Abstract
Habitat modifying species can play crucial roles in ecosystem function. Invasive engineers may assume these roles where native engineers have been lost from the system. We compared the dynamics of the foraging pits of an invasive engineer, the European rabbit (Oryctolagus cuniculus) with two native mammals, the greater bilby (Macrotis lagotis) and the burrowing bettong (Bettongia lesueur). Foraging pits are small surface depressions created by animals when they forage for seeds, bulbs, roots, invertebrates and fungi. We measured foraging pit density and turnover, and density and richness of plant seedlings in pits and adjacent surfaces across three landforms representing a gradient in resource availability inside (bilbies and bettongs), and outside (rabbits only) a reserve in an arid Australian shrubland over 2 years. Pits of the native engineers contained 80% more seedlings (11.2 plants m−2) than rabbit pits (6.22 plants m−2). Further, rabbit pits supported 3.6-times fewer seedlings than equivalent non-pit surfaces outside the exclosure. Only one plant species was restricted entirely to pits. The reserve had more foraging pits and greater turnover than outside, but contrary to prediction, pit effects on seedling density were no greater in the more resource-limited dunes. There were some strong temporal and landscape effects on pit density and species composition, but generally trends were similar inside and outside the reserve. Overall, despite their functional similarities, invasive rabbits created fewer pits that were less favourable patches for seedlings than those of native engineers. Our work suggests that a suite of ecosystem processes associated with fertile patch creation has potentially been lost with the extirpation of bilbies and bettongs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exotic species are a major cause of extinctions and population declines, and their negative impacts on species, communities and ecosystems are likely to be exacerbated with projected changes in global climates as species become more susceptible to environmental stress and native species are pushed further into suboptimal habitat (Lafferty and Gerber 2002). It is predicted that the invaders that are likely to have the greatest effects will be those that modify ecosystem-level resource flows, which is typically achieved through the modification of habitat (Crooks 2002). By altering the flow of resources, these habitat-modifying species, also known as ecosystem engineers (sensu Jones et al. 1997) may alter habitat availability, nutrient cycling, hydrology and ultimately, ecosystem-level productivity. Because they alter both the physical and biological characteristics of their adopted environment, invasive engineers have a greater potential to influence ecosystem-wide processes than non-engineering species (Vitousek 1990).
At the scale of the modified patch, invasive engineers that increase ecosystem complexity are predicted to increase ecosystem-wide species density and richness, while those that reduce complexity are predicted to cause declines in species density and richness (Crooks 2002). Engineering effects on patch-scale species richness also appear related to productivity. In harsh or unproductive environments, engineers that increase productivity are expected to increase species richness as they reduce abiotic stress, whereas in productive or more benign environments, engineers that increase productivity are expected to decrease species richness as certain species exert competitive dominance in the highly productive patches (Hacker and Gaines 1997; Wright et al. 2004). At the landscape level, engineers are predicted to increase complexity and species richness as the landscape becomes a mosaic of modified, unmodified and recovering patches (Jones et al. 1997). The actual outcome is complex, and depends on both the degree of modified habitat and how well species can utilize the newly created patches (Wright et al. 2004). For example, American beavers (Castor canadensis) introduced into the world’s southernmost forested ecosystem in Chile increased species richness and abundance of native and exotic plants by reducing canopy cover, allowing an increase in herbaceous species within the newly created beaver meadows (Anderson et al. 2006). Although introduced beavers created a new patch type, they did not create a beaver-specific plant assemblage as they do in their native North American range (Naiman et al. 1988; Wright et al. 2004). Rather, they substantially reduced the tree seedling bank as tree species were not adapted to beaver disturbance (Anderson et al. 2006).
A substantial review by Wright and Jones (2004) supports the notion that engineering effects on species richness are regulated by system productivity. Irrespective of the species of engineer or the type of disturbance created, Wright and Jones (2004) showed that there was a significant relationship between the productivity of unmodified patches and the effect of ecosystem engineering on species richness. To our knowledge, the only studies comparing native and invasive engineers have all examined the relationship between invasive plants and resident fauna that use the plants as habitat (e.g. Slobodchikoff and Doyen 1977; Brusati and Grosholz 2006). For example, Brusati and Grosholz (2006) compared Spartina foliosa, a saltmarsh plant that affects habitat structure and community composition, with an invasive hybrid (S. foliosa × S. alterniflora). While both species provided structure within the marine ecosystem, the invasive species created such a highly structured habitat that it excluded invertebrates, and transformed open mudflats into vegetated saltmarsh. Thus, although the invasive engineer increased complexity at the patch scale, it did not increase species richness or abundance as predicted.
But what if the invasive engineer modifies the environment in a similar way to that of its native analogue? Comparisons between functionally similar native and invasive engineers provide an opportunity to test predictions about the effects of ecosystem engineers, and are particularly important if the native engineer has been lost from the system. This scenario is increasingly likely as climate change is likely to cause range shifts of many vertebrate engineers. Invasive engineers that create structurally similar habitat to former native engineers may play crucial roles in ecosystem function that need to be understood, particularly prior to any eradication program. Ecosystems that have lost native species can respond in unexpected ways as a result of complex interactions between invasive and native species, and the fact that invasives can replace the functional roles of natives within systems (Zavaleta et al. 2001).
