Abstract
The diversity of native species is assumed to limit invasion by alien species, but there is a negative relationship between native and alien species diversity at fine spatial scales and a positive one at broad scales. This contradiction has been termed the invasion paradox, and it ensues from various processes operating at different spatial scales, such as species interactions and environmental heterogeneity. We investigated the relationship between native and alien plant species diversity components (α-, β- and γ-diversity) and their response to environmental factors at three spatial scales (1, 50 m2, 0.25 km2) in semi-natural agricultural habitats in Finland. Native and alien species diversity components were positively correlated across spatial scales, and the beta diversity contributed most to the total observed alien and native species richness (γ-diversity). The diversities of native and alien species were positively associated with productivity at the 1 m2 scale. At broader scales, alien and native species diversity responded similarly to geographical location, but differently to the productivity, disturbance and landscape diversity. Alien species diversity was positively correlated with disturbance regime, whereas native species were more strongly related to habitat type, and decreasing land-use intensity. Native and alien diversities were affected by both average and variability in local habitat conditions. Thus, both favourable conditions and heterogeneity in environmental conditions may contribute to the diversity–invasibility relationships. Disturbance regime typical of agricultural habitats may create open niches for both native and alien species, limit species competition even at fine spatial scales and lead to a positive diversity–invasibility relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the factors that determine the vulnerability of habitats and communities to plant invasions is crucial for managing biodiversity conservation. Species richness has been regarded as a potential factor controlling community invasibility. The classic theory of species invasions hypothesizes that communities with high native species diversity are less vulnerable to invasions than relatively simple communities due to competition-driven biotic resistance (Elton 1958). Later, biotic resistance was recognized to be scale-dependent: small-scale experiments frequently supported the prediction (e.g. Levine 2000; Naeem et al. 2000; Kennedy et al. 2002), whereas most of the broader scale observational studies established a contrasting pattern, indicating that native and alien species diversity was positively correlated (e.g. Stohlgren et al. 1999, 2003; Deutschewitz et al. 2003; Davies et al. 2005; Gilbert and Lechowicz 2005). This contradiction has been termed the invasion paradox (Fridley et al. 2007). Competing hypotheses have been put forward to explain the phenomena at fine and broad spatial scales and the invasion paradox has been proposed to result from different processes operating at different spatial scales, including niche exploitation, resource availability, disturbance and competitive abilities of the species (e.g. Fridley et al. 2007).
The biotic acceptance hypothesis suggests that environmental factors that promote native species diversity also promote alien species diversity, leading to a positive correlation between native and alien diversity (e.g. Stohlgren et al. 2003; Gilbert and Lechowicz 2005; Stohlgren et al. 2006). This hypothesis holds true also at the finest spatial scales, assuming the same conditions promote both native and alien species diversity, and the species diversity is not limited by competition (e.g. Gilbert and Lechowicz 2005; Stohlgren et al. 2006). The spatial heterogeneity hypothesis proposes that landscapes with greater spatial heterogeneity of environmental conditions are able to maintain more native and alien species, leading to a positive relationship between native and alien species at broader spatial scales (Davies et al. 2005). In addition, the relationship between native and alien species may be influenced by competitive or facilitative interactions among species (e.g. Elton 1958; Richardson et al. 2000), propagule pressure (e.g. Levine 2000), productivity (Davies et al. 2007) and disturbance regimes (e.g. Belote et al. 2008; Clark and Johnston 2011) at different spatial scales. For instance, a positive correlation can be expected at sites with low productivity and a negative correlation at high-productivity sites at fine spatial scales (Davies et al. 2007; Sandel and Corbin 2010), whereas disturbance can result in a positive correlation across spatial scales (Belote et al. 2008).
Shea and Chesson (2002) proposed a theoretical framework for examining correlation between native and alien species richness and for solving the invasion paradox (i.e. the shift from a fine-scale negative to a broad-scale positive native-alien relationship) by reconciling the issues of biotic resistance and niche processes, such as resource availability and spatial heterogeneity (Fridley et al. 2007). The within plot heterogeneity at fine spatial scales is expected to be low, and the effects of competition more apparent, whereas at broader spatial scales the heterogeneity in species composition and environmental conditions are responsible for the positive relationship between native and alien species (Levine 2000; Shea and Chesson 2002; Davies et al. 2005).
