Abstract
Current avian invasions are often the result of exotic birds accidentally escaping from cages. It has been hypothesised that the higher invasiveness of wild-caught cage-birds compared to captive-bred ones could be related to the loss of ability in captive-bred birds to cope with new environments. The acute stress response plays an important role in how animals cope with challenges because elevated corticosterone (CORT) levels can mediate learning and memory consolidation and help to increase their survival prospects. We experimentally tested whether exotic wild-caught and captive-bred cage-birds differ in their responses to acute stress using a representative sample of parrots. Wild-caught individuals showed longer CORT responses to acute stress than captive-bred ones, both at inter- and intra-specific levels when comparing wild-birds to the first generation born in captivity. Captive-bred birds may have attenuated their CORT responses due to acclimation, while high mortality rates during the international trade of wild-caught birds could have selected those individuals that are better able to cope with stress. We suggest that the longer acute response found in wild-caught parrots could help them to escape from cages and survive when facing challenges in new wild environments, possibly contributing to their higher invasiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since alien species invasions are considered among the major threats to biodiversity worldwide, scientists are keenly interested in investigating introduction pathways (Hulme 2009) as well as specific traits that may help to predict invaders (Kolar and Lodge 2001). Deliberate bird introductions have been reported since ancient times (Tella 2011) and offer a useful model to identify factors that might explain differences in invasion success among species, such as propagule pressure, ecological plasticity, and behavioural flexibility (Sol et al. 2005; Blackburn et al. 2009). Nowadays, however, globalization has resulted in greater transport and commercial trade of exotic species (Hulme 2009), and recent avian invasions often arise from traded birds that have escaped from cages rather than from deliberate introductions (Lever 2005; Carrete and Tella, in prep.). This introduction pathway has contributed to more than 200 species of exotic birds recently observed in the wild in European countries such as Spain (Carrete and Tella 2008) and Germany (Bauer and Woog 2008). Nonetheless, exotic cage-bird species differ widely in their invasion success. Carrete and Tella (2008) examined the availability in the pet market of 202 species of parrots and songbirds (which together constituted 99 % of all individuals recorded in markets) in relation to their origin (i.e., wild-caught vs captive-bred) and invasiveness in peninsular Spain. Captive-bred birds greatly outnumbered wild-caught birds as pets, but the latter were more successful invaders: all species reported breeding in the wild or showing spreading populations were available as wild-caught but not as captive-bred birds in markets. These results are applicable to other countries (Carrete and Tella, in prep.) and suggest that captive-bred birds may have lost their ability to cope with new environments after escaping from cages.
Captive breeding may relax natural selection, while artificial selection in the form of domestication may result in less active and aggressive animals that are easy to catch and handle (Archard and Braithwaite 2010). Endocrine mechanisms are involved in how animals cope with environmental and social challenges (Sapolsky et al. 2000; Wingfield and Ramenofsky 1999) and captivity may affect the ways they deal with stress (Archard and Braithwaite 2010; Mason 2010). In birds, the hormone corticosterone (CORT) is secreted by adrenal glands after the activation of the hypothalamic–pituitary–adrenal (HPA) axis (Sapolsky et al. 2000). This short-term acute stress response correlates with fitness components and is believed to improve an animal’s immediate survival (Cabezas et al. 2007; Sapolsky et al. 2000; Wingfield and Ramenofsky 1999). However, if a stressor persists or a series of acute stressors initiate multiple consecutive acute responses, the animal would become chronically stressed with detrimental consequences to its health (Wingfield et al. 1998; Wingfield and Romero 2001; Blas et al. 2007).
In the present paper, we experimentally tested whether captivity may have altered the physiological response to stressors, thus contributing to the lower invasiveness of captive-bred cage-birds (Carrete and Tella 2008). We used inter- and intra-specific approaches to assess the individual variation in acute stress response between a variety of wild-caught and captive-bred parrots usually traded as cage-birds.