Many of Australia’s medium-sized mammals have suffered massive range declines following European settlement and the subsequent introduction of exotic predators and herbivores (Short and Smith 1994; Johnson 2006). Two of these species, the greater bilby (Macrotis lagotis Reid) and the burrowing bettong (Bettongia lesueur Quoy & Gaimard) have been identified as ecosystem engineers (James and Eldridge 2007). Associated with the decline of the bilby and bettong has been the range expansion of the European rabbit (Oryctolagus cuniculus L.), which has become Australia’s most successful vertebrate pest because they are so widespread and numerous (Myers et al. 1994; Courchamp et al. 2003). The European rabbit now occupies most of the former range of both the bilby and bettong, and has been implicated in their decline through increased competition for forage, and by providing an abundant food source for two exotic predators, the red fox (Vulpes vulpes L.) and the feral cat (Felis catus L.) (Johnson 2006). Rabbits are in the same size class as bilbies and bettongs and also live in communal burrow systems (Strahan 1995).
Alterations to the soil-surface by ecosystem engineers in arid systems may have relatively large effects on plant communities, as these systems are limited by water and nutrients, which generally travel through overland flow. Rabbits, bilbies and bettongs all construct pits on the soil surface while foraging for seeds, bulbs, roots, invertebrates and fungi (Southgate 1990a; Robley et al. 2001; Myers et al. 1994). The foraging pits they create act as traps for organic matter and seed, becoming patches with enhanced nutrient levels, with greater levels of plant-available nitrogen and carbon than the surrounding soil matrix (James and Eldridge 2007; James et al. 2009). Rabbit pits are relatively shallow, however, compared with bilby and bettong pits, and may be unable to retain litter for long periods (James et al. 2009). Further, rabbits create fewer pits, and thus their per capita pit production is likely substantially less than that of the native engineers (James and Eldridge 2007; James et al. 2009).
This study compares the dynamics of bilby and bettong foraging pits with those of the European rabbit, among three landforms (dunes, ecotones, swales) representing a gradient in resource availability and productivity from lower resources (e.g. lower concentrations of organic carbon and nitrogen; lower water holding capacity) in the dunes to higher resources in the swales. Our primary aim was to determine whether invasive European rabbits have assumed the ecosystem engineering roles of the locally-extinct greater bilby and burrowing bettong in terms of pit creation and provision of sites for germinating plant. Further, we hypothesized that the engineering effect on seedlings would be greater in the resource-poor dunes than the relatively resource-richer swales.
Materials and methods
Study site
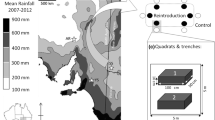
The study was conducted at Arid Recovery, a reserve 20 km north of Roxby Downs in arid South Australia. Arid Recovery is an 86 km2 reserve from which feral cats, red foxes and European rabbits have been eradicated and are prevented from reinvading by a 1.8 m vermin-proof fence. Locally-extinct mammals were reintroduced into the exclosure between 1999 and 2005. The exclosure is unique as its large size accommodates free ranging populations of reintroduced animals. The climate of the area is arid, with rainfall that is both spatially and temporally variable and fails to reach the long term annual average of 166 mm in 60% of years. The mean annual maximum temperature exceeds 35°C, and the mean annual minimum is 14°C (Olympic Dam Operations 1994).
The landscape is characterised by linear, west-east trending sand dunes about 200 m wide and inter-dunal swales about 500 m wide. Dunes have sandy topsoils (5–10% clay) that support an open shrubland of sandhill wattle (Acacia ligulata A. Cunn ex Benth) and narrow-leaved hopbush (Dodonaea viscosa (L.) Jacq.). The intervening gibber swales have sandy-clay topsoils (35–40% clay) often with a variable surface cover of stones (‘gibbers’) up to 5 cm in diameter. Swales are dominated by shrubs from the family Chenopodiaceae (Atriplex vesicaria Heward ex Bent., Maireana astrotricha (L.A.S. Johnson) Paul G. Wilson). The ecotones, intermediate areas between the swales and dunes, are 10–20 m wide, characterised by fine sandy topsoils (25% clay), and vegetated by species found in both the dunes and swales.
The study was conducted at four sites within the reserve and four sites outside the reserve. The sites inside the reserve were located in the ‘Main Exclosure’, henceforth referred to as ‘inside’, a 14 km2 paddock where the locally extinct burrowing bettong, greater bilby, greater stick-nest rat (Leporillus conditor Sturt, 1848) and western barred bandicoot (Perameles bougainville Quoy & Gaimard, 1824) have been reintroduced. As the latter two species do not forage extensively in the soil, they were not included in the current study. The sites outside the reserve (termed ‘outside’) were located on a mining lease operated by Olympic Dam Operations. The area had been grazed intermittently by cattle at relatively low stocking rates, with no grazing at the four outside sites since the reserve was established in 1999. Outside sites were subject to higher levels of grazing by kangaroos (Macropus spp.) and rabbits than inside the reserve, and the exclusion of these species from inside the reserve has resulted in higher vegetation cover inside compared to outside (Edwards 2001). The two paddocks were relatively similar in their vegetation structure and composition, and would represent typical, though slightly degraded, landscapes within which the reintroduced species would have occurred prior to European settlement (James et al. 2009).
