Abstract
Conservation of biodiversity is a major aim of marine reserves; however the effects of reserves on non-native species, a major threat to biodiversity globally, is not widely known. Marine reserves could resist non-native species due to enhanced native diversity and biomass that heightens biotic resistance. Or non-native species could be enhanced by reserves by at least three mechanisms, including protection from harvesting, increased fishing pressure outside reserves facilitating invasions at a regional scale and increasing the exposure of reserves to more potential invaders, and increased propagule pressure from human visitation. We exhaustively searched the literature and found 13 cases that contained quantitative data on non-native species inside and outside marine reserves. In no cases did reserves resist non-native species. Of the seven cases where reserves were established prior to the arrival of the non-native species, five had no effect on the non-native species and two enhanced non-native species. Of the six cases where reserves were established in areas that had pre-existing non-native species, two had no effect on the non-native species and four enhanced the non-native species. These results suggest that while non-native species do equally well or better within marine reserves, too few data are currently available to draw broad, general conclusions regarding the effects of marine reserves on non-native species. Management plans for marine reserves rarely include guidelines for preventing or managing non-native species. If the trends we have detected here are supported by future studies, non-native species should be a priority for management of marine reserves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In terrestrial reserves non-native species have long been documented, absorbing substantial portions of management budgets for control and prevention efforts (Usher 1988; Westman 1990; Pimentel et al. 2005; Allen et al. 2009). In the marine environment, reserves have only recently become a favored management option for conservation (Lubchenco et al. 2003). Like their terrestrial counterparts, marine reserves can effectively protect ecosystems from the effects of harvesting, but their ability to protect against other threats, such as pollution and non-native species, may be more limited (Simberloff 2000). In fact, marine non-native species are abundant (Bax et al. 2003); however, few studies (e.g. Simberloff 2000; Byers 2005; Klinger et al. 2006; Kellner and Hastings 2009) have discussed the extent or potential consequences of non-native species in marine reserves.
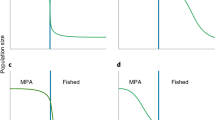
There are several mechanisms by which marine reserves could affect non-native species that lead to opposing predictions of their overall influence (Fig. 1). On one hand, marine reserves may limit non-native species. Marine reserves usually support a greater number of species than non-protected areas (Lester et al. 2009) and the higher biomass and species diversity within a reserve may limit the successful establishment and proliferation of non-native species whose success often stems from unused resources (Davis et al. 2000; Stachowicz et al. 2002). For example, at least at small spatial scales (settlement tiles), species-rich communities sometimes appear to withstand the establishment of non-native species (Fig. 1; Elton 1958; Stachowicz et al. 1999, 2002), leading to speculation that reserves may better resist invasion by non-native species (Kellner and Hastings 2009). However, the hypothesis that species richness confers “invasion resistance” is often not supported on larger spatial scales (Byers and Noonburg 2003; Stohlgren et al. 2003; Fridley et al. 2007), including marine reserves, which in some cases have been documented to contain greater densities of non-native species than non-protected areas (e.g. Byers 2005; Klinger et al. 2006). While it could be argued that the addition of a new species may increase local diversity, it has been found that the introduction of a new species does not compensate for loss of native biodiversity (although this finding may be scale-dependent; Worm et al. 2006).