Methods
Selection criteria for experimental individuals
A wide range of parrots and songbirds are available in the pet market and have the potential to become invaders upon escape from cages (Carrete and Tella 2008). We focused this study on parrot species of relatively similar body size in order to minimize potential phylogenetic and body-size effects that could contribute to differences in stress physiology among species (Romero 2004). Rather than comparing large sample sizes of wild-caught versus captive-bred birds from a single species, we chose to sample as many species as possible to try to generalize results at the cost of losing statistical power within species. Wild-caught parrots destined for pet-shop distribution were purchased from importers. Import documents demonstrated that they were legally caught in the wild for commerce. Acquisition of wild-caught individuals was not possible after 2006, when a European trade ban was established (Carrete and Tella 2008). Other species have been bred in captivity for decades (e.g., those originally from Australia), so there were no doubts about their captive-bred origin. Captive-bred birds born as a first generation (F1) from wild-caught parents were provided by parrot breeders who had previously collaborated with our research team. This ensured an accurate classification of the generation’s origin and that these captive-bred birds were unrelated to wild-caught conspecifics used in this study. We excluded hand-raised individuals, including only those birds naturally raised by parents. After all these restrictive criteria, sample sizes were also constrained by time required to conduct the capture-restraint protocols in only two experimental seasons, to minimize sampling effects. Therefore, we ultimately used 47 parrots from 11 species for this study (Table 1). All captive-bred and wild-caught individuals were full-grown birds and had been living in captivity for at least 1 year before our study, enough time for the birds to be habituated to captivity (Archard and Braithwaite 2010). We housed all birds in standard cages randomly placed at similar locations within the same indoor room (thus avoiding differences in behavioural responses associated with location, Feenders and Bateson 2011), and provided commercial parrot food and water ad libitum for 1 month before the start of experiments. JLT held the Spanish certificates that legally allow the design (Certificate C) and conduct of experimental research work using live animals (Certificate B), and work in captivity was done under institutional approval of the competent Spanish wildlife agency (Consejeria de Medio Ambiente, Junta de Andalucía).
Sample collection
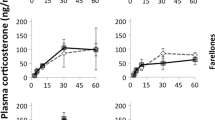
Blood collection was performed during the daytime in August 2006 and January 2008 on 29 and 18 different individuals, respectively. Each bird was quickly captured (usually within 1–3 s) by JLT, beginning with the cage closest to the entrance to the room and finishing with the most distant cage to minimize disturbance to the rest of the birds. The bird’s head was immediately covered with an opaque cloth to avoid producing distress calls that could stress other birds. The bird was then quickly moved to a distant room where we conducted the restraint experiment. The fast capture, together with the fact that birds were habituated to daily visits by the same researcher for routine provision of food and water inside the cages, make us confident that the birds waiting in their cages were not progressively more stressed as the experiment advanced. Indeed, CORT levels clearly increased after 3 min. from capture and there was a low variance among birds at the first bleeding instance (Fig. 1), thus supporting that we actually measured CORT baseline levels in unstressed individuals.
Blood samples were collected following standard capture and restraint protocols (Wingfield and Romero 2001) with the first sample taken within 3 min (1.6 min ± 0.6 SD) of initial capture (baseline levels; Romero and Reed 2005), and at 10 and 45 min post-capture. Birds were kept individually in dark boxes between successive bleedings, and captured, handled and bled by the same person (JLT). Blood samples were obtained from the jugular vein and centrifuged immediately after sampling. Plasma was stored at −20 °C until assayed. We separated a sample of about 0.02 ml of whole blood and preserved it in 90 % ethanol for molecular sexing (Vögeli et al. 2007). Each bird was weighed after the last bleeding.
Hormone assays
Plasma CORT concentration was determined using radioimmunoassay (RIA) following extraction with ethyl ether as described previously (Blas et al. 2005). Extraction efficiency, measured in samples spiked with 3H-corticosterone, was greater than 90 %. Antiserum and purified CORT for standards were purchased from Sigma Chemicals and 3H-CORT from Amersham Bioscience. The minimum detection limit of the RIA was 9.67 pg per tube. All plasma CORT samples were analyzed in two separate assays and the intra- and inter-assay coefficients of variation were 5.1 and 8.7, respectively.
Statistical analyses
We compared 47 individuals (20 wild-caught and 27 captive-bred) belonging to 11 species in an inter-specific approach, and a subsample of 26 individuals (15 wild-caught and 11 F1 captive-bred) belonging to 3 species (those with larger sample sizes and at least two wild-caught and two captive-bred individuals) in an intra-specific approach (Table 1). Within each approach, individual acute stress responses (i.e., changes in plasma CORT levels) were assessed through a repeated measures analysis, testing whether differences in individual changes in CORT levels over time (baseline, 10 and 45 min after capture) resulted from an effect of bird origin (i.e., wild-caught or captive-bred) or from different responses of individuals related to sex, body mass, or date of sampling and time of day (thus controlling for potential daily and seasonal variation in CORT, Romero and Remage-Healey 2000). We also accounted for potential differences in stress physiology among species by fitting this taxonomic category as a fixed factor in the repeated measures analysis. Sphericity tests (all P > 0.23) indicated the correct use of the F statistic for our data set. Models were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Birds used in this study displayed the expected increase in blood CORT levels 10 and 45 min after capture, as shown by the repeated measures analysis at the inter-specific level (F 2, 22 = 3.86; P = 0.036; Fig. 1a). However, the significant interaction of ‘time*origin’ (F 2, 22 = 4.62; P = 0.021) signifies that CORT levels are changing over time but that this change differs between wild-caught and captive-bred birds. In particular, differences were significant when comparing CORT changes between 10 and 45 min (F 1, 22 = 8.13; P = 0.009). Thus, all birds increased plasma CORT levels from baseline to 10 min after sampling, but after 45 min captive-bred individuals reduced CORT levels while wild-caught birds continued at levels comparable to those of the second sample (Fig. 1a). Models were performed by controlling for date of sampling (F 2, 22 = 3.86, P = 0.036), time of day, sex, body mass and species (all P > 0.05) as potential confounding variables.
