Abstract
The most common invasive mammals—mice, rats, and cats—have been introduced to islands around the world, where they continue to negatively affect native biodiversity. The eradication of those invasive mammals has had positive effects on many species of seabirds. However, the removal of one invasive mammal species may result in abundance changes of other species due to trophic and competitive interactions among species. Understanding the overall impact of several invasive species is a key challenge when evaluating the possible effects of eradication programmes. Here we assess the influence of the three most common invasive mammals on nest survival of Cory’s shearwater (Calonectris diomedea). We monitored six breeding colonies over 3 years and measured the activity of mice, rats and cats to examine the influence of invasive mammals on nest survival. We found that nest survival showed a similar temporal trend in all years, with lowest weekly survival probabilities shortly after chicks hatched. Cats were identified as major predators of chicks, but no measure of colony-specific cat activity was able to adequately explain variation in shearwater nest survival. Nest survival was on average 0.38 (95 % confidence interval 0.20–0.53) and varied among colonies as well as over time. We found a small positive influence of rats on nest survival, which may indicate that the presence of small rodents as alternative prey may reduce cat predation of chicks. Our findings suggest that the eradication of rodents alone may exacerbate the adverse effects of cats on shearwater nest survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assisted by humans, the most common invasive mammals, namely rats (Rattus spp.), mice (Mus domesticus), and cats (Felis catus) have successfully colonised the vast majority of islands around the world (Jeschke and Genovesi 2008). These invasive alien mammal species are widely recognised as a principal threat to the survival of many seabird species around the world (Jones et al. 2008; Bonnaud et al. 2009; Towns et al. 2009).
Many islands have been invaded by more than one invasive mammal species, and their effects on seabirds may differ on islands that host different assemblages of introduced mammals, especially because cats and rats are opportunistic species that consume prey according to relative availability (Clark 1981; Fitzgerald 1988). Many studies have examined the impact of mice (Wanless et al. 2007, 2009), rats (Thibault 1995; Igual et al. 2006; Ruffino et al. 2009; Brooke et al. 2010) or cats (Keitt et al. 2002; Bonnaud et al. 2009; Medina et al. 2011) on the productivity of seabirds on islands, but few studies have examined the effects of multiple invasive mammals on seabirds (Cuthbert 2002; Bonnaud et al. 2010). However, understanding the relative importance of coexisting invasive mammal species on seabird reproductive success is important to guide conservation management (Rayner et al. 2007).
Over the past decades much progress has been made to remove invasive alien mammals from islands where they have negative effects on biodiversity (Towns and Broome 2003; Nogales et al. 2004; Howald et al. 2007). Successful eradications have safeguarded many seabird populations, and eradications are considered for an increasing number of islands around the world (Brooke et al. 2007; Aguirre-Muñoz et al. 2009; Capizzi et al. 2010; Oppel et al. 2011; Veitch et al. 2011). Due to trophic and competitive interactions among mammal species (Courchamp et al. 1999, 2000), the removal of only one invasive species may result in abundance changes of other species (Caut et al. 2007; Bergstrom et al. 2009; Bonnaud et al. 2010), which can alter predation rates on breeding seabirds (Rayner et al. 2007; Hughes et al. 2008).
All islands in the Azores Archipelago (Portugal) have been invaded by mice, rats, and cats. The Azores were first colonised by humans in the 15th century, and seabird populations have experienced strong declines due to human exploitation and predation by introduced mammals (Monteiro et al. 1996). Some species became extinct on the nine main islands, or are confined to inaccessible cliffs on islands where they still persist (Monteiro et al. 1996), but larger species such as the Cory’s shearwater (Calonectris diomedea borealis) still nest on all the nine main islands. The Cory’s shearwater estimated population in the Azores is 188000 pairs (BirdLife International 2004), but invasive mammals and other human threats are likely to cause ongoing population declines (Fontaine et al. 2011). However, very little is known about the relative importance of mice, rats, and cats on the reproductive output of Cory’s shearwater, thus impeding the prioritisation of species that should be targeted for eradication and/or control.
The goal of the present study was to evaluate the relative importance of mice, rats and cats on the breeding success of Cory’s shearwaters. We studied Cory’s shearwater breeding success in six colonies that varied in their habitat structure, elevation, and distance to human habitation, and thus provided conditions under which the abundance of invasive mammals was expected to vary. We used different approaches over 3 years to determine rodent and cat activity in colonies, and estimated the effects of predator activity indices on nest survival. Our study thus provides an assessment of which invasive mammal species exerts the most influence on Cory’s shearwater breeding success in a situation where multiple invasive mammal species co-exist and interact.
Methods
Study area
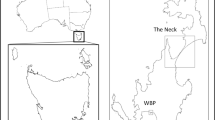
This study was conducted on Corvo, the smallest (1,700 ha) inhabited island of the Azores located half-way between the European continent and North America (39°40′14.62″N, 31°7′11.60″W). Corvo is of volcanic origin and the maximum elevation is 718 m, with steep cliffs >200 m in height surrounding most of the island (Fig. 1).