On the other hand, marine reserves may enhance non-native species. Marine reserves, particularly those created to protect areas of natural beauty, can be a drawcard for tourists (Edgar et al. 2010). If they attract higher rates of visitation this could promote the introduction of non-native species through increased disturbance and vectors (e.g. boat anchors, SCUBA equipment, bilge water, hull fouling) and subsequent dispersal of propagules (Lonsdale 1999; Minchinton and Bertness 2003; West et al. 2007; Britton-Simmons and Abbott 2008; Clark and Johnson 2009). Additionally, unlike terrestrial protected areas, most marine reserves are not managed to control non-native species within their boundaries. For non-native species that are harvested (recreationally or commercially), marine reserves may, ironically, act as a refuge from harvesting, resulting in the non-native species being more prevalent inside, than outside, the reserve (Byers 2005; Klinger et al. 2006). Another mechanism that may lead to a greater performance of invaders in reserves is higher diversity and abundance of predators and parasites that reduce the dominance and competitive abilities of potential native competitors (predator mediated competition; Liu and Stiling 2006). Additionally, it has been hypothesized that protection from fishing may facilitate the establishment of non-native species within a region by creating conditions for non-native species to establish (Kellner and Hastings 2009). By banning harvesting within reserves, fishing effort within the region is concentrated into smaller areas outside the reserve boundaries, increasing the fishing intensity per unit area (Byers and Noonburg 2007; Kellner and Hastings 2009). Concentrating fishing effort creates areas where densities of native species are reduced, which may increase the availability of resources and reduce interspecific competition (Kellner and Hastings 2009), creating an opportunity for non-native species to establish in a new region (Daskalov et al. 2007). Although inside the reserve the biomass at the highest tropic levels should remain intact maintaining biotic resistance, the presence of the reserve increases the ability of non-native species to establish at a regional level (Kellner and Hastings 2009). Additionally the reserves themselves may be more vulnerable compared to before the reserve was created because the establishment of new non-native species within a region may expose reserves to a greater abundance and diversity of potential invaders that are now established in the region (Kellner and Hastings 2009). This mechanism results in more non-native species overall in a region; however, at a smaller scale there would still likely be fewer non-native species inside reserves than outside due to stronger biotic resistance inside.
Finally, it is possible that marine reserves may have no effect on the persistence of non-native species. Many non-native species have dispersal mechanisms that would allow them to easily distribute across marine reserve boundaries, and if post-recruitment processes (e.g. predation, competition for resources or space) are not different between reserve and non-reserve locations, then there is unlikely to be a reserve effect.
In this study we conducted a systematic review of the literature to determine: (1) if marine reserves reduce, enhance or have no influence on the likelihood of successful colonization of non-native species; (2) if the implementation of a reserve affects the prevalence of existing non-native species.
Methods
General approach
We systematically searched the peer-reviewed literature in ISI Web of Science (covering 1898 to June 2010). Articles returned by the searches were indexed according to the non-native species studied, the geographic location of the study, the year the species was first detected, the year the no-take reserve was established, the response variable measured (e.g. abundance, biomass), whether the non-native species is harvested, and the non-native species’ performance in the reserve relative to control areas. We verified the location of the reserve, its no-take status, and the year it was established using the MPA Global database (Wood 2007). To be included in our analysis, studies needed to have quantitative measures of occurrence of the non-native species (e.g. abundance, relative abundance or biomass,) inside and outside (i.e. in control areas) of one or more marine reserves. The marine reserves needed to be zoned as ‘no-take’ (i.e. all extractive activities prohibited) to be included. There was one exception where studies of intertidal bivalves and an alga from the San Juan Islands, USA, (Byers 2005; Klinger et al. 2006) were included even though limited degrees of fishing for salmon and herring were permitted (Klinger et al. 2006). The performance of a species within a marine reserve was categorized as:
-
Enhanced—non-native species that were more prevalent inside than outside reserves,
-
Resisted—species absent from, or less prevalent inside than outside reserves, or
-
Neutral—species that had similar patterns of occurrence, or showed no consistent pattern inside and outside reserves.
Literature search strategies
We first paired the search terms “introduced species”, “invasive species”, “NIS”, “exotic”, “non-native”, “non-indigenous species”, “nonindigenous species”, “alien species”, “introduction”, and “foreign species” with every possible combination of “marine park”, “marine protected area”, “marine reserve”, “no-take marine”, and “MPA”. This search produced 336 papers on non-native species in marine reserves, resulting in six case studies. We then searched by a combination of species name for the 15 most common non-native species mentioned in the first set of papers (Online Resource 1), and the previously mentioned five search terms for marine reserves. This search expanded on the previous search by capturing papers listing only species names, and not if a species was non-native in that area. This search identified an additional two case studies.