The test for the hypotheses regarding the invasion paradox calls for the exploration of ecological processes operating at different spatial scales. However, the research related to correlation between native and alien plant species diversity in relation to environmental conditions has mainly focused on examining either favourable conditions for both native and alien species (e.g. Stohlgren et al. 1999, 2003; Brown and Peet 2003; Gilbert and Lechowicz 2005) or spatial heterogeneity (e.g. Deutschewitz et al. 2003; Kumar et al. 2006), and only few studies have estimated the effect of these two hypotheses at multiple spatial scales (e.g. Davies et al. 2005; Stohlgren et al. 2006; Belote et al. 2008). Furthermore, the quantification of species diversity components at multiple spatial scales has been rare in previous studies of diversity–invasibility relationships (but see Davies et al. 2005; Capers et al. 2007). Generally, researchers have been concerned with cumulative diversity (gamma diversity) when studying diversity–invasibility relationships (e.g. Stohlgren et al. 1999; Deutschewitz et al. 2003; Gilbert and Lechowicz 2005; Davies et al. 2007; Belote et al. 2008; Chen et al. 2010), whereas the model of Shea and Chesson (2002) only accounted for patterns of mean diversity (alpha diversity).
When the diversity components are partitioned additively (γ = α + β) or multiplicatively (γ = αβ), the gamma diversity rises as diversity within communities (alpha diversity) or differences among communities (beta diversity) increase (e.g. Lande 1996; Capers et al. 2007). The relationship between native and alien gamma diversity depends on the relative contribution of alpha and beta diversity to gamma diversity (e.g. Davies et al. 2005). Since, beta diversity is a measure of spatial heterogeneity in species composition and species turnover (see Anderson et al. 2011), it indicates the spatial heterogeneity in environmental conditions. Beta diversity and spatial heterogeneity of the environmental conditions may be responsible for the positive relationship between native and alien diversity broader spatial scales (Davies et al. 2005). Nevertheless, beta diversity has been seldom included in studies of native-alien diversity relationships (but see Davies et al. 2005; Capers et al. 2007). In agricultural habitats, beta diversity contribute most to the gamma diversity indicating considerable differences in community composition among different spatial scales due to environmental heterogeneity (Gabriel et al. 2006).
We examined the relationship between native and alien plant species diversity components in semi-natural agricultural habitats at three spatial scales: 1, 50 m2 and 0.25 km2. We expected to detect a positive relationship between native and alien plant species at broad spatial scales (50 m2 and 0.25 km2), and tested whether it results from spatial heterogeneity of the environmental conditions or favorable conditions for both native and alien species. At the fine spatial scale (1 m2), we hypothesized that low spatial heterogeneity of the agricultural habitats and species interactions leads to a negative relationship between native and alien species (see e.g. Elton 1958; Shea and Chesson 2002; Davies et al. 2005; Gilbert and Lechowicz 2005). In order to understand the drivers of these processes we included alpha, beta and gamma diversity in the analyses. Partitioning gamma diversity into within-community (alpha diversity) and between-community diversity (beta diversity) and exploration their relationship to variables of biogeographical location, productivity, disturbance regime and landscape structure helps to understand the processes that contribute to the native-alien diversity relationships.
Materials and methods
Study area and design
In order to sample typical agricultural landscapes, a total of 52 sites (1 km2) in four geographic regions in Finland were selected using stratified random sampling in 2001 (see details of the sampling design in Kuussaari et al. 2004; Jauni and Hyvönen 2010). The study regions, which vary in species richness (e.g. Tarmi et al. 2002), were situated within the southern and central boreal vegetation zone. These included the three most important and intensively cultivated areas of Finland in the south, south-west and west, but also a less intensively cultivated area in eastern Finland.
Each 1 km2 site was divided into four 0.25 km2 squares. Among these four squares, two most divergent squares were selected in order to represent a contrast in landscape heterogeneity within the 1 km2 site. Vascular plants were recorded along up to six individual 50 m long and 1 m wide transect lines in each 0.25 km2 square (see details in Kuussaari et al. 2004; Jauni and Hyvönen 2010). All transects were situated in non-crop habitats representing five distinct habitat types: (1) field margin (margin between two agricultural fields), (2) forest margin (margin of an agricultural field adjacent to a forest), (3) road margin (including margins of an agricultural field next to a road and road verges within an agricultural landscape), (4) grassland (including patches of uncultivated meadow, abandoned fields and cultivated or natural pastures), and (5) other habitats (including margins of an agricultural field next to a waterway, cart-tracks and other habitats few in number; details Jauni and Hyvönen 2010). In addition, species were recorded from three 1 m2 quadrats along each transect. Thus, the grid comprised five spatial scales: (1) three quadrats in each transect (quadrat: 1 m2), (2) up to six transects in each square (transect: 50 m2), (3) two squares in each site (square: 0.25 km2), (4) from 8 to 16 sites in each region (site: 1 km2), and four regions within the grid. A total of four regions, 52 sites, 104 squares, 580 transects and 1,740 quadrats were included in this study (see details Jauni and Hyvönen 2010). The analyses were conducted in three of the finest scales: 1 m2 quadrats, 50 m2 transects and 0.25 km2 squares.
The plant species nomenclature follows that of Hämet-Ahti et al. (1998). The species were categorized as native or alien according to Hämet-Ahti et al. (1998) and Suominen and Hämet-Ahti (1993). Archaeophytes and neophytes were pooled as alien species due to the low number of neophytes (Jauni and Hyvönen 2010). Neophytes were introduced into Finland after the early seventeenth century (Hämet-Ahti et al. 1998), whereas in Central Europe species are classified as neophytes if introduced after the sixteenth century (e.g. Pyšek et al. 2004).