The island has been permanently occupied by humans since 1580 (Branco et al. 2008), and is currently inhabited by 435 people living in one village at the southern tip of the island. Corvo was one of the last islands of the Azores archipelago to be colonized by rodents (~late 17th century: brown rat Rattus norvegicus, black rat R. rattus, house mouse) and cats (Fructuoso 1591). However, we did not record the presence of brown rat during our project (100 % of 69 captured individuals were black rat). Human exploitation, habitat loss and predation have led to the decline of Cory’s shearwaters on Corvo (Monteiro et al. 1996), but human exploitation ceased in the late 1990s and no longer accounts for low breeding success.
Cory’s shearwater nest monitoring
Fieldwork was carried out during three successive breeding seasons, from 2009 to 2011. In March and April 2009, we conducted nocturnal acoustic surveys to detect breeding areas and locate shearwater nests. The nests found with signs of occupation (faeces or feathers at entrance of burrows) were grouped into six spatially segregated colonies that differed by habitat characteristics (Fig. 1; Table 1). Nest cavities were marked individually and checked every week from 15th May (some days prior to laying) to 31st October (when juveniles leave the colonies). At each visit, nests were checked using a torch and a burrow-scope (elongated remote camera) for burrows that did not allow a straight line of sight to the nest chamber. In each week, we recorded the presence of adult, egg or chick, and examined the burrow surroundings for evidence of predation if a previously existing egg or chick had disappeared. Predation was assumed when remains of eggs or dead chicks were found inside or at the entrance of burrows (Igual et al. 2006). To eliminate the possibility that chicks had died naturally and were scavenged by opportunistic mammalian predators, we considered chicks only as predated if they contained conclusive teeth marks. Cats usually kill prey with a bite directed at the nape, which inflicts a rapid death and avoids injury to the predator (Biben 1979; Lyver 2000) and we used teeth marks on eggs and chicks to determine the species of predator.
In 2011, we used infrared-triggered camera traps (Bushnell TrophyCam 8MP) at nest entrances to identify predators and verify that cats and rats were the only nest predators in our study area. Nests were considered successful if fully grown chicks disappeared after 22 weeks.
Environmental determinants of nest survival and predation risk
Nest success of Cory’s shearwaters is known to vary with the abundance of introduced mammal predators (Thibault 1995; Igual et al. 2006) and the characteristics of nesting burrows (Granadeiro 1991; Thibault 1995; Igual et al. 2006; Bourgeois and Vidal 2007). To identify the most important factors influencing variation in nest survival on Corvo we measured physical variables of nesting burrows and activity of potential mammalian predators in each colony.
For each nest cavity we measured four continuous variables referring to the cavity dimensions and vegetation cover around the nest entrance and eight categorical variables related to the presence of protecting structures, nest substrate and orientation. We measured the maximum width of the nest entrance at ground level (nest width), the maximum height of the nest entrance (nest height), the maximum distance from the entrance to the back of the nest cavity (nest length), and visually assessed the proportion of ground vegetation cover within 1 m of the nest cavity entrance. In addition, we recorded the presence or absence of rock walls around a nest site, and whether the nest contained a chamber and a curved entrance. We classified the nest substrate as either soil or rock, and determined the orientation of the nest entrance into one of the four cardinal directions. The elevation and the distance to the village were measured for each nest using a GPS. The surrounding habitat of the nest site was classified into five categories: coastal rocks, semi-natural grassland, pasture, riparian woodland, and invasive Giant Reed (Arundo donax).
The examination of shearwater tissues in the diet of cats would be an ideal metric of predation pressure, however we used a measure of predator activity because the scats (or any other metric of diet) found in a given colony may not reflect the diet consumed in that colony, since the spatial scale of shearwater colonies is insufficient to contain a cat’s entire home range in our study system.
Rodent activity
To determine the presence and relative abundance of rodents, we used tracking tunnels in 2009 and wax blocks in 2010 and 2011. In 2009, 45 rodent footprint-tracking tunnels (King and Edgar 1977) were placed across the colonies, at 50 m intervals on nine lines (five tracking tunnels per line). We used two lines in colonies (Fajã, Fonte and Pão) with higher spatial separation between nests in order to estimate abundance for the nest area, and only one line in the remaining colonies. The tracking tunnels were baited with peanut butter and rodent activity was recorded for one active night in May (beginning of Cory’s breeding season) and one in October (at the end of the breeding season). The identification of footprints from different species was determined using descriptions given by Gillies and Williams (2002). The relative abundance of rodents per colony was calculated as the number of tracking-tunnels with footprints (rat or mouse) divided by the number of active tracking-tunnels in each colony. Because the tracking tunnels performed poorly under climatic conditions on Corvo, we used wax blocks in 2010 and 2011 to record rodent abundance (Thomas 1999). A regular grid with 20 wax-blocks spaced 50 m apart was established in each colony. To track changes in rodent activity over the breeding season, we set wax blocks for one night per month from May to October 2010 and 2011. Wax-blocks were made of a non-toxic mix of paraffin with peanut butter as an attractant (9:1), moulded in plastic ice cube trays, dipped in red colorant to improve visibility in the field, and anchored to the ground with wire. The relative abundance indices were estimated as the number of wax-block with bite-marks of either rats or mice divided by the total number of wax blocks used per colony.