Studies of non-native species undertaken incidentally in marine reserves but without a focus on reserves were unlikely to have been identified using our previous search strategies. We therefore undertook a detailed geographic search of countries occurring in 11 key regions (Online Resource 2). We searched the International Union for Conservation of Nature (IUCN) Global Invasive Species Database for invasive marine species that occurred in each country within each of the 11 regions. If a country bordered more than one region (e.g. Canada was included in both the west and east coast of North America regions), then the appropriate state/province that occurred in each region was searched instead of the country. It was important to search countries bordering more than one region separately as different invasive species occur in each region (e.g. Spartina alterniflora is native on the east coast of North America, but non-native on the west coast). Then we searched each species and location combination within ISI Web of Science to determine the species/location combinations with the greatest number of publications. Species that returned more than 30 papers for that region were included in regional searches (4–7 species per region). In the Caribbean, South America, southeastern Africa, Asia, Australia, New Zealand and Hawai’i, all non-native species occurring in the IUCN Global Invasive Species Database for that region were used, as there were few non-native species with greater than 30 studies. Studies were then searched for quantitative data on the occurrence of non-native species at two or more locations, where at least one of the locations could have been a reserve. In suitable papers, we searched study locations in the MPA Global database to determine if studies took place within reserves. Where necessary, authors were contacted to verify if studies were conducted within no-take reserves. Pelagic species were excluded, as they are likely to have short residence times in reserves as adults. We identified a further five case studies with this search strategy.
Case studies were divided into two categories: (1) reserves that were established before the non-native species was introduced, and (2) reserves that were established in areas where non-native species were already detected. If statistical comparisons of the occurrence of non-native species inside and outside reserves were not reported, we extracted relevant data and tested for differences inside and outside reserves. The statistical analyses and evidence used to categorize the effect of the reserve on the non-native species for each case study are presented in Table 1. Where statistical tests were possible but showed no significant difference inside and outside reserves, we calculated the statistical power of the test to detect differences. Power was calculated using G*Power v3 with alpha set at 0.05 and the effect size calculated using the mean and standard deviation of each group. The effect sizes therefore reflected the differences between means inside and outside reserves found in the study as opposed to us setting a standard consistent across the studies. Thus, effect sizes varied among studies, but were typically large (e.g. mean inside reserve >25 % different to outside; Online Resource 3). Two case studies (H. stipulacea from St. Lucia and Dominica) only had one reserve and one non-reserve location and therefore did not have enough data for formal statistical analysis (see Table 1). Our search revealed only 13 studies that met our strict criteria. A formal meta-analysis was not, therefore, possible but trends in the data were described.
Results
Our search through more than 13,000 papers found 13 cases from 8 papers containing quantitative data on non-native species inside and outside marine reserves. In no cases did reserves resist non-native species (Table 1). Where reserves were established before the non-native species was introduced, there were five cases where reserves exhibited neutral effects on non-native species and two cases where reserves enhanced non-native species (Table 2). The first of these latter two cases was the sponge Chalinula nematifera which, in a survey of 150 locations (9 reserve and 141 non-reserve) along the west coast of Mexico, occurred only in two reserves and none of the 141 non-reserve locations (Avila and Carballo 2009). The second case was the alga Undaria pinnatifida in Tinderbox Reserve in Tasmania, Australia, where the alga had an average of 10 % cover in spring 2001 (the first year the species was detected in the area) but was absent in two adjacent control sites (Barrett et al. 2009). For marine reserves that were established after an non-native species had been established, there were two cases where marine reserves had neutral effects on non-native species and four cases where marine reserves enhanced non-native species (Table 3). Of the four cases where marine reserves enhanced non-native species, two of these species were harvested (both bivalves, Crassostera gigas and Venerupis philippinarum) and two were not harvested (the barnacle Elminius modestus and the alga Sargassum muticum). Estimates of statistical power in neutral cases established that power of tests to detect differences inside and outside reserves was low (all values <0.32; Table 1); this low power, even at relatively large effect sizes, reflects the small sample size used in many tests of reserve effects.