Environmental variables
We measured several variables related to geographical location, productivity, landscape structure and disturbance regime. The local habitat variables measured at the 1 m2 quadrats included (1) the total coverage of vegetation, (2) the coverage of bare ground as a measure of disturbance, and (3) rockiness. The coverage of bare ground and rocks were estimated as continuous cover percentages (0–100%). The total vegetation cover was measured as the sum of percent cover of all species in a quadrat, and it was considered as local measure of productivity (see e.g. Davies et al. 2007).
Environmental variables measured at 0.25 km2 squares, included geographical location and landscape diversity. The mid-point (longitude and latitude) of each 0.25 km2 square was used as a measure of geographical location. In Finland, climate is strongly related to geographical location, and temperature has a decreasing trend toward north (e.g. Kivinen et al. 2006). The landscape composition was analysed by calculating percentages of particular land-use types in the 0.25 km2 squares, derived from aerial photographs as described by Kivinen et al. (2006). Land-use types were classified into five groups: (1) cultivated fields (including cereal and fodder fields), (2) semi-natural agricultural habitats (including fallow fields, non-cropped, open agricultural land, mainly grazed and ungrazed semi-natural grasslands and abandoned fields and field margins, (3) forested areas, (4) water (inland watercourses and marine water) and (5) built-up areas (urban elements and other man-made structures; Kivinen et al. 2006). Landscape diversity was measured by Shannon–Wiener diversity index calculated on land use type data. The values of Shannon–Wiener diversity index were negatively correlated with the area of cultivated field (rs = −0.609, P < 0.001), whereas the areas of semi-natural agricultural habitats (rs = 0.539, P < 0.001) and forested areas (rs = 0.419, P < 0.001) were positively correlated. Landscape diversity was considered as a measure of low-intensity land-use and spatial heterogeneity at landscape level.
Diversity partitioning
At three spatial scales (1, 50 m2 and 0.25 km2), we portioned gamma diversity (i.e. the observed total species richness) into alpha and beta diversity using the additive partitioning approach γ = α + β (Allan 1975; Lande 1996; Veech et al. 2002; Gabriel et al. 2006). The additive partitioning of species diversity allows direct comparison of alpha and beta diversities and it can be used at multiple spatial scales (Veech et al. 2002). The gamma diversity was defined as the total number of species observed within each spatial scale (quadrat, transects, squares). The alpha diversity (within-unit diversity) was the mean number of species observed at the lower spatial scale. For instance, for the 0.25 km2 squares alpha diversity was defined as the mean species richness observed in 50 m2 transects. Beta diversity can be estimated by subtracting alpha diversity from gamma diversity (β = γ − α; e.g. Lande 1996; Veech et al. 2002). Mean gamma diversity at the given scale is equivalent to alpha diversity at the next larger scale (e.g. Gabriel et al. 2006). For instance, the mean of total species richness (=gamma diversity) in 50 m2 transects is equivalent to the mean alpha diversity for the 0.25 km2 squares. Thus, the total species richness at one scale results from the beta diversity and the total species richness (i.e. a sum of alpha and beta diversity) of the next lower scale. Because beta diversity requires information on the variation of the diversity at the lower scale, we could not calculate beta diversities for our lowest spatial scale, i.e. the 1 m2 quadrat.
Statistical analyses
We used generalized linear mixed models with Poisson distribution to determine whether positive or negative correlation existed between native and alien species at different spatial scales. In the models, an alien species diversity component (alpha, beta or gamma diversity) was used as the response variable and native diversity component as the explanatory variable. In the models, ID number of the upper scale was used as a random term to reduce the spatial dependence of the data points. For instance, in the models for 50 m2 transects, the ID number of 0.25 km2 squares was used as a random term. If over-dispersion was detected in the generalized linear models, it was corrected with a quasi-poisson distribution. The significance of the explanatory variable was tested with the Wald t test for generalized linear mixed models.