Cat activity
The activity of feral cats was studied in 2010 using camera traps in the same area used to assess rodent activity in each of the six colonies. Estimating the abundance of solitary carnivores is challenging (Gardner et al. 2010; Obbard et al. 2010; Can et al. 2011), and hence, we tested multiple approaches in 2010 to measure cat activity in Cory’s shearwater colonies.
We used passive infra-red motion sensor cameras (Bushnell TrophyCam 8MP), programmed with similar settings (normal sensor; three photos at each trigger approximately 2 s apart; 10 s delay between each trigger), and located in the centre of randomly chosen 15 m by 15 m grid cells in each colony. Cats recorded by camera traps were individually identified based on habitus and coat colour pattern (Sarmento et al. 2010). We aimed to maximise spatial coverage in each colony by rotating camera traps among 120 random locations distributed throughout colonies (Foster and Harmsen 2012). We deployed each camera trap for 2 weeks at each random location, and all camera locations were recorded with a GPS to the nearest 5 m. Initially, we attempted to estimate cat density using spatially explicit capture-recapture models (Efford et al. 2009), but because the spatial extent of shearwater colonies was less than an average home range of feral cats (Konecny 1987; Moseby et al. 2009; Bengsen et al. 2012), the density estimates were uninformative. We explored three alternative simple indices of cat activity to explain variation in Cory’s shearwater nest survival in 2010, namely the number of cats per active camera trap day in each colony, the number of individual cats recorded in each colony during the breeding season, and the number of individual cats recorded each month as a temporally varying measure of cat activity.
Analysis of breeding success and nest survival
We present the following reproductive parameters to facilitate comparison with other studies: hatching success (n eggs hatched/n eggs laid), fledging success (n chicks fledged/n eggs hatched) and breeding success (n chicks fledged/n breeding pairs). All values are given as mean ± SD.
The approaches described above examine only the final outcome of nests, but given the long breeding season of Cory’s shearwaters it is likely that nest survival varies over time. In order to assess whether temporally fluctuating predator activity coincided with temporal changes in nest survival, we estimated weekly survival probabilities of Cory’s shearwater nests for the 21 weekly intervals spanning the breeding season of Cory’s shearwaters on Corvo. We used Program MARK (White and Burnham 1999) to evaluate biologically plausible scenarios explaining variation in Cory’s shearwater weekly nest survival (Dinsmore et al. 2002). These models allowed us to test whether there was support for temporal variation in weekly nest survival probabilities over the breeding season, and examine which of the environmental covariates had the greatest influence on weekly nest survival probabilities.
Our modelling approach proceeded in two steps: we first constructed ten models examining different temporal variation in weekly nest survival probabilities, and then used the most parsimonious temporal model structure as basis for further models examining the influence of environmental covariates. The ten temporal model structures considered (1) constant weekly nest survival throughout the breeding season, (2) different survival between egg and chick stage, (3) a linear trend, (4) a quadratic trend, or (5) weekly varying nest survival probabilities. These five model structures describing within-year variation were considered to be either equal among years or different for each year, resulting in ten candidate models of temporal variation in nest survival.
Prior to the second step, we tested whether environmental variables were correlated, and we did not include highly correlated (Spearman r s > 0.6) variables in the same model (Zuur et al. 2009). We then constructed nine candidate models representing different biological hypotheses to explain variation in Cory’s shearwater nest survival. Specifically, we tested whether nest survival varied with elevation and distance to human habitation (elevation model), among colonies (colony model), among habitat types (habitat model), with physical characteristics of the burrow influencing access for predators (height and presence of a chamber: burrow model), or between substrates and vegetation cover (vegetation model). In addition, we tested whether rat activity (rat model) and mouse activity (mouse model) explained variation in nest survival. Lastly, we included two models that examined rat or mouse activity and variation among colonies (rat colony and mouse colony model, respectively) to account for differences in habitat, elevation, and distance to human habitation.
We tested whether any cat activity metric explained variation in Cory’s shearwater nest survival for a subset of data from the 2010 season when we were able to measure cat activity in multiple ways. We analysed nest survival for the 2010 data by adding three competing models with different cat activity measures to the candidate model set outlined above. The three models describing cat activity included either the number of cats/trap night, the number of individual cats, or the number of individual cats/month, respectively. We report the support for each of those models in terms of evidence ratio and the Akaike weight ωAIC c .
Analysis of nest predation risk
As the majority of nest failures were due to predation (see “Results”), we examined whether any of the physical environmental variables associated with each burrow could explain which burrows were more vulnerable to predation. In this analysis we contrasted predated and non-predated nests, as opposed to the previous analysis which contrasted successful and failed nests. We identified important variables distinguishing between predated and non-predated nests using a machine learning approach based on ensembles of regression trees (RandomForest; Breiman 2001). This approach was appropriate for our small data set (n = 287 nests with known fate) with non-independent observations, and a large number (18) of explanatory variables that are likely to interact (Cutler et al. 2007; Hochachka et al. 2007; Olden et al. 2008; Grömping 2009; Oppel et al. 2009). We used the R package ‘randomForest’ and the extensions for variable selection provided by Murphy et al. (2010) to identify the most important variables. We averaged variable importance over 500 bootstrap replications, and considered all variables with an average relative importance value >50 % to be influential.