Although the bilbies and bettongs were enclosed within the Main Exclosure, estimated densities of 14 individuals of each species km−2 (Arid Recovery 2007) were within the range of estimated natural population densities ranging from more than 70 bettongs km−2 (Noble et al. 1995) to eight bilbies km−2 made by Le Soeuf and Burrell on the Nullarbor Plain in 1921 (Southgate 1990b). Rabbits are found outside the reserve at densities that fluctuated between 10 and 70 individuals km−2 over the period of the study in response to rainfall and outbreaks of Rabbit Haemorrhagic Disease (Arid Recovery 2007).
Experimental design
Each of the four sites within a paddock was separated by distances of about 1.5 km. However, sites were closer across the paddock boundary (inside and outside were ~100 m apart), and therefore sites were naturally paired across the boundary into four blocks. As such, the design is pseudo-replicated and hence interpretation of the effects of ecosystem engineers should not be generalised beyond the study site. However, home range analysis of bilbies and bettongs suggests that these sites are independent with respect to treatment effects (Moseby and O’Donnell 2003; Finlayson and Moseby 2004). Additionally, there is only one Arid Recovery and this single replicate of a unique ecosystem represents a valuable opportunity to gain information about the effects of both locally extinct and invasive species.
Each of the eight sites comprised three landform elements; dune, swale, and the intervening ecotone. Measurements were made along 50 m × 2 m transects in each landform element within each site. Transects were aligned parallel to the direction of the dunes to assess the density of foraging pits of each species group at the 24 landform element sites. The x- and y-coordinates of each pit encountered along each transect were recorded, and the depth, length and width through the centre of each pit measured. The species responsible for the construction of the pit was determined (bilby/bettong), based on pit shape, depth and excavation angle (James and Eldridge 2007). Consequently, pits constructed by bilbies and bettongs were pooled because their pits could not reliably be distinguished from each other due to similar morphologies. In each pit we recorded the number of seedlings of each plant species and estimated the percent cover of litter. The same variables were measured on the surface in 0.25 m2 quadrats placed every metre along the edge of the same transect. Algorithms in James et al. (2009) were used to calculate soil mass from measurements of pit diameter and depth.
Transects were sampled at four time periods; August 2006, December 2006, March 2007 and March 2008. During this time there was one large rainfall event in January 2007 and rainfall remained below the long-term annual average of 160 mm in all years (Fig. 1). We used the unique location of seedlings (x- and y-coordinates within each transect) to identify whether pits and seedlings were new or were present at previous samplings. We acknowledge however the problems that tracking seedling (and pits) over longer time periods might have on underestimating, to some extent, calculations of turnover. For example, it is possible that some seedlings may have germinated and died between our field recording visits thereby underestimating seedling recruitment. We believe that the chances of this occurring were very small as permanent field staff monitored the transects continually for signs of recruitment during the non-recording periods.
Univariate analyses
Differences in pit density, cover and volume were examined using a mixed-models ANOVA structure. Fixed effect factors were Paddock (inside, outside), Landform (dune, ecotone, swale) and Time of observation (n = 4). The nested nature of the sampling design (based on Time of observation within Landform within Paddock within Block) required the formation of three residual terms. The Block and Paddock factors formed the main stratum, Landscape and its interaction with Paddock the second stratum, and Time and its 2-, 3-way interactions with Paddock and Landform produced the third stratum. Differences in the distribution of pit volumes inside and outside the reserve were tested using χ2 analysis. We used algorithms in James et al. (2009) to calculate the volumes of pits, based on measurements of their diameters and depth. Pits were placed into one of eight classes based on volume: <250, 250–500, 500–750, 750–1,000, 1,000–2,000, 2,000–5,000, 5,000–10 000, >10,000 cm3.
We used the sum of appearances and disappearances of pits between successive sampling periods as an index of the variation in pit formation and infilling over time. Differences in this pit index in relation to Paddock, Landform, Time (n = 3 time intervals) and their interactions were analysed using a similar mixed-models ANOVA structure.
A mixed-models ANOVA was used to examine possible differences in community structure (number of species (diversity), richness (Margalef’s index), abundance (no. of individuals)) of plant seedlings growing in the pits and on surfaces in relation to the fixed factors Paddocks, Landform, Microsite (pit, surface) and Time (n = 3 samplings, as no seedlings were found at the fourth sampling in May 2008). These community measures were calculated using the DIVERSE function of PRIMER-E and were based on a balanced set of 144 observations. Again, the nested nature of the sampling design (based on Time within Microsite within Landform within Paddock within Block) required the analysis to specify four residual terms. Since the design was balanced, main effects and their interactions could all be confidently estimated. For all univariate analyses, data were checked for homogeneity of variance, independence, and normality using Levene’s test and other diagnostic tools within the GenStat statistical package (Payne et al. 2008) and transformed, where appropriate (ln) prior to ANOVA. The ANOVA directive of GenStat was used to analyse these data. In cases of a significant F statistic for treatment effects, significant differences between means were compared using Tukey’s Least Significant Difference (LSD) methods.