We analyzed how the environmental variables describing geographical location, productivity, disturbance, landscape structure affected diversity partitions of alien and native plant species at three different spatial scales (1, 50 m2, and 0.25 km2) using generalized linear mixed models with Poisson distribution. If overdispersion occurred, it was corrected with the quasi-poisson distribution. At 1 m2 quadrats, we included local habitat variables (i.e. total vegetation cover, bare ground and rockiness) in our model selection procedures. In addition, the ID numbers of 50 m2 transects were used as random terms for 1 m2 quadrats. To test whether the correlation between native and alien species diversity results from the spatial heterogeneity of the environmental conditions or favourable environmental conditions, we conducted two sets of generalized linear models at the broad spatial scales (50 m2 transects and 0.25 km2 squares). First, the mean values of the local habitat variables were used as explanatory variables in models testing the favourable-conditions hypothesis. Second, we analysed the relationship between diversity components and standard deviation (SD) of the local habitat variables to investigate the importance of variability in local habitat conditions in determining diversity–invasibility relationships. In addition, we included habitat type as a factor in the models and the ID numbers of 0.25 km2 squares was used as random term in the models for 50 m2 transects. At 0.25 km2 squares, geographical location (longitude and latitude) and the landscape diversity (Shannon–Wiener diversity index) were included as explanatory variables, as well. Since there were two 0.25 km2 squares in each 1 km2 site, the ID number of the 1 km2 site was fitted as a random term to overcome the problems of pseudoreplication at the broadest spatial scale. At the 0.25 km2 scale, one outlier was detected and it was omitted from the analysis. Prior to analyses, non-normally distributed environmental variables were log-transformed if the transformation improved normality. All linear models were performed using R 2.10.0 (R Development Core Team 2009).
Results
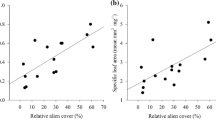
In total, 304 plant species were recorded, of which 103 (33.9%) were alien species. Most of the alien species (82.5%) were archaeophytes. The total species richness (gamma diversity) was higher for native than for alien plant species at all spatial scales (Fig. 1). At the 50 m2 transects, beta diversity contributed most to the total species richness, being 60.9% of total alien species richness and 63.7% of total native species richness. At the 0.25 km2 squares, beta diversity contributed slightly less to the total richness of alien and native species (56.2 and 60.1%, respectively) than at the 50 m2 transects. A positive relationship between native and alien diversity was detected at all spatial scales and for all diversity components (Fig. 2). The correlation between native and alien beta diversity was slightly lower than for alpha and gamma diversity.
Mean values and standard deviations for gamma (γ) diversity in 1 m2 quadrats, and for alpha (α) and beta (β) diversity in 50 m2 transects and 0.25 km2 squares. For 50 m2 transects and 0.25 km2 squares γ = α+β. The alpha (α) diversity of an upper scale equals that for gamma (γ) diversity of the next lower scale. For instance, alpha (α) diversity of 0.25 km2 squares equals that for gamma (γ) diversity of 50 m2 transects
Relationships between native and alien species diversity for gamma, alpha and beta diversity (β = γ − α), at tree spatial scales (1 m2 quadrats, 50 m2 transects and 0.25 km2 squares). Lines were drawn according to the results of generalized linear mixed models. The values of Wald t-test for generalized linear mixed models, P values are as, and Pearson’s correlation coefficients are as follows: a t = 10.70, P < 0.001, r = 0.50***, b t = 11.70, P < 0.001, r = 0.58***, c t = 10.98, P < 0.001, r = 0.54***, d t = 9.15, P < 0.001, r = 0.48***, e t = 5.10, P < 0.001, r = 0.59***, f t = 7.40, P < 0.001, r = 0.72***, g t = 4.13, P < 0.001, r = 0.48. For the correlation values *P < 0.05; **P < 0.01; ***P < 0.001
Effect of environmental variables on diversity partitions
At the 1 m2 quadrats, both native and alien species richness (gamma diversity) was positively related to total vegetation cover (P = 0.001 and P < 0.001, respectively), which indicates local productivity (Fig. 3). In addition, native species were positively associated with the rockiness of the habitat (P = 0.031; Table 1). The native species diversity was highest in 1 m2 quadrats with high vegetation cover and rockiness, whereas alien species diversity was highest in quadrats with high vegetation cover.
At the 50 m2 transects, alien beta and gamma diversity were positively correlated with mean of bare ground (P = 0.001 and P = 0.002, respectively) and all alien diversity components were positively associated with the variability in bare ground (Tables 1, 2). In addition, alien alpha diversity was positively correlated with mean vegetation cover (P = 0.023). The diversity components for native species were positively associated with the mean values of total vegetation coverage (P < 0.001), but native alpha and gamma diversity were negatively related to variability (SD) in total vegetation cover (P < 0.001 and P = 0.045, respectively). Thus, the alien diversity increased with increasing variability in the coverage of bare ground, whereas native alpha and gamma diversity increased with decreasing variability in the vegetation cover. The beta and gamma diversity of native species was positively related to the average rockiness (P = 0.033 and 0.036, respectively), whereas alien species diversities were uncorrelated with rockiness. The beta and gamma diversity of native species increased with increasing rockiness. Native species diversity was more strongly associated with habitat type than alien species diversity. The diversity of native species was significantly higher in forest margins than in field margins, whereas no difference was detected for alien species diversity. The species diversity of native and alien species was higher in road margins and grasslands than in field margins.