Results
Breeding success and predation rate
Overall breeding success of Cory’s shearwater on Corvo from 2009 to 2011 was 39 % (Table 2). Hatching success ranged from 0.66 to 0.82 among colonies, and was generally higher than fledging success (0.39–0.70; Table 2). The number of egg-neglect was low, 17 eggs were abandoned in total, and we found six dead chicks without predation signs in 2010.
Most causes of breeding failure were due to predation. Out of 287 recorded nest failures, 232 (81 %) showed obvious signs of predation by either cats or rats, and in 32 (11 %) nests the cause of failure remained unknown. Chick predation mainly occurred soon after hatching, when chicks were between six and 14 days old. Most predated chicks were found at the entrance of the nests, some of them were not eaten but showed incisor-marks at the neck. Of the 232 predated nests where sufficient evidence existed to positively identify the species of predator, 195 (84 %) were predated by cats and 37 (16 %) by rats. In 2011, camera traps recorded four predation events by cats and one predation by a rat (n = 11 predated nests with cameras, but at six nests the camera malfunctioned).
Environmental determinants of nest survival and predation risk
Rodent activity
Rodent activity varied over the course of the breeding season, with generally higher activity of rats during the chick rearing stage than during incubation, while mouse activity was higher during incubation (Table 3). As expected, rodent activity also varied among colonies, with the colony closest to the sea (Fajã) and without any vegetation having the lowest rodent activity indices (Table 3).
Cat activity
We identified 53 individual cats in 213 unique detections over the course of the 2010 breeding season. The different cat activity metrics ranked the colonies consistently (Table 3). The colony closest to the communal rubbish tip (Pão) had the highest cat detection rate and number of individual cats, whereas the colony furthest away from the village (Cancela) had the lowest cat detection rate and number of individual cats (Table 3). Moreover, the images showed that some of the identifiable cats visited more than one colony, and that some cats that visited shearwater colonies were domestic.
Analysis of variation nest survival
The temporal model structure accounting for weekly variation in nest survival probability and varying among years received overwhelming support from the data (ωAIC c = 0.9997), and was used in all models exploring the influence of environmental covariates. In all years, nest survival was high at the start and at the end of the breeding season, with a marked decrease shortly after hatching (Fig. 2). Nest survival varied among colonies, and the three most parsimonious models included ‘colony’ as the explanatory variable (Table 4). However, there was model selection uncertainty among those three models, with models including rat or mouse activity receiving similar support from the data (Table 4). These models indicated that nest survival increased with higher rat activity (rat colony model, b = 0.55 ± 0.44 SE), but decreased slightly with higher mouse activity (mouse model, b = −0.05 ± 0.43). The only other model that received some support by the data indicated that nest survival was lowest in rocky coastal habitat, and highest in areas characterised by invasive Giant Reed (habitat model, Table 4). Model-averaged mean nest survival across all years was 0.38 (95 % confidence interval 0.20–0.53), and the most parsimonious model explained 95 % of the variation in Cory’s shearwater nest survival.
For 2010, we additionally estimated support for each of three cat metrics on variation in Cory’s shearwater nest survival. The models using the simple indices of number of cats per trap night and the number of individual cats per colony performed best out of the three cat activity metrics that we tested, but none of the three cat models received much support from the data (ωAIC c = 0.07, Table 5). Model averaged nest survival in 2010 was 0.46 (95 % confidence interval 0.17–0.68), and the burrow model received the most support from the data and explained 83.5 % of the variation (Table 5). This model indicated that nest survival increased with lower nest height (b = −0.008 ± 0.006) and with the presence of a chamber inside the nest (b = 0.98 ± 0.38).
Analysis of nest predation risk
As most nest failures were due to predation, we assessed which environmental variables had the largest influence on predation risk. The most influential variables explaining the probability of a nest being predated were nest height and elevation (Table 6). Predation probability was higher for nests with a higher entrance and at lower elevations (Fig. 3).
Discussion
Breeding success and predation effect
Cory’s shearwaters on Corvo had one of the lowest values of breeding success among all available studies of this species, and the main cause of nest failure was predation by introduced mammalian predators. Cats were the most destructive invasive mammal, accounting for >80 % of predated nests. Chick mortality due to predators could be difficult to distinguish from natural chick mortality if chicks were subsequently scavenged by cats or rats. We are confident that most dead chicks were actually killed by mammals, because they exhibited distinctive incisor marks on the neck (Biben 1979). Although a small number of dead chicks may have died naturally and may have been scavenged subsequently, we believe that this would affect only a very small proportion of chicks. The fat levels of chicks we considered depredated suggested that none of them had died of starvation (Lyver 2000) and we never observed any scavenging on naturally deceased chicks.
Other studies of Cory’s shearwater have found breeding success ranging from 0.37 (Granadeiro 1991) to 0.52 (Faial, n = 47; J. Bried unpublished data) on islands with introduced rats or cats in the Atlantic, and from 0.44 (Thibault 1995) to 0.64 in the Mediterranean (Genovart 2001). However, on islands without introduced predators breeding success can be substantially higher (Fontaine et al. 2011), reaching 0.86 (Pascal et al. 2008). Natural limits to breeding success can be competition for nest sites (Ramos et al. 1997), or lack of experience of breeding birds (Mougin et al. 2000). The latter two causes are unlikely to majorly affect the breeding success on Corvo, where the vast majority of nest failures were due to predation by introduced mammals.