Discussion
Our results must be interpreted cautiously because of the limited sample size, but they do indicate that marine reserves do not resist non-native species, and that the implementation of a reserve reduce the persistence of existing non-native species. Indeed reserves appear to have little influence on non-native species as seven of the 13 cases showed no effect of protection. In all cases, however, the statistical power to detect a difference, should one have existed, was low. In areas where reserves were established after the non-native species was introduced, there were more cases where the non-native species performed better within reserves. This observation applied to some species that were harvested (Crassostrea gigas, Venerupis phillppinarum) and, therefore, benefited from the cessation of harvesting once the marine reserve was established (Byers 2005; Klinger et al. 2006). Two of the other positively influenced species (S. muticum and E. modestus) are not harvested themselves, but likely benefited by commonly living as epibionts on the harvested non-native oyster C. gigas (Klinger et al. 2006; Witte et al. 2010). The other species that were positively affected presumably benefited because many species generally perform better within marine reserves (Lester et al. 2009), and the same factors promoting the abundance of native species might also have aided the non-native species.
The limited studies available suggest that non-native species are often abundant and co-occur with other non-native species inside reserves (Byers 2005; Klinger et al. 2006; Abdulla et al. 2008). Indeed, even isolated or reasonably pristine reserves hosted multiple non-native species (PMNM 2008). It is important to acknowledge that there is often spatial and taxonomic bias in reporting the occurrence of non-native species (Ruiz et al. 2000); therefore, given that many marine reserves are the subjects of research it may be more likely that there are a higher number of non-native species reported in marine reserves than unprotected areas. However, any such bias would most likely affect data on the occurrence or presence/absence of invaders as opposed to the pairwise comparisons of the relative performance of non-native species inside versus outside of reserves that we focused on here. From a conservation perspective what remains as a potentially troubling issue is that the presence of non-native species may facilitate the colonization of subsequent non-native species (i.e. invasion meltdown; Simberloff and Von Holle 1999). Because a primary goal of many marine reserves is to maintain high biodiversity, their conservation value is potentially undermined since non-native species may ultimately reduce biodiversity (Carlton 1999; Bax et al. 2003).
Any relationship between reserve status and invasion success may be very complex. This relationship is likely to be a function of the attributes of the reserve (e.g. habitat, location, size, and age of the reserve) and the dynamics within the reserve (e.g. condition of the native community, disturbance regime, magnitude of invasion pressure). However, we need a much larger data set than is currently available to tease out the impact of each of these factors.
Even with our exhaustive review, we found very few empirical comparisons of non-native species between marine reserves and appropriate control areas. Additionally, of the 13 case studies included in this paper there were six different response variables measured: percent cover (4 case studies), biomass (2), CPUE (2), density (2), average maximum abundance (1), and presence/absence (1). The most rigorous approach to testing our hypothesis would be to undertake a formal meta-analysis but the paucity of data and the diversity of response variables measured in the case studies made a formal meta-analysis impracticable (see Harrison 2011). There were only two studies representing five case studies (Byers 2005; Klinger et al. 2006), of the 13,000 we searched, that explicitly examined the role of marine reserves on non-native species, which serves to highlight a critical need for more research on non-native species in marine reserves. It is also worth noting that there was a non-random geographic distribution of the case studies that we found. There were no case studies from Africa, South America, or Asia. While non-native species are identified in these regions, and marine reserves are present, there were fewer studies to interrogate from these regions.
Control, detection, and eradication of non-native species are a common priority issue for management of terrestrial reserves (Thomas 1988; Usher 1988; Macdonald et al. 1989; Westman 1990). For example, >3,700 non-native plant species have been catalogued within US National Parks (Allen et al. 2009). However, in the marine realm, non-native species have received little attention in reserve planning processes. In some marine reserves the occurrence of non-native species has been quantified (deRivera et al. 2005; Abdulla et al. 2008) yet these studies have not led to substantive changes to the ways in which those reserves are managed. Indeed, the role of non-native species (Simberloff 2000) and potential consequences to the effectiveness of marine reserves in maintaining biodiversity (Kellner and Hastings 2009) are rarely considered in reserve planning. While there has been some discussion among scientists of incorporating management of non-native species into management plans for marine reserves (Wasson et al. 2002), there are very few practical examples of this being done. The management plan for Papahānaumokuākea Marine National Monument in Hawai’i (PMNM 2008) is unique in its comprehensive management strategies for non-native species. For example, they have implemented strict quarantine and inspection protocols for vessels and equipment entering the marine reserve. Furthermore, they have developed an non-native species action plan to detect, control, and eradicate (where possible) non-native species occurring within the marine reserve. We acknowledge that the reserve’s extreme isolation (200 km from the nearest populated island, Kaua’i) may create a unique opportunity to incorporate strict vessel and gear inspection. However, it is important that all reserves incorporate strategies to limit the introduction of non-native species and manage existing non-native species as part of the reserve planning process.