The intra-specific approach (Fig. 1b) showed similar results. The hormone response differed between wild-caught birds and their captive-bred F1 conspecifics (time*origin: F 2, 13 = 5.39; P = 0.020; Fig. 1b), with significant differences when comparing individual CORT changes between the last two samples (F 1, 13 = 9.87; P = 0.007). Nearly significant differences among species were detected (F 4, 26 = 2.71, P = 0.052), while sex, body mass, date and time of day were not related to individual responses to stress (all P > 0.11).
Discussion
Differences in stress physiology between wild-caught and captive-bred birds
Parrots in this study differed in their acute stress responses from 10 to 45 min after restraint. Despite our relatively small sample sizes, wild-caught individuals consistently showed a more prolonged CORT response than the captive-bred ones while controlling for other individual traits, sampling schedules and slight differences among species. These results seem to physiologically support recent findings by Feenders and Bateson (2011): after human disturbance to caged European starlings (Sturnus vulgaris), wild-caught individuals were slower at decreasing their fear responses and activity than hand-reared birds. Differences between the responses of wild-caught and captive-bred individuals could be explained by factors such as physiological acclimation and/or facilitation (Romero 2004). With acclimation, animals reduce their CORT response to repeated or chronic exposure to noxious stimuli as a consequence of no longer perceiving the stressor to be deleterious (Romero 2004). CORT elevation interacts with receptors in the hypothalamus to down-regulate the intensity of HPA activity through negative feedback and to avoid the detrimental effect of chronic CORT release (Dallman et al. 1992).
In our study, a potential explanation is that both wild-caught and captive-bred parrots were exposed to the same sources of stress but birds born in captivity had a greater degree of physiological acclimation to captivity and human presence than wild-caught birds (Romero 2004). Experimental handling of parent-raised parrot chicks in a previous study (Collete et al. 2000) caused a decrease in CORT levels and an increase of tameness. Captive-bred birds are normally exposed to more frequent human handling than wild-caught ones, such as close-banding and periodical health checks since hatching. Therefore, acclimation may explain why captive-bred birds in our study did not mount a prolonged endocrine response to handling and restraint. On the other hand, wild-caught birds not yet acclimated to the stress of captivity could show facilitation, whereas captive-bred birds did not. Facilitation occurs when animals acclimate to one stressor to improve subsequent CORT responses to new and unpredictable stimuli (Romero 2004). In this scenario, wild-caught birds would show a higher CORT response to a novel challenge such as the experimental restraint protocol. This is an unlikely explanation, however, since wild-caught parrots were repeatedly handled by humans since their capture, restrained in small cages (the same that were used for experiments) for long time periods (even days) during internal and international transport, and handled again by sellers and buyers. Therefore, our experimental protocol would not likely be a novel challenge for them.
There is also the possibility that particular phenotypes could be selected through the international trade process. Cantú et al. (2007) estimated a cumulative mortality approaching 77 % of wild-caught parrots through capture, acclimatisation to captivity, national and international trade, quarantine, and distribution to pet shops, a figure quite similar to that for wild-caught traded birds as a whole (Thomsen et al. 1992). This high mortality could select for individuals better able to cope with stress and that are finally available in the pet market (Carrete et al. 2012). On the other hand, captive breeding can select lines of birds with a particular stress physiology (Martins et al. 2007), given that individuals differ in stress-induced CORT levels which are heritable (Evans et al. 2006). This selection could be unintentional if only the tamest wild-caught individuals, producing lower levels of CORT (Collete et al. 2000), would be able to reproduce in captive conditions. In fact, it is well known by parrot breeders that many wild-caught parrots fail to breed throughout their lives, whereas others from the same species breed soon after capture. This would lead to captive-bred birds differing in stress physiology and behaviour from the original pool of wild-caught conspecifics. Further experimental work is needed to assess these hypotheses for explaining differences in stress physiology between wild-caught and captive bred-birds.