Multi-variate analyses
We constructed a matrix of 144 observations (2 Paddocks × 3 Landforms × 2 Microsites × 4 replicate sites × 3 Times) by 48 plant species and tested for possible differences in plant species composition among paddocks, landforms, microsites and times (and their interactions) using permutational multi-variate analysis of variance (PERMANOVA, Anderson and Gorley 2008). The PERMANOVA package allowed us to partition the multivariate variation using the complicated multi-strata experimental design described above for the univariate analyses. The matrix of plant abundance was converted to a similarity matrix using the Bray Curtis similarity coefficients contained within the PRIMER-E statistical package. Pair-wise a posteriori comparisons were made, where necessary, using a multi-variate analogue of the t statistic, the probability levels being obtained by permutations. Thus it is unnecessary to correct for a large number of multiple comparisons.
Where we detected a significant difference in composition we used two methods to determine which plant species were responsible for the observed difference. First, the SIMPER subroutine in Primer-E was used to determine which species contributed most to the dissimilarity between the relevant groups e.g. differences between sampling times. Second, the ability of individual species to discriminate between different groups e.g. pit and surface was measured using Indicator-Species Analysis (Dufréne and Legendre 1997). Indicator values combine information on relative abundance and frequency of species. For example, when all individuals of a given species are restricted to a particular microsite, and all samples from the particular microsite contain an occurrence of that species, then the indicator value is maximal (IV = 100). Species data were randomized among the two microsites and a Monte Carlo randomization procedure performed with 1,000 iterations in order to determine the statistical significance of the indicator values. Indicator value analysis was performed using PC-ORD (McCune and Mefford 1999).
Results
Pit density, cover and volume of excavated soil
There were more than twice as many pits inside the reserve, which contained bilbies and bettongs (5,100 pits ha−1) than outside the reserve (2,137 pits ha−1; P = 0.01; Supplementary Table 1), which contained only rabbits. This effect was consistent across all sampling times except for the third sampling, when the difference between inside and outside the reserve was less marked (Paddock by Time interaction: P = 0.023; Fig. 2). The cover of pits (inside: 0.99%, outside: 0.33%) and the volume of excavated soil were also significantly greater inside the reserve (2.36 m3 ha−1) than outside (1.38 m3 ha−1; pit cover: P = 0.02; pit volume: P = 0.002). There was a significant difference in the distribution of pit volumes inside and outside the exclosure (χ2 = 42.3, df = 7, P < 0.001), largely due to more smaller pits inside the exclosure (Fig. 3).
The density, cover and volume of pits changed markedly over time, with less than half of the number of pits recorded at the final sampling in March 2008 (1,715 ha−1) than at other samplings (mean = 4,205 pits ha−1). This was consistent between the exclosure and outside (Supplementary Table 1).
Pit density varied markedly among landforms, with 30% fewer pits in the swales (3,969 pits ha−1) than in the dunes or ecotones (mean = 5,120 pits ha−1; Fig. 2), but the differences were not significant (P = 0.15). Similarly, there were no significant differences in cover (P = 0.56) nor volume of excavated soil (P = 0.37) between landforms (Supplementary Table 1). The lack of a significant Landform by Paddock interaction indicates that bilbies/bettongs and rabbits do not differ in their use of different landforms (P = 0.73).
Pit appearance and attrition
Pit turnover, calculated as the sum of appearing and disappearing pits, was greater inside the reserve (P = 0.035; Fig. 4). However, while turnover inside the reserve generally declined from dune through ecotone to swale, there was substantially less turnover in the dunes outside (Paddock by Landform interaction: P = 0.027). There were two markedly different periods in terms of pit turnover; with higher rates for the first two sampling periods (August 2006 to March 2007), and substantially lower rates for the final period of 12 months as the rate of activity declined. In the first period, the rate of appearance of new pits varied from 0.033 pits m−2 month−1 inside to 0.023 pits m−2 month−1 outside the reserve. Pit attrition rates were twice as high inside (0.046 pits m−2 month−1) than outside (0.021 pits m−2 month−1; Fig. 4). However for the final sampling period (March 2007 to March 2008), the rate of both pit appearance (0.006 and 0.004 pits m−2 month−1) and attrition (0.014–0.009 pits m−2 month−1 for inside and outside respectively) were markedly similar.
Seedling density
Averaged over the first three samplings that yielded seedlings, we recorded about 3.5–times more seedlings in the pits (26.6 seedlings m−2 pit) than an equivalent area of surface (7.7 seedlings m−2; P < 0.001, Supplementary Table 2). Although there were no differences in seedling density on the surface inside and outside the reserve (mean = 5.72 seedlings m−2, P = 0.25), there were about 80% more seedlings in the pits inside (11.2 m−2) than in the pits outside the reserve (6.22 m−2; Paddock by Microsite interaction: P = 0.003, Fig. 5).
We detected large differences across both paddocks in the total seedling density (both pits and surface) among all samplings, from an average of about 15.9 seedlings m−2 in August 2006 (Time 1) to 1.7 seedlings m−2 at Time 2 (December 2006: P < 0.001). There was no significant paddock by time interaction (P = 0.75). No plants were found at the fourth sampling in May 2008. There were also 5.5-times more seedlings in the pits than the surface in August 2006, but only a twofold increase in the pits compared with the surface at subsequent periods (Microsite by Time interaction: P < 0.001, Supplementary Table 2). There were about 2.6-times more seedlings in the dunes than the swales and ecotones in August 2006, but no significant differences between Landforms in subsequent periods (Landform by Time interaction: P < 0.001).