To our knowledge there are no studies on Cory’s shearwater breeding success on islands with both rats and cats. However, cats are well known to prey on breeding shearwaters (Keitt and Tershy 2003; Martínez-Gómez and Jacobsen 2004; Bonnaud et al. 2009) and our study confirms that low breeding success was mostly due to high cat predation rates. Some authors have argued that cats may have a beneficial effect on seabird colonies because they reduce the abundance of smaller predators like rodents (Courchamp et al. 1999). Cats are however opportunistic predators and will consume shearwater eggs and chicks when these are available and readily accessible (Bonnaud et al. 2007; Peck et al. 2008).
Rats are also known to be predators of seabirds (Jones et al. 2008), and affect Cory’s shearwaters on several islands (Granadeiro 1991; Thibault 1995; Genovart 2001; Igual et al. 2006). Our study, conducted on an island with cats and rats, showed that nest survival appeared to increase in colonies with a higher rat abundance index. Our interpretation of this counter-intuitive result is that rats may serve as main food source for cats (Bonnaud et al. 2007), and that higher availability of rats may therefore limit cat predation of Cory’s shearwater chicks (see Dumont et al. 2010) rather than increase cat predation due to the attraction of cats to areas with higher rat abundance. Such an interaction would have important implications for the planning of predator control or eradication operations (Collins et al. 2009). As Corvo is an inhabited island, the local community must embrace any eradication plans (Oppel et al. 2011). While the eradication of rats is broadly supported by the local community, there is considerable scepticism to the eradication of all cats from Corvo. Our results indicate that rat eradication alone may however increase cat predation on Cory’s shearwater nests, and may thus have an undesirable negative conservation outcome.
Environmental determinants of nest survival and predation risk
Our analysis revealed that nest survival varied over time and in the different colonies that we studied. Variation among colonies is most likely due to differences in habitat, abundance of invasive mammals, microclimate, and potentially factors that we were not able to measure. Our measures of rodent activity explained some variation in nest survival; however, we were not able to identify a measure that would adequately quantify the predation pressure of cats as the main predator. We found a striking temporal pattern in the weekly survival probability of Cory’s Shearwater nests that was consistent across all years. The lowest survival probabilities occurred shortly after hatching, indicating heavy predation pressure on young chicks, as has been documented for other colonies (Catry et al. 2009). It is likely that cats prey mainly on small chicks rather than on eggs (Imber et al. 2000), as incubating adults may be large enough to deter burrow intruders.
Despite cats being the most important nest predators, our measures of cat activity explained very little of variation in nest survival in 2010. Due to the length of the Cory’s shearwater breeding season (Granadeiro 1991; Ramos et al. 2003) there is considerable potential for cat activity to fluctuate over the breeding season. Hence, two of our cat activity metrics that were constant across the whole breeding season were likely inappropriate to reflect the temporally varying cat pressure on Cory’s shearwater nests. We expect that the correlation between cat activity indices and shearwater nest survival could be improved if cat activity were measured at a higher temporal resolution; however, this would entail a significant logistical effort that was not feasible in our study.
Environmental variables influencing predation risk
We found that higher burrows that were at lower elevation had the highest risk of being predated. This pattern is consistent with cats being the key nest predator for Cory’s shearwaters on Corvo. Very low nest heights presumably limit access to cats, and are therefore more protected from predation. Elevation may adequately reflect the accessibility of burrows for cats on Corvo, because most cats live around the village near sea level. Nests near sea level had the highest predation rates, whereas nests that were at higher elevations, and mostly further away from the village, had lower predation risk. We believe that the relationship with elevation is an island-specific phenomenon, which may not be transferrable to other islands where cats prey on seabirds.
Implications for future studies and conservation management
Predation is the main cause of breeding failure of Cory’s shearwater colonies on Corvo; however, activity or abundance metrics did not always explain most variation in nest survival in our study and may therefore not accurately portray predation risk. Measuring mammalian predators is difficult and the measurements we used at colony level may not be adequate to describe the risk of each individual nest to predation. Future studies measuring the relationship between mammalian predators and nest survival may benefit from accurate indices of mammal abundance at high temporal resolution to allow matching of mammal phenology to the temporal variation in nest survival. Although our simple metrics of breeding success and the more sophisticated analysis of nest survival yielded the same estimates, the analysis of weekly nest survival facilitates examination of temporal variation in nest survival and mammalian activity.
For long-lived seabird species like Cory’s Shearwater adult survival is likely to have a much stronger influence on population growth rate than nest success. However, current adult and juvenile survival rates of Cory’s Shearwaters in the Azores may be too low to maintain stable populations (Fontaine et al. 2011), yet the management actions required to improve adult survival are extremely challenging. Hence, increasing reproductive success may yield only a smaller change in population growth rate, but may be much more feasible from a political and socioeconomic perspective (Bentzen and Powell 2012).