In summary, it is surprising how little is known about the extent and performance of non-native species in marine reserves compared to their terrestrial counterparts. We encourage scientists to undertake quantitative and rigorous studies of non-native species by sampling non-native species inside marine reserves and at appropriate control areas, and replicating their studies through time. If the trend of marine reserves exerting positive effects on non-native species continues to be supported by future studies, non-native species should be a priority for management of marine reserves, much as they already are in terrestrial reserves.
References
Abdulla A, Gomei M, Maison E, Piante C (2008) Status of marine protected areas in the Mediterranean Sea. IUCN, Malaga and WWF, France
Allen JA, Brown CS, Stohlgren TJ (2009) Non-native plant invasions of the United States National Parks. Biol Invasions 11:2195–2207
Avila E, Carballo JL (2009) A preliminary assessment of the invasiveness of the Indo-Pacific sponge Chalinula nematifera on coral communities from the tropical Eastern Pacific. Biol Invasions 11:257–264
Barrett NS, Buxton CD, Edgar GJ (2009) Changes in invertebrate and macroalgal populations in Tasmanian marine reserves in the decade following protection. J Exp Mar Biol Ecol 370:104–119
Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W (2003) Marine invasive alien species: a threat to global biodiversity. Mar Policy 27:313–323
Britton-Simmons KH, Abbott KC (2008) Short- and long-term effects of disturbance and propagule pressure on a biological invasion. J Ecol 96:68–77
Byers JE (2005) Marine reserves enhance abundance but not competitive impacts of a harvested nonindigenous species. Ecology 86:487–500
Byers JE, Noonburg EG (2003) Scale dependent effects of biotic resistance to biological invasion. Ecology 84:1428–1433
Byers JE, Noonburg EG (2007) Poaching, enforcement, and the efficacy of marine reserves. Ecol App 17:1851–1856
Carlton JT (1999) Scale and ecological consequences of biological invasions in the world’s oceans. In: Sandlund OT, Schei PJ, Viken A (eds) Invasive species and biodiversity management. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 195–212
Clark GF, Johnson EL (2009) Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 118:1679–1689
Daskalov GM, Grishin AN, Rodionov S, Mihneva V (2007) Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. PNAS 104:10518–10523
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
deRivera CE, Ruiz GM, Crooks JA, Wasson K, Lonhart SI, Fofonoff P, Steves BP, Rumrill SS, Brancato MS, Pegau WS, Bulthuis DA, Preisler RK, Schooch GC, Browlby E, DeVogelaere A, Crawford MK, Gittings SR, Hines AH, Takata L, Larson K, Huber T, Leyman AM, Collinetti E, Pasco T, Shull S, Anderson M, Powell S (2005) Broad-scale non-indigenous species monitoring along the west coast in national marine sanctuaries and national estuarine research reserves. Aquatic Invasions Institute, Portland, Oregon
Edgar GJ, Last PR, Barrett NS, Gowlett-Holmes K, Driessen M, Mooney P (2010) Conservation of natural wilderness values in the Port Davey marine and estuarine protected area, south-western Tasmania. Aquatic Conserv Marine Freshw Eco 20:297–311
Elton CS (1958) The ecology of invasion by animals and plants. Methuen, London
Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, Von Holle B (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17
Grosholz ED, Ruiz GM (1995) Spread and potential impact of the recently introduced European green crab, Carcinus maenas, in central California. Mar Biol 122:239–247
Halpern BS (2003) The impact of marine reserves: do reserves work and does reserve size matter? Ecol App 13(1):S117–S137
Harrison F (2011) Getting started with meta-analysis. Meth Ecol Evol 2:1–10
Kellner JB, Hastings A (2009) A reserve paradox: introduced heterogeneity may increase regional invasability. Conserv Lett 2:115–122
Klinger T, Padilla DK, Britton-Simmons K (2006) Two invaders achieve higher densities in reserves. Aquat Conserv Mar Freshw Eco 16:301–311