Seedling diversity and composition
Outside the reserve we detected about 3.6-times more species on the surface than in the pits, but very little difference inside (Paddock by Microsite interaction: P < 0.001; Supplementary Table 2, Fig. 5). Bilby/bettong pits (inside) supported significantly more species than rabbit pits (outside; P < 0.001). More species were found at the third sampling (5.48 species) than the first (3.92 species) or second (1.50 species; P < 0.001). There was a marked suppression in diversity of plants in the swales compared with the dunes or ecotones at the final sampling (Landform by Time interaction: P = 0.025; Supplementary Table 2). Predictably, trends in Margalef’s richness mirrored those for diversity.
We recorded 48 plant species across all transects and times. Thirty-eight species were found in the pits and 47 on the surface (Supplementary Table 3). The shrub Acacia ligulata was the only species found exclusively in the pits, while 10 taxa comprising a mixture of grasses, forbs and sub-shrubs (Calotis hispidula, Carrichtera annua, Euphorbia eremophila, Enneapogon avenaceus, Maireana astrotricha, Rhodanthe stricta, Sclerolaena sp.1, Senecio gregorii, Swainsonia formosa, Trichodesma zeylanicum) were found only on the surface. Nine of these were native, with a mix of perennial and annuals. Seven taxa (Calotis hispidula, Goodenia cycloptera, Asteraceae sp., Abutilon otocarpum, Aristida holothera, Boerhavia dominii and Brassica tournefortii) made up 50% of the total seedling density, when data were converted to the number of seedlings m−2.
Multi-variate analyses indicated significant differences in species composition in relation to Landform (P (perm) = 0.003), Microsite: (P (perm) = 0.004) and Time (P (perm) = 0.001), but no Treatment effect (P (perm = 0.35). There were no strong indicators of pits or non-pits, but Brassica tournefortii was a weak indicator of pit microsites (IV = 21%, P = 0.043) and Maireana astrotricha (IV = 8.3%, P = 0.026) a weak indicator of non-pit microsites.
Discussion
Although foraging pits of the native engineers bilbies and bettongs, and their invasive analogue the European rabbit were both preferred sites for vascular plant establishment, clear differences were apparent at both patch (pit, surface) and landscape (reserve, outside) scales. The density of foraging pits was lower outside the reserve where rabbits occurred, than inside the reserve, which contained bilbies and bettongs, and rabbit pits contained fewer seedlings. Contrary to predictions, we failed to detect any differences in seedling density or species diversity between pits and surface in resource-limited dunes compared with the swales.
Pit density and turnover
Across all landforms and sampling times foraging pit density was greater inside the reserve, which supported native animals, than outside the reserve where the invasive rabbit occurred (Fig. 2). This is consistent with previous studies showing both greater pit densities and higher per capita rates of digging by native than exotic engineers at a single point in time (James and Eldridge 2007). Overall we measured no significant differences in either pit cover or volume of excavated soil inside or outside because of the greater number of smaller, shallower pits inside the reserve (Fig. 3).
Fluctuations in pit density over time (Fig. 2) showed similar patterns for native and invasive engineers, with up to twofold variation among time periods. Declines in pit density over time likely corresponded to the persistent drought extending over the latter half of the study (Fig. 1), though the small sample size prevented us from exploring possible relationships between rainfall and pit creation. Temporal variation in pit density is likely related to the length of time since the last effective rain event, as rainfall is a strong driver of primary productivity in deserts (Noy-Meir 1979; Le Houerou 1984). The activity of granivorous desert mammals is known to be closely aligned with rainfall events and therefore availability of annual plant seeds (Predavec 2000), and soil moisture can alter the efficiency of olfactory detection of buried seeds (Vander Wall 1998). Thus reductions in pit density with increasing time since rainfall could be due to either declines in available food resources, declines in the density of engineers, or both. Shachak et al. (1991) demonstrated that annual densities of Indian crested porcupine (Hystrix indica) foraging digs were significantly correlated with total rainfall in the 2 years prior to disturbance, while density was greatest at sites with the greatest rainfall, corresponding to maximum production of underground corms of lilies, the porcupine’s main forage item (Yair and Rutin 1981).
While pit creation will largely be a function of animal density and available resources, abiotic processes such as wind erosion and surface insolation will likely affect soil stability and transport and therefore govern how quickly pits infill. Greater pit density results in a greater supply of mobile, excavated sediment. As expected, sites supporting native ecosystem engineers (inside) were more dynamic, with greater rates of pit turnover than sites outside the reserve (Fig. 4). Our prediction of greater pit turnover in the dunes, given that dune soils are sandier, easier to excavate, but more erodible and therefore more likely to infill, was not supported by the data because of the strong interaction with Paddock. We found that turnover inside the reserve was greatest in the dunes and least in the swales, but outside, turnover was substantially less in the dunes relative to the swales (Fig. 4). We do not believe that this can be explained by differences in abiotic processes across the sites because of their close proximity. Rather, it is more likely related to the foraging behaviour of bilbies and bettongs (inside) compared with rabbits (outside). Our observations of foraging behaviour by rabbits at sites in western NSW suggest that they preferentially forage on soil surfaces which are relatively compact, which would have been typical of plains and swales in this study. It is not unexpected, therefore, that there would have been fewer digging by rabbits in the dune landscapes where the soils are very sandy. Compared with the other landforms, dunes contained more short-lived pits, which would have less time to sequester mobile leaf litter before they infill. The combination of both more pits and abundant shrubs on the dunes means that there is both a greater chance that litter will encounter a pit, and a higher probability of any litter being trapped by mobile dune soil.