Given the high levels of nest predation on Cory’s shearwaters, the eradication of all invasive mammals from Corvo would likely increase reproductive success. However, we believe that the eradication of all cats on Corvo is complicated by the co-existence of feral and domestic cats. Domestic cats were observed in our study to visit shearwater colonies and kill shearwater chicks, hence removing only feral cats will not entirely solve the problem of cat predation. In addition, because there is no sterilisation of all domestic cats to prevent the birth and subsequent release of unwanted kittens, the eradication of the feral cat population would yield only temporary benefits and a feral population would quickly re-establish. There is also no legislation that controls the introduction of new cats to the island. We recommend the implementation of a domestic cat register, and mandatory identification by microchip and sterilization of domestic cats before any feral cat eradication is attempted on Corvo (Ratcliffe et al. 2010; Calver et al. 2011). Given socio-political realities and practical limitations (Oppel et al. 2011), the eradication of feral cats may never find the support of local cat owners, and potential consequences need to be carefully considered before only some of the trophically linked mammals (e.g. rats) are removed (Courchamp et al. 1999; Le Corre 2008; Dumont et al. 2010). The removal of only rats could increase seabird predation by either cats, or potentially by mice (Caut et al. 2007; Wanless et al. 2007). For Corvo, we advise against the eradication of rats unless cats can be eradicated at the same time.
References
Aguirre-Muñoz A, Croll DA, Donlan CJ, Henry RW, Hermosillo MA, Howald GR, Keitt BS, Luna-Mendoza L, Rodríguez-Malagón M, Salas-Flores LM, Samaniego-Herrera A, Sanchez-Pacheco JA, Sheppard J, Tershy BR, Toro-Benito J, Wolf S, Wood B (2009) High-impact conservation: invasive mammal eradications from the islands of Western México. Ambio 37:101–107
Bengsen AJ, Butler JA, Masters P (2012) Applying home-range and landscape-use data to design effective feral-cat control programs. Wildl Res 39:258–265
Bentzen RL, Powell AN (2012) Population dynamics of king eiders breeding in northern Alaska. J Wildl Manag. doi:10.1002/jwmg.335
Bergstrom DM, Lucieer A, Kiefer K, Wasley J, Belbin L, Pedersen TK, Chown SL (2009) Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol 46:73–81
Biben M (1979) Predation and predatory play behaviour of domestic cats. Anim Behav 27:81–94
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. In BirdLife Conservation Series No. 12. BirdLife International Cambridge, UK
Bonnaud E, Bourgeois K, Vidal E, Kayser Y, Tranchant Y, Legrand J (2007) Feeding ecology of a feral cat population on a small Mediterranean island. J Mammal 88:1074–1081
Bonnaud E, Bourgeois K, Vidal E, Legrand J, Le Corre M (2009) How can the Yelkouan shearwater survive feral cat predation? A meta-population structure as a solution? Popul Ecol 51:261–270
Bonnaud E, Zarzoso-Lacoste D, Bourgeois K, Ruffino L, Legrand J, Vidal E (2010) Top-predator control on islands boosts endemic prey but not mesopredator. Anim Conserv 13:556–567
Bourgeois K, Vidal E (2007) Yelkouan shearwater nest-cavity selection and breeding success. Compt Rend Biol 330:205–214
Branco CC, Bento MS, Gomes CT, Cabral R, Pacheco PR, Mota-Vieira L (2008) Azores Islands: genetic origin, gene flow and diversity pattern. Ann Hum Biol 35:65–74
Breiman L (2001) Random forests. Mach Learn 45:5–32
Brooke MD, Hilton GM, Martins TLF (2007) Prioritizing the world’s islands for vertebrate-eradication programmes. Anim Conserv 10:380–390
Brooke M, O’Connell TC, Wingate D, Madeiros J, Hilton GM, Ratcliffe N (2010) Potential for rat predation to cause decline of the globally threatened Henderson petrel Pterodroma atrata: evidence from the field, stable isotopes and population modelling. Endanger Species Res 11:47–59
Calver MC, Grayson J, Lilith M, Dickman CR (2011) Applying the precautionary principle to the issue of impacts by pet cats on urban wildlife. Biol Conserv 144:1895–1901
Can ÖE, Kandemir I, Togan I (2011) The wildcat Felis silvestris in northern Turkey: assessment of status using camera trapping. Oryx 45:112–118
Capizzi D, Baccetti N, Sposimo P (2010) Prioritizing rat eradication on islands by cost and effectiveness to protect nesting seabirds. Biol Conserv 143:1716–1727
Catry P, Matias R, Vicente L, Granadeiro JP (2009) Brood-guarding behaviour in Cory’s Shearwaters Calonectris diomedea. J Orn 150:103–108
Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, Courchamp F (2007) Rats dying for mice: modelling the competitor release effect. Austral Ecol 32:858–868
Clark DA (1981) Foraging patterns of black rats across a desert-montane forest gradient in the Galapagos Islands. Biotropica 13:182–194
Collins PW, Latta BC, Roemer GW (2009) Does the order of invasive species removal matter? The case of the eagle and the pig. PLoS One 4:6
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68:282–292