At the broadest scale (0.25 km2 squares), alien alpha diversity was positively associated with average vegetation cover and bare ground. Alien beta and gamma diversity were positively correlated with mean rockiness and all alien diversity components were positively associated with variability in rockiness (Tables 1, 2). Native species diversity was positively related to landscape diversity (Tables 1, 2). Native species diversity tended to increase with increasing landscape diversity, whereas alien diversity tended to even decrease with increasing landscape diversity (Fig. 4). Native alpha and gamma diversity were positively related to average vegetation cover. In addition, native diversity was positively correlated with the mean and variance of rockiness. Alien and native species diversity components were positively correlated with longitude, and they were negatively associated with latitude (Tables 1,2; Fig. 4). Alien and native species diversity decreased with increasing latitude and decreasing longitude. Both alien and native species diversity were more strongly associated with biogeographical location than with landscape diversity and local habitat variables (total vegetation cover, bare ground, and rockiness).
Relationship between landscape diversity and a native and b alien beta diversity, and relationship between latitude and c native and d alien beta diversity at 0.25 km2 scale. Slope, Wald t and P values are given in Table 1
Discussion
Contrary to our expectations, we did not observe a change from negative to positive in the alien-native relationship with increasing spatial scale (see e.g. Fridley et al. 2007). Our results showed that native and alien plant diversity had a positive relationship in semi-natural agricultural habitats regardless of the spatial scale, and that competition did not limit invasibility even at the finest spatial scale.
Our results showed that the relationship between native and alien species was positive at broad spatial scales (50 m2 and 0.25 km2) for all studied diversity components, which was consistent with our expectation and other observational studies (e.g. Stohlgren et al. 1999, 2006; Davies et al. 2005; Fridley et al. 2007). Our expectation that the relationship between native and alien species would be negative at the fine spatial scale (1 m2) was not supported. The theory of biotic resistance suggests that a negative relationship between native and alien species is expected in sites where species interactions, such as competition, limit the invasibility and extrinsic factors are relatively constant (see Elton 1958; Shea and Chesson 2002; Davies et al. 2005). However, we detected only positive relationships between alien and native species diversity. Thus, we did not find evidence that species interactions such as competition limit the diversity of alien and native species in agricultural habitats even at a fine spatial scale. In these circumstances, the positive correlation between alien and native species diversity can be explained by favorable conditions for both native and alien species (e.g. Stohlgren et al. 2006) or spatial heterogeneity in environmental conditions (e.g. Davies et al. 2005).
Environmental conditions that promote native species diversity can also favor alien species diversity, when species richness is not limited by competition (e.g. Gilbert and Lechowicz 2005; Stohlgren et al. 2006). Due to improved conditions, including increased disturbance, resource availability and propagule pressure, a positive relationship between native and alien diversity may be expected, especially in agricultural habitats even at the fine spatial scale (see e.g. Brown and Peet 2003; Fridley et al. 2007). According to our results, alien and native species diversity responded similarly to geographical location, but differently to the landscape diversity and productivity at 0.25 km2 squares. In addition, the responses of native and alien diversity components varied in terms of productivity, disturbance and habitat at 50 m2 transects, and in terms of rockiness at both 1 m2 quadrats and 50 m2 transects. Thus, our results suggest that alien and native species are not favored by similar environmental conditions, but it is more likely that the spatial heterogeneity in resource availability, productivity, and disturbance regime and propagule pressure create suitable niches for both alien and native species, when competitive interactions do not limit the diversity of native and alien species. This will lead to coexistence and a positive relationship between native and alien species even at a fine spatial scale (see Davies et al. 2005, 2011; Fridley et al. 2007; Sandel and Corbin 2010).
The spatial heterogeneity hypothesis has been usually applied to broad spatial scales because the environmental and disturbance-based drivers of community composition are expected to be relatively homogeneous at the finer spatial scales (e.g. Davies et al. 2005; Fridley et al. 2007). However, highly disturbed habitats, such as agricultural habitats, have high spatial heterogeneity (e.g. Simonova and Lososova 2008), which could be perceived as differences in habitat structure, productivity and disturbance even at the finer spatial scales. Unfortunately, we could not measure variability in local habitat conditions at the finest spatial scale, but our results from the broad spatial scales showed that alien and native diversity were affected by both average and variability in local habitat conditions. For instance at 0.25 km2 squares, greater variability in rockiness supported more native and alien species, but higher average rockiness also resulted in more native and alien species. Thus, the favourable conditions hypothesis and spatial heterogeneity hypothesis may not be mutually exclusive (e.g. Belote et al. 2008).