Plant germination in foraging pits
In our study more than three-times more seedlings germinated in the pits than on the surface (Fig. 5), similar to observations of foraging pits of Indian crested porcupines in the Negev Desert (Gutterman et al. 1990; Boeken et al. 1995), the Cape porcupine (Hystrix africaeastralis) in South Africa (Bragg et al. 2005), and a range of heteromyid rodents in the Chihuahuan Desert in the United States (Duval et al. 2005). Additionally, there were almost twice as many seedlings in the pits of native engineers (11.2 seedlings m−2) than those of exotics (6.2 seedlings m−2) of a similar volume. We attribute this to differences in morphology between the deeper bilby-bettong pits and the shallower pits of rabbits. Bilby and bettong pits have a greater capacity therefore to collect and retain litter, and given that pit-resident litter would be shaded in the deeper pits, the chances of photo-oxidation would be reduced. This would likely influence plant-available nutrients, infiltration and soil moisture, and diurnal and night-time temperatures (Gutterman 1997; Alkon 1999; Eldridge and Mensinga 2007; James and Eldridge 2007; Eldridge and Whitford 2009; James et al. 2009). These results are consistent with previous studies of greater plant-available nutrient concentrations in native animal pits compared with those of invasive rabbits (James and Eldridge 2007).
Greater seedling density in the pits of native engineers cannot be explained by a greater availability of seed inside the reserve, nor by differences in post-germination herbivory, as density of the surface was similar inside and outside the reserve (Fig. 5a). Given the greater pit density inside the reserve, we might have expected that, had the reserve been seed-limited rather than microsite-limited, there would have been fewer seeds trapped per pit inside than outside. In contrast, however, there were more seedlings per pit inside the reserve than outside. Further, the fact that most species we recorded had annual growth responses, suggests that rainfall was the principal trigger for germination (Levine et al. 2008).
A number of studies has shown that animal disturbances lead to increased plant species diversity and abundance (Inouye et al. 1987; Huntly and Reichman 1994; Wright et al. 2004). Pits of native engineers in our study supported more seedlings and more than twice the diversity as those of exotics, even in an environment where both density and diversity on non-pit surfaces was relatively similar inside and out (Fig. 5a). Indeed, germinants of one species, the shrub Acacia ligulata, were only found in the pits in our study. There are a number of reasons why the pits of native engineers supported a greater diversity and density than those of the exotic rabbit. Firstly, species differences may lie in the marked contrasts in pit morphology between those of native and exotic engineers. Pits of bilbies and bettongs were more variable in shape and volume (standard deviation of volume; 3,423 cm3 for bilbies cf. 1,335 cm3 for rabbits), displaying a wider range of physical attributes such as depth, aperture size and orientation than those of rabbits. Potentially, therefore, this would be expected to provide a greater range of temperatures and moisture levels, advantaging different plant functional types at different times (Gutterman et al. 1990; Boeken et al. 1995), and supporting a greater number of plant growth forms. In particular, rabbit pits were shallower and more elongated than bilby and bettong pits, which were typically deep, cylindrical-shaped excavations. The ability of rabbit pits to retain resources such as seed and litter would therefore be much less than that of the pits of bilbies and bettongs. Second, we would expect a greater range of pit types (e.g. nose-pokes, shallow depressions, deep conical pits, Newell 2008), sizes and shapes inside than outside simply because we were comparing two soil disturbing animals inside with only one outside. Our inability to differentiate between bilby and bettong pits does not mean that these two animals do not have a differential effect on plant species.
Rabbit pits are a relatively new disturbance type in arid Australia (~200 years), and plant species may lack the adaptive capabilities to utilise rabbit pits. Further, we found no differences in seedling density between pit and surface outside the reserve (Fig. 5a) suggesting a relative diminution of the pit effect in the presence of exotic animals, or alternatively, a greater area of surface that is likely to support more plants. We cannot account for these differences, though grazing of pit-resident plants, which likely have higher levels of tissue nitrogen (Travers 2008), is the most parsimonious explanation.
Engineering effect is moderated by landscape
Ecological theory predicts that the effects of ecosystem engineers on plant species richness should increase as landscapes become more resource-limited (Wright and Jones 2004). This is predicted because species are located at the extremes of their physiological tolerances, and small increases in resource availability are likely to have relatively larger effects than in more productive environments. Given that dunes have lower nutrient and water availability and lower productivity (Whitford 2002), we predicted that the effect of pits on seedling density and diversity would be greater in the dunes than in the swales. Our results, however, did not support this hypothesis as the pit effect on seedling density was similar among landforms (Electronic Appendix Table 1) and plant diversity was markedly suppressed in the swales compared with the dunes or ecotones at the final sampling.