Courchamp F, Grenfell B, Clutton-Brock T (2000) Impact of natural enemies on obligately cooperative breeders. Oikos 91:311–322
Cuthbert R (2002) The role of introduced mammals and inverse density-dependent predation in the conservation of Hutton’s shearwater. Biol Conserv 108:69–78
Cutler DR, Edwards TC, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ (2007) Random forests for classification in ecology. Ecology 88:2783–2792
Dinsmore SJ, White GC, Knopf FL (2002) Advanced techniques for modeling avian nest survival. Ecology 83:3476–3488
Dumont Y, Russell JC, Lecomte V, Le Corre M (2010) Conservation of endangered endemic seabirds within a multi-predator context: the Barau’s petrel in Réunion Island. Nat Resour Model 23:381–436
Efford MG, Dawson DK, Borchers DL (2009) Population density estimated from locations of individuals on a passive detector array. Ecology 90:2676–2682
Fitzgerald B (1988) Diet of domestic cats and their impact on prey populations. In: Turner DC, Bateson P (eds) The domestic cat: the biology of its behavior. Cambridge University Press, Cambridge
Fontaine R, Gimenez O, Bried J (2011) The impact of introduced predators, light-induced mortality of fledglings and poaching on the dynamics of the Cory’s shearwater (Calonectris diomedea) population from the Azores, northeastern subtropical Atlantic. Biol Conserv 144:1998–2011
Foster RJ, Harmsen BJ (2012) A critique of density estimation from camera-trap data. J Wildl Manag 76 (in press)
Fructuoso G (1591) Saudades da Terra VI. Instituto Cultural de Ponta Delgada, Ponta Delgada
Gardner B, Reppucci J, Lucherini M, Royle JA (2010) Spatially explicit inference for open populations: estimating demographic parameters from camera-trap studies. Ecology 91:3376–3383
Genovart M (2001) Seguiment de la colònia de cria de virot Calonectris diomedea a l’illot des Pantaleu. Anuari Ornitòlogic de les Balears 16:23–28
Gillies C, Williams D (2002) Using tracking tunnels to monitor rodents and other small mammals. Department of Conservation unpublished report (HAMRO-60778) Northern Regional Office, Hamilton
Granadeiro JP (1991) The breeding biology of Cory’s shearwater Calonectris diomedea borealis on Berlenga Island, Portugal. Seabird 13:30–39
Grömping U (2009) Variable importance assessment in regression: linear regression versus random forest. Am Stat 63:308–319
Hochachka WM, Caruana R, Fink D, Munson ART, Riedewald M, Sorokina D, Kelling S (2007) Data-mining discovery of pattern and process in ecological systems. J Wildl Manag 71:2427–2437
Howald G, Donlan CJ, Galvan JP, Russell JC, Parkes J, Samaniego A, Wang YW, Veitch D, Genovesi P, Pascal M, Saunders A, Tershy B (2007) Invasive rodent eradication on islands. Conserv Biol 21:1258–1268
Hughes BJ, Martin GR, Reynolds SJ (2008) Cats and seabirds: effects of feral domestic cat Felis silvestris catus eradication on the population of Sooty Terns Onychoprion fuscata on Ascension Island, South Atlantic. Ibis 150:122–131
Igual JM, Forero MG, Gomez T, Orueta JF, Oro D (2006) Rat control and breeding performance in Cory’s shearwater (Calonectris diomedea): effects of poisoning effort and habitat features. Anim Conserv 9:59–65
Imber M, Harrison M, Harrison J (2000) Interactions between petrels, rats and rabbits on Whale Island, and effects of rat and rabbit eradication. NZ J Ecol 24:153–160
Jeschke JM, Genovesi P (2008) Do biodiversity and human impact influence the introduction or establishment of alien mammals? Oikos 120:57–64
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26
Keitt BS, Tershy BR (2003) Cat eradication significantly decreases shearwater mortality. Anim Conserv 6:307–308
Keitt BS, Wilcox C, Tershy BR, Croll DA, Donlan CJ (2002) The effect of feral cats on the population viability of black-vented shearwaters (Puffinus opisthomelas) on Natividad Island, Mexico. Anim Conserv 5:217–223
King CM, Edgar R (1977) Techniques for trapping and tracking stoats (Mustela erminea); a review, and a new system. N Z J Ecol 4:193–212
Konecny MJ (1987) Home range and activity patterns of feral house cats in the Galapagos Islands. Oikos 50:17–23
Le Corre M (2008) Cats, rats and seabirds. Nature 451:134–135
Lyver POB (2000) Identifying mammalian predators from bite marks: a tool for focusing wildlife protection. Mamm Rev 30:31–44
Martínez-Gómez JE, Jacobsen JK (2004) The conservation status of Townsend’s shearwater Puffinus auricularis auricularis. Biol Conserv 116:35–47
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Josh Donlan C, Keitt BS, Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol 17:3503–3510
Monteiro LR, Ramos JA, Furness RW (1996) Past and present status and conservation of the seabirds breeding in the Azores Archipelago. Biol Conserv 78:319–328
Moseby KE, Stott J, Crisp H (2009) Movement patterns of feral predators in an arid environment—implications for control through poison baiting. Wildl Res 36:422–435
Mougin JL, Jouanin C, Roux F, Zino F (2000) Fledging weight and juvenile survival of Cory’s Shearwaters Calonectris diomedea on Selvagem Grande. Ring Mig 20:107–110
