J Mar Res 57:579–589
Tegner MJ, Levin LL (1982) Do urchins and abalone compete in the California kelp communities? In: Lawrence J (ed) International echinoderms conference. A.A. Balkema, Rotterdam, pp 265–271
Todd CD, Keough MJ (1994) Larval settlement in hard substratum epifaunal assemblages: a manipulative field study of the effects of substratum filming and the presence of incumbents. J Exp Mar Biol Ecol 181:159–187
Valentine JP, Johnson C (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295:63–90
Vanderklift MA, Kendrick GA (2005) Contrasting influence of urchins on attached and drift macroalgae. Mar Ecol Prog Ser 299:101–110
Vanderklift MA, Wernberg T (2008) Detached kelps from distant sources are a food subsidy for urchins. Oecologia 157:327–335
Walther G (2010) Community and ecosystem responses to recent climate change. Philos Trans R Soc B 365:2019–2024
Williams AH (1977) Three-way competition in a patchy back reef environment. PhD thesis University of North Carolina
Williams SL, Grosholz ED (2008) The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coasts 31:3–20
Acknowledgments
We thank those who assisted with fieldwork, particularly Ryan Downie, Richard Holmes and David Sinn. We thank Dr. Graham Edgar and Dr. Adriana Villamor for their useful comments on an earlier draft of the manuscript. This study was part of a Commonwealth Scientific and Industrial Research Organization Joint PhD Program in Quantitative Marine Science and supported by an Australian Postgraduate Award. The research was supported by Tasmanian Aquaculture and Fisheries Institute, and Tasmanian Abalone Council grants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strain, E.M.A., Johnson, C.R. The effects of an invasive habitat modifier on the biotic interactions between two native herbivorous species and benthic habitat in a subtidal rocky reef ecosystem. Biol Invasions 15, 1391–1405 (2013). https://doi.org/10.1007/s10530-012-0378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0378-7