There is considerable variability in the effects of ecosystem engineers in low productivity systems (Wright and Jones 2004), as demonstrated by our study. Both plant density and diversity may also be influenced by factors other than increased resource availability. For example, species that germinate in pits in the dunes may benefit from the disturbance of the physical soil crust that would otherwise be difficult to penetrate (Zaady et al. 1997). Similarly, the availability of loose soil may enhance the degree of soil-seed contact for dune-resident species. These and other stochastic effects could likely have been responsible for some of the trends we observed.
Conclusions
The largest difference between the invasive engineer and its native analogues in our study was in the magnitude of patch creation i.e. the density of pits. Although both native and invasive engineers created functionally similar foraging pits that enhanced plant germination, rabbits created fewer pits that supported fewer species than those of native engineers. Contrary to expectation, the relative effect of engineers did not vary with increasing resource limitation, i.e. pit-moderated increases in plant density and diversity were similar across all landforms. Our study suggests that a suite of ecosystem processes associated with the creation of fertile patches has potentially been lost with the extirpation of bilbies and bettongs. Native engineers create a more dynamic landscape where greater numbers of pits are being both excavated and filled in. This likely leads to increased amounts of litter and seed being buried in the soil, and over time may change the nature of the soil matrix, creating a patchwork of ‘recovered pits’. Over time this may lead to increases in nutrient levels across in the matrix, and has implications for the distribution and longevity of the soil seed bank.
As well as altering resource distribution, pits may be preventing seeds from desiccating, thus allowing them to persist for longer between large and temporally-variable rainfall events that induce large-scale germination in deserts. Longer-term studies are needed to determine the relationships between pit dynamics and rainfall, and between pits and larger fertile patches, and how these relationships influence resource distribution, plant populations and soil seed banks. Further studies of the interactions between trophic and engineering roles of natives and invasives and potential feedbacks to the engineers themselves will improve our understanding of whether invasive engineers can assume the ecosystem engineering roles of native species.
References
Alkon PU (1999) Microhabitat to landscape impacts: crested porcupine digs in the Negev Desert highlands. J Arid Environ 41:183–202
Anderson MJ, Gorley RN (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Anderson CB, Griffith CR, Rosemond AD, Rozzi R, Dollenz O (2006) The effects of invasive North American beavers on riparian plant communities in Cape Horn, Chile: do exotic beavers engineer differently in sub-Antarctic ecosystems? Biol Conserv 128:467–474
Boeken B, Shachak M, Gutterman Y, Brand S (1995) Patchiness and disturbance: plant community responses to porcupine diggings in the central Negev. Ecography 18:410–421
Bragg CJ, Donaldson JD, Ryan PG (2005) Density of Cape porcupines in a semi–arid environment and their impact on soil turnover and related ecosystem processes. J Arid Environ 61:261–275
Brusati ED, Grosholz ED (2006) Native and introduced ecosystem engineers produce contrasting effects on estuarine infaunal communities. Biol Conserv 8:683–695
Courchamp F, Chapuis JL, Pascal M (2003) Mammal invaders on islands: impact control and control impact. Biol Rev 78:347–383
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Dufréne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monog 67:345–366
Duval BD, Jackson E, Whitford WG (2005) Mesquite (Prosopis glandulosa) germination and survival in black–grama (Bouteloua eriopoda) grassland: relations between microsite and heteromyid rodent (Dipodomys spp.) impact. J Arid Environ 62:541–554
Edwards J (2001) Monitoring vegetation change under different arid management regimes, near Olympic Dam, South Australia, using Landsat TM imagery. BSc Thesis, University of Adelaide, South Australia
Eldridge DJ, Mensinga A (2007) Foraging pits of the short-beaked echidna (Tachyglossus aculeatus) as small-scale patches in a semi-arid Australian box woodland. Soil Biol Biochem 39:1055–1065
Eldridge DJ, Whitford WG (2009) Badger (Taxidea taxus) disturbances increase soil heterogeneity in a degraded shrub-steppe ecosystem. J Arid Environ 73:66–73
Finlayson GR, Moseby KE (2004) Managing confined populations: the influence of density on the home range and habitat use of reintroduced burrowing bettongs (Bettongia lesueur). Wildl Res 31:457–463
Gutterman Y (1997) Spring and summer daily subsurface temperatures in three microhabitats in a flat natural loess area in the Negev Desert Israel. J Arid Environ 63:225–235
Gutterman Y, Golan T, Garsani M (1990) Porcupine diggings as a unique ecological system in a desert environment. Oecologia 85:122–127
Hacker SD, Gaines SD (1997) Some implications of direct positive interactions for community species diversity. Ecology 78:1990–2003
Huntly N, Reichman OJ (1994) Effects of subterranean mammalian herbivores on vegetation. J Mammal 75:852–859
Inouye RS, Huntly NJ, Tilman D, Tester JR (1987) Pocket gophers (Geomys bursarius), vegetation, and soil nitrogen along a successional sere in east central Minnesota. Oceologia 73:178–184