It is worth noting that, whatever the physiological mechanism responsible for attenuating the acute stress response in captive-bred birds, it is acting on the first generation of birds born into captivity. This is in accordance with fear responses recorded by Feenders and Bateson (2011) and Feenders et al. (2011), and with the loss of anti-predatory behaviours shown by the first captive-bred generation of 17 species of parrots and songbirds (Carrete and Tella, unpublished data).
Stress physiology and current avian invasions
Our results may provide a physiological explanation for the lower invasiveness of captive-bred cage-birds (Carrete and Tella 2008). Wild-caught starlings show greater fear responses to people managing the aviary and prolonged attempts to escape the cage (Feenders and Bateson 2011; Feenders et al. 2011), thus increasing their likelihood to escape to the wild when owners clean the cages, feed the birds or try to move them between cages. Moreover, higher levels of circulating CORT following stress result in greater exploratory behavior and greater risk taking in zebra finches (Taenopygia guttata) (Martins et al. 2007), which could result in higher escape abilities. Thereafter, once escaped, wild-caught birds may also have better abilities to face new challenges that would be crucial to their survival. After escaping, exotic birds are often preyed upon by domestic and wild vertebrates. Elevated CORT concentrations during the stress response are associated with an increase in locomotion and mobilization of energy stores (Breuner et al. 1998; Wingfield et al. 1998) and can mediate learning and memory consolidation (Roozendaal 2000) that should allow animals to better escape during these emergency situations, like an attempt of predation, and thus enhance survival (Cabezas et al. 2007; Cote et al. 2006). For example free-ranging tree lizards (Urosaurus ornatus) with higher CORT levels after a predator encounter showed greater initiation flight distances and longer hiding durations (Thaker et al. 2009). Accordingly, fence lizards (Sceloporus undulatus) with elevated CORT concentrations were capable of rapidly learning about novel attackers (Thaker et al. 2010). Thus, if the captive-bred birds had lower responses to stressors, they could be more limited in their ability than wild-caught birds to learn from the experience.
The above explanations are consistent with the observation that around 90 % of captive-bred birds, but only 5 % of wild-caught ones, are preyed upon or recaptured soon after escaping from cages (Carrete and Tella, Unpublished data). Moreover, the prolonged CORT response of wild-caught birds might make them more able to cope with a number of other stressors they may face when trying to establish themselves in new environments, such as competition with native species for food or nest sites (Strubbe and Matthysen 2009). We suggest that the prolonged CORT response found in wild-caught birds may at least partially explain their high probabilities of escape, survival and establishment in new environments and predict their ability as successful invaders (Carrete and Tella 2008). The existence of consistent individual differences in behavioral strategies (personalities, behavioral syndromes or coping styles) and their links with physiological responses to stress have been reported in several animal species, although these links are still not fully understood (Martins et al. 2007). In contrast to the assumed links reported by the personality literature (Koolhaas et al. 1999; Blas et al. 2007), Martins et al. (2007) found that birds with stronger CORT responses to stress tend to enhance their exploratory and risk-taking behaviors, indicating they have proactive or bold personalities which could boost their invasive skills. More research is clearly needed to understand the role of inter-individual variability in physiology and behavior in the invasion of novel environments (Réale et al. 2007; Carrete and Tella 2011; Carrete et al. 2012).