We found that at fine spatial scale (1 m2) both native and alien gamma diversity were positively related to productivity, whereas at broader spatial scale (50 m2) native species diversity components were more often related to both average and variability of productivity and alien species diversity components to both average and variability of disturbance. At fine spatial scales, where competitive interactions are more apparent, and may vary with productivity, Davies et al. (2007) found positive relationship between native and alien diversity at low-productivity sites, and negative at high-productivity sites. We studied agricultural habitats, which generally have high productivity, resources availability and disturbance, and which are often invaded by alien plant species (e.g. Chytrý et al. 2005; Simonova and Lososova 2008; Pyšek et al. 2010). Our previous results from the same data revealed that among semi-natural agricultural habitats, alien species favour frequently disturbed and intensively managed habitats, whereas native species thrive in more natural habitats in the vicinity of forested areas (Jauni and Hyvönen 2010). Belote et al. (2008) found a positive correlation between native and alien species following disturbance across spatial scales (from 1 m2 scale to 2 ha), thus more alien species may invade species-rich communities compared to species-poor communities after disturbance. Different intensity and frequency of disturbance can increase resource availability and/or propagule pressure, limit species competition and maintain an open vegetation canopy (e.g. Celesti-Grapow et al. 2006; Belote et al. 2008; Simonova and Lososova 2008; Clark and Johnston 2011), hence creating suitable niches for both native and alien species in disturbed agricultural habitats. Thus, disturbance may create more spatial heterogeneity and reduce competitive interactions even in productive agricultural habitats resulting in positive relationship between native and alien species.
At the broadest spatial scale (0.25 km2 squares), native alpha, beta and gamma diversity were negatively related to land-use intensity and positively to the landscape diversity and forested areas. Previous studies have indicated that both native and alien diversity is positively associated with spatial heterogeneity at the landscape level (Deutschewitz et al. 2003; Pino et al. 2005; Poggio et al. 2010), and that alien plant species favour intensively managed, human-dominated landscapes (e.g. Chytrý et al. 2005; Lososová and Cimalová 2009; Polce et al. 2011; Vilà and Ibáñez 2011). However, we found that alien species diversity components were insignificantly associated with landscape diversity, which describes the spatial heterogeneity at the landscape level. There may be several possible explanations for this phenomenon. First, the alien species richness in Finnish agricultural habitats is rather low compared to native species richness (e.g. Jauni and Hyvönen 2010), and therefore there are more native species available to occupy niches in specific habitat types than alien species. For instance, native plant species are more strongly related to the vicinity of forest than alien plant species (Jauni and Hyvönen 2010). This may cause more variability in species richness between different landscapes. Generally, boreal agricultural landscape is a mosaic of arable land and forest (e.g. Luoto 2000), and complex farmland mosaics can contribute to maintain plant diversity even at intensively managed agroecosystems (e.g. Poggio et al. 2010). Second, farming practices in boreal regions are usually not as intensive as in temperate regions in Europe (Ekroos et al. 2010), where positive effects of spatial heterogeneity at landscape level have been found among alien plant species (e.g. Deutschewitz et al. 2003; Pino et al. 2005). Third, arable landscapes are dominated by spring cereal fields in Finland and they are not considered as benign habitat for alien plant species, especially for neophytes (e.g. Hyvönen and Jalli 2011; Lososová and Cimalová 2009). Thus, in intensively managed agricultural landscapes the share of benign habitat for alien species (e.g. Jauni and Hyvönen 2010) may actually decline.
In addition, factors, which we did not consider in our study, may have contributed to the positive relationship between alien and native species. These factors include for instance propagule pressure and the facilitative interactions between native and alien species. In agricultural habitats, the propagule pressure is high (e.g. Chytrý et al. 2008), and disturbance may create openings that allow more alien and native propagules to arrive and establish at a new site (Hobbs and Huenneke 1992; Belote et al. 2008). Thus, increased propagule pressure and dispersal-driven communities can generate a positive relationship between native and alien species even at fine spatial scales (Levine 2000; Brown and Peet 2003; Fridley et al. 2007). In addition, the low number of invasive alien species and high proportion of archaeophytes in our data (see Jauni and Hyvönen 2010) may explain why only a positive relationship between native and alien species was found. Ortega and Pearson (2005) suggested that the negative correlation between native and alien species is not driven by biotic resistance, but by strong invaders that out-compete the native species. In our data, most of the alien species were archaeophytes, i.e. they had established their populations a long time ago (Jauni and Hyvönen 2010), which responded like native species to increasing agricultural intensification and propagule pressure (Pyšek et al. 2005). This could also lead to a positive relationship between alien and native species.