James AI, Eldridge DJ (2007) Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biol Conserv 138:351–359
James AI, Eldridge DJ, Hill BM (2009) Foraging animals create fertile patches on an Australian desert shrubland. Ecography 32:723–732
Johnson C (2006) Australia’s mammal extinctions: a 50000 year history. Cambridge University Press, Melbourne
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78:1946–1957
Lafferty KD, Gerber LR (2002) Good medicine for conservation biology: the intersection of epidemiology and conservation theory. Conserv Biol 16:593–604
Le Houerou HN (1984) Rain use efficiency: a unifying concept in arid-land ecology. J Arid Environ 7:213–247
Levine JM, McEachern AK, Cowan C (2008) Rainfall effects on rare annual plants. J Ecol 96:795–806
McCune B, Mefford MJ (1999) PC-ORD: multivariate analysis of ecological data. Version 4. User’s guide. MjM Software Design, Gleneden Beach
Moseby KE, O’Donnell E (2003) Reintroduction of the greater bilby, Macrotis lagotis (Reid) (Marsupalia: Thylacomyidae), to northern South Australia: survival, ecology and notes on reintroduction protocols. Wildl Res 30:15–27
Myers K, Parer I, Wood D, Cooke BD (1994) The rabbit in Australia. In: Thompson V, King CM (eds) The European rabbit: the history and biology of a successful colonizer, vol 5. Oxford University Press, New York, pp 108–157
Naiman RJ, Johnston CA, Kelley JC (1988) Alteration of North American streams by beaver. Bioscience 38:753–762
Newell J (2008) The role of the reintroduction of Greater Bilbies (Macrotis lagotis) and Burrowing Bettongs (Bettongia lesueur) in the ecological restoration of an arid ecosystem: foraging diggings, diet, and soil seed banks. PhD Thesis, University of Adelaide, South Australia
Noble JC, Hik DS, Sinclair ARE (1995) Landscape ecology of the burrowing bettong: fire and marsupial biocontrol of shrubs in semi-arid Australia. Rangel J 29:107–119
Noy-Meir I (1979) Structure and function of desert ecosystems. Israel J Bot 28:1–19
Olympic Dam Operations (1994) Environmental management program annual report 1994. Olympic Dam Operations, Olympic Dam
Payne RW, Harding SA, Murray DA, Soutar DM, Baird DB, Glaser AI, Channing IC, Welham SJ, Gilmour AR, Thompson R, Webster R (2008) The guide to GenStat release 11, Part 2: statistics. VSN International, Hemel Hempstead
Predavec M (2000) Food limitation in Australian desert rodents: experiments using supplementary feeding. Oikos 91:512–522
Recovery Arid (2007) Arid recovery annual report, 2006. Arid Recovery, Roxby Downs
Robley AJ, Short J, Bradley S (2001) Dietary overlap between the burrowing bettong and the European rabbit in semi–arid coastal Western Australia. Wildl Res 28:341–349
Shachak M, Brand S, Gutterman Y (1991) Porcupine disturbances and vegetation pattern along a resource gradient in a desert. Oecologia 88:141–147
Short J, Smith A (1994) Mammal decline and recovery in Australia. J Mammal 75:288–297
Slobodchikoff CF, Doyen JT (1977) Effects of Ammophila arenaria on sand dune arthropod communities. Ecology 58:1171–1175
Southgate R (1990a) Habitat and diet of the Greater Bilby Macrotis lagotis Reid (Marsupalia: Peramelidae). In: Seebeck JH, Brown PR, Wallis RI, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty, Sydney, pp 303–309
Southgate R (1990b) Distribution and abundance of the greater bilby Macrotis lagotis Reid (Marsupialia: Peramelidae). In: Seebeck JH, Brown PR, Wallis RI, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty, Sydney, pp 293–302
Strahan RS (1995) The mammals of Australia, 2nd edn. Reed New Holland, Sydney
Travers S (2008) Foraging pit soil enhances the growth and production of an annual native grass. BSc (Hons.) Thesis, University of New South Wales, Sydney
Vander Wall SB (1998) Foraging success of granivorous rodents: effects of variation in seed and soil water on olfaction. Ecology 79:233–241
Vitousek PM (1990) Biological invasions and ecosystem processes: towards an integration of population biology and ecosystem studies. Oikos 57:7–13
Whitford WG (2002) Ecology of desert systems. Elsevier, London
Wright JP, Jones CG (2004) Predicting effects of ecosystem engineers on patch-scale species richness from primary productivity. Ecology 85:2071–2081
Wright JP, Gurney WSC, Jones CG (2004) Patch dynamics in an engineered landscape. Oikos 105:336–348
Yair A, Rutin J (1981) Some aspects of the regional variation in the amount of available sediment produced by isopods and Porcupines, northern Negev, Israel. Earth Surf Process Landf 6:221–234
Zaady E, Gutterman Y, Boeken B (1997) The germination of mucilaginous seeds of Plantago coronopus, Reboudia pinnata, and Carrichtera annua on cyanobacterial soil crust from the Negev Desert. Plant Soil 190:247–252
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgments
We thank Adam Bester and staff and volunteers of Arid Recovery for support in the field, Steve Shoobert for calculations of pit turnover, and Frank Hemmings for assistance with plant identification. This study was supported by Arid Recovery, a joint conservation initiative between BHP Billiton, University of Adelaide, S.A. Department for Environment and Heritage and the Friends of Arid Recovery. The research was carried out under appropriate University of NSW ethics guidelines.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, A.I., Eldridge, D.J., Koen, T.B. et al. Can the invasive European rabbit (Oryctolagus cuniculus) assume the soil engineering role of locally-extinct natives?. Biol Invasions 13, 3027–3038 (2011). https://doi.org/10.1007/s10530-011-9987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-9987-9