Lawson J, Davenport J, Whitaker A (2004) Barnacle distribution in Lough Hyne Marine nature reserve: a new baseline and an account of invasion by the introduced Australasian species Elminius modestus Darwin. Est Coast Shelf Sci 60:729–735
Lester SE, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, Airame S, Warner RR (2009) Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog Ser 384:33–46
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 7:1535–1545
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536
Lubchenco J, Palumbi SR, Gaines SD, Andelman S (2003) Plugging a hole in the ocean: the emerging science of marine reserves. Ecol App 13:S3–S7
Macdonald IAW, Loope LL, Usher MB, Hamann O (1989) Wildlife conservation and the invasion of nature reserves by introduced species: a global perspective. In Drake JA, Mooney HA, di Castri F, Groves RH, Kruger EJ, Rejmanek M, Williamson M (eds) Biological invasions: a global perspective. Scope 37. Wiley, Chichester, England, pp 215–256
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecol Appl 13:1400–1416
Papahānaumokuākea Marine National Monument (PMNM) (2008) Papahānaumokuākea Marine National Monument managment plan. Papahānaumokuākea Marine National Monument, Honolulu, Hawai’i
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Simberloff D (2000) No reserve is an island: marine reserves and nonindigenous species. Bull Mar Sci 66:567–580
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Stachowicz JJ, Whitlatch RB, Osman RW (1999) Species diversity and invasion resistance in a marine ecosystem. Science 286:1577–1579
Stachowicz JJ, Fried H, Osman RW, Whitlatch RB (2002) Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83:2575–2590
Steiner SC, Willette DA (2010) Distribution and size of benthic marine habitats in Dominica, Lesser Antilles. Rev Biol Tropo 58:589–602
Stohlgren TJ, Barnett DT, Kartesz JT (2003) The rich get richer: patterns of plant invasions in the United States 1:11–14
Thomas LK (1988) Management of exotic species in natural communities. Conference on science in the National Parks, volume 5. The George Wright Society and the USDA National Park Service, Ft. Collins, Colorado, USA
Thresher R, Proctor C, Ruiz G, Gurney R, MacKinnon C, Walton W, Rodriguez L, Bax N (2003) Invasion dynamics of the European shore crab, Carcinus maenas, in Australia. Mar Biol 142:867–876
Usher MB (1988) Biological invasions of nature reserves: a search for generalisations. Biol Consv 44:119–135
Wasson K, Lohrer D, Crawforf M, Rumrill S (2002) Non-native species in our nation’s estuaries: a framework for an invasion monitoring program, National Esturine Research Reserve Technical Report Series 2002:1
West EJ, Barnes P, Wright J, Davis A (2007) Anchors aweigh: fragment generation of invasive Caulerpa taxifolia by boat anchors and its resistance to desiccation. Aquat Bot 87:196–202
Westman WE (1990) Park management of exotic plant species: problems and issues. Cons Biol 4:251–260
Willette DA, Ambrose RF (2009) The distribution and expansion of the invasive seagrass Halophila stipulacea in Dominica, West Indies, with a preliminary report from St. Lucia. Aquat Bot 91:137–142
Witte S, Buschbaum C, van Beusekom JEE, Reise K (2010) Does climatic warming explain why an introduced barnacle finally takes over after a lag of more than 50 years? Biol Invasions 12:3579–3589
Wood LJ (2007) MPA Global: A database of the world’s marine protected areas. Sea Around Us Project, UNEP-WCMC & WWF. Available from www.mpaglobal.org. Accessed August 2010
Worm B, Barbier EB, Beaumont N, Duffy E, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790
Acknowledgments
We thank the many authors we contacted for providing additional information. Funding was provided by an Australian Research Council Grant (LP0989796) to R.M.C. and K.A.P. We would also like to acknowledge the anonymous reviewers for their comments, which improved the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burfeind, D.D., Pitt, K.A., Connolly, R.M. et al. Performance of non-native species within marine reserves. Biol Invasions 15, 17–28 (2013). https://doi.org/10.1007/s10530-012-0265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0265-2