Murphy MA, Evans JS, Storfer A (2010) Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 91:252–261
Nogales M, Martin A, Tershy BR, Donlan CJ, Veitch D, Puerta N, Wood B, Alonso J (2004) A review of feral cat eradication on islands. Conserv Biol 18:310–319
Obbard ME, Howe EJ, Kyle CJ (2010) Empirical comparison of density estimators for large carnivores. J Appl Ecol 47:76–84
Olden JD, Lawler JJ, Poff NL (2008) Machine learning methods without tears: a primer for ecologists. Q Rev Biol 83:171–193
Oppel S, Strobl C, Huettmann F (2009) Alternative methods to quantify variable importance in ecology. Department of Statistics, Ludwig-Maximilians Universität, München, Germany, p 7
Oppel S, Beaven B, Bolton M, Vickery JA, Bodey TW (2011) Eradication of invasive mammals on islands inhabited by humans and domestic animals. Conserv Biol 25:232–240
Pascal M, Lorvelec O, Bretagnolle V, Culioli JM (2008) Improving the breeding success of a colonial seabird: a cost-benefit comparison of the eradication and control of its rat predator. Endanger Species Res 4:267–276
Peck DR, Faulquier L, Pinet P, Jaquemet S, Le Corre M (2008) Feral cat diet and impact on sooty terns at Juan de Nova Island, Mozambique Channel. Anim Conserv 11:65–74
Ramos JA, Monteiro LR, Sola E, Moniz Z (1997) Characteristics and competition for nest cavities in burrowing Procellariiformes. Condor 99:634–641
Ramos JA, Moniz Z, Solá E, Monteiro LR (2003) Reproductive measures and chick provisioning of Cory’s Shearwater Calonectris diomedea borealis in the Azores: timing of breeding influenced wing-length at fledging, and egg size may be an indicator of fledging weight and the amount of food received by chicks. Bird Study 50:47–54
Ratcliffe N, Bell MB, Pelembe T, Boyle D, Benjamin R, White R, Godley BJ, Stevenson J, Sanders S (2010) The eradication of feral cats from Ascension Island and its subsequent recolonization by seabirds. Oryx 44:20–29
Rayner MJ, Clout MN, Stamp RK, Imber MJ, Brunton DH, Hauber ME (2007) Predictive habitat modelling for the population census of a burrowing seabird: a study of the endangered Cook’s petrel. Biol Conserv 138:235–247
Ruffino L, Bourgeois K, Vidal E, Duhem C, Paracuellos M, Escribano F, Sposimo P, Baccetti N, Pascal M, Oro D (2009) Invasive rats and seabirds after 2,000 years of an unwanted coexistence on Mediterranean islands. Biol Invasions 11:1631–1651
Sarmento PB, Cruz JP, Eira CI, Fonseca C (2010) Habitat selection and abundance of common genets Genetta genetta using camera capture-mark-recapture data. Eur J Wildl Res 56:59–66
Thibault J (1995) Effect of predation by the black rat Rattus rattus on the breeding success of Cory’s shearwater Calonectris diomedea in Corsica. Mar Ornithol 23:1–10
Thomas MD (1999) Feasibility of using wax-blocks to measure rodent and possum abundance and changes in population size. In: Progress in mammal pest control on New Zealand conservation lands. ed. D.o. Conservation, pp 39–48, Christchurch, New Zealand
Towns D, Broome K (2003) From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. N Z J Ecol 30:377–398
Towns DR, Wardle DA, Mulder CPH, Yeates GW, Fitzgerald BM, Parrish GR, Bellingham PJ, Bonner KI (2009) Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118:420–430
Veitch CR, Clout MN, Towns DR (eds) (2011) Island invasives: eradication and management. IUCN and CBB, Gland, Switzerland and Auckland, New Zealand
Wanless RM, Angel A, Cuthbert RJ, Hilton GM, Ryan PG (2007) Can predation by invasive mice drive seabird extinctions? Biol Lett 3:241–244
Wanless RM, Ryan PG, Altwegg R, Angel A, Cooper J, Cuthbert R, Hilton GM (2009) From both sides: dire demographic consequences of carnivorous mice and longlining for the critically endangered Tristan albatrosses on Gough Island. Biol Conserv 142:1710–1718
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139
Zuur AF, Ieno EN, Elphick CS (2009) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
This work was included in the project LIFE07 NAT/P/000649 ‘Safe Islands for Seabirds’, coordinated by the Portuguese Society for the Study of Birds and co-financed by the European Commission. We thank A. Díez, J. Benedicto, J. García, J. Katzenberger, J. Landschoff, J. Roma, K. Cunningham, K. Puttick and S. Monforte for help in fieldwork, and P. Domingos for his friendly and material support. J. Bried, M. Bolton, M. Brooke, T. Bodey and Y. van Heezik provided stimulating discussions on the design, analysis, and interpretation of results. We appreciate the input of G. Cielniak who filtered a multitude of cat images and all the volunteers for processing these images. This manuscript was benefited by the constructive comments made by the editor and two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hervías, S., Henriques, A., Oliveira, N. et al. Studying the effects of multiple invasive mammals on Cory’s shearwater nest survival. Biol Invasions 15, 143–155 (2013). https://doi.org/10.1007/s10530-012-0274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0274-1