References
Archard GA, Braithwaite VA (2010) The importance of wild populations in studies of animal temperament. J Zool 281:149–160
Bauer H-G, Woog F (2008) Nichtheimische Vogelarten (Neozoen) in Deutschland, Teil I: Auftreten, Bestände und Status. Vogelwarte 46:157–194
Blackburn TM, Lockwood JL, Cassey P (2009) Avian invasions. The ecology and evolution of exotic birds. Oxford University Press, Oxford
Blas J, Baos R, Bortolotti GR, Marchant T, Hiraldo H (2005) A multi-tier approach to identifying environmental stress in altricial nestling birds. Funct Ecol 19:315–322
Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA (2007) Stress response during development predicts fitness in a wild, long-lived vertebrate. Proc Natl Acad Sci USA 104:8880–8884
Breuner CW, Greenberg AL, Wingfield JC (1998) Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen Comp Endocrinol 111:386–394
Cabezas S, Blas J, Marchant TA, Moreno S (2007) Physiological stress levels predict survival probabilities in wild rabbits. Horm Behav 51:313–320
Cantú JC, Sánchez ME, Grosselet M, Silva J (2007) Tráfico ilegal de pericos en México. Una evaluación detallada. Defenders of Wildlife, NW
Carrete M, Tella JL (2008) Wild-bird trade and exotic invasions: a new link of conservation concern? Front Ecol Environ 6:207–211
Carrete M, Tella JL (2011) Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS ONE 6(4):e18859
Carrete M, Edelaar P, Blas J, Serrano D, Potti J, Dingemanse NJ, Tella JL (2012) Don’t neglect pre-establishment individual selection in deliberate introductions. Trends Ecol Evol 27:67–68
Collete JC, Millam JR, Klasing KC, Wakenell PS (2000) Neonatal handling of Amazon parrots alters the stress response and immune function. Appl Anim Behav Sci 66:335–349
Cote J, Clobert J, Meylan S, Fitze PS (2006) Experimental enhancement of corticosterone levels positively affects subsequent male survival. Horm Behav 49:320–327
Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS (1992) Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–526
Evans MR, Roberts ML, Buchanan KL, Goldsmith AR (2006) Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol 19:343–352
Feenders G, Bateson M (2011) Hand-rearing reduces fear of humans in European starlings. Sturnus vulgaris. PLoS ONE 6(2):e17466
Feenders G, Klaus K, Bateson M (2011) Fear and exploration in European starlings (Sturnus vulgaris): a comparison of hand-reared and wild-caught birds. PLoS ONE 6(4):e19074
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–19
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935
Lever C (2005) Naturalised birds of the world. T, Poyser AD, London
Martins TLF, Roberts ML, Giblin I, Huxham R, Evans MR (2007) Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Hormon Behav 52:445–453
Mason G (2010) Species differences in responses to captivity: stress, welfare and the comparative method. Trends Ecol Evol 25:713–721
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:1–28
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A 140:73–79
Romero LM, Remage-Healey L (2000) Daily and seasonal variation in response to stress in captive starlings (Sturnus vulgaris): corticosterone. Gen Comp Endocrin 119:52–59
Roozendaal B (2000) Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25:213–238
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endoc Rev 21:55–89
Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L (2005) Big brains, enhanced cognition, and response of birds to novel environments. Proc Natl Acad Sci USA 102:5460–5465
Strubbe D, Matthysen E (2009) Experimental evidence for nest-site competition between invasive ring-necked parakeets (Psittacula krameri) and native nuthatches (Sitta europaea). Biol Conserv 142:1588–1594
Tella JL (2011) The unknown extent of ancient bird introductions. Ardeola 58:399–404
Thaker M, Lima SL, Hews DK (2009) Alternative antipredator tactics in tree lizard morphs: hormonal and behavioural responses to a predator encounter. Anim Behav 77:395–401
Thaker M, Vanak AT, Lima SL, Hews DK (2010) Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor. Am Nat 175:50–60
Thomsen JB, Edwards SR, Mulliken TA (1992) Perceptions, conservation and management of wild birds in trade. TRAFFIC International, WWF and IUCN, Cambridge
Vögeli M, Serrano D, Tella JL, Méndez M, Godoy JA (2007) Sex determination of dupont’s lark Chersophilus duponti using molecular sexing and discriminant functions. Ardeola 54:69–80
Wingfield JC, Ramenofsky M (1999) Hormones and the behavioral ecology of stress. In: Balm PHM (ed) Stress physiology in animals. Academic Press, Sheffield, pp 1–51
Wingfield JC, Romero LM (2001) Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen BS, Goodman HM (eds) The endocrine system, coping with the environment: neural and endocrine mechanisms. Handbook of physiology. Oxford University Press, New York, pp 211–234
Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Sharon L, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone-behavior interactions: the “Emergency Life History Stage”. Am Zool 38:191–206
Acknowledgments
We thank V. Fachal, I. Luque, G. Fairhurst, M. Manogué and J.A. Arriola for their assistance, and two anonymous reviewers for their suggestions. Funding was provided by NSERC and the Stuart and Mary Houston Professorship in Ornithology (to GRB), Excellence Project P07RNM02918 (to JLT and MC), and a postdoctoral fellowship from the Spanish MEC (to SC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gary R. Bortolotti: Deceased.
Rights and permissions
About this article
Cite this article
Cabezas, S., Carrete, M., Tella, J.L. et al. Differences in acute stress responses between wild-caught and captive-bred birds: a physiological mechanism contributing to current avian invasions?. Biol Invasions 15, 521–527 (2013). https://doi.org/10.1007/s10530-012-0304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0304-z