In accordance with previous studies, our study suggests that additive diversity partitioning contributes to the understanding of spatial patterns of alien and alien species diversity, and spatial scale dependence of diversity components (e.g. Veech et al. 2002; Gabriel et al. 2006; Klimek et al. 2008). At broad spatial scales, beta diversity contributed most to the total observed species richness (gamma diversity) indicating considerable differences in species composition among 50 m2 transects and 0.25 km2 squares. This is consistent with previous studies from agricultural habitats, showing that overall diversity of arable weeds is strongly determined by beta diversity (Gabriel et al. 2006; Klimek et al. 2008; Poggio et al. 2010). The relative contribution of beta diversity was slightly lower for total alien species richness than for native species richness, which may be explained by higher dispersal abilities of the alien plant species. Dispersal between spatial units acts as a homogenizing force reducing the beta diversity and increasing alpha diversity since it contributes to maintenance of local coexistence of the species (e.g. Loreau 2000; Gabriel et al. 2006). This may lead to a stronger diversity–invasibility relationship in alpha and gamma diversity than beta diversity. Beta diversity of native and alien plant species results from environmental heterogeneity in space, time, resources, disturbance regime and niche differences among species (Loreau 2000; Gabriel et al. 2006). In addition, higher beta diversity of both native and alien species can ensue from greater spatial heterogeneity in resource availability and disturbance regime (Davies et al. 2005; Belote et al. 2008; Chen et al. 2010).
To conclude, we detected no evidence of the biotic resistance in agricultural habitats. The positive relationship across spatial scales tended to result from both spatial heterogeneity and favourable conditions for alien and native diversity. Disturbance regime, typical to agricultural habitats, can reduce species competition, create suitable niches and increase resources availability and propagule pressure even at fine spatial scales.
References
Allan JD (1975) Components of diversity. Oecologia 18:359–367
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegan JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the predicting ecologist. Ecol Lett 14:19–28
Belote RT, Jones RH, Hood SM, Wender BW (2008) Diversity-invasibility across an experimental disturbance gradient in Appalachian forests. Ecology 89:183–192
Brown RL, Peet RK (2003) Diversity and invasibility of southern Appalachian plant communities. Ecology 84:32–39
Capers RS, Selsky R, Bugbee GJ, White JC (2007) Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 88:3135–3143
Celesti-Grapow L, Pyšek P, Jarošík V, Blasi C (2006) Determinants of native and alien species richness in the urban flora of Rome. Divers Distrib 12:490–501
Chen H, Qian H, Spyreas G, Crossland M (2010) Native-exotic species richness relationships across spatial scales and biotic homogenization in wetland plants communities of Illinois, USA. Divers Distrib 16:737–743
Chytrý M, Pyšek P, Tichý L, Knollova I, Danihelka J (2005) Invasions by alien plants in the Czech Republic: a quantitative assessment across habitats. Preslia 77:339–354
Chytrý M, Jarošík V, Pyšek P, Hájek O, Knollová I, Tichý L, Danihelka J (2008) Separating habitat invisibility by alien plants from the actual level of invasion. Ecology 89:1541–1553
Clark GF, Johnston EL (2011) Temporal change in the diversity-invasibility relationships in the presence of disturbance regime. Ecol Lett 14:52–57
Davies KF, Chesson P, Harrison S, Inouye BD, Melbourne BA, Rice KJ (2005) Spatial heterogeneity explains the scale dependence of the native-exotic diversity relationship. Ecology 86:1602–1610
Davies K, Harrison S, Safford HD, Viers JH (2007) Productivity alters the scale dependence of the diversity-invasibility relationship. Ecology 88:1940–1947
Davies KF, Cavender-Bares J, Deacon N (2011) Native communities determine the identity of exotic invaders even at scales at which communities are unsaturated. Divers Distrib 17:35–42
Deutschewitz K, Lausch A, Kühn I, Klotz S (2003) Native and alien plant species richness in relation to spatial heterogeneity on a regional scale in Germany. Global Ecol Biogeogr 12:299–311
Ekroos J, Hyvönen T, Tiainen J, Tiira M (2010) Responses in plant and carabid communities to farming practices in boreal landscapes. Agric Ecosyst Environ 135:288–293
Elton CS (1958) The ecology of invasions by animals and plants. The University of Chicago Press, Chicago
Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, Von Holle B (2007) The invasion paradox: reconciling pattern and process on species invasions. Ecology 88:3–17
Gabriel D, Roschewitz I, Tscharntke T, Thies C (2006) Beta diversity at different spatial scales: plant communities in organic and conventional agriculture. Ecol Appl 16:2011–2021
Gilbert B, Lechowicz MJ (2005) Invasibility and abiotic gradients: the positive correlation between native and exotic plant diversity. Ecology 86:1848–1855
Hämet-Ahti L, Suominen J, Ulvinen T, Uotila P (eds) (1998) Retkeilykasvio (field flora of Finland), 4th edn. Finnish Museum of Natural History, Botanical Museum, Helsinki
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity and invasion. Implications for conservation. Conserv Biol 6:324–337
Hyvönen T, Jalli H (2011) Alien species in the Finnish weed flora. Agric Food Sci 20:86–95
Jauni M, Hyvönen T (2010) Invasion level of alien plants in semi-natural agricultural habitats in boreal region. Agric Ecosyst Environ 138:109–115
Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P (2002) Biodiversity as barrier to ecological invasion. Nature 417:636–638
Kivinen S, Luoto M, Kuussaari M, Helenius J (2006) Multi-species richness of boreal agricultural landscapes: effect of climate, biotope, soil and geographical location. J Biogeogr 33:862–875
Klimek S, Marini L, Hofmann M, Isselstein J (2008) Additive partitioning of plant diversity with respect to grassland management regime, fertilization and abiotic factors. Basic Appl Ecol 9:626–634
Kumar S, Stohlgren TJ, Chong GW (2006) Spatial heterogeneity influences native and non-native plant species richness. Ecology 87:3186–3199
Kuussaari M, Heliölä J, Luoto M (2004) Farmland biodiversity indicators and monitoring in Finland. In: Groom G (ed) Developments in strategic landscape monitoring for the Nordic countries. Nordic Council of Ministers, Copenhagen, pp 29–40
Lande R (1996) Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76:5–13
Levine JM (2000) Species diversity and biological invasions: relating local process to community pattern. Science 288:852–854
Loreau M (2000) Are communities saturated? On the relationship between α, β and γ diversity. Ecol Lett 3:73–76
Lososová Z, Cimalová Š (2009) Effects of different cultivation types on native and alien weed species richness and diversity in Moravia (Czech Republic). Basic Appl Ecol 10:456–465
Luoto M (2000) Spatial analysis of landscape ecological characteristics of five agricultural areas in Finland by GIS. Fennia 178:15–54
Naeem S, Knops JMH, Tilman D, Howe KM, Kennedy T, Gale S (2000) Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91:97–108
Ortega Y, Pearson DE (2005) Weak vs. strong invaders of natural plant communities: assessing invasibility and impact. Ecol Appl 15:651–661
Pino J, Font X, Carbó J, Jové L, Pallarès L (2005) Large-scale correlates of alien plant invasion in Catalonia. Biol Conserv 122:339–350
Poggio SL, Chaneton EJ, Ghersa CM (2010) Landscape complexity differentially affects alpha, beta, and gamma diversities of plants in fencerows and crop fields. Biol Conserv 143:2477–2486
Polce C, Kunin WE, Biesmeijer JC, Dauber J, Phillips OL, the ALARM Field Site Network (2011) Alien and native plants show contrasting responses to climate and land use in Europe. Global Ecol Biogeogr 20:367–379
Pyšek P, Richardson DM, Rejmánek M, Grady LW, Williamson M, Kirscher J (2004) Alien plant in checklists and floras: towards better communication between taxonomists and ecologists. Taxon 53:131–143
Pyšek P, Jarošík V, Chytrý M, Kropač Z, Tichý L, Wild J (2005) Alien plants in temperate weed communities: prehistoric and recent occupy different habitats. Ecology 86:772–785
Pyšek P, Chytrý M, Jarošík V (2010) Habitats and land-use as determinants of plant invasions in the temperate zone of Europe. In: Perrings C, Mooney HA, Williamson M (eds) Bioinvasions and globalization: ecology, economics, management and policy. Oxford University Press, Oxford, pp 66–79
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M (2000) Plant invasions, the role of mutualisms. Biol Rev 75:65–93
Sandel B, Corbin JD (2010) Scale, disturbance and productivity control the native-exotic richness relationship. Oikos 119:1281–1290
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Simonova D, Lososova Z (2008) Which factors determine plant invasions in man-made habitats in the Czech Republic? Perspect. Plant Ecol Evol Syst 10:89–100
Stohlgren TJ, Binkley D, Chong GW, Kalkhan MA, Schell LD, Bull KA, Otsuki Y, Newman G, Bashkin M, Son Y (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69:25–46
Stohlgren TJ, Barnett DT, Kartesz JT (2003) The rich get richer: patterns of plant invasions in the United States. Front Ecol Environ 1:11–14
Stohlgren TJ, Jarnevich C, Chong GW, Evangelista PH (2006) Scale and plant invasions: a theory of biotic acceptance. Preslia 78:405–426
Suominen J, Hämet-Ahti L (1993) Archaeophytes in the flora of Finland. Norrlinia 4:1–90
Tarmi S, Tuuri H, Helenius J (2002) Plant communities of field boundaries in Finnish farmland. Agric Food Sci Finland 11:121–135
Veech JA, Summerville KS, Crist TO, Gering JC (2002) The additive partitioning of species diversity: recent revival of an old idea. Oikos 99:3–9
Vilà M, Ibáñez I (2011) Plant invasions in the landscape. Landscape Ecol 26:461–472
Acknowledgments
We are grateful to Professor Juha Helenius and Mikko Kuussaari for providing the data, anonymous reviewers for their comments and Jonathan Robinson for the language revision. We acknowledge funding from the Jenny and Antti Wihuri Foundation (M.J.), the Academy of Finland (T.H.; the project number 122477), and MTT Agrifood Research Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jauni, M., Hyvönen, T. Positive diversity–invasibility relationships across multiple scales in Finnish agricultural habitats. Biol Invasions 14, 1379–1391 (2012). https://doi.org/10.1007/s10530-011-0163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0163-z